Recent from talks
Nothing was collected or created yet.
Alkoxide
View on Wikipedia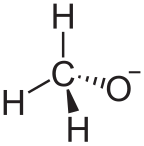

In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases[citation needed] and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.[1][2] Transition metal alkoxides are widely used for coatings and as catalysts.[3][4]
Enolates are unsaturated alkoxides derived by deprotonation of a C−H bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols.
Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a phenyl group. Phenol is more acidic than a typical alcohol; thus, phenoxides are correspondingly less basic and less nucleophilic than alkoxides. They are, however, often easier to handle and yield derivatives that are more crystalline than those of the alkoxides.[citation needed]
Structure
[edit]Alkali metal alkoxides are often oligomeric or polymeric compounds, especially when the R group is small (Me, Et).[3][page needed] The alkoxide anion is a good bridging ligand, thus many alkoxides feature M2O or M3O linkages. In solution, the alkali metal derivatives exhibit strong ion-pairing, as expected for the alkali metal derivative of a strongly basic anion.

Preparation
[edit]From reducing metals
[edit]Alkoxides can be produced by several routes starting from an alcohol. Highly reducing metals react directly with alcohols to give the corresponding metal alkoxide. The alcohol serves as an acid, and hydrogen is produced as a by-product. A classic case is sodium methoxide produced by the addition of sodium metal to methanol:[citation needed]
- 2 CH3OH + 2 Na → 2 CH3ONa + H2
Other alkali metals can be used in place of sodium, and most alcohols can be used in place of methanol. Generally, the alcohol is used in excess and left to be used as a solvent in the reaction. Thus, an alcoholic solution of the alkali alkoxide is used. Another similar reaction occurs when an alcohol is reacted with a metal hydride such as NaH. The metal hydride removes the hydrogen atom from the hydroxyl group and forms a negatively charged alkoxide ion.
Properties
[edit]Reactions with alkyl halides
[edit]The alkoxide ion and its salts react with primary alkyl halides in an SN2 reaction to form an ether via the Williamson ether synthesis.[1][2]
Hydrolysis and transesterification
[edit]Aliphatic metal alkoxides decompose in water as summarized in this idealized equation:
- Al(OR)3 + 3 H2O → Al(OH)3 + 3 ROH
In the transesterification process, metal alkoxides react with esters to bring about an exchange of alkyl groups between metal alkoxide and ester. With the metal alkoxide complex in focus, the result is the same as for alcoholysis, namely the replacement of alkoxide ligands, but at the same time the alkyl groups of the ester are changed, which can also be the primary goal of the reaction. Sodium methoxide in solution, for example, is commonly used for this purpose, a reaction that is used in the production of biodiesel.
Formation of oxo-alkoxides
[edit]Many metal alkoxide compounds also feature oxo-ligands. Oxo-ligands typically arise via the hydrolysis, often accidentally, and via ether elimination:[citation needed]
- RCO2R' + CH3O− → RCO2CH3 + R'O−
Thermal stability
[edit]Many metal alkoxides thermally decompose in the range ≈100–300 °C.[citation needed] Depending on process conditions, this thermolysis can afford[clarification needed] nanosized powders of oxide or metallic phases. This approach is a basis of processes of fabrication of functional materials intended for aircraft, space, electronic fields, and chemical industry: individual oxides, their solid solutions, complex oxides, powders of metals and alloys active towards sintering. Decomposition of mixtures of mono- and heterometallic alkoxide derivatives has also been examined. This method represents a prospective approach possessing an advantage of capability of obtaining functional materials with increased phase and chemical homogeneity and controllable grain size (including the preparation of nanosized materials) at relatively low temperature (less than 500–900 °C) as compared with the conventional techniques.[citation needed]
Illustrative alkoxides
[edit]| name | molecular formula | comment |
|---|---|---|
| Tetraethyl orthosilicate | Si(OEt)4 | for sol-gel processing of Si oxides; Si(OMe)4 is avoided for safety reasons |
| Aluminium isopropoxide | Al4(OiPr)12 | reagent for Meerwein–Ponndorf–Verley reduction |
| Potassium tert-butoxide, | K4(OtBu)4 | basic reagent in alcohol solution for organic elimination reactions |
Sodium methoxide
[edit]Sodium methoxide, also called sodium methylate and sodium methanolate, is a white powder when pure.[6] It is used as an initiator of an anionic addition polymerization with ethylene oxide, forming a polyether with high molecular weight.[citation needed] Both sodium methoxide and its counterpart prepared with potassium are frequently used as catalysts for commercial-scale production of biodiesel. In this process, vegetable oils or animal fats, which chemically are fatty acid triglycerides, are transesterified with methanol to give fatty acid methyl esters (FAMEs).
