Recent from talks
Nothing was collected or created yet.
Thioflavin
View on Wikipedia | |
 | |
| Names | |
|---|---|
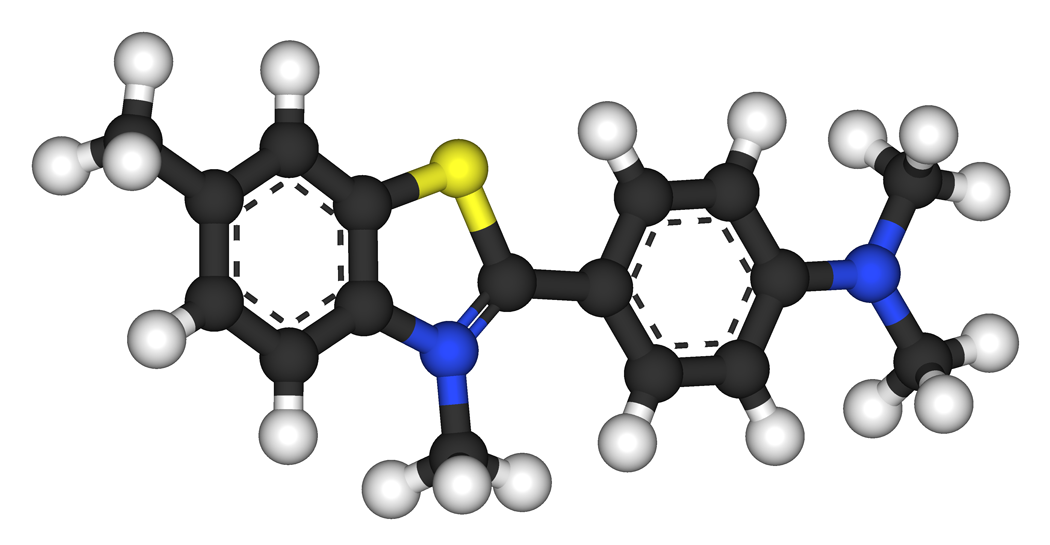
| Preferred IUPAC name
2-[4-(Dimethylamino)phenyl]-3,6-dimethyl-1,3-benzothiazol-3-ium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.482 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H19ClN2S | |
| Molar mass | 318.86 g/mol |
| Density | 1.301 g/cm3 |
| Melting point | 137.9 °C (decomp.) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thioflavins are fluorescent dyes that are available as at least two compounds, namely Thioflavin T and Thioflavin S. Both are used for histology staining and biophysical studies of protein aggregation.[1] In particular, these dyes have been used since 1959 to investigate amyloid formation.[2][3] They are also used in biophysical studies of the electrophysiology of bacteria.[4] Thioflavins are corrosive, irritant, and acutely toxic, causing serious eye damage.[5] Thioflavin T has been used in research into Alzheimer's disease and other neurodegenerative diseases.
Thioflavin T
[edit]Thioflavin T (Basic Yellow 1, Methylene yellow, CI 49005, or ThT) is a benzothiazole salt obtained by the methylation of dehydrothiotoluidine with methanol in the presence of hydrochloric acid. The dye is widely used to visualize and quantify the presence of misfolded protein aggregates called amyloid, both in vitro and in vivo (e.g., plaques composed of amyloid beta found in the brains of Alzheimer's disease patients).[1]
When it binds to beta sheet-rich structures, such as those in amyloid aggregates, the dye displays enhanced fluorescence and a characteristic red shift of its emission spectrum.[6][7] Additional studies also consider fluorescence changes as result of the interaction with double stranded DNA.[8] This change in fluorescent behavior can be caused by many factors that affect the excited state charge distribution of thioflavin T, including binding to a rigid, highly-ordered nanopocket, and specific chemical interactions between thioflavin T and the nanopocket.[9][10]
Prior to binding to an amyloid fibril, thioflavin T emits weakly around 427 nm. Quenching effects of the nearby excitation peak at 450 nm is suspected to play a role in minimizing emissions.
When excited at 450 nm, thioflavin T produces a strong fluorescence signal at approximately 482 nm upon binding to amyloids. Thioflavin T molecule consists of a phenylamine and a benzothiazole ring connected through a carbon-carbon bond. These two rings can rotate freely when the molecule is in solution. The free rotation of these rings results in quenching of any excited state generated by photon excitation. However, when thioflavin T binds to amyloid fibrils, the two rotational planes of the two rings become immobilized and therefore, this molecule can maintain its excited state.[1]
Thioflavin T fluorescence is often used as a diagnostic of amyloid structure, but it is not perfectly specific for amyloid. Depending on the particular protein and experimental conditions, thioflavin T may[9] or may not[11] undergo a spectroscopic change upon binding to precursor monomers, small oligomers, unaggregated material with a high beta sheet content, or even alpha helix-rich proteins. Conversely, some amyloid fibers do not affect thioflavin T fluorescence,[12] raising the prospect of false negative results.
