Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Welcome to the community hub built to collect knowledge and have discussions related to Androstane.
Nothing was collected or created yet.
Androstane
View on Wikipediafrom Wikipedia
 | |
| Names | |
|---|---|
| IUPAC name
5ξ-Androstane
| |
| Systematic IUPAC name
(3aS,3bS,5aΞ,9aS,9bS,11aS)-9a,11a-Dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Other names
Etioallocholane; 10β,13β-Dimethylgonane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H32 | |
| Molar mass | 260.465 g·mol−1 |
| Density | 0.95 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
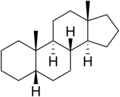
Androstane is a C19 steroidal hydrocarbon with a gonane core. Androstane can exist as either of two isomers, known as 5α-androstane and 5β-androstane.
-
5α-Androstane
-
5β-Androstane
Pharmacology
[edit]5α-Androstane is reported to be effective as an androgen, in spite of having no oxygen containing functional groups.[1][2]
Androstanes
[edit]Androstanes are steroid derivatives with carbons present at positions 1 through 19.
See also
[edit]References
[edit]- ^ Wilson JD (1996). "Role of dihydrotestosterone in androgen action". Prostate Suppl. 6 (S6): 88–92. doi:10.1002/(SICI)1097-0045(1996)6+<88::AID-PROS17>3.0.CO;2-N. PMID 8630237. S2CID 41352599.
- ^ Segaloff A, Gabbard RB (1960). "5α-Androstane—An Androgenic Hydrocarbon". Endocrinology. 67 (6): 887–889. doi:10.1210/endo-67-6-887. ISSN 0013-7227. PMID 13749674.
Androstane
View on Grokipediafrom Grokipedia
Androstane is a saturated tetracyclic C19 steroid hydrocarbon with the molecular formula C19H32, serving as the fundamental parent structure for androstane-class steroids, including androgens.[1][2] It features a gonane core with angular methyl groups at positions 10 and 13, distinguishing it from other steroid hydrocarbons like pregnane, which possess an additional side chain at C17.[1] Androstane exists in two principal isomeric forms—5α-androstane and 5β-androstane—differing in the stereochemistry at the A/B ring junction, with the 5α form being more prevalent in mammalian biochemistry due to its association with 5α-reductase-mediated metabolites.[1] As the skeletal framework for key endogenous androgens such as testosterone, dihydrotestosterone, and androsterone, androstane derivatives play critical roles in male sexual development, muscle anabolism, and metabolic regulation, underscoring their foundational importance in endocrinology and steroid hormone signaling.[2][3] Synthetic modifications of the androstane nucleus have yielded anabolic-androgenic steroids used therapeutically for conditions like hypogonadism and cachexia, though they are also implicated in performance-enhancing doping due to their potent effects on protein synthesis and secondary sexual characteristics.[4] The compound's saturated nature contrasts with unsaturated precursors like androstene, enabling diverse functionalization at positions such as C3 and C17 for biological activity.[1]
Chemical Structure and Nomenclature
Core Framework
Androstane is a saturated C19H32 hydrocarbon serving as the parent structure for the androstane series of steroids, characterized by a tetracyclic ring system.[1] This core framework, derived from the gonane skeleton, features four linearly fused rings: three six-membered cyclohexane rings designated A, B, and C, and a five-membered cyclopentane ring D, with angular methyl groups attached at positions C10 (C19 methyl) and C13 (C18 methyl).[5] The fusion between rings follows a trans configuration at most junctions, contributing to the rigid, planar nature of the molecule essential for steroid functionality.[1] Standard steroid numbering begins at C1 in ring A, proceeding clockwise around the rings to C17 in ring D, with side chains or substituents typically at C17 for higher steroids, though androstane lacks an extended chain beyond C17.[1] The systematic IUPAC name for the 5α-isomer is (8S,9S,10S,13S,14S)-10,13-dimethylhexadecahydro-1H-cyclopentaphenanthrene, reflecting the perhydrocyclopentanophenanthrene backbone with specified stereochemistry.[5] In steroid nomenclature, "androstane" denotes the fully saturated C19 parent, distinguishing it from unsaturated variants like androstene or oxygenated derivatives such as androstanediols.[1] The core framework's stereochemistry at C5 defines the two primary isomers: 5α-androstane (with trans A/B fusion) and 5β-androstane (with cis A/B fusion), influencing molecular conformation and biological activity in derivatives.[1] This structural rigidity and specific chirality at eight asymmetric centers (C8, C9, C10, C13, C14, and potentially C5, C17) underpin the scaffold's role in androgenic and other steroid hormones.[5]Isomers and Stereochemistry
