Recent from talks
Nothing was collected or created yet.
Colossal squid
View on Wikipedia
| Colossal squid | |
|---|---|
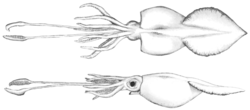
| |
| Depiction with an inflated mantle | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Cephalopoda |
| Order: | Oegopsida |
| Family: | Cranchiidae |
| Subfamily: | Taoniinae |
| Genus: | Mesonychoteuthis Robson, 1925 |
| Species: | M. hamiltoni
|
| Binomial name | |
| Mesonychoteuthis hamiltoni | |

| |
| Global range of M. hamiltoni | |
The colossal squid (Mesonychoteuthis hamiltoni) is a species of very large squid belonging to the family Cranchiidae, that of the cockatoo squids or glass squids. It is sometimes called the Antarctic cranch squid or giant squid (not to be confused with the giant squid in genus Architeuthis) and is believed to be the largest squid species in terms of mass.[3] It is the only recognized member of the genus Mesonychoteuthis.[4]
The species is confirmed to reach a mass of at least 495 kilograms (1,091 lb), though the largest specimens—known only from beaks found in sperm whale stomachs—may perhaps weigh as much as 600–700 kilograms (1,300–1,500 lb),[5][6] making it the largest extant invertebrate.[3] The maximum total length is ~4.2 metres (14 ft).[7] Larger estimates exist; however, these include the feeding tentacles measured on dead specimens. In life the squid's tentacles are hidden, only released when capturing prey. If tentacles are considered, lengths of 10 metres (33 ft) and 14 metres (46 ft) exist, but the former estimate is more likely.[8][9] The colossal squid has the largest eyes of any known creature ever to exist, with an estimated diameter of 27–30 cm (11–12 in)[10] to 40 cm (16 in) for the largest collected specimen.
The species has similar anatomy to other members of its family, although it is the only member of Cranchiidae to display hooks on its arms, suckers, and tentacles.[11][12] It is known to inhabit the circumantarctic Southern Ocean.[3] It is presumed to be an ambush predator, with a diet including various fish, and is likely a key prey item of the sperm whale.[13][14]
Morphology
[edit]The colossal squid shares features common to all squids: a mantle for locomotion, one pair of gills, a beak or tooth, and certain external characteristics like eight arms and two tentacles, a head, and two fins.[11] In general, the morphology and anatomy of the colossal squid are the same as any other squid.[11] However, there are certain morphological characteristics that separate the colossal squid from other squids in its family: the colossal squid is the only squid in its family whose arms and tentacles are equipped with hooks, either swiveling or three-pointed.[12] There are squids in other families that also have hooks, but no other squid in the family Cranchiidae.[11]

Unlike most squid species, the colossal squid exhibits abyssal gigantism, as it is the heaviest living invertebrate species, reaching weights up to 495 kg (1,091 lb).[3] For comparison, squids typically have a mantle length of about 30 cm (12 in) and weigh about 100–200 g (3+1⁄2–7 oz).[11]
The colossal squid also has the largest eyes documented in the animal kingdom, with a diameter of 27–30 cm (11–12 in).[15][16]
Distribution and habitat
[edit]The squid's known range extends thousands of kilometres north of Antarctica to southern South America, southern South Africa, and the southern tip of New Zealand, making it primarily an inhabitant of the circumantarctic Southern Ocean.[3] Colossal squid are also often sighted near Cooperation Sea and Ross Sea because of its prey and competitor, the Antarctic toothfish.[17] The region between the Weddell Sea and the western Kerguelen archipelago has been deemed a "hotspot" based on characteristics of the habitat.[18] The squid's vertical distribution appears to correlate directly with age. Young squid are found between 0–500 m (0–1,640 ft), adolescent squid are found 500–2,000 m (1,600–6,600 ft) and adult squid are found primarily within the mesopelagic and bathypelagic regions of the open ocean.[3]
Behavior
[edit]Feeding
[edit]
While little is known about their behavior, colossal squid are believed to feed primarily on small fish, such as lanternfish and deep-sea smelt, which have been found as stomach contents in adult specimens.[19] They also attack larger fish; of 8,000 Antarctic toothfish brought aboard by trawlers between 2011 and 2014, seventy-one showed clear signs of attack by colossal squid.[20] A study in the Prydz Bay region of Antarctica found squid remains in a female colossal squid's stomach, suggesting the possibility of cannibalism within this species.[21] Studies measuring the δ15N content of the chitinous beaks of cephalopods to determine trophic ecology levels have demonstrated that the colossal squid is a top predator that is positively correlated with its increased size.[22] This new confirmation of the colossal squid's trophic level suggests that it likely preys on large fishes, such as the Patagonian toothfish, and smaller squids, according to its size, and that its predators include sperm whales and sleeper sharks.[22][23]