Sodium methoxide is produced on an industrial scale and is available from a number of chemical companies.
Potassium methoxide
[edit]Potassium methoxide in alcoholic solution is commonly used as a catalyst for transesterification in the production of biodiesel.[7]
References
[edit]- ^ a b Williamson, Alexander (1850). "Theory of Ætherification". Phil. Mag. 37 (251): 350–356. doi:10.1080/14786445008646627. (excerpt)
- ^ a b Boyd, Robert Neilson; Morrison, Robert Thornton (1992). Organic Chemistry (6th ed.). Englewood Cliffs, N.J.: Prentice Hall. pp. 241–242. ISBN 9780136436690.
- ^ a b Bradley, Don C.; Mehrotra, Ram C.; Rothwell, Ian P.; Singh, A. (2001). Alkoxo and Aryloxo Derivatives of Metals. San Diego: Academic Press. ISBN 978-0-08-048832-5.
- ^ Turova, Nataliya Y.; Turevskaya, Evgeniya P.; Kessler, Vadim G.; Yanovskaya, Maria I. (2002). The Chemistry of Metal Alkoxides. Dordrecht: Kluwer Academic Publishers. ISBN 9780792375210.
- ^ Unkelbach, Christian; O'Shea, Donal F.; Strohmann, Carsten (2014). "Insights into the Metalation of Benzene and Toluene by Schlosser's Base: A Superbasic Cluster Comprising PhK, PhLi, and tBuOLi". Angew. Chem. Int. Ed. 53 (2): 553–556. doi:10.1002/anie.201306884. PMID 24273149.
- ^ "Sodium Methoxide Material Safety Data Sheet (MSDS)". NOAA.gov. Archived from the original on 2009-02-18. Retrieved 2010-04-13.
- ^ G. Knothe; J. Krahl; J. Van Gerpen, eds. (2010), The Biodiesel Handbook (2nd ed.), AOCS Press, ISBN 978-1-893997-62-2
Further reading
[edit]- Turova, Nataliya Y. (2004). "Metal oxoalkoxides. Synthesis, properties and structures". Russian Chemical Reviews. 73 (11): 1041–1064. Bibcode:2004RuCRv..73.1041T. doi:10.1070/RC2004v073n11ABEH000855. S2CID 250920020.
Alkoxide
View on GrokipediaDefinition and Structure
General Definition
Alkoxides are the anionic conjugate bases of alcohols, characterized by an alkyl group (R) bonded to a negatively charged oxygen atom, with the general formula . These species typically exist as salts paired with metal cations, denoted as , where is commonly an alkali metal ion such as sodium () or potassium (). This ionic nature arises from the deprotonation of the hydroxyl group in alcohols (ROH), rendering alkoxides highly reactive due to the localized negative charge on oxygen.[4][5] The preparation and properties of alkoxides were first systematically explored in the 19th century through reactions of alcohols with alkali metals, which generate the corresponding alkoxide salts and hydrogen gas. This foundational work laid the groundwork for their use in organic synthesis, highlighting their role as versatile reagents.[6] In chemical reactions, alkoxides function primarily as strong bases, with basicity exceeding that of hydroxide ion due to the lower acidity of their conjugate acids (alcohols). Their nucleophilicity varies by solvent: in protic solvents like water or alcohols, solvation through hydrogen bonding reduces their nucleophilic effectiveness, favoring basic behavior such as deprotonation; in contrast, polar aprotic solvents like dimethyl sulfoxide (DMSO) minimize solvation, enhancing their nucleophilic attack on electrophiles. This solvent-dependent duality makes alkoxides essential for selective transformations in synthesis.[7][8]Molecular Structure
Alkoxides of alkali metals, such as sodium methoxide (NaOCH₃), exhibit largely ionic character due to the high electropositivity of the metal, resulting in the dissociation into Na⁺ and RO⁻ ions in polar solvents.[9] In these environments, the alkoxide anion (RO⁻) undergoes solvation, where solvent molecules coordinate to the oxygen atom, forming solvated species represented by the equation: This solvation stabilizes the anion through hydrogen bonding or electrostatic interactions, as evidenced by spectroscopic studies showing shifts in vibrational frequencies.[10] In contrast, transition metal alkoxides display more covalent bonding characteristics, with polar M-OR bonds influenced by the metal's electronegativity and coordination preferences. For instance, titanium(IV) ethoxide (Ti(OCH₂CH₃)₄) often forms polynuclear structures featuring bridging OR groups (μ₂-OR or μ₃-OR), which link metal centers into oligomeric clusters like [Ti(OEt)₄]₃.[9] These bridging ligands contribute to the overall geometry, with terminal OR groups typically adopting a tetrahedral arrangement around the metal in monomeric units, as seen in Ti(OR)₄ complexes. Steric effects from the R group significantly influence the degree of oligomerization; smaller alkyl substituents (e.g., ethyl in OEt) promote bridging and oligomeric forms due to reduced spatial hindrance, while bulkier groups (e.g., isopropyl in O iPr) favor monomeric structures by inhibiting close metal-metal approaches, as demonstrated in Ti(O iPr)₄.[11] Spectroscopic techniques provide evidence for these structural features: infrared (IR) spectroscopy reveals O-R stretching frequencies around 1000-1100 cm⁻¹, indicative of single C-O bonds, while nuclear magnetic resonance (NMR) data, including ¹H and ¹³C shifts, confirm the integrity of the OR ligand and its coordination environment.[12] X-ray crystallography further supports O-R bond lengths of approximately 1.4 Å, consistent with typical alkoxide C-O bonds.[10]Nomenclature
