Recent from talks
Contribute something
Nothing was collected or created yet.
Stable nuclide
View on WikipediaThis article needs additional citations for verification. (December 2018) |

Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay.[1] When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes.
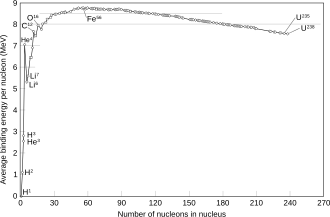
The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element.
Definition of stability, and naturally occurring nuclides
[edit]Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (total of 286) are known to be radioactive with long enough half-lives (also known) to occur primordially. If the half-life of a nuclide is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the formation of the Solar System, and then is said to be primordial. It will then contribute in that way to the natural isotopic composition of a chemical element. Primordial radioisotopes are easily detected with half-lives as short as 700 million years (e.g., 235U). This is the present limit of detection,[citation needed] as shorter-lived nuclides have not yet been detected undisputedly in nature except when recently produced, such as decay products or cosmic ray spallation.
Many naturally occurring radioisotopes (another 53 or so, for a total of about 339) exhibit still shorter half-lives than 700 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium), or from ongoing energetic reactions, such as cosmogenic nuclides produced by present bombardment of Earth by cosmic rays (for example, 14C made from nitrogen).
Some isotopes that are classed as stable (i.e. no radioactivity has been observed for them) are predicted to have extremely long half-lives (sometimes 1018 years or more).[2] If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of stable nuclides to the radioactive category, once their activity is observed. For example, 209Bi and 180W were formerly classed as stable, but were found to be alpha-active in 2003. However, such nuclides do not change their status as primordial when they are found to be radioactive.
Most stable isotopes on Earth are believed to have been formed in processes of nucleosynthesis, either in the Big Bang, or in generations of stars that preceded the formation of the Solar System. However, some stable isotopes also show abundance variations in the earth as a result of decay from long-lived radioactive nuclides. These decay-products are termed radiogenic isotopes, in order to distinguish them from the much larger group of 'non-radiogenic' isotopes.
Isotopes per element
[edit]Of the known chemical elements, 80 elements have at least one stable nuclide. These comprise the first 82 elements from hydrogen to lead, with the two exceptions, technetium (element 43) and promethium (element 61), that do not have any stable nuclides. As of 2024, there are total of 251 known "stable" nuclides. In this definition, "stable" means a nuclide that has never been observed to decay against the natural background. Thus, these elements have half-lives too long to be measured by any means, direct or indirect.
Stable isotopes:
- 1 element (tin) has 10 stable isotopes
- 5 elements have 7 stable isotopes apiece
- 7 elements have 6 stable isotopes apiece
- 11 elements have 5 stable isotopes apiece
- 9 elements have 4 stable isotopes apiece
- 5 elements have 3 stable isotopes apiece
- 16 elements have 2 stable isotopes apiece
- 26 elements have 1 single stable isotope.
These last 26 are thus called monoisotopic elements.[3] The mean number of stable isotopes for elements which have at least one stable isotope is 251/80 = 3.1375.
Physical magic numbers and odd and even proton and neutron count
[edit]Stability of isotopes is affected by the ratio of protons to neutrons, and also by presence of certain magic numbers of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. As in the case of tin, a magic number for Z, the atomic number, tends to increase the number of stable isotopes for the element.
Just as in the case of electrons, which have the lowest energy state when they occur in pairs in a given orbital, nucleons (both protons and neutrons) exhibit a lower energy state when their number is even, rather than odd. This stability tends to prevent beta decay (in two steps) of many even–even nuclides into another even–even nuclide of the same mass number but lower energy (and of course with two more protons and two fewer neutrons), because decay proceeding one step at a time would have to pass through an odd–odd nuclide of higher energy. Such nuclei thus instead undergo double beta decay (or are theorized to do so) with half-lives several orders of magnitude larger than the age of the universe. This makes for a larger number of stable even–even nuclides, which account for 150 of the 251 total. Stable even–even nuclides number as many as three isobars for some mass numbers, and up to seven isotopes for some atomic numbers.
Conversely, of the 251 known stable nuclides, only five have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, nitrogen-14, and tantalum-180m. Also, only four naturally occurring, radioactive odd–odd nuclides have a half-life >109 years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Odd–odd primordial nuclides are rare because most odd–odd nuclei beta-decay, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.[4]
Yet another effect of the instability of an odd number of either type of nucleon is that odd-numbered elements tend to have fewer stable isotopes. Of the 26 monoisotopic elements (those with only one stable isotope), all but one have an odd atomic number, and all but one has an even number of neutrons: the single exception to both rules is beryllium.
The end of the stable elements occurs after lead, largely because nuclei with 128 neutrons—two neutrons above the magic number 126—are extraordinarily unstable and almost immediately alpha-decay.[5] This contributes to the very short half-lives of astatine, radon, and francium. A similar phenomenon occurs to a much lesser extent with 84 neutrons—two neutrons above the magic number 82—where various isotopes of lanthanide elements alpha-decay.