Androstane possesses six chiral centers at carbons 5, 8, 9, 10, 13, and 14, enabling multiple stereoisomers, though the biologically relevant forms adhere to specific configurations.[6] The principal distinction among androstane isomers lies in the stereochemistry at C5, yielding 5α-androstane and 5β-androstane.[7][8] In 5α-androstane, the A/B ring fusion is trans, characterized by the α-orientation of the hydrogen at C5 relative to the β-methyl group at C10, promoting a more extended conformation.[9] Conversely, 5β-androstane exhibits a cis A/B fusion, with the hydrogen at C5 in the β-position, resulting in a bent structure more typical of certain metabolites.[9] Both isomers maintain trans fusions at the B/C and C/D junctions, with standard orientations including β-methyl groups at C10 and C13, and α-hydrogens at C9 and C14.[2] The absolute configuration for 5α-androstane is designated as (5S,8R,9S,10S,13S,14S), reflecting the specific spatial arrangement derived from biosynthetic pathways.[10] This stereochemistry influences metabolic processing, with 5α-androstane serving as a human metabolite in androgen pathways.[7] Variations at other chiral centers, such as epi-isomers, occur but are less prevalent in natural contexts.[11]Physicochemical Properties
Physical Characteristics
5α-Androstane appears as a white to off-white crystalline solid.[12] It has a melting point of 50–51 °C and a boiling point of 336 °C at 760 mmHg.[13] The density is predicted to be 0.95 g/cm³.[14] As a non-polar hydrocarbon, it shows low solubility in polar solvents like methanol but is slightly soluble in chloroform at approximately 50 mg/mL.[12][14] 5β-Androstane, the epi-isomer, is also a solid with a higher melting point of 78–80 °C and a comparable boiling point of 336 °C at 760 mmHg.[15] Its density is similarly around 0.95 g/cm³.[15] Like its 5α counterpart, it exhibits hydrophobic character, with negligible solubility in water and preferential dissolution in non-polar organic solvents.[15] Both isomers are lipophilic due to their fully saturated C19 steroid framework, rendering them insoluble in aqueous media but compatible with apolar environments such as chloroform or hexane.[14][15] Refractive index values around 1.508 have been reported for 5α-androstane.[13]Chemical Reactivity and Stability
Androstane, as a fully saturated C19 polycyclic hydrocarbon, displays minimal chemical reactivity characteristic of alkanes, lacking sites for facile electrophilic addition, substitution, or oxidation under ambient conditions.[16] Its trans-fused ring system and angular methyl groups confer rigidity, limiting conformational flexibility that might otherwise expose reactive sites, though free-radical halogenation can occur preferentially at tertiary carbons (e.g., C-17 or methyl groups) under UV irradiation or high temperatures.[17] Both 5α-androstane and 5β-androstane isomers are chemically stable, showing no tendency for thermal decomposition, hazardous polymerization, or spontaneous reactions in air or moisture.[18] They resist hydrolysis and mild oxidizing agents, with incompatibility primarily limited to strong oxidizers or reactive metals that could initiate combustion or peroxidation at elevated temperatures above 300°C. In steroid synthesis, the androstane core withstands acidic or basic conditions used for functional group manipulations elsewhere in derivatives, underscoring its robustness as a scaffold.[16]Synthesis and Production
Biosynthetic Pathways
The biosynthesis of the androstane skeleton, the C19 core structure of androgens, occurs primarily in the gonads and adrenal cortex through steroidogenesis, starting from cholesterol. Cholesterol is transported into mitochondria via the steroidogenic acute regulatory protein (StAR) and undergoes side-chain cleavage by the enzyme cytochrome P450 side-chain cleavage (CYP11A1) to form pregnenolone, the universal precursor for all steroids.[19] This initial step requires NADPH and molecular oxygen and is rate-limiting, regulated by adrenocorticotropic hormone (ACTH) in adrenals and luteinizing hormone (LH) in gonads.[20] Conversion to the androstane nucleus involves transformation of C21 pregnane intermediates to C19 steroids via 17,20-lyase activity, cleaving the C17-C20 bond to remove the two-carbon side chain. The bifunctional enzyme cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) performs sequential 17α-hydroxylation followed by lyase action, with activity enhanced by cytochrome b5 in the zona reticularis of the adrenal cortex. Two parallel pathways exist: the Δ5 pathway, yielding dehydroepiandrosterone (DHEA) from 17α-hydroxypregnenolone, and the Δ4 pathway, yielding androstenedione from 17α-hydroxyprogesterone (itself from progesterone via 3β-hydroxysteroid dehydrogenase type 2, HSD3B2).