Recent from talks
Nothing was collected or created yet.
Factor X
View on Wikipedia
| F10 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | F10, FX, FXA, coagulation factor X | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 613872; MGI: 103107; HomoloGene: 30976; GeneCards: F10; OMA:F10 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Coagulation factor X (EC 3.4.21.6), or Stuart factor, is an enzyme of the coagulation cascade, encoded in humans by F10 gene.[5] It is a serine endopeptidase (protease group S1, PA clan). Factor X is synthesized in the liver and requires vitamin K for its synthesis.
Factor X is activated, by hydrolysis, into factor Xa by both factor IX with its cofactor, factor VIII in a complex known as intrinsic pathway; and factor VII with its cofactor, tissue factor in a complex known as extrinsic pathway.[6] It is therefore the first member of the final common pathway or thrombin pathway.
It acts by cleaving prothrombin in two places (an Arg-Thr and then an Arg-Ile bond), which yields the active thrombin. This process is optimized when factor Xa is complexed with activated co-factor V in the prothrombinase complex.
Factor Xa is inactivated by protein Z-dependent protease inhibitor (ZPI), a serine protease inhibitor (serpin). The affinity of this protein for factor Xa is increased 1000-fold by the presence of protein Z, while it does not require protein Z for inactivation of factor XI. Defects in protein Z lead to increased factor Xa activity and a propensity for thrombosis. The half life of factor X is 40–45 hours.
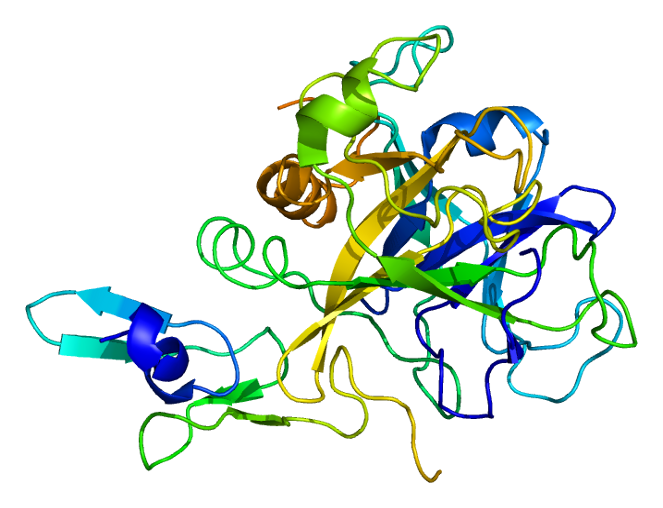
Structure
[edit]The first crystal structure of human factor Xa was deposited in May 1993. To date, 191 crystal structures of factor Xa with various inhibitors have been deposited in the protein data bank. The active site of factor Xa is divided into four subpockets as S1, S2, S3 and S4. The S1 subpocket determines the major component of selectivity and binding. The S2 sub-pocket is small, shallow and not well defined. It merges with the S4 subpocket. The S3 sub-pocket is located on the rim of the S1 pocket and is quite exposed to solvent. The S4 sub-pocket has three ligand binding domains: the "hydrophobic box", the "cationic hole" and the water site. Factor Xa inhibitors generally bind in an L-shaped conformation, where one group of the ligand occupies the anionic S1 pocket lined by residues Asp189, Ser195, and Tyr228, and another group of the ligand occupies the aromatic S4 pocket lined by residues Tyr99, Phe174, and Trp215. Typically, a fairly rigid linker group bridges these two interaction sites.[7]
Genetics
[edit]The human factor X gene is located on chromosome 13 (13q34).
Role in disease
[edit]Inborn deficiency of factor X is very rare (1:1,000,000), and may present with epistaxis (nosebleeds), hemarthrosis (bleeding into joints) and gastrointestinal blood loss. Apart from congenital deficiency, low factor X levels may occur occasionally in a number of disease states. For example, factor X deficiency may be seen in amyloidosis, where factor X is adsorbed to the amyloid fibrils in the vasculature.
Deficiency of vitamin K or antagonism by warfarin (or similar medication) leads to the production of an inactive factor X. In warfarin therapy, this is desirable to prevent thrombosis. As of late 2007, four out of five emerging anti-coagulation therapeutics targeted this enzyme.[8]
Inhibiting Factor Xa would offer an alternate method for anticoagulation. Direct Xa inhibitors are popular anticoagulants.