Metabolism
[edit]The colossal squid is thought to have a very slow metabolic rate, needing only around 30 grams (1 oz) of prey daily for an adult with a mass of 500 kilograms (1,100 lb).[24] Estimates of its energy requirements suggest it is a slow-moving ambush predator, using its large eyes primarily for prey-detection rather than engaging in active hunting.[24][14]
Predation
[edit]Many sperm whales have scars on their backs that are believed to be caused by the hooks of colossal squid. Colossal squid are a major prey item for sperm whales in the Antarctic; 14% of the squid beaks found in the stomachs of these sperm whales are those of the colossal squid, which indicates that colossal squid likely make up 77% of the biomass consumed by these whales.[25] Many other animals also feed on colossal squid, including the beaked whales, such as southern bottlenose whales, and Cuvier's and Baird's beaked whales. Other possible predators include the pilot whale, killer whales, larger southern elephant seals, Patagonian toothfish,[26] southern sleeper sharks (Somniosus antarcticus), Antarctic toothfish, and albatrosses (e.g., the wandering and sooty albatrosses).[3] Beaks from mature adults have only been recovered from large predators (i.e. sperm whales and southern sleeper sharks), while other predators only eat juveniles or young adults.[27]
Reproduction
[edit]Not much is known about the colossal squid's reproductive cycle, although it does have two distinct sexes. Many species of squid, however, develop sex-specific organs as they age and develop.[28] The adult female colossal squid has been discovered in much shallower waters, which likely implies that females spawn in shallower waters, rather than their usual depth.[3] Additionally, the colossal squid has a high possible fecundity reaching over 4.2 million oocytes, which is quite high compared to other squids in such cold waters.[28] Colossal squid oocytes have been observed at sizes ranging from as large as 3.2 mm × 2.1 mm to as small as 1.4 mm × 0.5 mm. Sampling of colossal squid ovaries show an average of 2,175 eggs per gram.[19] Young squid are thought to spawn near the summer time at surface temperatures from −0.9 to 0 °C (30.4 to 32.0 °F).[17]
Vision
[edit]For pelagic organisms of similar weight to the colossal squid, such as the swordfish, the average eye diameter required for visual detection is 10 cm, but the colossal squid's are as large as 30 cm (12 in).[29][30] This allows for an increase in visual detection strategies, including reduced diffraction blurring and greater contrast distinction, which must be extremely beneficial to the colossal squid to justify the large energetic expenses to grow, move, camouflage, and maintain these eyes.[29] The colossal squid's increased pupil size has been mathematically proven to overcome the visual complications of the pelagic zone (the combination of downwelling daylight, bioluminescence, and light scattering with increasing distance), especially by monitoring larger volumes of water at once and by detecting long-range changes in plankton bioluminescence via the physical disruption of large moving objects (e.g., sperm whales).[29]
The colossal squid's eyes glow in the dark via long, rectangular light-producing photophores located next to the lens on the front of both eyeballs.[31] Symbiotic bacteria reside within these photophores and luminesce through chemical reaction.[32]
It is hypothesized that the colossal squid's eyes can detect predator movement beyond 120 m (390 ft), which is the upper limit of the sperm whale's sonar range.[29]
Hearing
[edit]Squid have been found to detect the movement of sound waves via organs called statocysts (similar to the human cochlea).[33] Squid statocysts likely respond to low-frequency sounds less than 500 Hz, similar to pelagic fish.[33] Colossal squid are likely essentially deaf to high frequencies, such as whale sonar, so they rely largely on visual detection mechanisms to avoid predation.[29][34]
History of knowledge
[edit]The colossal squid, species Mesonychoteuthis hamiltoni, was discovered in 1925.[35] This species belongs to the class Cephalopoda and family Cranchiidae.[36]
In 1981, an adult specimen was discovered; in 2003, a second specimen was collected.[37][38] Captured in 2007, the largest colossal squid weighed 495 kilograms (1,091 lb),[39] and is now on display at the Museum of New Zealand Te Papa Tongarewa.[40][41]
Most of the time, full colossal squid specimens are not collected; as of 2015, only 12 complete colossal squids had ever been recorded, with only half of these being full adults.[4] Beak remnants of the colossal squid are commonly collected; 55 beaks of colossal squids have been recorded in total.[4] Less commonly (four times), a fin, mantle, arm, or tentacle of a colossal squid has been collected.[4]