Naming Conventions
Alkoxides are typically named in a straightforward manner by combining the name of the metal cation with the name of the alkoxide anion. In common nomenclature, the anion is designated using terms such as "methoxide" for the CH₃O⁻ ion derived from methanol, "ethoxide" for CH₃CH₂O⁻ from ethanol, and similarly "propoxide" or "butoxide" for more complex variants, resulting in names like sodium methoxide (NaOCH₃) or potassium ethoxide (KOCH₂CH₃).[13] The International Union of Pure and Applied Chemistry (IUPAC) retains the common anion names (methoxide, ethoxide, propoxide, butoxide) as preferred IUPAC names for the simplest straight-chain cases. For alkoxides with branched or longer alkyl chains, a systematic approach is used for the anion portion, replacing the "-ol" suffix of the parent alcohol with "-olate," such as sodium 2-methylpropan-2-olate for the tert-butoxide ion derived from 2-methylpropan-2-ol. This method ensures precision in complex cases.[14][13][15] Naming conventions vary by metal type to reflect coordination and oxidation states. For alkali and alkaline earth metals, the simple metal name suffices, as in magnesium methoxide (Mg(OCH₃)₂). In contrast, for transition metals, the oxidation state is indicated using Roman numerals, such as titanium(IV) ethoxide for Ti(OCH₂CH₃)₄, emphasizing the metal's valency in the compound.[16] The full IUPAC name for such complexes may list the anion multiple times, as in tetraethanolate titanium(4+).[16] Historically, these compounds were termed "alcoholates," referring to salts where alcohol molecules were incorporated analogously to water in hydrates, but this usage has largely been supplanted by the more specific "alkoxide" in contemporary chemical literature to denote the RO⁻ ligand directly.[17][18]Variations for Different Metals
The nomenclature of alkoxides varies significantly depending on the metal involved, reflecting differences in valency, coordination geometry, and structural complexity such as oligomerization or bridging ligands. For alkali and alkaline earth metals, naming remains straightforward, typically following the pattern "metal alkoxide" to denote the simple ionic or polymeric structures formed. For instance, magnesium methoxide is designated as Mg(OMe)₂, highlighting the divalent metal and methoxide ligands without needing to specify coordination details, as these compounds often exist as tetramers or polymers.[19] In contrast, transition metal alkoxides require more precise nomenclature to account for stoichiometry, coordination numbers, and potential polynuclear assemblies, which arise from the metals' tendency to achieve higher coordination through bridging alkoxo groups. Titanium(IV) isopropoxide, for example, is commonly named as titanium tetraisopropoxide for the monomeric Ti(OiPr)₄, though it can form tetranuclear clusters like [Ti₄(OiPr)₁₆] in solution or solid state. Aluminum alkoxides often adopt binuclear forms, such as [Al(OR)₂(μ-OR)₂Al(OR)₂], named as dialuminum tetraalkoxide with bridging ligands to emphasize the dimeric structure and tetrahedral coordination around each aluminum center. These naming conventions are essential for transition metal derivatives, which play pivotal roles in catalysis due to their tunable reactivity and structural diversity.[19][20] Lanthanide and actinide alkoxides are less common and their nomenclature explicitly incorporates the oxidation state to clarify the metal's electronic configuration, given the variability in +3 and +4 states. Cerium(IV) tert-butoxide, represented as Ce(OtBu)₄, exemplifies this approach, often stabilized by coordination with solvents like THF in [Ce(OtBu)₄(THF)₂], and may form clusters such as trinuclear [Ce₃(OtBu)₉(tBuOH)₂] due to the large ionic radius and high coordination numbers (up to 9) typical of these metals. Such detailed naming underscores their rarity and specialized applications in advanced materials synthesis.[19]Preparation
Reaction with Alkali Metals
