Recent from talks
Contribute something
Nothing was collected or created yet.
Neocortex
View on Wikipedia| Neocortex | |
|---|---|
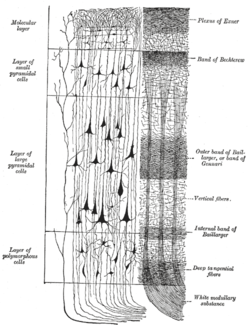
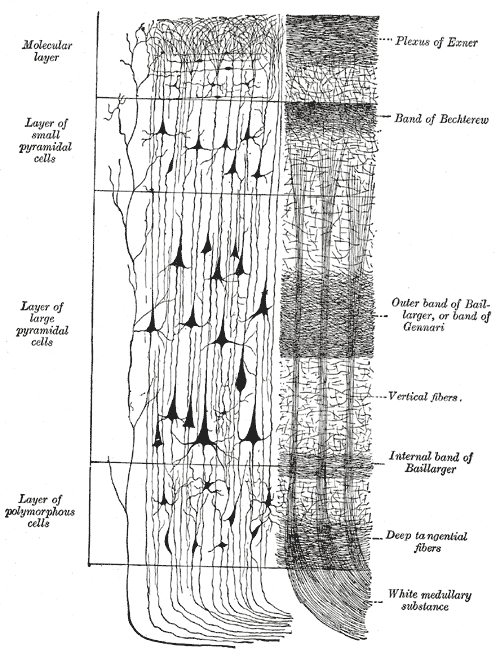
 A representative column of neocortex. Cell body layers are labeled on the left, and fiber layers are labeled on the right. | |
| Identifiers | |
| MeSH | D019579 |
| NeuroNames | 2314 |
| NeuroLex ID | birnlex_2547 |
| TA98 | A14.1.09.304 A14.1.09.307 |
| TA2 | 5532 |
| FMA | 62429 |
| Anatomical terms of neuroanatomy | |
The neocortex, also called the neopallium, isocortex, or the six-layered cortex, is a set of layers of the mammalian cerebral cortex involved in higher-order brain functions such as sensory perception, cognition, generation of motor commands,[1] spatial reasoning, and language.[2] The neocortex is further subdivided into the true isocortex and the proisocortex.[3]
In the human brain, the cerebral cortex consists of the larger neocortex and the smaller allocortex, respectively taking up 90% and 10%.[4] The neocortex is made up of six layers, labelled from the outermost inwards, I to VI.
Etymology
[edit]The term is from cortex, Latin, "bark" or "rind", combined with neo-, Greek, "new". Neopallium is a similar hybrid, from Latin pallium, "cloak". Isocortex and allocortex are hybrids with Greek isos, "same", and allos, "other".
Anatomy
[edit]The neocortex is the most developed in its organisation and number of layers, of the cerebral tissues.[5] The neocortex consists of the grey matter, or neuronal cell bodies and unmyelinated fibers, surrounding the deeper white matter (myelinated axons) in the cerebrum. This is a very thin layer though, about 2–4 mm thick.[6] There are two types of cortex in the neocortex, the proisocortex and the true isocortex. The pro-isocortex is a transitional area between the true isocortex and the periallocortex (part of the allocortex). It is found in the cingulate cortex (part of the limbic system), in Brodmann's areas 24, 25, 30 and 32, the insula and the parahippocampal gyrus.
Of all the mammals studied to date (including humans), a species of oceanic dolphin known as the long-finned pilot whale has been found to have the most neocortical neurons.[7]
Geometry
[edit]The neocortex is smooth in rodents and other small mammals, whereas in elephants, dolphins and primates and other larger mammals it has deep grooves (sulci) and ridges (gyri). These folds allow the surface area of the neocortex to be greatly increased. All human brains have the same overall pattern of main gyri and sulci, although they differ in detail from one person to another.[8] The mechanism by which the gyri form during embryogenesis is not entirely clear, and there are several competing hypotheses that explain gyrification, such as axonal tension,[9] cortical buckling[10] or differences in cellular proliferation rates in different areas of the cortex.[11]
Layers
[edit]
The neocortex contains both excitatory (~80%) and inhibitory (~20%) neurons, named for their effect on other neurons.[12] The human neocortex consists of hundreds of different types of cells.[13] The structure of the neocortex is relatively uniform (hence the alternative names "iso-" and "homotypic" cortex), consisting of six horizontal layers segregated principally by cell type and neuronal connections.[14] However, there are many exceptions to this uniformity; for example, layer IV is small or missing in the primary motor cortex. There is some canonical circuitry within the cortex; for example, pyramidal neurons in the upper layers II and III project their axons to other areas of neocortex, while those in the deeper layers V and VI often project out of the cortex, e.g. to the thalamus, brainstem, and spinal cord. Neurons in layer IV receive the majority of the synaptic connections from outside the cortex (mostly from thalamus), and themselves make short-range, local connections to other cortical layers.[12] Thus, layer IV is the main recipient of incoming sensory information and distributes it to the other layers for further processing.