Polymorphisms in Factor X have been associated with an increased prevalence in bacterial infections, suggesting a possible role directly regulating the immune response to bacterial pathogens.[9]
Therapeutic use
[edit]Factor X is part of fresh frozen plasma and the prothrombinase complex. There are two commercially available Factor X concentrates: "Factor X P Behring" manufactured by CSL Behring,[10] and high purity Factor X Coagadex produced by Bio Products Laboratory and approved for use in the United States by the FDA in October 2015, and in the EU in March 2016, after earlier acceptance by CHMP and COMP.[11][12][13][14]
Kcentra, manufactured by CSL Behring, is a concentrate containing coagulation Factors II, VII, IX and X, and antithrombotic Proteins C and S.[15]
Use in biochemistry
[edit]The factor Xa protease can be used in biochemistry to cleave off protein tags that improve expression or purification of a protein of interest. Its preferred cleavage site (after the arginine in the sequence Ile-Glu/Asp-Gly-Arg, IEGR or IDGR) can easily be engineered between a tag sequence and the protein of interest. After expression and purification, the tag is then proteolytically removed by factor Xa.
Factor Xa
[edit]
Factor Xa is the activated form of the coagulation factor X, also known as thrombokinase. Factor X is an enzyme, a serine endopeptidase, which plays a key role at several stages of the coagulation system. Factor X is synthesized in the liver. The most commonly used anticoagulants in clinical practice, warfarin and the heparin series of anticoagulants and fondaparinux, act to inhibit the action of Factor Xa in various degrees.
Traditional models of coagulation developed in the 1960s envisaged two separate cascades, the extrinsic (tissue factor (TF)) pathway and the intrinsic pathway. These pathways converge to a common point, the formation of the Factor Xa/Va complex which together with calcium and bound on a phospholipids surface, generate thrombin (Factor IIa) from prothrombin (Factor II).
A new model, the cell-based model of anticoagulation appears to explain more fully the steps in coagulation. This model has three stages: 1) initiation of coagulation on TF-bearing cells, 2) amplification of the procoagulant signal by thrombin generated on the TF-bearing cell and 3) propagation of thrombin generation on the platelet surface. Factor Xa plays a key role in all three of these stages.[16]
In stage 1, Factor VII binds to the transmembrane protein TF on the surface of cells and is converted to Factor VIIa. The result is a Factor VIIa/TF complex, which catalyzes the activation of Factor X and Factor IX. Factor Xa formed on the surface of the TF-bearing cell interacts with Factor Va to form the prothrombinase complex which generates small amounts of thrombin on the surface of TF-bearing cells.
In stage 2, the amplification stage, if enough thrombin has been generated, then activation of platelets and platelet-associated cofactors occurs.
In stage 3, thrombin generation, Factor XIa activates free Factor IX on the surface of activated platelets. The activated Factor IXa with Factor VIIIa forms the "tenase" complex. This "tenase" complex activates more Factor X, which in turn forms new prothrombinase complexes with Factor Va. Factor Xa is the prime component of the prothrombinase complex which converts large amounts of prothrombin—the "thrombin burst". Each molecule of Factor Xa can generate 1000 molecules of thrombin. This large burst of thrombin is responsible for fibrin polymerization to form a thrombus.
Factor Xa also plays a role in other biological processes that are not directly related to coagulation, like wound healing, tissue remodelling, inflammation, angiogenesis and atherosclerosis.
Inhibition of the synthesis or activity of Factor X is the mechanism of action for many anticoagulants in use today. Warfarin, a synthetic derivative of coumarin, is the most widely used oral anticoagulant in the US. In some European countries, other coumarin derivatives (phenprocoumon and acenocoumarol) are used. These agents known as vitamin K antagonists (VKA), inhibit the vitamin K-dependent carboxylation of Factors II (prothrombin), VII, IX, X in the hepatocyte. This carboxylation after the translation is essential for the physiological activity.[17]
Heparin (unfractionated heparin) and its derivatives low molecular weight heparin (LMWH) bind to a plasma cofactor, antithrombin (AT) to inactivate several coagulation factors IIa, Xa, XIa and XIIa. The affinity of unfractionated heparin and the various LMWHs for Factor Xa varies considerably. The efficacy of heparin-based anticoagulants increases as selectivity for Factor Xa increases. LMWH shows increased inactivation of Factor Xa compared to unfractionated heparin, and fondaparinux, an agent based on the critical pentasacharide sequence of heparin, shows more selectivity than LMWH. This inactivation of Factor Xa by heparins is termed "indirect" since it relies on the presence of AT and not a direct interaction with Factor Xa.