Nuclear isomers, including a "stable" one
[edit]The 251 known stable nuclides include tantalum-180m, since even though its decay is automatically implied by it being "metastable", this has not been observed. All "stable" isotopes (stable by observation, not theory) are the ground states of nuclei, except for tantalum-180m, which is a nuclear isomer or excited state. The ground state, tantalum-180, is radioactive with half-life 8 hours; in contrast, the decay of the nuclear isomer is extremely strongly forbidden by spin-parity selection rules. It has been reported by direct observation that the half-life of 180mTa to gamma decay must be >1015 years. Other possible modes of 180mTa decay (beta decay, electron capture, and alpha decay) have also never been observed.
Still-unobserved decay
[edit]It is expected that improvement of experimental sensitivity will allow discovery of very mild radioactivity of some isotopes now considered stable. For example, in 2003 it was reported that bismuth-209 (the only primordial isotope of bismuth) is very mildly radioactive, with half-life (1.9 ± 0.2) × 1019 yr,[6][7] confirming earlier theoretical predictions[8] from nuclear physics that bismuth-209 would very slowly alpha decay.
Isotopes that are theoretically believed to be unstable but have not been observed to decay are termed observationally stable. Currently there are 105 "stable" isotopes which are theoretically unstable, 40 of which have been observed in detail with no sign of decay, the lightest in any case being 36Ar.[citation needed] Many "stable" nuclides are "metastable" in that they would release energy if they were to decay,[9] and are expected to undergo very rare kinds of radioactive decay, including double beta decay.
146 nuclides from 62 elements with atomic numbers from 1 (hydrogen) to 66 (dysprosium) except 43 (technetium), 61 (promethium), 62 (samarium), and 63 (europium) are theoretically stable to any kind of nuclear decay — except for the theoretical possibility of proton decay, which has never been observed despite extensive searches for it; and spontaneous fission (SF), which is theoretically possible for the nuclides with atomic mass numbers ≥ 93,[10] that is all those with atomic numbers ≥ 41.
Besides SF, other theoretical decay routes for heavier elements include:[10]
- alpha decay – 70 heavy nuclides (the lightest two are cerium-142 and neodymium-143)
- double beta decay – 55 nuclides
- beta decay – tantalum-180m
- electron capture – tellurium-123, tantalum-180m
- double electron capture
- isomeric transition – tantalum-180m
These include all nuclides of mass 165 and greater. Argon-36 is the lightest known "stable" nuclide which is theoretically unstable.[10]
The positivity of energy release in these processes means they are allowed kinematically (they do not violate conservation of energy) and, thus, in principle, can occur.[10] They are not observed due to strong but not absolute suppression, by spin-parity selection rules (for beta decays and isomeric transitions) or by the thickness of the potential barrier (for alpha and cluster decays and spontaneous fission).
Summary table for numbers of each class of nuclides
[edit]This is a summary table from List of nuclides. Numbers are not exact and may change slightly in the future, as nuclides are observed to be radioactive, or new half-lives are determined to some precision.
| Type of nuclide by stability class | Number of nuclides in class | Running total of nuclides in all classes to this point | Notes |
|---|---|---|---|
| Theoretically stable according to known decay modes, including alpha decay, beta decay, isomeric transition, and double beta decay | 146 | 146 | All the first 66 elements, except technetium, promethium, samarium, and europium. If spontaneous fission is possible for the nuclides with mass numbers ≥ 93, then all such nuclides are unstable, so that only the first 40 elements would be stable. If protons decay, then there are no stable nuclides. |
| Energetically unstable to one or more known decay modes, but no decay yet seen. Considered stable until radioactivity confirmed. | 105[2][11] | 251 | Total is the observationally stable nuclides. All elements up to lead (except technetium and promethium) are included. |
| Radioactive primordial nuclides. | 35 | 286 | Includes bismuth, thorium, and uranium |
| Radioactive nonprimordial, but occur naturally on Earth. | ~61 significant | ~347 significant | Cosmogenic nuclides from cosmic rays; daughters of radioactive primordials such as francium, etc. |
List of stable nuclides
[edit]The primordial radionuclides are included for comparison; they are italicized and offset from the list of stable nuclides proper.