Cortical columns
[edit]
The neocortex is often described as being arranged in vertical structures called cortical columns, patches of neocortex with a diameter of roughly 0.5 mm (and a depth of 2 mm, i.e., spanning all six layers). These columns are often thought of as the basic repeating functional units of the neocortex, but their many definitions, in terms of anatomy, size, or function, are generally not consistent with each other, leading to a lack of consensus regarding their structure or function or even whether it makes sense to try to understand the neocortex in terms of columns.[15]
Function
[edit]The neocortex is derived embryonically from the dorsal telencephalon, which is the rostral part of the forebrain. The neocortex is divided into regions demarcated by the cranial sutures in the skull above, into frontal, parietal, occipital, and temporal lobes, which perform different functions. For example, the occipital lobe contains the primary visual cortex, and the temporal lobe contains the primary auditory cortex. Further subdivisions or areas of neocortex are responsible for more specific cognitive processes. In humans, the frontal lobe contains areas devoted to abilities that are enhanced in or unique to our species, such as complex language processing localized to the ventrolateral prefrontal cortex (Broca's area).[12] In humans and other primates, social and emotional processing is localized to the orbitofrontal cortex.
The neocortex has also been shown to play an influential role in sleep, memory and learning processes. Semantic memories appear to be stored in the neocortex, specifically the anterolateral temporal lobe of the neocortex.[16] It is also involved in instrumental conditioning; responsible for transmitting sensory information and information about plans for movement to the basal ganglia.[16] The firing rate of neurons in the neocortex also has an effect on slow-wave sleep. When the neurons are at rest and are hyperpolarizing, a period of inhibition occurs during a slow oscillation, called the down state. When the neurons of the neocortex are in the excitatory depolarizing phase and are firing briefly at a high rate, a period of excitation occurs during a slow oscillation, called the up state.[16]
Clinical significance
[edit]Lesions that develop in neurodegenerative disorders, such as Alzheimer's disease, interrupt the transfer of information from the sensory neocortex to the prefrontal neocortex. This disruption of sensory information contributes to the progressive symptoms seen in neurodegenerative disorders such as changes in personality, decline in cognitive abilities, and dementia.[17] Damage to the neocortex of the anterolateral temporal lobe results in semantic dementia, which is the loss of memory of factual information (semantic memories). These symptoms can also be replicated by transcranial magnetic stimulation of this area. If damage is sustained to this area, patients do not develop anterograde amnesia and are able to recall episodic information.[18]
Evolution
[edit]The neocortex is the newest part of the cerebral cortex to evolve (hence the prefix neo meaning new); the other part of the cerebral cortex is the allocortex. The cellular organization of the allocortex is different from the six-layered neocortex. In humans, 90% of the cerebral cortex and 76% of the entire brain is neocortex.[12]
For a species to develop a larger neocortex, the brain must evolve in size so that it is large enough to support the region. Body size, basal metabolic rate and life history are factors affecting brain evolution and the coevolution of neocortex size and group size.[19] The neocortex increased in size in response to pressures for greater cooperation and competition in early ancestors. With the size increase, there was greater voluntary inhibitory control of social behaviors resulting in increased social harmony.[20]
The six-layer cortex appears to be a distinguishing feature of mammals; it has been found in the brains of all mammals, but not in any other animals.[2] There is some debate,[21][22] however, as to the cross-species nomenclature for neocortex. In avians, for instance, there are clear examples of cognitive processes that are thought to be neocortical in nature, despite the lack of the distinctive six-layer neocortical structure.[23] Evidence suggest the avian pallium to be broadly equivalent to the mammalian neocortex.[24][25][26] In a similar manner, reptiles, such as turtles, have primary sensory cortices. A consistent, alternative name has yet to be agreed upon.
Neocortex ratio
[edit]The neocortex ratio of a species is the ratio of the size of the neocortex to the rest of the brain. A high neocortex ratio is thought to correlate with a number of social variables such as group size and the complexity of social mating behaviors.[27] Humans have a large neocortex as a percentage of total brain matter when compared with other mammals. For example, there is only a 30:1 ratio of neocortical gray matter to the size of the medulla oblongata in the brainstem of chimpanzees, while the ratio is 60:1 in humans.[28]
See also
[edit]- List of regions in the human brain
- Blue Brain, a project to produce a computer simulation of a neocortical column and eventually a whole neocortex
- Memory-prediction framework, a theory of the neocortex function by Jeff Hawkins and related software models
- Claustrum
References
[edit]- ^ Lodato S, Arlotta P (2015-11-13). "Generating neuronal diversity in the mammalian cerebral cortex". Annual Review of Cell and Developmental Biology. 31 (1): 699–720. doi:10.1146/annurev-cellbio-100814-125353. PMC 4778709. PMID 26359774.
The neocortex is the part of the brain responsible for execution of higher-order brain functions, including cognition, sensory perception, and sophisticated motor control.
- ^ a b Lui JH, Hansen DV, Kriegstein AR (July 2011). "Development and evolution of the human neocortex". Cell. 146 (1): 18–36. doi:10.1016/j.cell.2011.06.030. PMC 3610574. PMID 21729779.
- ^ "BrainInfo". braininfo.rprc.washington.edu.
- ^ Saladin, K (2012). Anatomy & physiology : the unity of form and function (6th ed.). New York, NY: McGraw-Hill. p. 417. ISBN 9780073378251.
- ^ Dorland's Illustrated Medical Dictionary (32nd ed.). Elsevier Saunders. 2012. p. 1238. ISBN 978-1-4160-6257-8.
- ^ Kandel E (2006). Principles of neural science (5th ed.). Appleton and Lange: McGraw Hill. ISBN 978-0071390118.