Alkoxides of alkali metals can be prepared by the direct reaction of the corresponding alcohols with alkali metals such as sodium or potassium. The general equation for this process is where is an alkyl group and is the alkali metal. This reaction is highly exothermic, releasing hydrogen gas, and is typically conducted under an inert atmosphere, such as argon or nitrogen, to avoid interference from oxygen or moisture.[21] The mechanism involves single electron transfer (SET) from the alkali metal surface to the adsorbed alcohol molecule, resulting in heterolytic cleavage of the O-H bond to directly form the alkoxide ion (RO⁻), a metal cation (M⁺), and a hydrogen radical (H•). The hydrogen radicals combine to produce H₂ gas.[22] This process is supported by observations of solvated electrons, analogous to alkali metal-water interactions. For secondary or tertiary alcohols, the reaction is slower and may produce minor alkene byproducts due to dehydration under the reaction conditions, but the primary pathway remains alkoxide formation. Practical conditions emphasize anhydrous environments to prevent hydrolysis of the product. For instance, sodium methoxide is commonly prepared by adding small pieces of freshly cut sodium metal to refluxing methanol (approximately 65°C) in a flask equipped with a reflux condenser and a drying tube filled with calcium sulfate to exclude moisture. The reaction proceeds vigorously with evolution of hydrogen gas until the sodium is fully consumed, typically yielding a 25-30% solution of sodium methoxide in methanol with high efficiency (around 80% based on sodium). Moisture contamination can reduce purity by promoting partial hydrolysis to sodium hydroxide.[23] This approach extends to alkaline earth metals like magnesium and calcium, though these are less reactive and often require elevated temperatures, catalysts (e.g., iodine for magnesium), or activated metal forms to initiate the reaction. Magnesium alkoxides, for example, can be synthesized by refluxing magnesium turnings with the alcohol under inert conditions, producing and , albeit with potentially lower yields due to slower kinetics compared to alkali metals.[24]Deprotonation of Alcohols
Alkoxides of alkali metals are commonly prepared by the deprotonation of alcohols using strong bases such as metal hydrides or alkyllithiums. A representative method involves reacting an alcohol (ROH) with sodium hydride (NaH) in an inert solvent like tetrahydrofuran (THF), yielding the sodium alkoxide (RONa) and hydrogen gas according to the equation: This approach is straightforward and widely employed in laboratory syntheses due to the commercial availability of NaH as a dispersion in mineral oil, which facilitates handling.[25][26] Similarly, organolithium reagents like n-butyllithium (BuLi) serve as effective deprotonating agents for preparing lithium alkoxides, particularly when high reactivity or anhydrous conditions are required. The reaction proceeds as: This method is preferred for lithium alkoxides in organic synthesis, as BuLi provides clean deprotonation without introducing additional metal ions, and the byproduct butane is a gas that evolves readily.[27] Another preparation route is transalkoxylation, an equilibrium-driven exchange between an existing metal alkoxide (MOR') and a different alcohol (ROH), resulting in the desired alkoxide (MOR) and the displaced alcohol (R'OH): This technique is useful for synthesizing alkoxides with sterically hindered or functional-group-containing alkyl chains, where direct deprotonation might be challenging; the equilibrium can be shifted by removing the more volatile alcohol or using excess ROH. It is particularly applied in the preparation of magnesium and aluminum alkoxides for catalytic applications.[28][24] For transition metal alkoxides, deprotonation often involves reacting metal halides or amides with excess alcohol in the presence of a base to neutralize the acid byproduct. A classic example is the synthesis of tetraalkoxytitanium compounds from titanium tetrachloride (TiCl₄) and an alcohol (ROH), typically with ammonia (NH₃) as the base: This method allows control over the alkoxide ligands and is scalable for producing precursors in materials science, such as those used in sol-gel processes for ceramics.[29] These deprotonation strategies offer advantages over alternative preparations, including reduced handling risks compared to reactive alkali metals—NaH dispersions, for instance, are safer and less prone to ignition—and enhanced scalability for industrial production of complex alkoxides without generating excessive heat or redox byproducts. The evolution of hydrogen gas is managed through controlled addition and venting, minimizing hazards in both lab and large-scale settings. Post-2000, hydride-based methods have become more prevalent in laboratories for their convenience and compatibility with sensitive substrates.[26]Physical Properties
Solubility and State