- Hydrogen-1
- Hydrogen-2 (deuterium)
- Helium-3
- Helium-4
- no mass number 5
- Lithium-6
- Lithium-7
- no mass number 8
- Beryllium-9
- Boron-10
- Boron-11
- Carbon-12
- Carbon-13
- Nitrogen-14
- Nitrogen-15
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Fluorine-19
- Neon-20
- Neon-21
- Neon-22
- Sodium-23
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Aluminium-27
- Silicon-28
- Silicon-29
- Silicon-30
- Phosphorus-31
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Chlorine-35
- Chlorine-37
- Argon-36 (2E)
- Argon-38
- Argon-40
- Potassium-39
- Potassium-40 (B, E) – long-lived primordial radionuclide
- Potassium-41
- Calcium-40 (2E)*
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46 (2B)*
- Calcium-48 (2B) – long-lived primordial radionuclide (B also predicted possible)
- Scandium-45
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Vanadium-50 (B, E) – long-lived primordial radionuclide
- Vanadium-51
- Chromium-50 (2E)*
- Chromium-52
- Chromium-53
- Chromium-54
- Manganese-55
- Iron-54 (2E)*
- Iron-56
- Iron-57
- Iron-58
- Cobalt-59
- Nickel-58 (2E)*
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Copper-63
- Copper-65
- Zinc-64 (2E)*
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70 (2B)*
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Germanium-76 (2B) – long-lived primordial radionuclide
- Arsenic-75
- Selenium-74 (2E)
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80 (2B)
- Selenium-82 (2B) – long-lived primordial radionuclide
- Bromine-79
- Bromine-81
- Krypton-78 (2E) – long-lived primordial radionuclide
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86 (2B)
- Rubidium-85
- Rubidium-87 (B) – long-lived primordial radionuclide
- Strontium-84 (2E)*
- Strontium-86
- Strontium-87
- Strontium-88
- Yttrium-89
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94 (2B)*
- Zirconium-96 (2B) – long-lived primordial radionuclide (B also predicted possible)
- Niobium-93
- Molybdenum-92 (2E)*
- Molybdenum-94
- Molybdenum-95
- Molybdenum-96
- Molybdenum-97
- Molybdenum-98 (2B)*
- Molybdenum-100 (2B) – long-lived primordial radionuclide
- Technetium – no stable isotopes
- Ruthenium-96 (2E)*
- Ruthenium-98
- Ruthenium-99
- Ruthenium-100
- Ruthenium-101
- Ruthenium-102
- Ruthenium-104 (2B)
- Rhodium-103
- Palladium-102 (2E)
- Palladium-104
- Palladium-105
- Palladium-106
- Palladium-108
- Palladium-110 (2B)*
- Silver-107
- Silver-109
- Cadmium-106 (2E)*
- Cadmium-108 (2E)*
- Cadmium-110
- Cadmium-111
- Cadmium-112
- Cadmium-113 (B) – long-lived primordial radionuclide
- Cadmium-114 (2B)*
- Cadmium-116 (2B) – long-lived primordial radionuclide
- Indium-113
- Indium-115 (B) – long-lived primordial radionuclide
- Tin-112 (2E)*
- Tin-114
- Tin-115
- Tin-116
- Tin-117
- Tin-118
- Tin-119
- Tin-120
- Tin-122 (2B)*
- Tin-124 (2B)*
- Antimony-121
- Antimony-123
- Tellurium-120 (2E)*
- Tellurium-122
- Tellurium-123 (E)*
- Tellurium-124
- Tellurium-125
- Tellurium-126
- Tellurium-128 (2B) – long-lived primordial radionuclide
- Tellurium-130 (2B) – long-lived primordial radionuclide
- Iodine-127
- Xenon-124 (2E) – long-lived primordial radionuclide
- Xenon-126 (2E)
- Xenon-128
- Xenon-129
- Xenon-130
- Xenon-131
- Xenon-132
- Xenon-134 (2B)*
- Xenon-136 (2B) – long-lived primordial radionuclide
- Caesium-133
- Barium-130 (2E) – long-lived primordial radionuclide
- Barium-132 (2E)*
- Barium-134
- Barium-135
- Barium-136
- Barium-137
- Barium-138
- Lanthanum-138 (B, E) – long-lived primordial radionuclide
- Lanthanum-139
- Cerium-136 (2E)*
- Cerium-138 (2E)*
- Cerium-140
- Cerium-142 (α, 2B)*
- Praseodymium-141
- Neodymium-142
- Neodymium-143 (α)
- Neodymium-144 (α) – long-lived primordial radionuclide
- Neodymium-145 (α)*
- Neodymium-146 (α, 2B)*
- no mass number 147§
- Neodymium-148 (α, 2B)*
- Neodymium-150 (2B) – long-lived primordial radionuclide
- Promethium - no stable isotopes
- Samarium-144 (2E)
- Samarium-146 (α) – probable long-lived primordial radionuclide
- Samarium-147 (α) – long-lived primordial radionuclide
- Samarium-148 (α) – long-lived primordial radionuclide
- Samarium-149 (α)*
- Samarium-150 (α)
- no mass number 151§
- Samarium-152 (α)
- Samarium-154 (2B)*
- Europium-151 (α) – long-lived primordial radionuclide
- Europium-153 (α)*
- Gadolinium-152 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Gadolinium-154 (α)
- Gadolinium-155 (α)
- Gadolinium-156