First collected specimens
[edit]The species was first discovered in the form of two arm crowns found in the stomach of a sperm whale in the winter of 1924–1925.[35] This species, named Mesonychoteuthis hamiltoni after E. Hamilton who made the initial discovery, was formally described by Guy Coburn Robson in 1925.[35]
Entire collected specimens
[edit]In 1981, a Soviet Russian trawler in the Ross Sea, off the coast of Antarctica, caught a large squid with a total length of over 4 m (13 ft), which was later identified as an immature female of M. hamiltoni.[37] In 2003, a complete specimen of a subadult female was found near the surface with a total length of 6 m (20 ft) and a mantle length of 2.5 m (8 feet 3 inches).[38] In 2005, the first full living specimen was captured at a depth of 1,625 m (5,331 ft) while taking a toothfish from a longline off South Georgia Island.[42] Although the mantle was not brought aboard, its length was estimated at over 2.5 m (8 feet 3 inches), and the tentacles measured 2.3 metres (7 feet 7 inches).[42] The animal is thought to have weighed between 150 and 200 kg (330 and 440 lb).[42]
Largest known specimen
[edit]
The largest recorded specimen was a female, which are thought to be larger than males, captured in February 2007 by a New Zealand fishing boat in the Ross Sea off Antarctica.[16] The squid was close to death when it was captured and subsequently was taken back to New Zealand for scientific study.[43] The specimen was initially estimated to measure about 10 metres in total length and weigh about 450 kg.[43]
Defrosting and dissection, April–May 2008
[edit]Thawing and dissection of the specimen took place at the Museum of New Zealand Te Papa Tongarewa.[44] AUT biologist Steve O'Shea, Tsunemi Kubodera, and AUT biologist Kat Bolstad were invited to the museum to aid in the process, joined by marine ecologist Mark Fenwick and Dutch scientist Olaf Blaauw.[44] Media reports suggested scientists at the museum were considering using a giant microwave to defrost the squid because thawing it at room temperature would take several days and it would likely begin to decompose on the outside while the core remained frozen.[45] However, they later opted for the more conventional approach of thawing the specimen in a bath of salt water.[46] After thawing, it was found that the specimen was 495 kg with a mantle length of 2.5 m and a total length of only 4.2 m, probably because the tentacles shrank once the squid was dead.[39]
Parts of the specimen have been examined:
- The beak is considerably smaller than some found in the stomachs of sperm whales,[47][48] suggesting that some other colossal squid are much larger than this one.[47][48]
- The eye is 27 cm (10+1⁄2 in) wide, with a lens 12 cm (4+1⁄2 in) across. This is the largest eye of any known animal.[15] These measurements are of the partly collapsed specimen; when the squid was alive, the eye was probably 30[16] to 40 cm (12 to 16 in) across.[49]
- Inspection of the specimen with an endoscope revealed ovaries containing thousands of eggs.[16]
Exhibition
[edit]
The Museum of New Zealand Te Papa Tongarewa began displaying this specimen from 13 December 2008. The exhibition was closed between 2018 and 2019, but it was reopened for public viewing at Te Papa.[40]
Filming in natural habitat
[edit]In 2022–23 there were several attempts made by scientists, including an ocean exploration non-profit called KOLOSSAL, to find and film the colossal squid in its natural habitat for the first time to learn more about its biology and ecological behavior.[50][51] The science team used a tourism vessel[52][53] to survey 36 locations throughout the Southern Ocean and may have filmed a small juvenile colossal squid for the first time. Researchers have confirmed that that video is of a species of glass squid, but due to marine snow, the footage has been harder to confirm without a DNA analysis, and may instead represent Galiteuthis glacialis or a new species of glass squid unknown to science.[54][55][56][57]

On 9 March 2025, for the first time, a confirmed colossal squid was filmed in its natural environment during an expedition near the South Sandwich Islands in the South Atlantic Ocean. The squid, a juvenile measuring around 30 cm (12 in) long, was captured on video at a depth of 600 m (2,000 ft) by the Schmidt Ocean Institute's remotely operated vehicle (ROV) SuBastian.[58][59]
Conservation status
[edit]The colossal squid has been assessed as a species of "least concern" on the IUCN Red List.[1] Colossal squid are not targeted by fishers; rather, they are only caught when they attempt to feed on fish caught on hooks.[60] Additionally, due to their habitat, interactions between humans and colossal squid are considered rare.[61]
See also
[edit]References
[edit]- ^ a b Barratt, I.; Allcock, L. (2014). "Mesonychoteuthis hamiltoni". IUCN Red List of Threatened Species. 2014 e.T163170A980001. doi:10.2305/IUCN.UK.2014-1.RLTS.T163170A980001.en. Retrieved 15 September 2022.