Recently a new series of specific, direct acting inhibitors of Factor Xa has been developed. These include the drugs rivaroxaban, apixaban, betrixaban, LY517717, darexaban (YM150), edoxaban and 813893. These agents have several theoretical advantages over current therapy. They may be given orally. They have rapid onset of action. And they may be more effective against Factor Xa in that they inhibit both free Factor Xa and Factor Xa in the prothrombinase complex.[18]
History
[edit]American and British scientists described deficiency of factor X independently in 1953 and 1956, respectively. As with some other coagulation factors, the factor was initially named after these patients, a Mr Rufus Stuart (1921) and a Miss Audrey Prower (1934). At that time, those investigators could not know that the human genetic defect they had identified would be found in the previously characterized enzyme called thrombokinase.
Thrombokinase was the name coined by Paul Morawitz in 1904 to describe the substance that converted prothrombin to thrombin and caused blood to clot.[19] That name embodied an important new concept in understanding blood coagulation – that an enzyme was critically important in the activation of prothrombin. Morawitz believed that his enzyme came from cells such as platelets yet, in keeping with the state of knowledge about enzymes at that time, he had no clear idea about the chemical nature of his thrombokinase or its mechanism of action. Those uncertainties led to decades during which the terms thrombokinase and thromboplastin were both used to describe the activator of prothrombin and led to controversy about its chemical nature and origin.[20]
In 1947, J Haskell Milstone isolated a proenzyme from bovine plasma which, when activated, converted prothrombin to thrombin. Following Morawitz’s designation, he called it prothrombokinase [21] and by 1951 had purified the active enzyme, thrombokinase. Over the next several years he showed that thrombokinase was a proteolytic enzyme that, by itself, could activate prothrombin. Its activity was greatly enhanced by addition of calcium, other serum factors, and tissue extracts,[22] which represented the thromboplastins that promoted the conversion of prothrombin to thrombin by their interaction with thrombokinase. In 1964 Milstone summarized his work and that of others: “There are many chemical reactions which are so slow that they would not be of physiological use if they were not accelerated by enzymes. We are now confronted with a reaction, catalyzed by an enzyme, which is still too slow unless aided by accessory factors.” [23]
Interactions
[edit]Factor X has been shown to interact with Tissue factor pathway inhibitor.[24]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000126218 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031444 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "F10 gene: MedlinePlus Genetics". medlineplus.gov.
- ^ Camire RM (August 2021). "Blood coagulation factor X: molecular biology, inherited disease, and engineered therapeutics". Journal of Thrombosis and Thrombolysis. 52 (2): 383–390. doi:10.1007/s11239-021-02456-w. PMC 8531165. PMID 33886037.
- ^ "Presentation on Direct Factor Xa Inhibitors". Archived from the original on 2016-03-03. Retrieved 2010-04-08.
- ^ Ron Winslow, Avery Johnson (2007-12-10). "Race Is on for the Next Blood Thinner". The Wall Street Journal. p. A12. Archived from the original on 2016-03-10. Retrieved 2008-01-06.
The flurry of interest reflects increasing understanding of what doctors call the coagulation cascade... Four new blood thinners target an enzyme called factor Xa, one of several enzymes that play an important role in the cascade.
- ^ Choby JE, Monteith AJ, Himmel LE, Margaritis P, Shirey-Rice JK, Pruijssers A, et al. (March 2019). "A Phenome-Wide Association Study Uncovers a Pathological Role of Coagulation Factor X during Acinetobacter baumannii Infection". Infection and Immunity. 87 (5): IAI.00031–19. doi:10.1128/IAI.00031-19. PMC 6479028. PMID 30782860.
- ^ Brooker M (April 2008). Registry of Clotting Factor Concentrates (Eighth ed.). World Federation of Hemophilia.