- Gadolinium-157
- Gadolinium-158
- Gadolinium-160 (2B)*
- Terbium-159
- Dysprosium-156 (α, 2E)*
- Dysprosium-158 (α)
- Dysprosium-160 (α)
- Dysprosium-161 (α)
- Dysprosium-162 (α)
- Dysprosium-163
- Dysprosium-164
- Holmium-165 (α)
- Erbium-162 (α, 2E)*
- Erbium-164 (α, 2E)
- Erbium-166 (α)
- Erbium-167 (α)
- Erbium-168 (α)
- Erbium-170 (α, 2B)*
- Thulium-169 (α)
- Ytterbium-168 (α, 2E)*
- Ytterbium-170 (α)
- Ytterbium-171 (α)
- Ytterbium-172 (α)
- Ytterbium-173 (α)
- Ytterbium-174 (α)
- Ytterbium-176 (α, 2B)*
- Lutetium-175 (α)
- Lutetium-176 (B) – long-lived primordial radionuclide (α, E also predicted possible)
- Hafnium-174 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Hafnium-176 (α)
- Hafnium-177 (α)
- Hafnium-178 (α)
- Hafnium-179 (α)
- Hafnium-180 (α)
- Tantalum-180m (α, B, E, IT)* ^
- Tantalum-181 (α)
- Tungsten-180 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Tungsten-182 (α)*
- Tungsten-183 (α)*
- Tungsten-184 (α)*
- Tungsten-186 (α, 2B)*
- Rhenium-185 (α)
- Rhenium-187 (B) – long-lived primordial radionuclide (A also predicted possible)
- Osmium-184 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Osmium-186 (α) – long-lived primordial radionuclide
- Osmium-187 (α)
- Osmium-188 (α)
- Osmium-189 (α)
- Osmium-190 (α)
- Osmium-192 (α, 2B)*
- Iridium-191 (α)
- Iridium-193 (α)
- Platinum-190 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Platinum-192 (α)*
- Platinum-194 (α)
- Platinum-195 (α)*
- Platinum-196 (α)
- Platinum-198 (α, 2B)*
- Gold-197 (α)
- Mercury-196 (α, 2E)*
- Mercury-198 (α)
- Mercury-199 (α)
- Mercury-200 (α)
- Mercury-201 (α)
- Mercury-202 (α)
- Mercury-204 (2B)
- Thallium-203 (α)
- Thallium-205 (α)
- Lead-204 (α)*
- Lead-206 (α)*
- Lead-207 (α)*
- Lead-208 (α)*
- Bismuth ^^ and above –
- no stable isotopes
- no mass number 209 and above
- Bismuth-209 (α) – long-lived primordial radionuclide
- Thorium-232 (α, SF) – long-lived primordial radionuclide (2B also predicted possible)
- Uranium-235 (α, SF) – long-lived primordial radionuclide
- Uranium-238 (α, 2B, SF) – long-lived primordial radionuclide
- Plutonium-244 (α, SF) – probable long-lived primordial radionuclide (2B also predicted possible)
- Bismuth ^^ and above –
Abbreviations for predicted unobserved decay:[12][2][11]
α for alpha decay, B for beta decay, 2B for double beta decay, E for electron capture, 2E for double electron capture, IT for isomeric transition, SF for spontaneous fission, * for the nuclides whose half-lives have lower bound. Double beta decay has only been listed when beta decay is not also possible.
^ Tantalum-180m is a "metastable isotope", meaning it is an excited nuclear isomer of tantalum-180. See isotopes of tantalum. However, the half-life of this nuclear isomer is so long that it has never been observed to decay, and it thus is an "observationally stable" primordial nuclide, a rare isotope of tantalum. This is the only nuclear isomer with a half-life so long that it has never been observed to decay. It is thus included in this list.
^^ Bismuth-209 was long believed to be stable, due to its half-life of 2.01×1019 years, which is more than a billion times the age of the universe.
§ Europium-151 and samarium-147 are primordial nuclides with very long half-lives of 4.62×1018 years and 1.066×1011 years, respectively.
See also
[edit]- Isotope geochemistry
- List of elements by stability of isotopes
- List of nuclides (991 nuclides in order of stability, all with half-lives over one hour)
- Mononuclidic element
- Periodic table
- Primordial nuclide
- Radionuclide
- Stable isotope ratio
- Table of nuclides
- Valley of stability
References
[edit]- ^ "DOE explains ... Isotopes". Department of Energy, United States. Archived from the original on 14 April 2022. Retrieved 11 January 2023.
- ^ a b c Belli, P.; Bernabei, R.; Danevich, F. A.; et al. (2019). "Experimental searches for rare alpha and beta decays". European Physical Journal A. 55 (8): 140–1–140–7. arXiv:1908.11458. Bibcode:2019EPJA...55..140B. doi:10.1140/epja/i2019-12823-2. ISSN 1434-601X. S2CID 201664098.
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brook haven National Laboratory. Archived from the original on 2018-10-10. Retrieved 2008-06-06.
- ^ Various (2002). Lide, David R. (ed.). Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 978-0-8493-0486-6. OCLC 179976746. Archived from the original on 2017-07-24. Retrieved 2008-05-23.