Alkali metal alkoxides are typically obtained as white, hygroscopic solids at room temperature. For instance, sodium methoxide appears as a white amorphous powder, while potassium tert-butoxide forms a white crystalline solid.[30][31] In contrast, many transition metal alkoxides exhibit liquid or viscous states, facilitating their use in solution-based processes; titanium(IV) isopropoxide, for example, is a colorless, distillable liquid with a boiling point of 232 °C.[32] These differences in physical state arise from the varying degrees of ionic character and molecular complexity, with ionic alkali derivatives favoring crystalline lattices and covalent transition metal variants adopting oligomeric structures in the absence of coordinating solvents.[33] The solubility of metal alkoxides is highly dependent on the solvent's ability to solvate the alkoxide anion (RO⁻), with donor solvents playing a key role in stabilizing the ionic species. Alkali metal alkoxides, being predominantly ionic, display high solubility in polar protic solvents such as alcohols and polar aprotic solvents like ethers and tetrahydrofuran (THF); sodium methoxide is fully miscible in methanol and ethanol, while potassium tert-butoxide shows solubilities of 17.8 g/100 g in tert-butanol and 25 g/100 g in THF at 25–26 °C.[30][31] They exhibit low solubility in non-polar hydrocarbons, such as hexane (0.27 g/100 g for potassium tert-butoxide), due to insufficient solvation of the charged components. Transition metal alkoxides follow similar trends but often extend to additional organic solvents; titanium(IV) isopropoxide is soluble in ethanol, diethyl ether, benzene, and chloroform, though it reacts rapidly with water.[32][33] Factors influencing solubility include the lattice energy of the solid state for ionic alkoxides, which is modulated by the ionic radius of the metal cation. Smaller cations, such as Li⁺, result in higher lattice energies owing to closer ion packing with the large alkoxide anion, potentially reducing solubility in polar solvents compared to larger cations like K⁺ or Cs⁺, where lower lattice energies facilitate dissolution.[34] This radius-dependent effect parallels trends observed in other alkali salts, emphasizing the balance between lattice disruption and solvation energy in donor media.[35]Thermal Stability
Alkoxides exhibit varying degrees of thermal stability depending on the metal cation and the alkyl group, with decomposition typically occurring through pathways involving the elimination of organic fragments and formation of metal oxides, hydroxides, or carbonates. For alkali metal alkoxides such as sodium methoxide (NaOMe) and sodium ethoxide (NaOEt), thermal decomposition initiates at elevated temperatures, generally above 300–350°C, leading to gaseous hydrocarbons (both saturated and unsaturated) and solid residues comprising sodium hydroxide (NaOH), sodium carbonate (Na₂CO₃), and amorphous carbon.[36] This process is endothermic, with activation energies around 188 kJ/mol for NaOMe and 151 kJ/mol for NaOEt, indicating that longer-chain alkoxides like NaOEt decompose at slightly lower temperatures than shorter-chain analogs.[36] The thermal stability of alkoxides increases with the electronegativity of the metal cation, as higher electronegativity promotes more covalent M–O bonding, which resists thermal dissociation. For ionic alkali metal alkoxides, stability generally increases down the group, analogous to trends in other salts with large anions. Additionally, the nature of the R group influences stability; bulky alkyl substituents, such as tert-butoxide, enhance thermal resilience through steric hindrance that inhibits intermolecular associations leading to decomposition, making tert-butyl derivatives more stable than n-butyl or methyl analogs. Analytical techniques like thermogravimetric analysis (TGA) coupled with mass spectrometry (MS) and differential scanning calorimetry (DSC) reveal characteristic weight loss profiles corresponding to organic volatilization. For instance, NaOEt remains stable up to approximately 300°C under inert conditions, with subsequent rapid weight loss (20–30% by 400°C) attributed to hydrocarbon evolution, as confirmed by MS detection of ethylene and ethane fragments. DSC profiles show endothermic peaks around 300–350°C for these processes, providing insights into decomposition kinetics.[36]Chemical Properties and Reactivity
Basicity and Nucleophilicity
Alkoxides (RO⁻) are strong bases in organic chemistry, owing to the relatively high pKa values of their conjugate acids, alcohols (ROH), which typically range from 15 to 18 in aqueous or DMSO solutions.[37] This positions alkoxides as capable of deprotonating a variety of stronger acids (with pKa lower than that of the alcohol), such as carboxylic acids (pKa ≈ 4–5) or protonated amines (pKa ≈ 10–11), but not significantly affecting compounds with pKa values exceeding 18, like terminal alkynes (pKa ≈ 25). For instance, the reaction RO⁻ + RH → ROH + R⁻ illustrates their basic character when RH is an acid with a pKa lower than that of ROH, transferring the proton to form the alcohol and the conjugate base R⁻.