- ^ Bieler R, Bouchet P, Gofas S, Marshall B, Rosenberg G, La Perna R, Neubauer TA, Sartori AF, Schneider S, Vos C, ter Poorten JJ, Taylor J, Dijkstra H, Finn J, Bank R, Neubert E, Moretzsohn F, Faber M, Houart R, Picton B, Garcia-Alvarez O (eds.). "Mesonychoteuthis hamiltoni G. C. Robson, 1925". MolluscaBase. World Register of Marine Species. Retrieved 15 September 2022.
- ^ a b c d e f g h Rosa, Rui; Lopes, Vanessa M.; Guerreiro, Miguel; Bolstad, Kathrin & Xavier, José C. (30 March 2017). "Biology and ecology of the world's largest invertebrate, the colossal squid (Mesonychoteuthis hamiltoni): a short review" (PDF). Polar Biology. 40 (9): 1871–1883. Bibcode:2017PoBio..40.1871R. doi:10.1007/s00300-017-2104-5. S2CID 15480545.
- ^ a b c d McClain, Craig R.; Balk, Meghan A.; Benfield, Mark C.; Branch, Trevor A.; Chen, Catherine; Cosgrove, James; Dove, Alistair D.M.; Gaskins, Lindsay C.; Helm, Rebecca R.; Hochberg, Frederick G.; Lee, Frank B.; Marshall, Andrea; McMurray, Steven E.; Schanche, Caroline; Stone, Shane N. & Thaler, Andrew D. (2015). "Sizing ocean giants: patterns of intraspecific size variation in marine megafauna". PeerJ. 3 e715. doi:10.7717/peerj.715. PMC 4304853. PMID 25649000.
- ^ "How big is the colossal squid on display? | Te Papa". tepapa.govt.nz. Retrieved 9 April 2025.
- ^ "The beak of the colossal squid | Te Papa". tepapa.govt.nz. Retrieved 9 April 2025.
- ^ "The arms and tentacles of the colossal squid". tepapa. 25 March 2025.
- ^ Roper, C.F.E. & P. Jereb (2010). Family Cranchiidae. In: P. Jereb & C.F.E. Roper (eds.) Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No. 4, Vol. 2. FAO, Rome. pp. 148–178.
- ^ "Colossal Squid". Oceana. Retrieved 12 February 2024.
- ^ "The eyes of the colossal squid". Museum of New Zealand Te Papa Tongarewa, Wellington, NZ. 29 December 2016. Retrieved 15 September 2022.
- ^ a b c d e Jereb, P. & Roper, C.F.E. (2010). Cephalopods of the World. Food and Agriculture Organization of the U.N. Vol. 2. United Nations. pp. 6–10.
- ^ a b "Hooks and suckers". Te Papa (blog). 30 April 2008. Retrieved 30 September 2011 – via Blog.tepapa.govt.nz.
- ^ Nilsson, Dan-Eric; Warrant, Eric J.; Johnsen, Sönke; Hanlon, Roger; Shashar, Nadav (2012). "A Unique Advantage for Giant Eyes in Giant Squid". Current Biology. 22 (8): 683–688. Bibcode:2012CBio...22..683N. doi:10.1016/j.cub.2012.02.031. PMID 22425154.