- ^ Kelkar, N. G.; Nowakowski, M. (2016). "Signature of the N = 126 shell closure in dwell times of alpha-particle tunneling". Journal of Physics G: Nuclear and Particle Physics. 43 (105102). arXiv:1610.02069. Bibcode:2016JPhG...43j5102K. doi:10.1088/0954-3899/43/10/105102.
- ^ "WWW Table of Radioactive Isotopes". [permanent dead link]
- ^ Marcillac, Pierre de; Noël Coron; Gérard Dambier; Jacques Leblanc & Jean-Pierre Moalic (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ de Carvalho H. G., de Araújo Penna M. (1972). "Alpha-activity of 209Bi". Lett. Nuovo Cimento. 3 (18): 720–722. doi:10.1007/BF02824346.
- ^ "NNDC – Atomic Masses". www.nndc.bnl.gov. Archived from the original on 2019-01-11. Retrieved 2009-01-17.
- ^ a b c d "Nucleonica website". Archived from the original on 2017-02-19. Retrieved 2014-06-14.
- ^ a b Tretyak, V.I.; Zdesenko, Yu.G. (2002). "Tables of Double Beta Decay Data — An Update". At. Data Nucl. Data Tables. 80 (1): 83–116. Bibcode:2002ADNDT..80...83T. doi:10.1006/adnd.2001.0873.
- ^ "Nucleonica :: Web driven nuclear science". Archived from the original on 2017-02-19. Retrieved 2014-06-14.
Book references
[edit]- Various (2002). Lide, David R. (ed.). Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 978-0-8493-0486-6. OCLC 179976746. Archived from the original on 2017-07-24. Retrieved 2008-05-23.
External links
[edit]- The LIVEChart of Nuclides – IAEA
- AlphaDelta: Stable Isotope fractionation calculator
- National Isotope Development Center Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
- Isotope Development & Production for Research and Applications (IDPRA) U.S. Department of Energy program for isotope production and production research and development
- Isosciences Archived 2021-01-18 at the Wayback Machine Use and development of stable isotope labels in synthetic and biological molecules
Stable nuclide
View on GrokipediaDefinition and Occurrence
Definition of Stability
A stable nuclide is an atomic nucleus composed of a specific number of protons (denoted as Z) and neutrons (N) that does not undergo spontaneous radioactive decay, maintaining its composition unchanged indefinitely in the absence of external influences.[7] This stability arises from a balanced configuration where the strong nuclear force dominates over disruptive electromagnetic repulsion between protons, resulting in a bound state that persists for timescales far exceeding the age of the universe.[8] Archetypal examples include hydrogen-1 (^1H), which consists of a single proton with no neutrons, and helium-4 (^4He), with two protons and two neutrons, both of which exhibit no measurable decay.[1] The criterion for stability is operational and tied to observability: a nuclide is considered stable if its half-life exceeds approximately 46 billion years (4.6 × 10^{10} years), meaning no decay is detectable over human or even cosmic timescales, in contrast to radioactive nuclides with half-lives ranging from fractions of a second to billions of years.[9] This threshold ensures that stable nuclides, such as those forming the bulk of ordinary matter, remain unaltered throughout the 13.8-billion-year history of the universe, whereas even long-lived radioactive isotopes like uranium-238 (half-life 4.5 × 10^9 years) show measurable decay. Stable nuclides correspond to local minima in the nuclear binding energy curve, where the total binding energy per nucleon is maximized relative to neighboring configurations, making decay energetically unfavorable.[10] The semi-empirical mass formula (SEMF), developed by von Weizsäcker and others, approximates this binding energy B(A, Z) as a function of mass number A = Z + N, incorporating volume, surface, Coulomb, asymmetry, and pairing terms to predict stability valleys for given Z.[11] For instance, in isobaric sequences (fixed A, varying Z), the SEMF identifies the most stable nuclide at the energy minimum, often enhanced by shell effects such as magic numbers.[12]Naturally Occurring Nuclides
Stable nuclides constitute the majority of isotopes found in nature, persisting indefinitely without undergoing radioactive decay, in contrast to transient radioactive isotopes that decay over time. These stable nuclides form the foundational building blocks of matter on Earth and throughout the solar system, originating from ancient cosmic processes that predate planetary formation.[5] Primordial stable nuclides were produced either during Big Bang nucleosynthesis, which synthesized light elements in the first few minutes after the universe's inception, or through stellar nucleosynthesis in pre-solar stars, where heavier elements formed via fusion reactions. Examples include helium-4, the primary product of Big Bang nucleosynthesis with a primordial mass fraction of approximately 0.245, and carbon-12 and oxygen-16, which were generated in the cores of massive stars through the triple-alpha process and subsequent carbon-burning stages, respectively, before being dispersed into the interstellar medium. These nuclides have endured since the formation of the solar system around 4.6 billion years ago, comprising the bulk of the material incorporated into planets and meteorites.[13][14] In addition to primordial origins, a small number of stable nuclides occur naturally through ongoing cosmogenic production, where high-energy cosmic rays interact with atmospheric or surface atoms to create rare isotopes. Notable examples include helium-3 and neon-21, generated via spallation reactions on targets like oxygen, magnesium, and silicon in meteorites, lunar regolith, and Earth's atmosphere; these accumulate over geological timescales and serve as tracers for exposure histories. Although cosmogenic stable nuclides are present in trace amounts compared to primordial ones, they contribute to the isotopic diversity observed in extraterrestrial samples.