[38] The basicity of alkoxides increases with the degree of alkyl substitution on the carbon attached to the oxygen atom, following the order tert-butoxide (tBuO⁻) > ethoxide (EtO⁻) > methoxide (MeO⁻). This trend aligns with the pKa values of the corresponding alcohols: tert-butanol (pKa ≈ 18), ethanol (pKa ≈ 15.9), and methanol (pKa ≈ 15.5).[37] The inductive electron-donating effect of additional alkyl groups raises the pKa of the alcohol by increasing electron density on the oxygen, thereby stabilizing the neutral ROH relative to the anion RO⁻ and enhancing the basicity of RO⁻.[38] In addition to their basicity, alkoxides display significant nucleophilicity, particularly in bimolecular nucleophilic substitution (SN2) reactions, where they attack electrophilic centers like alkyl halides. Their nucleophilicity is notably high even in protic solvents, such as alcohols, due to the inherent electron richness of the oxygen anion, though hydrogen bonding with the solvent partially solvates the alkoxide and moderates its reactivity.[39] This solvation effect is diminished in polar aprotic solvents (e.g., DMSO or DMF), which lack hydrogen bond donors, leading to "naked" alkoxide ions with enhanced nucleophilicity and accelerated SN2 rates—often by orders of magnitude compared to protic media. Nucleophilicity generally parallels basicity for similar structures within the same solvent class, but decreases with increasing alkyl substitution due to steric hindrance, with less substituted alkoxides like methoxide being more effective in SN2 than bulkier ones like tert-butoxide, especially for unhindered substrates.[40]Reactions with Alkyl Halides
Alkoxides serve as nucleophiles in substitution reactions with alkyl halides, primarily through the Williamson ether synthesis, which forms ethers via an SN2 mechanism.[41] In this process, the alkoxide ion (RO⁻) attacks the carbon atom bearing the halogen in the alkyl halide (R'X), displacing the halide ion (X⁻) and yielding the ether product (ROR').[42] The general reaction is represented as: This synthesis is most effective with primary alkyl halides, where the SN2 pathway predominates due to minimal steric hindrance, allowing efficient backside attack by the nucleophile./09._Further_Reactions_of_Alcohols_and_the_Chemistry_of_Ethers/9.06:_Williamson_Ether_Synthesis) A classic example is the reaction of sodium ethoxide with ethyl bromide to produce diethyl ether and sodium bromide: This reaction proceeds under mild conditions, typically in ethanol solvent, and exemplifies the utility of alkoxides derived from simple alcohols.[43] The SN2 mechanism ensures stereochemical inversion at the carbon center of the alkyl halide, converting an (R)-configuration to (S) or vice versa, provided the substrate is chiral and secondary at most./Ethers/Synthesis_of_Ethers/Williamson_Ether_Synthesis) However, limitations arise with secondary or, especially, tertiary alkyl halides, where steric bulk favors E2 elimination over substitution, producing alkenes instead of ethers.[44] To address issues like the poor solubility of alkali metal alkoxides in nonpolar solvents, phase-transfer catalysis has been employed, using quaternary ammonium salts to transport the alkoxide into the organic phase and enhance reaction rates under milder, anhydrous-free conditions.[45] Modern variants, such as microwave-assisted Williamson reactions, further accelerate the process, often reducing reaction times to minutes while improving yields for challenging substrates like aryl alkyl ethers.[46]Specific Reactions
Hydrolysis
Hydrolysis of alkoxides involves the reaction with water, typically represented as , where M is a metal cation and R is an alkyl group. For alkali metal alkoxides, such as sodium or potassium derivatives, this process is rapid and proceeds via proton transfer from water to the alkoxide ion, owing to the strong basicity of the alkoxide. The reaction proceeds under mild conditions, often at room temperature, and is essentially irreversible, yielding the corresponding metal hydroxide and alcohol.[47] In contrast, hydrolysis of covalent metal alkoxides, such as those of transition metals, occurs more slowly and leads to the formation of oxo-hydroxo species through subsequent condensation reactions. This stepwise process generates reactive M-OH bonds that promote oligomerization and polymerization, forming larger metal-oxygen networks.[48] A representative example is the hydrolysis of titanium(IV) alkoxides, , which serves as a precursor in sol-gel synthesis of ; controlled addition of water produces hydroxo intermediates that condense to yield sols, enabling the fabrication of nanostructured metal oxide materials.[49] The kinetics of alkoxide hydrolysis are strongly pH-dependent, with the reaction rate exhibiting a minimum near neutral pH (around 7) and accelerating under acidic conditions. Acid catalysis protonates the alkoxide oxygen, enhancing the susceptibility of the M-OR bond to nucleophilic attack by water and thereby increasing the hydrolysis rate.[50][51] This pH sensitivity allows precise control over the reaction in applications like materials synthesis, where rapid hydrolysis under acidic media favors the formation of uniform oxide particles.[52]Transesterification and Ester Exchange