- ^ a b Bourton, Jody (7 May 2010). "Huge 'monster squid' not fearsome". BBC News. Retrieved 2 August 2015.
- ^ a b Ballance, Alison; Meduna, Veronika (16 September 2014). "Colossal squid to give up its secrets". Radio New Zealand. Retrieved 2 August 2015.
- ^ a b c d Black, Richard (30 April 2008). "Colossal Squid's big eye revealed". BBC News. BBC.
- ^ a b Remeslo, Alexander; Yukhov, Valentin; Bolstad, Kathrin & Laptikhovsky, Vladimir (1 May 2019). "Distribution and biology of the colossal squid, Mesonychoteuthis hamiltoni: New data from depredation in toothfish fisheries and sperm whale stomach contents". Deep Sea Research Part I: Oceanographic Research Papers. 147: 121–127. Bibcode:2019DSRI..147..121R. doi:10.1016/j.dsr.2019.04.008. S2CID 146043830.
- ^ Xavier, José C.; Raymond, Ben; Jones, Daniel C. & Griffiths, Huw (19 October 2015). "Biogeography of Cephalopods in the Southern Ocean Using Habitat Suitability Prediction Models" (PDF). Ecosystems. 19 (2): 220–247. doi:10.1007/s10021-015-9926-1. S2CID 14435325.
- ^ a b Remeslo, Alexander; Yukhov, Valentin; Bolstad, Kathrin & Laptikhovsky, Vladimir (May 2019). "Distribution and biology of the colossal squid, Mesonychoteuthis hamiltoni: New data from depredation in toothfish fisheries and sperm whale stomach contents". Deep Sea Research Part I: Oceanographic Research Papers. 147: 121–127. Bibcode:2019DSRI..147..121R. doi:10.1016/j.dsr.2019.04.008. S2CID 146043830.
A total of 107 M. hamiltoni stomachs (out of 849 examined) contained prey remains. These comprised remnants of small fish, some of which were tentatively identified as Myctophidae and Bathylagidae. Large fish scales were also observed, but no other remains facilitating exact species identification were found. No invertebrate remains were observed.
- ^ Remeslo, A.V.; Yakushev, M.R.; Laptikhovsky, V. (10 November 2015). "Alien vs. Predator: interactions between the colossal squid ( Mesonychoteuthis hamiltoni ) and the Antarctic toothfish ( Dissostichus mawsoni )". Journal of Natural History. 49 (41–42): 2483–2491. doi:10.1080/00222933.2015.1040477. ISSN 0022-2933.
- ^ Lu, C.C. & Williams, R. (June 1994). "Contribution to the biology of squid in the Prydz Bay region, Antarctica". Antarctic Science. 6 (2): 223–229. Bibcode:1994AntSc...6..223L. doi:10.1017/s0954102094000349. S2CID 130139281.
- ^ a b Cherel, Yves; Hobson, Keith A (7 August 2005). "Stable isotopes, beaks and predators: a new tool to study the trophic ecology of cephalopods, including giant and colossal squids". Proceedings of the Royal Society B: Biological Sciences. 272 (1572): 1601–1607. doi:10.1098/rspb.2005.3115. PMC 1559839. PMID 16048776.
- ^ "Mesonychoteuthis hamiltoni". Animal Diversity Web.
- ^ a b Rosa, Rui & Seibel, Brad A. (2010). "Slow pace of life of the Antarctic colossal squid". Journal of the Marine Biological Association of the United Kingdom. 90 (7): 1375–1378. Bibcode:2010JMBUK..90.1375R. doi:10.1017/S0025315409991494. S2CID 85063782.
- ^ Clarke, M.R. (1980). "Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology". Discovery Reports. 37: 1–324.
- ^ Remeslo, A. V.; Yakushev, M. R. & Laptikhovsky, V. (10 November 2015). "Alien vs. Predator: interactions between the colossal squid (Mesonychoteuthis hamiltoni) and the Antarctic toothfish (Dissostichus mawsoni)". Journal of Natural History. 49 (41–42): 2483–2491. Bibcode:2015JNatH..49.2483R. doi:10.1080/00222933.2015.1040477. S2CID 82152308.