[15] Approximately 254 stable nuclides have been observed in nature, representing the non-decaying isotopes of 80 elements out of thousands of possible proton-neutron combinations. These stable nuclides overwhelmingly dominate the elemental compositions both in the solar system, where isotopic ratios in the solar photosphere and chondritic meteorites reflect uniform primordial abundances (e.g., silicon and iron isotopes show near homogeneity across samples), and in Earth's crust, where they account for nearly all of the oxygen (primarily ^{16}O at 99.76%), silicon, aluminum, and iron present. This prevalence underscores their role in sustaining the chemical and geological stability of planetary environments.[5][16]Stability Factors
Magic Numbers
Magic numbers in nuclear physics denote specific counts of protons or neutrons—namely 2, 8, 20, 28, 50, 82, and 126—that result in exceptionally stable atomic nuclei by corresponding to the completion of nuclear shells.[17] These values were identified through observations of nuclear binding energies and stability patterns, highlighting discontinuities at these nucleon numbers where nuclei exhibit enhanced resistance to decay.[18] The underlying physical basis stems from the nuclear shell model, independently developed by Maria Goeppert Mayer and J. Hans D. Jensen in the late 1940s, which posits that protons and neutrons occupy quantized energy levels within the nucleus, similar to electrons in atomic orbitals but influenced by strong nuclear forces and spin-orbit coupling.[17][19] In this model, filling a complete subshell at a magic number leads to a closed-shell configuration, maximizing the nucleus's binding energy per nucleon and minimizing excitation energies, thereby reducing the likelihood of beta decay or other instability modes.[20] This shell closure effect is evident in the semi-empirical mass formula, where pairing and shell corrections contribute to steeper drops in decay probabilities beyond magic numbers.[18] Doubly magic nuclides, with both proton and neutron counts at magic numbers, demonstrate the most pronounced stability. Representative examples include helium-4 (Z=2, N=2), the lightest stable nuclide with no known decay pathway; oxygen-16 (Z=8, N=8), which forms a tightly bound core in heavier elements; and lead-208 (Z=82, N=126), the heaviest known doubly magic stable nuclide.[20] These configurations often result in the highest natural abundances for their elements, reflecting their thermodynamic favorability in nucleosynthesis processes: helium-4 accounts for virtually 100% of terrestrial helium, oxygen-16 comprises about 99.76% of natural oxygen, and lead-208 makes up roughly 52.4% of lead.[21] Such abundances underscore the role of magic numbers in shaping isotopic distributions observed in nature.[17]Odd-Even Proton-Neutron Effects
The pairing effect in nuclear physics arises from the tendency of nucleons to form pairs with opposite spins and angular momenta, leading to a lower total energy for nuclei where both protons and neutrons can fully pair up. This phenomenon, known as the odd-even proton-neutron effect, significantly influences nuclide stability by enhancing binding energies in certain configurations through the Pauli exclusion principle and attractive short-range interactions between like nucleons. Even-even nuclides, characterized by even numbers of protons (Z) and neutrons (N), exhibit the highest stability among stable isotopes because all nucleons participate in pairing, which increases the binding energy by approximately 1-2 MeV per pair compared to unpaired configurations, thereby inhibiting decay modes such as beta decay. This pairing reduces the nucleus's overall energy state, making even-even nuclides the most common type of stable nuclide and explaining their prevalence in the isotopic distribution across the periodic table.[22] In contrast, odd-odd nuclides, with odd Z and odd N, are the least stable among non-radioactive nuclides because only one proton and one neutron remain unpaired, resulting in higher energy states and greater susceptibility to decay; consequently, there are only five primordial stable odd-odd nuclides: deuterium (^2H), lithium-6 (^6Li), boron-10 (^10B), nitrogen-14 (^14N), and tantalum-180m (^180mTa), the long-lived isomer. These examples highlight how the absence of complete pairing destabilizes such nuclei relative to their even counterparts.[23] Nuclides with odd mass number A (A = Z + N odd), which include odd-even (odd Z, even N) and even-odd (even Z, odd N) configurations, display intermediate stability as only one type of nucleon remains unpaired, leading to binding energies between those of even-even and odd-odd cases and allowing for a moderate number of stable isotopes in these categories. This partial pairing provides sufficient energy gain to support stability but less than in fully paired systems, contributing to the observed trends in nuclear charts.[22] Statistically, based on the NUBASE2020 evaluation, there are 145 even-even stable nuclides, 101 odd-A stable nuclides (53 even Z-odd N and 48 odd Z-even N), and 5 odd-odd stable nuclides, underscoring the dominance of pairing symmetry in determining nuclear longevity. Note that exact counts can vary slightly depending on the classification of nuclides with extremely long half-lives as "stable." These effects can be amplified near magic numbers, where shell closures further enhance stability in even-even configurations.[24]Isotopic Distribution
