Recent from talks
Nothing was collected or created yet.
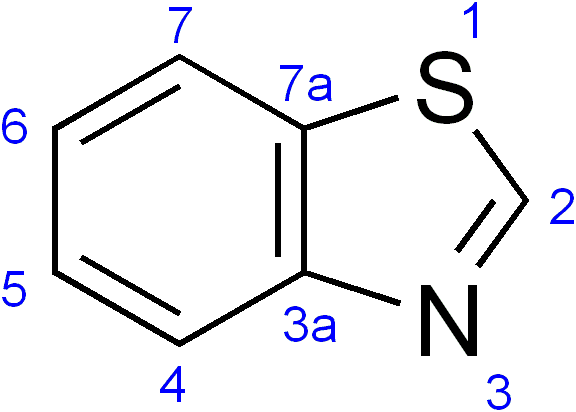
Benzothiazole
View on Wikipedia | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Benzothiazole | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.179 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H5NS | |||
| Molar mass | 135.1863 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 1.238 g/mL | ||
| Melting point | 2 °C (36 °F; 275 K) | ||
| Boiling point | 227 to 228 °C (441 to 442 °F; 500 to 501 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.[1] It has a sulfurous odor and meaty flavor.[2]
The three structural isomers of benzothizaole are 1,3-benzothiazole, 1,2-benzothiazole and 2,1-benzothiazole.
Structure and reactivity
[edit]Benzothiazoles consist of a 5-membered 1,3-thiazole ring fused to a benzene ring. The nine atoms of the bicycle and the attached substituents are coplanar. The heterocyclic core of the molecule is readily substituted at the methyne (CH) centre in the thiazole ring. Thiazole is electron-withdrawing.
Synthesis and biosynthesis
[edit]Benzothiazoles are typically prepared by treatment of 2-mercaptoaniline.[3] For example, acid chlorides are effective:[4]
- C6H4(NH2)SH + RC(O)Cl → C6H4(N)SCR + HCl + H2O
Many other precursors have been used, commonly aldehydes in the presence of oxidants. In some cases, benzothiazoles are prepared directly from anilines, a process that entails ortho functionalization.[5]
Naturally occurring benzothiazoles are proposed to arise by condensation of cysteine with quinones.[1]
Uses
[edit]Dyes
[edit]The dye thioflavin is a benzothiazole derivative.[6]
Food additives
[edit]Benzothiazole occurs naturally in some foods but is also used as a food additive.[1] It has a sulfurous odor and meaty flavor.[7] The European Food Safety Authority assessment had "no safety concern at estimated levels of intake as a flavouring substance".[8]
Rubber additive
[edit]Accelerators for the sulfur vulcanization of rubber are based on 2-mercaptobenzothiazoles.[9]
Pharmacology
[edit]
Benzothiazoles have been widely investigated for their bioactivity.[10][11] The benzothiazole moiety is, for example, seen in certain dopamine-acting drugs, e.g. riluzole and pramipexole. Moreover, benzothiazole derivatives act as monoamine oxidase inhibitors or dopamine antagonists:
- 6-oxoethers and derivatives of thiadiazole, thiazolyhydrazine that act selectively on either MAO-A or MAO-B depending on the O-sidechain[12][13][14]
- 1-alkylpiperidines that act on dopamine D4 receptor[15][16]
See also
[edit]- thiazoles, which lack the fused benzene ring.
- Benzoxazoles, which substitute an oxygen for the sulfur atom.
- 2-Aminobenzothiazoles, well-studied derivatives of benzothiazole
Safety and environmental considerations
[edit]Benzothiazoles are widely used in the vulcanization of rubber, so their possible role in the environment has attracted attention. Evidence suggests that they biodegrade readily.[17]
References
[edit]- ^ a b c Le Bozec, Lucille; Moody, Christopher J. (2009). "Naturally Occurring Nitrogen–Sulfur Compounds. The Benzothiazole Alkaloids". Australian Journal of Chemistry. 62 (7): 639. doi:10.1071/CH09126.
- ^ "Benzothiazole". The Good Scents Company. Retrieved 2020-10-06.
- ^ Gill, Rupinder K.; Rawal, Ravindra K.; Bariwal, Jitender (2015). "Recent Advances in the Chemistry and Biology of Benzothiazoles". Archiv der Pharmazie. 348 (3): 155–178. doi:10.1002/ardp.201400340. PMID 25682746.
- ^ T. E. Gilchrist "Heterocyclic Chemistry" 3rd Edition, Longman, 1992.
- ^ Würfel, Hendryk; Jakobi, Dörthe (2018). "Syntheses of Substituted 2-Cyano-benzothiazoles". Organic Syntheses. 95: 177–191. doi:10.15227/orgsyn.095.0177.
- ^ Gill, Rupinder K.; Rawal, Ravindra K.; Bariwal, Jitender (2015). "Recent Advances in the Chemistry and Biology of Benzothiazoles". Archiv der Pharmazie. 348 (3): 155–178. doi:10.1002/ardp.201400340. PMID 25682746. S2CID 10421792.
- ^ "Benzothiazole". The Good Scents Company. Retrieved 2020-10-06.
- ^ "Flavouring Group Evaluation 76, (FGE.76) - Consideration of sulphur-containing heterocyclic compounds evaluated by JECFA (59th meeting) structurally related to thiazoles, thiophene, thiazoline and thienyl derivatives from chemical group 29, miscellaneous". EFSA Journal. 6 (11): 875. 2008. doi:10.2903/j.efsa.2008.875.