- ^ Cherel, Yves & Duhamel, Guy (2004). "Antarctic jaws: cephalopod prey of sharks in Kerguelen waters". Deep Sea Research Part I: Oceanographic Research Papers. 51 (1): 17–31. Bibcode:2004DSRI...51...17C. doi:10.1016/j.dsr.2003.09.009.
- ^ a b Jereb, P & Roper, C.F.E. (2010). Cephalopods of the world: an annotated and illustrated catalogue of cephalopod species known to date. Food and Agriculture Organization of the United Nations. ISBN 978-92-5-106720-8.
- ^ a b c d e Nilsson, Dan-Eric; Warrant, Eric J.; Johnsen, Sönke; Hanlon, Roger & Shashar, Nadav (24 April 2012). "A Unique Advantage for Giant Eyes in Giant Squid". Current Biology. 22 (8): 683–688. Bibcode:2012CBio...22..683N. doi:10.1016/j.cub.2012.02.031. PMID 22425154. S2CID 6119783.
- ^ Nilsson, Dan-E; Warrant, Eric J.; Johnsen, Sönke; Hanlon, Roger T. & Shashar, Nadav (8 September 2013). "The giant eyes of giant squid are indeed unexpectedly large, but not if used for spotting sperm whales". BMC Evolutionary Biology. 13 (1): 187. Bibcode:2013BMCEE..13..187N. doi:10.1186/1471-2148-13-187. PMC 3854791. PMID 24010674.
- ^ Herring, Peter J.; Dilly, P. N. & Cope, Celia (September 2002). "The photophores of the squid family Cranchiidae (Cephalopoda: Oegopsida)". Journal of Zoology. 258 (1): 73–90. doi:10.1017/S095283690200122X.
- ^ "The eyes of the colossal squid". 29 December 2016.
- ^ a b "Scientists Find that Squid Can Detect Sounds". www.whoi.edu/. Retrieved 10 April 2022.
- ^ Partridge, Julian C. (24 April 2012). "Sensory Ecology: Giant Eyes for Giant Predators?". Current Biology. 22 (8): R268–R270. Bibcode:2012CBio...22.R268P. doi:10.1016/j.cub.2012.03.021. PMID 22537628. S2CID 16685449.
- ^ a b c Robson, G.C. (1925). "On Mesonychoteuthis, a new genus of oegopsid, Cephalopoda". Annals and Magazine of Natural History. 9 (16): 272–277. doi:10.1080/00222932508633309.
- ^ "ITIS - Report: Mesonychoteuthis hamiltoni". www.itis.gov. Retrieved 9 April 2025.
- ^ a b Ellis, R. 1998. The Search for the Giant Squid. The Lyons Press.
- ^ a b Griggs, Kim (2 April 2003). "Super squid surfaces in Antarctic". BBC News. Wellington. Retrieved 2 August 2015.
- ^ a b "The Colossal Squid Exhibition – The Squid Files – How big is the colossal squid?". 17 December 2008. Archived from the original on 17 December 2008. Retrieved 9 March 2020.
- ^ a b "The Colossal Squid". Te Papa. 21 December 2015. Retrieved 14 May 2019.
- ^ Tapaleao, Vaimoana (11 August 2014). "Is it a boy? Te Papa gets new colossal squid". New Zealand Herald. ISSN 1170-0777. Retrieved 2 August 2015.
- ^ a b c "Very Rare Giant Squid Caught Alive". South Georgia Island. Archived from the original on 5 June 2010. Retrieved 2 August 2015.
- ^ a b "NZ fishermen land colossal squid". BBC News. 22 February 2007. Retrieved 2 August 2015.
- ^ a b Te Papa's Specimen: The Thawing and Examination Archived 25 April 2008 at the Wayback Machine. Tepapa.govt.nz. Retrieved on 30 September 2011.
- ^ Marks, Kathy (23 March 2007). "NZ's colossal squid to be microwaved". The New Zealand Herald. Archived from the original on 29 September 2007. Retrieved 25 September 2011.
- ^ Black, Richard (28 April 2008). "Colossal squid comes out of ice". BBC News. Retrieved 2 August 2015.