Stable Isotopes per Element
The number of stable isotopes varies across the periodic table, with most elements featuring 1 to 5 such isotopes. Tin possesses the maximum of 10 stable isotopes, while xenon has 9, representing the extremes among naturally occurring elements. Hydrogen, in contrast, has only 2 stable isotopes.[25][26][27] Twenty-six elements are monoisotopic, exhibiting exactly one stable isotope; examples include beryllium-9 and fluorine-19. These elements are distributed throughout the periodic table but are more common among those with odd atomic numbers.[1] A clear pattern emerges in the distribution: heavier elements generally have more stable isotopes than lighter ones, as the valley of stability widens with increasing atomic number, accommodating a broader range of neutron-to-proton ratios for nuclear stability. This variation is partly explained by odd-even effects, whereby elements with even atomic numbers tend to have more stable isotopes due to enhanced binding from nucleon pairing.[28] No element exceeds 10 stable isotopes, establishing a practical theoretical limit observed in nature. Dysprosium exemplifies a high count among lanthanides with 7 stable isotopes, while bismuth represents a borderline case, as its sole isotope, bismuth-209, has an extraordinarily long half-life of approximately years, rendering it effectively stable for all practical purposes.[25][29]Nuclear Isomers in Stability
Nuclear isomers are long-lived excited states of atomic nuclei, distinct from typical short-lived excitations, where the nucleus remains in a metastable configuration due to hindered transition probabilities to the ground state. These states arise when nucleons occupy higher-energy configurations that are separated from the ground state by energy barriers, resulting in half-lives ranging from nanoseconds to billions of years or longer. In the context of stability, long-lived nuclear isomers are those with half-lives far exceeding geological or astronomical timescales, rendering their decay effectively unobservable.[30] A prime example of such a long-lived isomer is the metastable state of tantalum-180, denoted , which possesses an excitation energy of 76.79 keV and a half-life greater than years (90% confidence level, as of 2023).[31] This isomer is unique as the only naturally occurring long-lived excited nuclear state, comprising a significant fraction (approximately 0.012%) of primordial tantalum on Earth, since its ground state decays rapidly with a half-life of about 8 hours. Another notable long-lived isomer is with a half-life of years, illustrating how isomers can persist far longer than expected for excited states. These examples highlight how certain nuclear configurations achieve apparent stability despite being energetically higher than the ground state.[30][30] Nuclear isomers form through various nuclear reactions, including photon absorption where a ground-state nucleus captures a gamma ray to reach the isomeric level, or beta decay processes that populate the excited state in the daughter nucleus. For instance, is believed to originate primarily from neutrino-induced processes in supernovae, leaving the nucleus in its metastable configuration. The longevity of these isomers stems from decay inhibition mechanisms, particularly high angular momentum barriers; the isomeric state often has a significantly different spin and parity compared to the ground state, necessitating electromagnetic transitions of high multipolarity (e.g., E3 or higher), which have low probabilities due to the dependence on the angular momentum change . This spin hindrance is more pronounced in regions influenced by odd-even proton-neutron effects, where pairing interactions limit low-energy decay channels.[32][30] In contrast to truly stable ground-state nuclides, which exhibit no measurable decay over cosmic timescales, nuclear isomers are quasi-stable, as their eventual de-excitation to the ground state is theoretically inevitable, albeit hindered to practical immortality for the longest-lived cases. This distinction underscores that stability in nuclear physics refers to ground states for primordial nuclides, while isomers represent a metastable landscape within the same isotopic family.[30]Theoretical and Observational Limits
Unobserved Decay Modes
Certain stable nuclides, particularly even-even isotopes, are theoretically susceptible to rare decay modes such as neutrinoless double beta decay (0νββ), which would violate lepton number conservation if mediated by Majorana neutrinos. This process involves the simultaneous conversion of two neutrons into two protons and two electrons, without neutrino emission, and is predicted in extensions of the Standard Model but remains unobserved despite intensive experimental efforts. Searches in dedicated low-background detectors have established stringent lower limits on the half-lives, demonstrating that such decays, if they occur, are far slower than ordinary radioactive processes, thereby underscoring the practical stability of these nuclides.[33] A prominent example is xenon-136, a stable isotope comprising about 9% of natural xenon, for which 0νββ decay to barium-136 has been extensively probed using xenon-loaded detectors. The KamLAND-Zen experiment, employing over 700 kg of enriched xenon in a liquid scintillator, analyzed data from multiple phases and reported no evidence of the decay, setting a lower limit on the half-life of > 2.3 × 10^{26} years at 90% confidence level as of 2025.