[19] [20] These C19 Δ4-3-keto steroids represent the foundational androstane framework before further saturation or functionalization. Subsequent transformations produce active androgens bearing the androstane skeleton. Androstenedione is reduced to testosterone by 17β-hydroxysteroid dehydrogenase type 3 (17β-HSD3, encoded by HSD17B3) in the testes, while DHEA is isomerized to androstenedione via HSD3B enzymes. Testosterone is then 5α-reduced to the more potent dihydrotestosterone (DHT) by 5α-reductase types 1 and 2 (SRD5A1/2), saturating the Δ4 bond to form the canonical 5α-androstane structure. In peripheral tissues like prostate and adipose, intracrine metabolism activates circulating DHEA and androstenedione locally via these enzymes, bypassing gonadal testosterone.[19] An alternative "backdoor" pathway generates DHT directly from progesterone or 17α-hydroxyprogesterone without passing through testosterone, involving early 5α-reduction (SRD5A), oxidation to 17α-hydroxy dihydroprogesterone, and CYP17A1-mediated cleavage to androsterone, followed by oxidation to DHT via aldo-keto reductase 1C (AKR1C) enzymes. This route predominates in fetal development and certain pathologies like congenital adrenal hyperplasia, contributing up to 20-40% of DHT in some contexts. Adrenal production favors weak androgens like DHEA (sulfated to DHEAS for circulation) and 11-oxygenated derivatives (e.g., 11-ketotestosterone via CYP11B1), reflecting zona reticularis expression of CYP17A1 but limited HSD3B2.[20] [19]Chemical Synthesis Methods
Androstane, the saturated C19 steroid hydrocarbon, is primarily synthesized through partial reduction of naturally derived or semi-synthetic oxygenated precursors rather than de novo total synthesis, due to the complexity and inefficiency of constructing the tetracyclic core from simple starting materials. A common preparative route involves catalytic hydrogenation of Δ5-unsaturated androstane derivatives, such as 3β-hydroxy-5-androsten-17-one, using platinum or palladium catalysts under acidic conditions to selectively yield the 5α-epimer.[21] [22] This stereoselective reduction exploits the thermodynamic preference for the 5α-configuration in androstane systems, with reaction conditions typically involving ethanol or acetic acid solvents at room temperature and atmospheric pressure, achieving high yields of the saturated 5α-androstane skeleton after subsequent deoxygenation. Deoxygenation of functional groups in intermediates like androstan-3,17-dione or androsterone is accomplished via reductive methods such as Wolff-Kishner or Clemmensen reduction, converting carbonyl moieties to methylene groups while preserving the ring fusions.[23] For instance, androstan-17-ols are subjected to hydrogenolysis or tosylate formation followed by reduction to remove hydroxy groups, yielding the parent hydrocarbon.[24] These methods leverage commercially available precursors like dehydroepiandrosterone, enabling scalable production for research, though they inherit stereochemistry from the natural source material. Total syntheses of androstane, though less practical for bulk preparation, demonstrate the feasibility of assembling the skeleton from acyclic precursors and have advanced stereocontrol techniques. A notable 1979 route employs an SnCl₄-catalyzed Diels-Alder cycloaddition to forge ring C, establishing the β-methyl at C13 and H at C8 with high diastereoselectivity, followed by keto ester cyclization to complete the tetracycle.[25] Subsequent reductions and adjustments yield androstane derivatives. Alternative total approaches, such as those for (±)-androstane-2,17-dione, utilize intramolecular Diels-Alder reactions or metal-involved annulations to build rings A/B and D sequentially, highlighting the challenges in achieving the trans-anti-trans-anti-trans fusion.[26] These racemic syntheses, reported in the mid-20th century onward, prioritize proof-of-concept over enantiopurity, as chiral resolution or asymmetric variants remain underdeveloped for the unsubstituted hydrocarbon.Biological Significance
Role as Androgen Precursor
