Recent from talks
Contribute something
Nothing was collected or created yet.
Quinagolide
View on Wikipedia
 Structure without stereochemistry | |
| Clinical data | |
|---|---|
| Trade names | Norprolac |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| Drug class | Dopamine receptor agonist |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H33N3O3S |
| Molar mass | 395.56 g·mol−1 |
| |
Quinagolide (INN, BAN), sold under the brand name Norprolac, is a selective dopamine D2 receptor agonist which is used to manage hyperprolactinemia.[1] It has also been found to be effective in the treatment of breast pain.[2] It is used in the UK, but it is not available in US.
Chemistry
[edit]Quinagolide is a racemate composed of the following two enantiomers:[3]
| Enantiomeres of Quinagolide | |
|---|---|
 (+)-Quinagolid CAS number: 140630-79-1 |
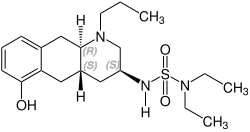 (-)-Quinagolid CAS number: 140630-80-4 |
Synthesis
[edit]Laboratory synthesis
[edit]The first synthesis of quinagolide was disclosed in patents filed by Sandoz.[4]
A sequence of nine steps is required to transform the starting material 5-methoxy-2-tetralone (1) into the octahydrobenzo[g]quinoline ring system with the correct stereochemistry required. This intermediate (11) is then converted in another five steps to the drug. Transformation of the ester (13) into the amine (15) is accomplished by a Curtius rearrangement in which an acyl hydrazide is treated with nitrosyl chloride.[4][5][6]
Manufacture
[edit]The laboratory route was not practical for the synthesis of quinagoline on a large scale. Therefore scientists at Novartis developed an improved process.[7]
The starting material is 1,6-dimethoxynaphthalene (1). This is selectively lithiated at the C-7 position and reacts with (2Z)-ethyl 2-cyano-3-ethoxyacrylate (2), to give the cyanoacrylate (3). Catalytic hydrogenation and hydrolysis produces (5). Birch reduction of (5) leads first to (6) which on acid work-up gives the imine (7), which is reduced with sodium borohydride to yield (8). Fischer esterification with methanol gives an ester that is next alkylated with 1-iodopropane to give (11). The required stereochemistry for quinagoline is set in the final steps.[7][8][9]
See also
[edit]References
[edit]- ^ Di Sarno A, Landi ML, Marzullo P, Di Somma C, Pivonello R, Cerbone G, et al. (July 2000). "The effect of quinagolide and cabergoline, two selective dopamine receptor type 2 agonists, in the treatment of prolactinomas". Clinical Endocrinology. 53 (1): 53–60. doi:10.1046/j.1365-2265.2000.01016.x. PMID 10931080. S2CID 31677949.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Pluchinotta AM (20 April 2015). The Outpatient Breast Clinic: Aiming at Best Practice. Springer. pp. 167–. ISBN 978-3-319-15907-2.
- ^ Rote Liste Service GmbH (Hrsg.) (2017). Rote Liste 2017 - Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte) [Red List 2017 - List of medicinal products for Germany (including EU approvals and certain medical devices)] (in German) (57th ed.). Frankfurt/Main: Rote Liste Service GmbH. p. 214. ISBN 978-3-946057-10-9.
- ^ a b EP patent 0077754, Nordmann R, Petcher TJ, "Novel pharmaceutically active 1,2,3,4,4a,5,10,10a-octahydrobenzo(g)quinoline derivatives", issued 1983-04-27, assigned to Sandoz
- ^ Nordmann R, Petcher TJ (March 1985). "Octahydrobenzo[g]quinolines: potent dopamine agonists which show the relationship between ergolines and apomorphine". Journal of Medicinal Chemistry. 28 (3): 367–375. doi:10.1021/jm00381a017. PMID 3973904.
- ^ "Quinagolide". Thieme. Retrieved 2024-07-07.[permanent dead link]
- ^ a b Bänziger M, Cercus J, Stampfer W, Sunay U (2000). "Practical and Large-Scale Synthesis of rac-(3 S,4a R,10a R)- 6-Methoxy-1-propyl-1,2,3,4,4a,5,10,10a-octahydrobenzo[g]quinoline-3-carboxylic Acid Methyl Ester". Organic Process Research & Development. 4 (6): 460–466. doi:10.1021/op0000531.
- ^ Comparini LM, Menichetti A, Favero L, Di Pietro S, Badalassi F, Ryberg P, et al. (August 2023). "Development of an asymmetric formal synthesis of (-)-quinagolide via enzymatic resolution and stereoselective iminium ion reduction". Organic & Biomolecular Chemistry. 21 (31): 6389–6396. doi:10.1039/D3OB00946G. PMID 37492953.
- ^ Chavan SP, Kadam AL, Kawale SA (May 2019). "Total Synthesis of (±)-Quinagolide: A Potent D2 Receptor Agonist for the Treatment of Hyperprolactinemia". ACS Omega. 4 (5): 8231–8238. doi:10.1021/acsomega.9b00903. PMC 6648496. PMID 31459911.
-Quinagolid_Structural_Formula_V1.svg/250px-(+-)-Quinagolid_Structural_Formula_V1.svg.png)
-Quinagolid_Structural_Formula_V1.svg/2000px-(+-)-Quinagolid_Structural_Formula_V1.svg.png)