[33] Similarly, for germanium-76, the GERDA collaboration achieved a half-life limit of > 1.8 × 10^{26} years, while the successor LEGEND-200 experiment, as of November 2025, has set > 1.9 × 10^{26} years; combining with prior data yields > 2.8 × 10^{26} years.[33][34][35] These limits not only affirm the stability of Xe-136 and Ge-76 but also constrain the effective Majorana neutrino mass to below 10-100 meV, depending on nuclear matrix element calculations.[36][37] Another class of unobserved decay modes involves proton decay within stable nuclei, predicted by grand unified theories that unify the fundamental forces but forbidden in the Standard Model. Modes such as p → e⁺ + π⁰ would destabilize ordinary matter if occurring on accessible timescales, yet no events have been detected in water Cherenkov detectors monitoring vast numbers of nucleons. The Super-Kamiokande experiment, with over 30 years of operation and exposure equivalent to hundreds of kiloton-years, has set lower limits on the proton lifetime exceeding 2.4 × 10^{34} years for this channel, confirming the long-term stability of nuclei like oxygen-16 in water. These bounds highlight the extreme rarity of such processes and provide critical tests for theories beyond baryon number conservation.[38] The absence of observed events in these searches, often leveraging underground facilities like Gran Sasso or Kamioka to suppress cosmic-ray backgrounds, illustrates the boundaries of current nuclear physics knowledge. For nuclides near magic numbers, such as those with closed shells, these decay modes are further suppressed due to selection rules, enhancing their stability. Ongoing experiments like CUORE for tellurium-130 and future ton-scale detectors aim to extend these limits by orders of magnitude, potentially revealing or further ruling out these elusive processes.[33]Classification and Counts
Stable nuclides are quantitatively classified into categories based on their observed or theoretical stability, drawing from nuclear data compilations. The core group consists of 254 nuclides that have not been observed to decay, even in principle, using current detection methods. These are complemented by primordial nuclides, numbering 80 across elements that were present at the formation of the solar system and have persisted due to their stability. Additionally, approximately 40 nuclides with half-lives exceeding 10^8 years are considered effectively stable for most practical and geological purposes, as their decay rates are negligible over human timescales.[23][39] The distribution of these stable nuclides follows patterns tied to atomic number Z. Elements with Z from 1 to 83 all possess at least one stable nuclide, except for technetium (Z=43) and promethium (Z=61), which lack any; for Z ≥ 84, only long-lived radioactive nuclides exist, such as ^{232}Th and ^{238}U. This breakdown reflects the limits of nuclear stability, where lighter elements (low Z) tend to have fewer stable isotopes, while mid-range elements like tin (Z=50) have up to 10. The odd-even effects briefly referenced here contribute to these patterns, favoring even numbers of protons and neutrons for greater binding energy.[40]| Classification (Proton-Neutron Parity) | Approximate Number of Stable Nuclides | Notes on Magic Involvement |
|---|---|---|
| Even-even (even Z, even N) | 157 | Predominant class; many involve magic numbers (e.g., ^{16}O with Z=8, N=8; ^{208}Pb with Z=82, N=126), enhancing stability through closed shells. |
| Even-odd (even Z, odd N) | 53 | Common in elements with even Z; examples near magic N like ^{138}La (Z=57, N=81 near 82). |
| Odd-even (odd Z, even N) | 50 | Symmetric to even-odd; stability boosted near magic Z or N (e.g., ^{115}In with Z=49 near 50). |
| Odd-odd (odd Z, odd N) | 4 | Rare (only ^{2}H, ^{6}Li, ^{10}B, ^{14}N); none directly at magic numbers due to pairing instability. |
Comprehensive Lists
Table of Nuclide Classes
The classification of nuclides into stability classes provides a framework for understanding their longevity and occurrence in nature. Stable nuclides are those for which no radioactive decay has been observed, with lifetimes effectively infinite compared to the age of the universe. Long-lived nuclides are radioactive isotopes with half-lives exceeding 10^8 years, allowing them to persist in measurable quantities. Extinct nuclides are those that were produced in the early solar system but have since fully decayed due to shorter half-lives, leaving no detectable remnants today. The following table summarizes counts across these classes, categorized by total numbers and proton-neutron parity (even or odd numbers of protons Z and neutrons N). Data for stable nuclides are based on comprehensive tabulations of observed isotopes as of 2025; long-lived and extinct classes include only primordial examples relevant to cosmochemistry, with parity distributions reflecting their scarcity and tendency toward odd-odd configurations due to pairing effects. Magic status (nuclides with Z or N equal to 2, 8, 20, 28, 50, 82, or 126) is noted separately, as these confer enhanced stability primarily within the stable class.| Class | Total | Even-Even | Even-Odd | Odd-Even | Odd-Odd | Magic Z or N (single) | Doubly Magic |
|---|---|---|---|---|---|---|---|
| Stable | 251 | 145 | 53 | 50 | 5 | 50 | 5 |
| Long-lived | 12 | 2 | 2 | 2 | 6 | 0 | 0 |
| Extinct | 15 | 2 | 3 | 2 | 8 | 0 | 0 |
.svg/250px-Table_of_nuclides_(mul).svg.png)
.svg/1517px-Table_of_nuclides_(mul).svg.png)