Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Prostaglandin.
Nothing was collected or created yet.
Prostaglandin
View on Wikipediafrom Wikipedia
Not found
Prostaglandin
View on Grokipediafrom Grokipedia
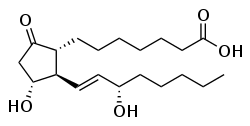
Prostaglandins are a family of bioactive lipid compounds, classified as eicosanoids, that are enzymatically derived from the 20-carbon polyunsaturated fatty acid arachidonic acid primarily through the cyclooxygenase (COX) pathway, acting as autacoids with paracrine and autocrine effects rather than circulating hormones.[1][2] These molecules are not stored in cells but synthesized rapidly in response to stimuli, binding to specific G-protein-coupled receptors to modulate diverse physiological processes including vasodilation, smooth muscle contraction, platelet aggregation inhibition, and cytokine-mediated responses.[2][3]
First identified in the early 1930s by Swedish physiologist Ulf von Euler through bioassays of human semen and sheep seminal vesicular gland extracts, which exhibited potent smooth muscle-stimulating activity, prostaglandins were so named due to their initial detection in prostate-related tissues, though they are produced ubiquitously across mammalian organs and tissues.[4] Subsequent structural elucidation in the 1960s by Sune Bergström and others revealed their cyclopentane ring core with side chains, paving the way for understanding their biosynthesis from endoperoxide intermediates like PGH2 via isomerases and synthases.[1] This foundational work, alongside discoveries on their roles in inflammation by John Vane, earned Bergström, Bengt Samuelsson, and Vane the 1982 Nobel Prize in Physiology or Medicine for prostaglandins and related substances.[4]
In physiology, prostaglandins such as PGE2 and PGI2 (prostacyclin) promote homeostasis by regulating renal blood flow, gastric mucosal protection, and parturition, while also driving pathogenic states like fever, pain sensitization, and chronic inflammation through COX-2 induction in response to injury or infection.[2][5] Their inhibition by COX-blocking nonsteroidal anti-inflammatory drugs underscores their centrality in therapeutic targeting, though this also explains side effects like gastrointestinal ulceration from reduced cytoprotective PGE2.[2] Synthetic analogs, including misoprostol for ulcer prevention and dinoprostone for labor induction, exploit these pathways for clinical utility.[3]