- ^ "FDA approves first Factor X concentrate to treat patients with rare hereditary bleeding disorder" (Press release). US FDA. October 20, 2015. Archived from the original on October 21, 2015. Retrieved October 21, 2015.
Until today's orphan drug approval, no specific coagulation factor replacement therapy was available for patients with hereditary Factor X deficiency.
- ^ "Coagadex". U.S. Food and Drug Administration. 28 June 2017. Archived from the original on 22 July 2017. Retrieved 2 April 2020.
- ^ "Coagadex". U.S. Food and Drug Administration. 21 September 2018. Archived from the original on 17 December 2019. Retrieved 2 April 2020.
- ^ "Coagadex EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 30 December 2019. Retrieved 21 April 2020.
- ^ "Kcentra- prothrombin, coagulation factor vii human, coagulation factor ix human, coagulation factor x human, protein c, protein s human, and water kit". DailyMed. 22 October 2018. Archived from the original on 25 March 2021. Retrieved 21 April 2020.
- ^ Hoffman M, Monroe DM (February 2007). "Coagulation 2006: a modern view of hemostasis". Hematology/Oncology Clinics of North America. 21 (1): 1–11. doi:10.1016/j.hoc.2006.11.004. PMID 17258114.
- ^ Golan DE (2012). Principles of Pharmacology The Pathophysiologic Basis of Drug Therapy. Philadelphia: Lippincott Williams & Wilkins. p. 387. ISBN 978-1-4511-1805-6.
- ^ Turpie AG (June 2007). "Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases". Arteriosclerosis, Thrombosis, and Vascular Biology. 27 (6): 1238–1247. CiteSeerX 10.1.1.536.872. doi:10.1161/ATVBAHA.107.139402. PMID 17379841. S2CID 2998452.
- ^ Morawitz P. "Beitrage zur Kenntnis der Blutgerinnung". Deutsches Archiv für Klinische Medizin. 79: 432–442.
- ^ Milstone JH (December 1952). "On the evolution of blood clotting theory". Medicine. 31 (4): 411–447. doi:10.1097/00005792-195212000-00004. PMID 13012730.
- ^ Milstone JH (December 1947). "Prothrombokinase and the Three Stages of Blood Coagulation". Science. 106 (2762): 546–547. Bibcode:1947Sci...106..546M. doi:10.1126/science.106.2762.546-a. PMID 17741228. S2CID 35643683.
- ^ Milstone LM (August 2021). "Factor Xa: Thrombokinase from Paul Morawitz to J Haskell Milstone". Journal of Thrombosis and Thrombolysis. 52 (2): 364–370. doi:10.1007/s11239-021-02387-6. PMID 33484373. S2CID 231682954.
- ^ Milstone JH, Oulianoff N, Milstone VK (November 1963). "Thrombokinase as prime activator of prothrombin: historical perspectives and present status". The Journal of General Physiology. 47 (2): 315–327. doi:10.1085/jgp.47.2.315. PMC 2195336. PMID 14080818.
- ^ Broze GJ, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP (February 1988). "The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action". Blood. 71 (2): 335–343. doi:10.1182/blood.V71.2.335.335. PMID 3422166.
Further reading
[edit]- Broze GJ, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP (February 1988). "The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action". Blood. 71 (2): 335–343. doi:10.1182/blood.V71.2.335.335. PMID 3422166.
- Cooper DN, Millar DS, Wacey A, Pemberton S, Tuddenham EG (July 1997). "Inherited factor X deficiency: molecular genetics and pathophysiology". Thrombosis and Haemostasis. 78 (1): 161–172. doi:10.1055/s-0038-1657520. PMID 9198147. S2CID 27129058.
- España F, Berrettini M, Griffin JH (August 1989). "Purification and characterization of plasma protein C inhibitor". Thrombosis Research. 55 (3): 369–384. doi:10.1016/0049-3848(89)90069-8. PMID 2551064.
- Fung MR, Hay CW, MacGillivray RT (June 1985). "Characterization of an almost full-length cDNA coding for human blood coagulation factor X". Proceedings of the National Academy of Sciences of the United States of America. 82 (11): 3591–3595. Bibcode:1985PNAS...82.3591F. doi:10.1073/pnas.82.11.3591. PMC 397831. PMID 2582420.