- ^ Mortensen HS, Pakkenberg B, Dam M, Dietz R, Sonne C, Mikkelsen B, Eriksen N (2014). "Quantitative relationships in delphinid neocortex". Frontiers in Neuroanatomy. 8: 132. doi:10.3389/fnana.2014.00132. PMC 4244864. PMID 25505387.
- ^ Moerel M, De Martino F, Formisano E (2006). "An anatomical and functional topography of human auditory cortical areas". Front. Neurosci. 8 (225): 225. doi:10.3389/fnins.2014.00225. PMC 4114190. PMID 25120426.
For example, in the human brain, the auditory cortex presents an expansion of cortical surface, with additional gyri and with a much larger inter-individual variability...
- ^ Van Essen DC (January 1997). "A tension-based theory of morphogenesis and compact wiring in the central nervous system" (PDF). Nature. 385 (6614): 313–8. doi:10.1038/385313a0. PMID 9002514. S2CID 4355025. Archived from the original (PDF) on 2014-07-14. Retrieved 2014-03-29.
- ^ Richman DP, Stewart RM, Hutchinson JW, Caviness VS (July 1975). "Mechanical model of brain convolutional development". Science. 189 (4196): 18–21. doi:10.1126/science.1135626. PMID 1135626.
- ^ Ronan L, Voets N, Rua C, Alexander-Bloch A, Hough M, Mackay C, Crow TJ, James A, Giedd JN, Fletcher PC (August 2014). "Differential tangential expansion as a mechanism for cortical gyrification". Cerebral Cortex. 24 (8): 2219–28. doi:10.1093/cercor/bht082. PMC 4089386. PMID 23542881.
- ^ a b c d Noback CR, Strominger NL, Demarest RJ, Ruggiero DA (2005). The Human Nervous System: Structure and Function (Sixth ed.). Totowa, NJ: Humana Press. ISBN 1-59259-730-0.
- ^ Berg, Jim; Sorensen, Staci A.; Ting, Jonathan T.; Miller, Jeremy A.; Chartrand, Thomas; Buchin, Anatoly; Bakken, Trygve E.; Budzillo, Agata; Dee, Nick; Ding, Song-Lin; Gouwens, Nathan W.; Hodge, Rebecca D.; Kalmbach, Brian; Lee, Changkyu; Lee, Brian R. (October 2021). "Human neocortical expansion involves glutamatergic neuron diversification". Nature. 598 (7879): 151–158. doi:10.1038/s41586-021-03813-8. ISSN 1476-4687. PMC 8494638. PMID 34616067.
- ^ Kurzweil R (2012). How to Create a Mind: The Secret of Human Thought Revealed. New York: Viking Penguin. p. 36. ISBN 978-0670025299.
- ^ Horton JC, Adams DL (April 2005). "The cortical column: a structure without a function". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 360 (1456): 837–62. doi:10.1098/rstb.2005.1623. PMC 1569491. PMID 15937015.
- ^ a b c Carlson N (2013). Physiology of Psychology (Eleventh ed.). Pearson. ISBN 978-0-205-239481.
- ^ Braak H, Del-Tredici K, Bohl J, Bratzke H, Braak E (2000). Annals of the New York academy of sciences, Vol. 911. New York, NY, US: New York Academy of Sciences. ISBN 1-57331-263-0.
- ^ Carlson N (2013). Physiology of Behavior. Pearson. ISBN 978-0-205-23948-1.
- ^ Dunbar RI, Shultz S (April 2007). "Understanding primate brain evolution". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 362 (1480): 649–58. doi:10.1098/rstb.2006.2001. PMC 2346523. PMID 17301028.
- ^ Bjorklund D, Kipp K (2002). Social cognition, inhibition, and theory of mind: The evolution of human intelligence. Mahwah, NJ: Lawrence Erlbaum Associate Publishers. ISBN 0-8058-3267-X.
- ^ Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, et al. (February 2005). "Avian brains and a new understanding of vertebrate brain evolution". Nature Reviews. Neuroscience. 6 (2): 151–9. doi:10.1038/nrn1606. PMC 2507884. PMID 15685220.
- ^ Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, et al. (May 2004). "Revised nomenclature for avian telencephalon and some related brainstem nuclei". The Journal of Comparative Neurology. 473 (3): 377–414. doi:10.1002/cne.20118. PMC 2518311. PMID 15116397.
- ^ Prior H, Schwarz A, Güntürkün O (August 2008). De Waal F (ed.). "Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition". PLOS Biology. 6 (8): e202. doi:10.1371/journal.pbio.0060202. PMC 2517622. PMID 18715117.
- Alison Motluk (19 August 2008). "Mirror test shows magpies aren't so bird-brained". New Scientist.
- ^ Stacho, Martin; Herold, Christina; Rook, Noemi; Wagner, Hermann; Axer, Markus; Amunts, Katrin; Güntürkün, Onur (2020-09-25). "A cortex-like canonical circuit in the avian forebrain". Science. 369 (6511). doi:10.1126/science.abc5534. ISSN 0036-8075. PMID 32973004.
- ^ Nieder, Andreas; Wagener, Lysann; Rinnert, Paul (September 25, 2020). "A neural correlate of sensory consciousness in a corvid bird". Science. 369 (6511): 1626–1629. Bibcode:2020Sci...369.1626N. doi:10.1126/science.abb1447. ISSN 0036-8075. PMID 32973028.
- ^ Herculano-Houzel, Suzana (September 25, 2020). "Birds do have a brain cortex—and think". Science. 369 (6511): 1567–1568. Bibcode:2020Sci...369.1567H. doi:10.1126/science.abe0536. ISSN 0036-8075. PMID 32973020.