In adult C. elegans, exposure to thioflavin T results "in a profoundly extended lifespan and slowed aging" at some levels, but decreased lifespan at higher levels.[13]
Thioflavin S
[edit]Thioflavin S is a homogenous mixture of compounds that results from the methylation of dehydrothiotoluidine with sulfonic acid. It is also used to stain amyloid plaques. Like thioflavin T it binds to amyloid fibrils but not monomers and gives a distinct increase in fluorescence emission. However unlike thioflavin T, it does not produce a characteristic shift in the excitation or emission spectra.[6] This latter characteristic of thioflavin S results in high background fluorescence, making it unable to be used in quantitative measurements of fibril solutions.[6] Another dye that is used to identify amyloid structure is Congo red.
See also
[edit]References
[edit]- ^ a b c Biancalana M, Koide S (July 2010). "Molecular mechanism of Thioflavin-T binding to amyloid fibrils". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1804 (7): 1405–12. doi:10.1016/j.bbapap.2010.04.001. PMC 2880406. PMID 20399286.
- ^ Vassar, P.S.; Culling, C.F. (1959). "Fluorescent stains, with special reference to amyloid and con- nective tissues". Arch. Pathol. 68: 487–498. PMID 13841452. Retrieved 2025-07-31.
- ^ Gade Malmos, Kirsten; Blancas-Mejia, Luis M.; Weber, Benedikt; Buchner, Johannes; Ramirez-Alvarado, Marina; Naiki, Hironobu; Otzen, Daniel (2017). "THT 101: A primer on the use of thioflavin T to investigate amyloid formation". Amyloid. 24 (1): 1–16. doi:10.1080/13506129.2017.1304905. PMID 28393556.
- ^ Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Süel GM (November 2015). "Ion channels enable electrical communication in bacterial communities". Nature. 527 (7576): 59–63. Bibcode:2015Natur.527...59P. doi:10.1038/nature15709. PMC 4890463. PMID 26503040.
- ^ "Thioflavin T". National Center for Biotechnology Information. PubChem.
- ^ a b c H. LeVine III, Methods in Enzymology. 309, 274 (1999)
- ^ Groenning M (March 2010). "Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status". Journal of Chemical Biology. 3 (1): 1–18. doi:10.1007/s12154-009-0027-5. PMC 2816742. PMID 19693614.
- ^ Ilanchelian M, Ramaraj R (2004). "Emission of thioflavin T and its control in the presence of DNA". Journal of Photochemistry and Photobiology A: Chemistry. 162 (1): 129–137. Bibcode:2004JPPA..162..129I. doi:10.1016/s1010-6030(03)00320-4.
- ^ a b c Wolfe LS, Calabrese MF, Nath A, Blaho DV, Miranker AD, Xiong Y (September 2010). "Protein-induced photophysical changes to the amyloid indicator dye thioflavin T". Proceedings of the National Academy of Sciences of the United States of America. 107 (39): 16863–8. Bibcode:2010PNAS..10716863W. doi:10.1073/pnas.1002867107. PMC 2947910. PMID 20826442.
- ^ Biancardi A, Biver T, Mennucci B (2017). "Fluorescent dyes in the context of DNA-binding: The case of Thioflavin T". Int. J. Quantum Chem. 117 (8) e25349. doi:10.1002/qua.25349.
- ^ LeVine H (March 1993). "Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution". Protein Science. 2 (3): 404–10. doi:10.1002/pro.5560020312. PMC 2142377. PMID 8453378.
- ^ Cloe AL, Orgel JP, Sachleben JR, Tycko R, Meredith SC (March 2011). "The Japanese mutant Aβ (ΔE22-Aβ(1-39)) forms fibrils instantaneously, with low-thioflavin T fluorescence: seeding of wild-type Aβ(1-40) into atypical fibrils by ΔE22-Aβ(1-39)". Biochemistry. 50 (12): 2026–39. doi:10.1021/bi1016217. PMC 3631511. PMID 21291268.
- ^ Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ (April 2011). "Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan". Nature. 472 (7342): 226–9. Bibcode:2011Natur.472..226A. doi:10.1038/nature09873. PMC 3610427. PMID 21451522.