- Gilgenkrantz S, Briquel ME, André E, Alexandre P, Jalbert P, Le Marec B, et al. (1986). "Structural genes of coagulation factors VII and X located on 13q34". Annales de Génétique. 29 (1): 32–35. PMID 3487272.
- Hassan HJ, Leonardi A, Chelucci C, Mattia G, Macioce G, Guerriero R, et al. (September 1990). "Blood coagulation factors in human embryonic-fetal development: preferential expression of the FVII/tissue factor pathway". Blood. 76 (6): 1158–1164. doi:10.1182/blood.V76.6.1158.1158. PMID 1698100.
- Heeb MJ, Rosing J, Bakker HM, Fernandez JA, Tans G, Griffin JH (March 1994). "Protein S binds to and inhibits factor Xa". Proceedings of the National Academy of Sciences of the United States of America. 91 (7): 2728–2732. Bibcode:1994PNAS...91.2728H. doi:10.1073/pnas.91.7.2728. PMC 43443. PMID 8146182.
- Inoue K, Morita T (November 1993). "Identification of O-linked oligosaccharide chains in the activation peptides of blood coagulation factor X. The role of the carbohydrate moieties in the activation of factor X". European Journal of Biochemistry. 218 (1): 153–163. doi:10.1111/j.1432-1033.1993.tb18361.x. PMID 8243461.
- Jagadeeswaran P, Reddy SV, Rao KJ, Hamsabhushanam K, Lyman G (December 1989). "Cloning and characterization of the 5' end (exon 1) of the gene encoding human factor X". Gene. 84 (2): 517–519. doi:10.1016/0378-1119(89)90529-5. PMID 2612918.
- Kaul RK, Hildebrand B, Roberts S, Jagadeeswaran P (1986). "Isolation and characterization of human blood-coagulation factor X cDNA". Gene. 41 (2–3): 311–314. doi:10.1016/0378-1119(86)90112-5. PMID 3011603.
- Krishnaswamy S (March 1990). "Prothrombinase complex assembly. Contributions of protein-protein and protein-membrane interactions toward complex formation". The Journal of Biological Chemistry. 265 (7): 3708–3718. doi:10.1016/S0021-9258(19)39652-8. PMID 2303476.
- Leytus SP, Chung DW, Kisiel W, Kurachi K, Davie EW (June 1984). "Characterization of a cDNA coding for human factor X". Proceedings of the National Academy of Sciences of the United States of America. 81 (12): 3699–3702. Bibcode:1984PNAS...81.3699L. doi:10.1073/pnas.81.12.3699. PMC 345286. PMID 6587384.
- Leytus SP, Foster DC, Kurachi K, Davie EW (September 1986). "Gene for human factor X: a blood coagulation factor whose gene organization is essentially identical with that of factor IX and protein C". Biochemistry. 25 (18): 5098–5102. doi:10.1021/bi00366a018. PMID 3768336.
- Marchetti G, Castaman G, Pinotti M, Lunghi B, Di Iasio MG, Ruggieri M, et al. (August 1995). "Molecular bases of CRM+ factor X deficiency: a frequent mutation (Ser334Pro) in the catalytic domain and a substitution (Glu102Lys) in the second EGF-like domain". British Journal of Haematology. 90 (4): 910–915. doi:10.1111/j.1365-2141.1995.tb05214.x. PMID 7669671. S2CID 29324903.
- McMullen BA, Fujikawa K, Kisiel W, Sasagawa T, Howald WN, Kwa EY, et al. (June 1983). "Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid". Biochemistry. 22 (12): 2875–2884. doi:10.1021/bi00281a016. PMID 6871167.
- Messier TL, Pittman DD, Long GL, Kaufman RJ, Church WR (March 1991). "Cloning and expression in COS-1 cells of a full-length cDNA encoding human coagulation factor X". Gene. 99 (2): 291–294. doi:10.1016/0378-1119(91)90141-W. PMID 1902434.
- Morgenstern KA, Sprecher C, Holth L, Foster D, Grant FJ, Ching A, et al. (March 1994). "Complementary DNA cloning and kinetic characterization of a novel intracellular serine proteinase inhibitor: mechanism of action with trypsin and factor Xa as model proteinases". Biochemistry. 33 (11): 3432–3441. doi:10.1021/bi00177a037. PMID 8136380.