- ^ Dunbar RI (1995). "Neocortex size and group size in primates: A test of the hypothesis". Journal of Human Evolution. 28 (3): 287–96. doi:10.1006/jhev.1995.1021.
- ^ Semendeferi K, Lu A, Schenker N, Damasio H (March 2002). "Humans and great apes share a large frontal cortex". Nature Neuroscience. 5 (3): 272–6. doi:10.1038/nn814. PMID 11850633. S2CID 5921065.
External links
[edit]- Comparative Neuroscience at Wikiversity
- "Model of the neocortex". Brain Engineering Laboratory. Dartmouth College.
- "Proisocortex". Brain Info. University of Washington. Archived from the original on 2006-10-23. Retrieved 2014-06-17.
Neocortex
View on GrokipediaDefinition and Etymology
Definition
The neocortex, also known as the isocortex, constitutes the six-layered outer region of the cerebral cortex in mammals, forming the bulk of the brain's outer mantle and enabling advanced neural processing. In humans, it accounts for approximately 90% of the cerebral cortex's surface area, spanning an unfolded expanse of roughly 2,000–2,500 cm², and harbors about 16 billion neurons, underscoring its central role in higher brain functions. This phylogenetically newer structure evolved alongside the emergence of mammals, providing a uniform architectural foundation for integrating diverse neural activities across species.[4][5][6] Distinct from the allocortex, which features a simpler three-layered organization primarily in regions like the hippocampus, and the paleocortex, characterized by transitional three- to five-layer arrangements in olfactory areas, the neocortex maintains a consistent six-layer cytoarchitecture throughout its extent. This uniformity facilitates efficient information processing and connectivity, setting it apart as the dominant cortical type in mammalian brains.[4][7] Fundamentally, the neocortex serves as the neural hub for synthesizing sensory inputs from the environment, orchestrating voluntary motor outputs, and underpinning complex cognitive processes such as perception, decision-making, and learning. Its expansive neuronal network supports the emergence of sophisticated behaviors unique to mammals, with its layered design—briefly, comprising molecular, granular, and pyramidal cell strata—enabling hierarchical signal propagation.[8]Etymology
The term "neocortex" is derived from the Greek prefix neo- ("new") and the Latin cortex ("bark" or "rind"), reflecting its identification as the phylogenetically recent outer layer of the mammalian cerebral hemispheres.[9] This nomenclature arose amid 19th-century efforts to categorize cerebral structures by evolutionary age, with Theodor Meynert playing a pivotal role in distinguishing the neocortex from more primitive regions. In 1867, Meynert published the first detailed microscopic analysis of the mammalian cerebral cortex, describing its horizontal layering and contrasting it with allocortical areas like the hippocampus, which he viewed as evolutionarily older.[10] His classification underscored the neocortex's relative novelty in vertebrate brain evolution.[11] Meynert elaborated on these ideas in his influential 1872 chapter "Vom Gehirn der Säugetiere" within Stricker's Handbuch der Lehre von den Geweben des Menschen und der Thiere, where he systematically outlined the cytoarchitecture and connectivity of cortical tissues, establishing a framework for recognizing the neocortex as a distinct, advanced formation.[12] By the early 20th century, the English term "neocortex" entered scientific literature, with its earliest documented use in 1909 by C. U. Ariëns Kappers in a comparative study of cortical evolution.[13] Around the same period, Korbinian Brodmann adopted "isocortex" in his 1909 cytoarchitectonic atlas to denote the six-layered, homotypical cortex, emphasizing structural uniformity rather than phylogenetic newness.[14] In contemporary usage, "neocortex" has supplanted "isocortex" as the standard designation, prioritizing evolutionary context while retaining the latter for specific architectural discussions.[15]Anatomy
Geometry and Organization
The neocortex forms a highly folded sheet of neural tissue that constitutes the outer layer of the cerebral hemispheres in mammals, particularly expanded in humans to accommodate increased computational capacity within the confined space of the skull. This folding pattern consists of ridges known as gyri and grooves called sulci, which dramatically expand the surface area while maintaining a relatively thin structure with an average thickness of 2-4 mm. In humans, the total cortical surface area measures approximately 2000 cm², with about two-thirds concealed within the sulci, allowing for a total cortical volume of around 458 cm³.[4][16] The neocortex is regionally organized into four primary lobes—frontal, parietal, temporal, and occipital—delineated by prominent sulci such as the central sulcus (separating frontal from parietal) and the lateral sulcus (separating frontal/parietal from temporal). These lobes encompass primary sensory and motor areas as well as extensive association areas that integrate information across modalities, with the association cortices predominantly located in the parietal, temporal, and frontal regions.[17][18] Cytoarchitectonic mapping further subdivides the neocortex into distinct regions based on variations in neuronal density, size, and layering, as pioneered by Korbinian Brodmann in the early 20th century. Brodmann's scheme identifies approximately 48 areas across the cerebral cortex, each with characteristic cellular architectures that correlate with functional specializations; for instance, area 17 corresponds to the primary visual cortex in the occipital lobe. Modern techniques, including myelin mapping and transcriptomics, estimate approximately 180-200 neocortical areas.[14][19][20] Underlying the neocortical gray matter are white matter tracts that facilitate inter-regional communication, including commissural fibers such as the corpus callosum, which interconnects homologous regions between the left and right hemispheres to enable bilateral integration.[21]Layered Structure