- ^ Engels, Hans-Wilhelm; Weidenhaupt, Herrmann-Josef; Pieroth, Manfred; Hofmann, Werner; Menting, Karl-Hans; Mergenhagen, Thomas; Schmoll, Ralf; Uhrlandt, Stefan (2004), "Rubber, 4. Chemicals and Additives", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a23_365.pub2, ISBN 3-527-30673-0
- ^ Rouf, Abdul; Tanyeli, Cihangir (2015). "Bioactive thiazole and benzothiazole derivatives". European Journal of Medicinal Chemistry. 97: 911–927. doi:10.1016/j.ejmech.2014.10.058. hdl:11511/43872. PMID 25455640.
- ^ Keri, Rangappa S.; Patil, Mahadeo R.; Patil, Siddappa A.; Budagumpi, Srinivasa (2015). "A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry". European Journal of Medicinal Chemistry. 89: 207–251. doi:10.1016/j.ejmech.2014.10.059. PMID 25462241.
- ^ le Roux, Anandie; Petzer, Anél; Cloete, Stephanus J.; Petzer, Jacobus P. (2025-03-01). "An investigation of the monoamine oxidase inhibition properties of benzothiazole derivatives". Results in Chemistry. 14 102142. doi:10.1016/j.rechem.2025.102142. ISSN 2211-7156.
- ^ Acar Çevik, Ulviye; Osmaniye, Derya; Sağlik, Begüm N.; Levent, Serkan; K. Çavuşoğlu, Betül; Karaduman, Abdullah B.; D. Özkay, Ümide; Özkay, Yusuf; Kaplancikli, Zafer A.; Turan, Gülhan (2020). "Synthesis of new benzothiazole derivatives bearing thiadiazole as monoamine oxidase inhibitors". Journal of Heterocyclic Chemistry. 57 (5): 2225–2233. doi:10.1002/jhet.3942. ISSN 1943-5193.
- ^ Turan, Gülhan; Osmaniye, Derya; Sağlik, Begüm Nurpelin; Çevik, Ulviye Acar; Levent, Serkan; Çavuşoğlu, Betül Kaya; Özkay, Ümide Demir; Özkay, Yusuf; Kaplancikli, Zafer Asım (2020-06-02). "Synthesis and monoamine oxidase A/B inhibitory evaluation of new benzothiazole-thiazolylhydrazine derivatives". Phosphorus, Sulfur, and Silicon and the Related Elements. 195 (6): 491–497. doi:10.1080/10426507.2020.1722667. ISSN 1042-6507.
- ^ Boateng, Comfort A.; Nilson, Ashley N.; Placide, Rebekah; Pham, Mimi L.; Jakobs, Franziska M.; Boldizsar, Noelia; McIntosh, Scot; Stallings, Leia S.; Korankyi, Ivana V.; Kelshikar, Shreya; Shah, Nisha; Panasis, Diandra; Muccilli, Abigail; Ladik, Maria; Maslonka, Brianna (2023-09-14). "Pharmacology and Therapeutic Potential of Benzothiazole Analogues for Cocaine Use Disorder". Journal of Medicinal Chemistry. 66 (17): 12141–12162. doi:10.1021/acs.jmedchem.3c00734. ISSN 0022-2623. PMC 10510399. PMID 37646374.
- ^ Sampson, Dinithia; Zhu, Xue Y.; Eyunni, Suresh V. K.; Etukala, Jagan R.; Ofori, Edward; Bricker, Barbara; Lamango, Nazarius S.; Setola, Vincent; Roth, Bryan L.; Ablordeppey, Seth Y. (2014-06-15). "Identification of a new selective dopamine D4 receptor ligand". Bioorganic & Medicinal Chemistry. 22 (12): 3105–3114. doi:10.1016/j.bmc.2014.04.026. ISSN 0968-0896. PMC 4096627. PMID 24800940.
- ^ Clarke, Bradley O.; Smith, Stephen R. (2011). "Review of 'emerging' organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids". Environment International. 37 (1): 226–247. Bibcode:2011EnInt..37..226C. doi:10.1016/j.envint.2010.06.004. PMID 20797791.
External links
[edit]Benzothiazole
View on GrokipediaChemical Properties
Molecular Structure
Benzothiazole has the molecular formula C₇H₅NS and a molar mass of 135.186 g/mol.[1][12] It is a bicyclic heterocyclic compound consisting of a six-membered benzene ring fused to a five-membered thiazole ring, where the thiazole is a heterocycle containing sulfur at position 1 and nitrogen at position 3.[1][13] The fusion occurs between the 4 and 5 positions of the thiazole ring and the adjacent carbons of the benzene ring, forming a planar bicyclic system. The nine atoms comprising the bicyclic core—six from the benzene ring and five from the thiazole ring, sharing two fusion atoms—are arranged in a coplanar configuration, which facilitates extended π-conjugation across the molecule.[1] The thiazole ring imparts an electron-withdrawing character to the overall structure due to the electronegative sulfur and nitrogen heteroatoms, influencing the electron density distribution and making certain positions susceptible to electrophilic substitution. In the standard IUPAC numbering system for benzothiazole, numbering begins at the sulfur atom as position 1, proceeds to the carbon between sulfur and nitrogen as position 2, nitrogen as position 3, and continues through the fused benzene ring at positions 4 through 7, with fusion points denoted as 7a and 3a.[13] Substituents are commonly referenced relative to this system, particularly at position 2 on the thiazole ring. The structural diagram of benzothiazole depicts the benzene ring fused linearly to the thiazole, with the heteroatoms positioned to maintain aromaticity in both rings: 4 5
/ \ / \
3a 6 7
| \ / 7a
S(1)-C(2)=N(3)
4 5
/ \ / \
3a 6 7
| \ / 7a
S(1)-C(2)=N(3)