- Padmanabhan K, Padmanabhan KP, Tulinsky A, Park CH, Bode W, Huber R, et al. (August 1993). "Structure of human des(1-45) factor Xa at 2.2 A resolution". Journal of Molecular Biology. 232 (3): 947–966. doi:10.1006/jmbi.1993.1441. PMID 8355279.
- Reddy SV, Zhou ZQ, Rao KJ, Scott JP, Watzke H, High KA, et al. (October 1989). "Molecular characterization of human factor XSan Antonio". Blood. 74 (5): 1486–1490. doi:10.1182/blood.V74.5.1486.1486. PMID 2790181.
- Sinha U, Wolf DL (February 1993). "Carbohydrate residues modulate the activation of coagulation factor X". The Journal of Biological Chemistry. 268 (5): 3048–3051. doi:10.1016/S0021-9258(18)53657-7. PMID 8428982.
External links
[edit]- "Summary for peptidase S01.216: coagulation factor Xa". MEROPS. European Molecular Biology Laboratory (EMBL).
- "Factor X deficiency". Canadian Hemophilia Society. 14 April 2018.































![1mq5: Crystal Structure of 3-chloro-N-[4-chloro-2-[[(4-chlorophenyl)amino]carbonyl]phenyl]-4-[(4-methyl-1-piperazinyl)methyl]-2-thiophenecarboxamide Complexed with Human Factor Xa](http://upload.wikimedia.org/wikipedia/commons/thumb/6/68/PDB_1mq5_EBI.jpg/250px-PDB_1mq5_EBI.jpg)
![1mq6: Crystal Structure of 3-chloro-N-[4-chloro-2-[[(5-chloro-2-pyridinyl)amino]carbonyl]-6-methoxyphenyl]-4-[[(4,5-dihydro-2-oxazolyl)methylamino]methyl]-2-thiophenecarboxamide Complexed with Human Factor Xa](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a4/PDB_1mq6_EBI.jpg/250px-PDB_1mq6_EBI.jpg)





![1v3x: Factor Xa in complex with the inhibitor 1-[6-methyl-4,5,6,7-tetrahydrothiazolo(5,4-c)pyridin-2-yl] carbonyl-2-carbamoyl-4-(6-chloronaphth-2-ylsulphonyl)piperazine](http://upload.wikimedia.org/wikipedia/commons/thumb/7/70/PDB_1v3x_EBI.jpg/250px-PDB_1v3x_EBI.jpg)
![1wu1: Factor Xa in complex with the inhibitor 4-[(5-chloroindol-2-yl)sulfonyl]-2-(2-methylpropyl)-1-[[5-(pyridin-4-yl) pyrimidin-2-yl]carbonyl]piperazine](http://upload.wikimedia.org/wikipedia/commons/thumb/1/17/PDB_1wu1_EBI.jpg/250px-PDB_1wu1_EBI.jpg)










![2d1j: Factor Xa in complex with the inhibitor 2-[[4-[(5-chloroindol-2-yl)sulfonyl]piperazin-1-yl] carbonyl]thieno[3,2-b]pyridine n-oxide](http://upload.wikimedia.org/wikipedia/commons/thumb/a/ae/PDB_2d1j_EBI.jpg/250px-PDB_2d1j_EBI.jpg)
![2fzz: Factor Xa in complex with the inhibitor 1-(3-amino-1,2-benzisoxazol-5-yl)-6-(2'-(((3r)-3-hydroxy-1-pyrrolidinyl)methyl)-4-biphenylyl)-3-(trifluoromethyl)-1,4,5,6-tetrahydro-7h-pyrazolo[3,4-c]pyridin-7-one](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e1/PDB_2fzz_EBI.jpg/250px-PDB_2fzz_EBI.jpg)
![2g00: Factor Xa in complex with the inhibitor 3-(6-(2'-((dimethylamino)methyl)-4-biphenylyl)-7-oxo-3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridin-1-yl)benzamide](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b8/PDB_2g00_EBI.jpg/250px-PDB_2g00_EBI.jpg)