The neocortex exhibits a characteristic six-layered, or laminar, organization that distinguishes it from other cortical regions. These layers, numbered I through VI from the pial surface (outermost) inward toward the white matter, vary in thickness, neuronal density, and composition across different cortical areas. Layer I, also known as the molecular layer, is the thinnest and contains few neurons, primarily horizontal cells and apical dendrites from deeper pyramidal neurons, serving mainly as a site for axonal arborizations.[4] Layers II and III, collectively termed the external granular layer, are dominated by small pyramidal neurons whose axons form intracortical associations, projecting to other cortical regions. These layers are rich in granule cells and support local connectivity within the cortex. Layer IV, the internal granular layer, is prominent in sensory areas and consists primarily of spiny stellate cells and smaller pyramidal cells that receive major thalamic inputs, acting as the primary site for sensory relay.[22][23] Layer V, the internal pyramidal layer, features large pyramidal neurons with long axons that project to subcortical targets such as the brainstem, spinal cord, and thalamus, facilitating output from the cortex. Layer VI, the multiform layer, contains a mix of pyramidal and fusiform neurons, with projections back to the thalamus and claustrum, and is involved in modulating thalamic activity. Throughout these layers, the neuronal population is predominantly excitatory glutamatergic neurons (about 80%), including pyramidal and spiny stellate cells, interspersed with inhibitory GABAergic interneurons such as basket and chandelier cells that regulate local circuits.[22][24][23] While the six-layer structure is canonical, variations exist based on cytoarchitectonic features, particularly the prominence of Layer IV. Agranular cortex, typical of motor areas like the primary motor cortex, lacks a distinct Layer IV due to sparse granule cells, emphasizing pyramidal projections. Granular cortex, found in primary sensory regions such as the primary visual or somatosensory cortex, has a well-developed Layer IV with high densities of stellate cells for sensory processing. Dysgranular cortex, intermediate between the two, features a poorly defined Layer IV and occurs in transitional areas like parts of the insular cortex. These types reflect adaptations to functional demands, with agranular regions prioritizing output and granular ones input integration.[25][26] The neocortex is densely packed with synapses, estimated at approximately per cubic centimeter, enabling complex information processing. This high synaptic density, with excitatory glutamatergic synapses outnumbering inhibitory GABAergic ones (approximately 80-90% excitatory), supports the balance necessary for cortical computation.[27][24][28]Cortical Columns
The neocortex exhibits a vertical columnar organization, where neurons are arranged in narrow, cylinder-like structures perpendicular to the cortical surface, spanning all six layers. This organization was first identified by Vernon Mountcastle through electrophysiological recordings in the somatic sensory cortex of cats, revealing that neurons within these vertical penetrations shared similar receptive fields and response properties to specific sensory modalities. Mountcastle's observations indicated that these columns serve as fundamental processing units, with adjacent columns responding to neighboring regions of the sensory periphery. Within this framework, the neocortex is composed of minicolumns, the smallest vertical units, each comprising approximately 80–100 neurons extending across the cortical layers, with a diameter of about 30–50 µm. These minicolumns form the basic replicative unit of cortical architecture, as proposed by Mountcastle in his synthesis of columnar principles. Groups of minicolumns, typically 50–100 in number, aggregate to form larger hypercolumns, which have a diameter of 300–500 µm and integrate related functions, such as processing specific stimulus orientations in the visual cortex. Cortical columns demonstrate functional modularity, acting as discrete units specialized for particular features of the sensory environment. In the primary visual cortex (V1), for instance, columns are tuned to specific attributes like edge orientation, with adjacent columns representing progressively different angles. This columnar tuning was elucidated by David Hubel and Torsten Wiesel through microelectrode recordings in cat visual cortex during the 1960s, where they observed that neurons in a single vertical penetration responded preferentially to bars of light at a consistent orientation. Columnar organization varies across neocortical regions, with minicolumn spacing and density differing systematically; for example, minicolumns are smaller and more densely packed in primary sensory areas compared to association cortices, where they are wider and sparser.[29] This regional variation reflects adaptations to the computational demands of different cortical territories, such as finer-grained feature detection in sensory processing zones.[29]Functions
Sensory and Motor Processing
The neocortex plays a central role in sensory processing by receiving relayed inputs from the thalamus to its primary sensory areas, enabling the initial detection and representation of environmental stimuli. The primary visual cortex (V1), situated in the occipital lobe, processes basic visual features such as edges and orientations from inputs via the lateral geniculate nucleus of the thalamus, with higher-order areas V2 through V5 in the same region handling progressively complex attributes like motion and color.[30][31] Similarly, the primary auditory cortex (A1), located in the superior temporal gyrus of the temporal lobe, receives tonotopically organized inputs from the ventral division of the medial geniculate nucleus, allowing for the encoding of sound frequencies and temporal patterns.[32][33] The primary somatosensory cortex (S1), found in the postcentral gyrus of the parietal lobe, integrates tactile, proprioceptive, and nociceptive signals relayed from the ventral posterolateral and ventral posteromedial nuclei of the thalamus, facilitating the perception of touch and body position.[34][35] Motor processing in the neocortex involves distinct regions in the frontal lobe that coordinate voluntary actions. The primary motor cortex (M1), positioned in the precentral gyrus, directly executes fine-grained voluntary movements by sending descending projections to the spinal cord, particularly for distal musculature like fingers and hands.