Transesterification, also known as ester exchange, is a reversible reaction in which an alkoxide ion facilitates the exchange of alkoxy groups between an ester and an alcohol, producing a new ester and a different alkoxide.[53] This process is base-catalyzed and relies on the nucleophilicity of the alkoxide to initiate the transformation.[53] The mechanism proceeds through a nucleophilic acyl substitution pathway. The alkoxide ion (RO⁻) attacks the carbonyl carbon of the ester (R'COOR''), forming a tetrahedral intermediate. This intermediate then collapses, reforming the carbonyl group and expelling the original alkoxy group (R''O⁻), yielding the new ester (R'COOR) and alkoxide (R''O⁻). The overall equilibrium is represented as: This stepwise process ensures the reaction's reversibility, necessitating careful control of conditions to favor the forward direction.[53] In practice, base-catalyzed transesterification employs alkoxides such as sodium methoxide (NaOMe) in methanol, typically at concentrations of 0.3–0.5% by weight of the ester substrate and temperatures below 60°C to prevent methanol boiling. A representative example is the conversion of vegetable oils, like soybean oil, into fatty acid methyl esters (FAME, or biodiesel) and glycerol, where NaOMe catalyzes the reaction with excess methanol (e.g., 100% molar excess) over 20 minutes to 1.5 hours under vigorous mixing.[54] This yields approximately 1004 kg of biodiesel per 1000 kg of soybean oil.[54] Industrially, the equilibrium is shifted toward product formation by using a large excess of alcohol (e.g., 6:1 molar ratio to triglycerides), in accordance with Le Chatelier's principle, which drives the reversible reaction forward and enhances conversion efficiency to over 94% under optimized conditions.[55] However, side reactions such as saponification can occur if free fatty acids are present or if excess catalyst is used, leading to soap formation that consumes reactants, reduces ester yields, and complicates separation of biodiesel from glycerol.[56] Mitigation involves precise catalyst dosing (e.g., 1.26% KOH equivalent) and elevated settling temperatures to minimize these effects.[56]Applications
Organic Synthesis
Alkoxides are widely employed as deprotonating agents in organic synthesis to generate enolates from active methylene compounds, facilitating carbon-carbon bond formations. A prominent example is their role in the Claisen condensation, where sodium ethoxide deprotonates the alpha hydrogen of an ester such as ethyl acetate, yielding an enolate ion that attacks the carbonyl carbon of a second ester molecule to produce a β-keto ester after elimination of the alkoxide leaving group. This reaction, first reported by Ludwig Claisen in 1887, relies on the equilibrium deprotonation driven by the higher acidity of the product β-keto ester (pKa ≈ 11) compared to the starting ester (pKa ≈ 25), ensuring complete conversion upon workup.[57] Beyond enolate formations, alkoxides participate in rearrangement reactions, such as the Favorskii rearrangement of α-halo ketones. In this process, an alkoxide base, often sodium methoxide or ethoxide, initiates the conversion of the halo ketone to a carboxylic ester via a semibenzilic mechanism involving cyclopropanone intermediate formation and subsequent migration, with the alkyl group from the alkoxide incorporated into the ester product. Originally described by Alexei Favorskii in 1895, this transformation is particularly useful for ring contractions in cyclic systems and proceeds under mild conditions in alcoholic solvents. Alkoxides also function as nucleophiles in the ring opening of epoxides under basic conditions, where the alkoxide attacks the less hindered carbon of the strained ring, leading to trans-1,2-alkoxy alcohols with high regioselectivity. This reaction is commonly performed with sodium or potassium alkoxides in alcoholic media and is valued for its stereospecificity in synthesizing polyols or ether derivatives.[58][59] The utility of alkoxides in these applications stems from their tunable basicity, which varies with the alkyl substituent—primary alkoxides like methoxide (conjugate acid pKa 15.5) are stronger bases than tertiary ones like tert-butoxide (pKa 18)—allowing selection based on substrate sensitivity, and their inherent compatibility with protic solvents like alcohols, which prevents side reactions in polar media.Materials Science and Catalysis