Androstane constitutes the core tetracyclic hydrocarbon skeleton (C19H32) underlying all androgen steroids, which exert primary masculinizing effects through binding to the androgen receptor.[4] In steroidogenesis, this skeleton emerges via enzymatic side-chain cleavage of C21 pregnane precursors, such as progesterone or 17α-hydroxyprogesterone, primarily in the Leydig cells of the testes, theca cells of the ovaries, and zona reticularis of the adrenal cortex.[27] The process begins with cholesterol conversion to pregnenolone by CYP11A1, followed by sequential hydroxylations and lyase actions (e.g., CYP17A1-mediated 17,20-lyase activity), yielding C19 intermediates like dehydroepiandrosterone (DHEA) and androstenedione that retain the androstane framework.[28] These androstane-derived precursors possess weak intrinsic androgenic activity but serve as substrates for conversion to potent androgens. Androstenedione, for instance, is reduced to testosterone by 17β-hydroxysteroid dehydrogenase type 3 (17β-HSD3) in the gonads, accounting for the majority of circulating testosterone in males (approximately 95% from testicular synthesis).[29] DHEA, secreted abundantly by the adrenals (up to 25 mg/day in young adults), undergoes peripheral transformation via 3β-HSD and 17β-HSD enzymes to androstenedione and testosterone, contributing 5-10% to total androgens in men and a larger fraction in women.[27] In target tissues like prostate and skin, testosterone is irreversibly reduced to dihydrotestosterone (DHT) by 5α-reductase isoforms (types 1 and 2), yielding a 5α-androstane derivative with 2-10 times greater potency due to higher receptor affinity and slower dissociation.[30] Alternative "backdoor" pathways bypass testosterone, directly generating DHT from 5α-reduced androstane intermediates such as 5α-androstane-3α,17β-diol or androsterone, particularly in conditions like congenital adrenal hyperplasia or castration-resistant prostate cancer where classic routes are impaired.[30] These pathways, involving enzymes like 3α-HSD and 17β-HSD5, highlight androstane's versatility as a scaffold for androgen production independent of the Δ4-androstenedione bottleneck, with evidence from isotopic tracer studies showing up to 20-40% of DHT deriving from backdoor routes in fetal development and certain pathologies.[31] Adrenal C19 steroids thus provide a reservoir for extra-gonadal androgen synthesis, influencing pubertal virilization, muscle anabolism, and spermatogenesis, though dysregulation elevates risks like polycystic ovary syndrome.[27]Endogenous Metabolism and Derivatives
In human physiology, endogenous androstane derivatives primarily arise from the 5α-reduction of testosterone and androstenedione, catalyzed by steroid 5α-reductase isoenzymes (SRD5A1 and SRD5A2), which saturate the Δ4-5 double bond in the A-ring to form the 5α-androstane nucleus.[32] This irreversible step occurs predominantly in androgen target tissues such as prostate, skin, and liver, with SRD5A2 predominating in genital tissues and SRD5A1 in nongenital sites like sebaceous glands.[32] The resulting 5α-dihydrotestosterone (DHT; 5α-androstan-17β-ol-3-one) is a more potent androgen than testosterone, amplifying androgen receptor signaling before undergoing further inactivation.[33] Subsequent metabolism of DHT involves oxidation at the 17β-position by 17β-hydroxysteroid dehydrogenases (17β-HSDs), yielding 5α-androstane-3,17-dione, followed by reduction at the 3-keto group by 3α-hydroxysteroid dehydrogenases (3α-HSDs) to produce androsterone (5α-androstan-3α-ol-17-one).[33] Alternatively, DHT can be reduced directly at the 3-keto group to 5α-androstane-3α,17β-diol (3α-diol), a weaker agonist of the androgen receptor that serves as a precursor to androsterone via 17β-HSD activity.[34] These transformations represent inactivation pathways, with androsterone and its 3β-epimer epiandrosterone excreted primarily in urine as glucuronide or sulfate conjugates after hepatic processing.[19] A minor 5β-reduction pathway, mediated by less specific reductases, generates 5β-androstane derivatives such as etiocholanolone (5β-androstan-3α-ol-17-one) from testosterone or androstenedione, though this route contributes only about 20-30% of total 17-ketosteroid excretion in adult males compared to the 5α-pathway.[19] Etiocholanolone lacks significant androgenic activity and is rapidly conjugated for elimination, reflecting its role in androgen catabolism rather than signaling.[35] Additionally, a "backdoor" pathway bypasses testosterone, converting progesterone derivatives to androsterone as an intermediate en route to DHT, particularly active in fetal development and certain tissues.[33] These metabolites collectively account for over 40% of daily androgen turnover in humans, with urinary 17-ketosteroids like androsterone and etiocholanolone serving as biomarkers of androgen production; daily excretion rates average 1-3 mg for androsterone and 2-5 mg for etiocholanolone in adult males.[19] Enzymatic deficiencies, such as in 5α-reductase type 2, disrupt this metabolism, leading to elevated testosterone-to-DHT ratios and conditions like 5α-reductase deficiency syndrome.[36]Derivatives and Applications