[36] Adjacent premotor cortex and supplementary motor area, both within Brodmann area 6, contribute to movement planning and sequencing, integrating sensory cues with internal goals to prepare actions such as reaching or grasping, often activating prior to M1 during complex tasks.[37][38] Sensory and motor functions exhibit hierarchical organization across neocortical areas, where primary regions detect elemental features before relaying to secondary areas for integration and contextual analysis. In sensory pathways, primary cortices like V1, A1, and S1 perform initial feature extraction—such as orientation selectivity in vision or frequency tuning in audition—while secondary areas combine these into coherent percepts, supported by feedforward connections that amplify relevant signals.[39][40] This progression ensures efficient processing from raw thalamic inputs to elaborated representations. A key feature of somatosensory and motor cortices is their somatotopic organization, mapping the body across the cortical surface in a distorted manner known as the homunculus. In S1 and M1, body parts are represented proportionally to their peripheral innervation density, with enlarged areas for the hands and face due to high receptor concentration, while trunk regions occupy smaller zones; this arrangement supports precise sensory discrimination and coordinated motor control.[41][42]Cognitive and Associative Roles
The neocortex's association cortices integrate sensory and motor information to support higher-order cognitive processes. The prefrontal association cortex, particularly its dorsolateral region, plays a central role in executive functions such as planning, decision-making, and cognitive control by maintaining goal-directed behavior and inhibiting irrelevant responses.[43] In contrast, the temporal-parietal association cortices contribute to spatial awareness and attentional modulation, enabling the prioritization of relevant environmental features for perception and action.[44] These regions form interconnected networks that facilitate abstract reasoning and adaptive behavior, drawing briefly on sensory inputs from primary areas to contextualize information.[45] Synaptic plasticity in the neocortex underpins learning and associative processes through mechanisms like long-term potentiation (LTP), an activity-dependent strengthening of synapses that enhances signal transmission between co-activated neurons. LTP, observed in neocortical pyramidal cells, supports the storage of associative memories by amplifying connections in response to correlated presynaptic and postsynaptic activity.[46] This process aligns with Hebbian learning principles, where "cells that fire together wire together," as proposed in foundational neurophysiological theory and evidenced in neocortical circuits for forming stable neural assemblies.[47] Such plasticity allows the neocortex to refine representations over time, enabling flexible adaptation to complex environments without altering core wiring. Distributed neocortical networks mediate working memory and episodic recall, essential for temporary information maintenance and autobiographical event reconstruction. The dorsolateral prefrontal cortex coordinates working memory by sustaining representations of task-relevant items through recurrent excitation and inhibition, facilitating manipulation and updating of mental content.[48] For episodic memory, neocortical regions like the temporal lobes integrate spatiotemporal details via hippocampal-neocortical interactions, supporting recall through pattern completion in distributed assemblies.[49] These networks contribute to aspects of consciousness by enabling unified awareness of internal states and external events, though the precise mechanisms remain under investigation. Recent connectomics studies from the 2020s, including large-scale reconstructions of mouse visual cortex, reveal dense recurrent connections within neocortical layers that enable predictive coding models. These loops allow neurons to anticipate sensory inputs by minimizing prediction errors, as seen in hierarchical circuits where feedback refines forward processing for efficient inference.[50] Such architectures, mapped at cellular resolution, underscore how recurrent motifs support associative integration and learning across cognitive domains.[51]Clinical Significance
Associated Disorders
The neocortex is implicated in a range of neurological and psychiatric disorders characterized by structural, functional, or connectivity disruptions within its layered architecture and columnar organization. These conditions often manifest through progressive degeneration, developmental anomalies, vascular insults, or aberrant neural signaling, leading to impairments in sensory processing, cognition, and behavior. Key examples include neurodegenerative diseases like Alzheimer's, developmental disorders such as autism spectrum disorders (ASD), vascular events like stroke, traumatic injuries, and psychiatric conditions including schizophrenia, each highlighting distinct vulnerabilities in neocortical regions.[52][53][54][55] In Alzheimer's disease, a primary neurodegenerative disorder, neocortical dysfunction arises from the accumulation of amyloid-β plaques and hyperphosphorylated tau tangles, which preferentially affect association areas such as the temporal and parietal cortices early in the disease progression. These pathological deposits disrupt synaptic integrity and neuronal connectivity, leading to cognitive decline, memory loss, and executive function deficits as the pathology spreads hierarchically from neocortical association zones to primary sensory and motor areas. Amyloid-β deposition is thought to initiate and facilitate the propagation of tau pathology throughout the neocortex, exacerbating neuronal loss and inflammation in these regions.[56][57][52] Autism spectrum disorders involve developmental alterations in neocortical organization, particularly affecting minicolumnar structure and connectivity, which are fundamental units of cortical processing. Postmortem studies reveal narrower minicolumn widths and disrupted peripheral neuropil in autistic brains, especially in prefrontal and superior temporal regions, contributing to atypical sensory integration, social cognition deficits, and repetitive behaviors. These changes suggest an imbalance in excitatory-inhibitory circuits and reduced long-range connectivity, potentially stemming from early prenatal disruptions in neuronal migration and synaptogenesis within the neocortex.