Alkoxides play a pivotal role in materials science through the sol-gel process, where metal alkoxides serve as precursors for synthesizing metal oxides and advanced ceramics at low temperatures. In this method, the alkoxide undergoes hydrolysis followed by condensation to form a sol that evolves into a gel network, ultimately yielding oxide materials upon drying and calcination. The general reaction for hydrolysis is represented as: where M is a metal cation, R is an alkyl group, and the stoichiometry adjusts based on the oxide's oxidation state. This approach enables precise control over composition, microstructure, and porosity, making it ideal for applications in coatings, fibers, and nanocomposites. A representative example is the use of tetraethoxysilane (TEOS, Si(OC₂H₅)₄) to produce silica gels, where controlled hydrolysis in ethanol-water mixtures forms amorphous SiO₂ networks with tunable pore sizes for use in optics and chromatography.[60] The sol-gel technique has been extensively applied to synthesize perovskite oxides, such as ABO₃ structures (e.g., BaTiO₃ or LaMnO₃), by combining metal alkoxides or alkoxide-salt mixtures as precursors. This method facilitates uniform doping and nanostructuring, enhancing properties like ferroelectricity and catalytic activity for energy storage devices. Recent advances in the 2020s have focused on optimizing sol-gel parameters—such as pH, precursor ratios, and chelating agents—to produce high-entropy perovskites and thin films with improved stability and performance in solid oxide fuel cells and photocatalysis, addressing the post-2010 surge in demand for multifunctional materials. For instance, alkoxide-based sol-gel routes have enabled the fabrication of SrTiO₃ nanoparticles with enhanced oxygen evolution reaction efficiency.[61] In catalysis, alkoxides function as homogeneous initiators or co-catalysts in polymerization reactions, leveraging their Lewis acidity and nucleophilicity to coordinate monomers and propagate chains. Aluminum alkoxides, such as Al(OiPr)₃, are particularly effective in the ring-opening polymerization (ROP) of cyclic esters like ε-caprolactone and L-lactide, yielding biodegradable polyesters such as polycaprolactone and polylactide with controlled molecular weights and low polydispersity. These catalysts operate via a coordination-insertion mechanism, where the alkoxide group initiates the ring opening, and the metal center stabilizes the growing chain. In Ziegler-Natta-type systems, soluble aluminum alkyl-titanium alkoxide combinations have been employed for the stereospecific polymerization of conjugated diolefins like butadiene and isoprene, producing cis-1,4-polybutadiene with high yield and tacticity suitable for synthetic rubber.[62] Recent developments highlight aluminum alkoxides in sustainable polymer synthesis, including stereoblock polylactides via binary systems with urea co-catalysts, advancing applications in biomedical materials.[63]Illustrative Examples
Sodium Methoxide
Sodium methoxide (CH₃ONa), also known as sodium methylate, is a prototypical alkoxide compound widely used in organic synthesis due to its strong basicity. It is typically prepared by the direct reaction of metallic sodium with anhydrous methanol, which proceeds as follows:This exothermic reaction requires careful control to manage hydrogen gas evolution and heat.[64] Commercially, sodium methoxide is often supplied as a 30 wt% solution in methanol to enhance stability and ease of handling, avoiding the challenges of isolating the pure solid.[65] As a white, amorphous, highly hygroscopic solid, sodium methoxide readily absorbs moisture from the air, which can lead to decomposition. It decomposes at approximately 127 °C without a distinct melting point, and it is highly soluble in methanol (with commercial solutions reaching 30 wt%), ethanol, and other polar solvents, but it reacts vigorously with water and is insoluble in hydrocarbons.[30] These properties necessitate storage under inert atmospheres or in alcoholic solutions to prevent hydrolysis.[66] In applications, sodium methoxide serves as a catalyst in the transesterification of vegetable oils or animal fats with methanol to produce biodiesel (fatty acid methyl esters), where it facilitates the conversion of triglycerides into esters and glycerol, achieving high yields under mild conditions.[67] It is also employed in the Claisen condensation, a key C-C bond-forming reaction for synthesizing β-keto esters from esters bearing α-hydrogens, typically using sodium methoxide in methanol for methyl ester substrates to drive the equilibrium toward the product. Safety considerations are critical, as sodium methoxide is corrosive to skin, eyes, and mucous membranes, causing severe burns upon contact. It reacts exothermically with water to generate sodium hydroxide and methanol, potentially leading to violent boiling or ignition if not controlled.[30] Handling requires protective equipment, inert conditions, and avoidance of moisture.[68]