- ^ a b Ballance, Alison (14 October 2014). "Colossal Squid Revealed". Radio New Zealand. Retrieved 2 August 2015.
- ^ a b "Massive squid may be just a babe". The Star. South Africa. 30 April 2008.
- ^ "World's biggest squid reveals 'beach ball' eyes". www.terradaily.com. Wellington: AFP. 30 April 2008. Retrieved 2 August 2015.
- ^ McGrath, Jenny. "Scientists went on a hunt for the elusive colossal squid — and brought cruise ship tourists with them". Business Insider. Retrieved 5 June 2024.
- ^ "Studying the World's Largest Invertebrate – the Colossal Squid, Mesonychoteuthis hamiltoni". Experiment – Moving Science Forward. Retrieved 5 June 2024.
- ^ ECO (22 November 2022). "Expedition Launching to Study the Colossal Squid in Antarctica". ECO Magazine. Retrieved 5 June 2024.
- ^ Graham, Myrah; Herbig, Jennifer; Jacobsen, Eugenie; Maldonado, Tatiana K.; Beck, Jared; Lackey, Brent; Mulrennan, Matthew (20 February 2024). "New methods of undertaking marine science in Antarctica using tourism vessels". PLOS Climate. 3 (2) e0000348. doi:10.1371/journal.pclm.0000348. ISSN 2767-3200.
- ^ Haro, Alexander (30 May 2024). "A Baby Colossal Squid Might Have Been Filmed for the First Time Ever". The Inertia. Retrieved 5 June 2024.
- ^ Magazine, Hakai. "Visiting the Kraken at Home". Hakai Magazine. Retrieved 5 June 2024.
- ^ ""Mystery" Glass Squid In Antarctica Could Be First-Ever Colossal Squid Baby Filmed". IFLScience. 29 May 2024. Retrieved 5 June 2024.
- ^ "For the First Time Ever, the Colossal Squid Might Have Shown Its Secret Face". Popular Mechanics. 5 June 2024. Retrieved 5 June 2024.
- ^ "First Confirmed Footage of a Colossal Squid—and it's a Baby!". Schmidt Ocean. 15 April 2025. Retrieved 16 April 2025.
- ^ Preston, Elizabeth (15 April 2025). "The First Ever Sighting of a Colossal Squid". The New York Times. ISSN 0362-4331. Retrieved 16 April 2025.
- ^ "Colossal Squid". Oceana. Retrieved 10 March 2020.
- ^ "Colossal Squid ~ MarineBio Conservation Society". Marine Bio. 18 May 2017.
Further reading
[edit]- Aldridge, A.E. (2009). "Can beak shape help to research the life history of squid?". New Zealand Journal of Marine and Freshwater Research. 43 (5): 1061–1067. Bibcode:2009NZJMF..43.1061A. doi:10.1080/00288330.2009.9626529. S2CID 85883651.
- (in Russian) Klumov, S.K. & V.L. Yukhov 1975. Mesonychoteuthis hamiltoni Robson, 1925 (Cephalopoda, Oegopsida). Antarktika Doklady Komission 14: 159–189. [English translation: TT 81–59176, Al Ahram Center for Scientific Translations]
- McSweeny, E.S. (1970). "Description of the juvenile form of the Antarctic squid Mesonychoteuthis hamiltoni Robson". Malacologia. 10: 323–332.
- Rodhouse, P.G.; Clarke, M.R. (1985). "Growth and distribution of young Mesonychoteuthis hamiltoni Robson (Mollusca: Cephalopoda): an Antarctic squid". Vie Milieu. 35 (3–4): 223–230.
External links
[edit]- "CephBase: Colossal squid". Archived from the original on 17 August 2005.
- Tree of Life web project: Mesonychoteuthis hamiltoni
- Museum of New Zealand Te Papa Tongarewa(Te Papa) Colossal Squid Specimen Information
- Museum of New Zealand Te Papa Tongarewa(Te Papa) Colossal Squid Images and Video
- Tonmo.com: Giant Squid and Colossal Squid Fact Sheet
- New Zealand Herald: Fishermen haul in world's biggest squid
- USA Today: Colossal Squid Caught in Antarctic Waters
- BBC: Super squid surfaces in Antarctic
- MarineBio: Mesonychoteuthis hamiltoni