[53][58][59] Vascular disorders like ischemic stroke, often resulting from occlusion of the middle cerebral artery (MCA), cause acute neocortical damage primarily in frontal and parietal regions, leading to contralateral motor and sensory deficits, aphasia, and neglect syndromes. MCA territory infarcts disrupt the blood supply to large expanses of lateral neocortex, resulting in selective neuronal loss and secondary gliosis in these association and sensorimotor areas. Traumatic brain injury (TBI), particularly through diffuse axonal injury (DAI), induces widespread shearing of neocortical white matter tracts and axonal pathology, impairing network communication and contributing to persistent cognitive and behavioral impairments. DAI in TBI predominantly affects neocortical projection fibers, triggering cytoskeletal changes and Wallerian degeneration that exacerbate long-term circuit dysfunction.[17][60][61][54][62] Schizophrenia, a psychiatric disorder with strong neocortical involvement, features prefrontal hypoactivity and disrupted thalamocortical loops, which underlie symptoms such as hallucinations, delusions, and cognitive disorganization. Hypoactivation in the dorsolateral prefrontal cortex impairs working memory and executive control, while aberrant thalamocortical connectivity disrupts sensory gating and attentional modulation across neocortical networks. These alterations reflect neurodevelopmental and neuroplastic changes in prefrontal and temporoparietal regions, contributing to the disorder's core deficits in reality testing and social functioning.[55][63][64] Epilepsy, particularly neocortical forms, involves hyperexcitability and structural malformations in the neocortex, such as focal cortical dysplasia (FCD), which disrupts laminar organization and leads to drug-resistant seizures originating from abnormal neuronal circuits. FCD type II, often linked to somatic mutations in the mTOR pathway, results in dysmorphic neurons and balloon cells in neocortical layers, causing aberrant synchronization and propagation of epileptiform activity across cortical columns. These changes impair sensory processing and cognitive functions, with surgical resections targeting epileptogenic zones to alleviate symptoms.[65][66] Major depressive disorder (MDD), a psychiatric condition, is associated with neocortical alterations including reduced prefrontal gray matter volume and dysfunctional connectivity in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex, contributing to mood dysregulation, anhedonia, and cognitive biases. Neuroimaging reveals hypoactivation in these association areas during emotional processing tasks, linked to imbalances in glutamatergic and GABAergic signaling within neocortical layers, which underlie impaired executive function and emotional regulation.[67][68]Diagnostic and Treatment Approaches
Diagnostic approaches to assessing neocortical integrity primarily rely on non-invasive imaging techniques that map functional, metabolic, and electrical activity across cortical layers and columns. Functional magnetic resonance imaging (fMRI) enables precise mapping of neocortical activation patterns during cognitive tasks, revealing disruptions in sensory-motor and associative processing relevant to disorders like epilepsy and dementia.[69] Positron emission tomography (PET) quantifies regional metabolic activity, such as glucose uptake in neocortical regions, which decreases in conditions involving cholinergic degeneration, providing biomarkers for early pathological changes.[70] Electroencephalography (EEG) and magnetoencephalography (MEG) capture spatiotemporal electrical and magnetic patterns originating from pyramidal neurons in neocortical layers II/III and V, offering high temporal resolution for detecting oscillatory abnormalities in cortical columns associated with seizures or cognitive decline.[71][72] Invasive methods, such as electrocorticography (ECoG), are employed during epilepsy surgery to localize seizure foci directly on exposed neocortical surfaces, recording high-fidelity signals from cortical layers to guide precise resections and improve outcomes in drug-resistant cases.[73] These recordings identify epileptogenic zones by analyzing interictal spikes and rhythmic activity in neocortical tissue, with meta-analyses confirming their utility in enhancing seizure freedom rates when integrated with preoperative imaging. For instance, intraoperative ECoG refines resection margins in temporal and frontal neocortical epilepsies, reducing recurrence by targeting residual abnormal discharges. Treatment strategies targeting neocortical dysfunction encompass pharmacological, neuromodulatory, and emerging genetic interventions tailored to specific pathologies. Cholinesterase inhibitors, such as donepezil and rivastigmine, address neocortical cholinergic loss in Alzheimer's disease by elevating acetylcholine levels, thereby ameliorating cognitive symptoms through enhanced transmission in affected cortical areas.[74] Transcranial magnetic stimulation (TMS) modulates prefrontal neocortical excitability in major depressive disorder, with repetitive high-frequency protocols over the dorsolateral prefrontal cortex yielding remission rates of 30-40% in treatment-resistant patients by normalizing dysfunctional connectivity.[68] These approaches address neocortical involvement in Alzheimer's disease, major depressive disorder, and epilepsy. Recent advances from 2023 to 2025 include AI-assisted analysis of connectome data for personalized neocortical diagnostics, leveraging machine learning to predict pathology from functional connectivity patterns in disorders like mild cognitive impairment.[75] Connectome-based predictive modeling integrates multimodal imaging to forecast neocortical tau accumulation and amyloid deposition, enabling early intervention with accuracies exceeding 80% in cohort studies.[76] Emerging gene therapies, such as AAV9-delivered constructs targeting mTOR pathways in focal cortical dysplasia, suppress neocortical hyperexcitability in preclinical models, paving the way for translational trials to correct malformations and reduce seizures.[77]Evolution and Comparative Aspects
Evolutionary Origins