Natural Androstanes
Natural androstanes comprise endogenous steroids featuring the saturated C19 androstane nucleus, formed predominantly through the peripheral metabolism of androgens like testosterone and androstenedione in mammals, including humans. These compounds arise via reductase enzymes that saturate the Δ4-ene bond and modify functional groups at C3 and C17, yielding primarily 17-ketosteroids excreted in urine as biomarkers of androgen turnover.[37][38] The principal natural androstane is androsterone (5α-androstan-3α-ol-17-one), generated from dihydrotestosterone through 3α-reduction followed by 17-oxidation, or alternatively from androstanedione via 3α-reduction; this pathway predominates in tissues expressing 5α-reductase type II, such as prostate and skin. Androsterone possesses weak androgenic potency but functions as a neurosteroid, modulating glycine and GABA_A receptors to influence neuronal excitability.[37][39][40] Etiocholanolone (5β-androstan-3α-ol-17-one), the 5β-isomer, emerges via parallel 5β-reduction of testosterone or androstenedione, primarily in hepatic tissues, and constitutes a major urinary etiocholanone alongside androsterone, with daily excretion varying by sex and age but typically comprising 1-3 mg in adult males. Like androsterone, it exhibits modest biological activity, including pyrogenic effects upon injection, historically exploited to assess marrow function.[38][41]| Compound | Systematic Name | Biosynthetic Origin | Key Biological Role |
|---|---|---|---|
| Androsterone | 3α-Hydroxy-5α-androstan-17-one | Metabolite of DHT and androstanedione | Neurosteroid, weak androgen, urinary excretion[37][39] |
| Etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | Metabolite of testosterone via 5β-pathway | Urinary metabolite, immunostimulant[38] |
| Androstanedione | 5α-Androstane-3,17-dione | Intermediate in 5α-androgen reduction | Precursor to androsterone[42] |
Synthetic Androstane Derivatives
Synthetic androstane derivatives are chemically engineered steroids built upon the C19 androstane nucleus, designed to amplify anabolic effects—such as protein synthesis and nitrogen retention—while modulating androgenic activity, pharmacokinetics, and receptor selectivity compared to endogenous androgens like testosterone. These compounds emerged from systematic structural modifications starting in the 1930s following the isolation of testosterone in 1935, with early efforts focused on enhancing therapeutic utility for conditions including hypogonadism, muscle atrophy, and anemia.[43] Key innovations included 17α-alkylation to enable oral administration by evading first-pass hepatic degradation, and 19-demethylation to reduce estrogenic side effects via impaired aromatization.[44] Over 100 such derivatives have been synthesized, though only a subset gained clinical approval due to efficacy-risk balances informed by empirical trials showing dose-dependent gains in lean body mass but elevated risks of hepatotoxicity and cardiovascular strain.[45] Prominent examples include nandrolone, a 19-norandrostane derivative (specifically 17β-hydroxyestr-4-en-3-one) first synthesized in 1950 and approved for medical use in 1959, which exhibits approximately 3-4 times the anabolic potency of testosterone with diminished androgenic effects, as measured by levator ani muscle assays in rodents.[44] Oxymetholone, a 17α-methylated dihydrotestosterone analog developed in the 1950s, promotes erythropoiesis in anemic patients via stimulation of bone marrow activity, with clinical data from 1960s trials demonstrating hemoglobin increases of 2-3 g/dL after 4-6 weeks at 50 mg/day doses.[46] Stanozolol, featuring a pyrazole ring fused at C2-C3 and synthesized in 1959, offers high oral bioavailability and has been used for hereditary angioedema, though long-term studies highlight risks like peliosis hepatis.[47]| Compound | Key Modification | Anabolic:Androgenic Ratio (approx., via Hershberger assay) | Approved Indications (examples) |
|---|---|---|---|
| Nandrolone | 19-Demethylation, Δ4-unsaturation | 10:1 | Anemia, cachexia[44] |
| Oxymetholone | 17α-Methyl, 2-hydroxymethylene | 3:1 | Aplastic anemia[46] |
| Stanozolol | 17α-Methyl, pyrazoline fusion | 3:1 | Angioedema, growth failure[47] |