The neocortex emerged approximately 300 million years ago in synapsid ancestors of mammals, evolving as an expansion of the reptilian dorsal cortex, a simpler three-layered structure in sauropsids.[78] This transition occurred in stem amniotes during the Carboniferous period, where the dorsal pallium began to develop increased cellular complexity and layering in the synapsid lineage, distinguishing it from the diapsid (reptilian) branch.[3] The neocortex's precursor thus represented an adaptive innovation for enhanced sensory integration in early mammal-like reptiles, setting the stage for further diversification in true mammals around 200 million years ago.[79] The defining six-layered architecture of the neocortex first appeared in early mammals during the late Triassic or early Jurassic periods, marking a key evolutionary milestone absent in non-mammalian vertebrates.[80] This laminar organization arose from the radial migration of neurons generated in the ventricular zone, transforming the uniform reptilian cortex into a radially and tangentially expanded sheet with specialized layers for processing.[81] Subsequent expansions occurred prominently in primates, where the neocortex grew disproportionately; the human neocortex has enlarged roughly 1,000-fold relative to that in small mammals like mice, reflecting dramatic expansion over mammalian evolution, with further increases in primates facilitating advanced cognitive capacities. This primate-specific proliferation involved increased numbers of basal progenitors during neurogenesis, driving both surface area and thickness. Genetic factors have played a pivotal role in neocortical enlargement and the development of gyrification, the folding that maximizes surface area within the skull. The human-specific gene ARHGAP11B, duplicated from ARHGAP11A around 5 million years ago, promotes the amplification of basal radial glia progenitors, leading to greater neuron production and cortical expansion observed in hominins.[82] Similarly, the FOXP2 gene, under positive selection in humans, influences neuronal migration and connectivity in the developing neocortex, contributing to gyrification patterns essential for increased computational density.[82] These molecular drivers underscore the rapid evolutionary tweaks that enhanced neocortical folding and volume in the hominin lineage. Fossil evidence from endocranial casts provides direct insights into early neocortical morphology, revealing the onset of folding in australopithecines around 4 million years ago. Endocasts of Australopithecus afarensis specimens, such as those from Hadar, Ethiopia, show impressions of nascent sulci and gyri on the frontal and parietal lobes, indicating the beginnings of neocortical expansion and convolution beyond the smoother brains of earlier apes. These features suggest that gyrification was underway by the Pliocene, correlating with bipedalism and environmental pressures that favored enhanced sensory-motor integration.[83] Such paleoneurological data highlight the neocortex's progressive adaptation in early hominins, bridging reptilian origins to modern complexity.[84]Neocortex Ratio and Species Comparisons
The neocortex ratio, defined as the ratio of neocortex volume to the volume of the rest of the brain (neocortex volume / (total brain volume minus neocortex volume)), serves as a quantitative measure of relative neocortical expansion and has been proposed as an indicator of cognitive capacity across mammalian species.[85] This volume-based metric, pioneered by R.I.M. Dunbar (1992), correlates with social complexity; recent neuron-count approaches (Herculano-Houzel, 2009) provide refined comparisons accounting for cellular density variations, emphasizing neuronal distribution over gross volume.[6] In humans, the neocortex constitutes about 80% of brain volume, yielding a neocortex ratio of approximately 4.1. This value reflects the significant expansion of the neocortex relative to subcortical regions in Homo sapiens, supporting advanced cognitive processing. In contrast, dolphins exhibit a higher ratio of about 5.3, driven by their large neocortical volumes relative to the rest of the brain—despite total brain sizes similar to humans. Elephants, however, maintain a ratio around 4.0, with a relatively smaller proportion of brain volume dedicated to the neocortex despite a total brain exceeding 4 kg, largely due to enlarged brainstem, diencephalon, and cerebellum adapted for their massive body size and sensory-motor demands.[6][86][87] Complementing volume ratios, direct neuron counts reveal further insights: the human neocortex contains approximately 16 billion neurons, while dolphins have 10–37 billion neocortical neurons depending on species (e.g., ~37 billion in the long-finned pilot whale), and elephants have only about 5.6 billion despite a total brain neuron count exceeding 257 billion. This ratio correlates with behavioral indicators of cognitive sophistication, including tool use and social complexity, as species with higher values demonstrate enhanced abilities to manipulate environments and maintain intricate social bonds. For instance, elevated neocortex ratios facilitate coalition formation and deception in social groups, key adaptations in species facing complex ecological pressures. Elephants, despite their vast total neuronal resources, show a lower effective cognitive leverage from the neocortex due to the disproportionate investment in non-neocortical structures for autonomic regulation in their large bodies, limiting relative capacity for abstract reasoning compared to high-ratio species like dolphins.[88][89][87] Among primates, the neocortex ratio exhibits a progressive increase along evolutionary lineages, underscoring the order's specialization in cognitive evolution. Prosimians and early anthropoids have ratios below 3.0, while great apes range from 3.5 to 4.0, with chimpanzees approaching 3.5 and reflecting their proficiency in tool use and proto-cultural behaviors. This trend culminates in humans at approximately 4.1, enabling unparalleled social and technological advancements.[6][90]| Species/Group | Approximate Neocortex Ratio | Key Neocortical Neurons (billions) | Notable Implications |
|---|---|---|---|
| Humans | 4.1 | 16 | Advanced tool use, complex societies[91] [6] |
| Bottlenose Dolphin | 5.3 | ~10–12 | High social intelligence, echolocation integration[86] |
| African Elephant | 4.0 | 5.6 | Memory and empathy, but brainstem-dominant[87] |
| Great Apes (e.g., Chimpanzees) | 3.5–4.0 | ~6–9 | Emergent tool use, group alliances[6] |