Recent from talks
Nothing was collected or created yet.
Dracunculus medinensis
View on Wikipedia
| Guinea worm | |
|---|---|
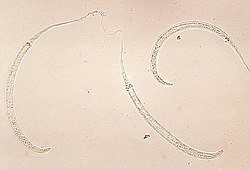
| |
| Photomicrograph of larvae | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Secernentea |
| Order: | Camallanida |
| Family: | Dracunculidae |
| Genus: | Dracunculus |
| Species: | D. medinensis
|
| Binomial name | |
| Dracunculus medinensis | |
| Synonyms | |
Dracunculus medinensis (Guinea worm, dragon worm, fiery serpent[1]) is a nematode that causes dracunculiasis, also known as Guinea worm disease.[2] The disease is caused by the female[3] which, at around 80 centimetres (31 inches) in length,[4] is among the longest nematodes infecting humans.[5] The length of specimens exhibits extreme sexual dimorphism, as the longest recorded male Guinea worm is only 4 cm (1+1⁄2 in).[4]
Guinea worm disease is on target to be the second infectious disease of humans to be eradicated, after smallpox, and the D. medinensis species would be made extinct to accomplish it. It was formerly endemic to a wide swath of Africa and Eurasia; as of 2023, it remains endemic in five countries: Chad, Mali, South Sudan, Angola and Ethiopia,[6] with most cases in Chad. Guinea worm spread to Angola c. 2018, and it is considered endemic there. Infection of domestic dogs is a serious complication in Chad.
The common name "Guinea worm" is derived from the Guinea region of Western Africa.
History
[edit]Dracunculus medinensis ("little dragon from Medina") was described in Egypt as early as the 15th century BCE and possibly was the "fiery serpent" afflicting the Israelites described in the Bible.[7]
In the mid-19th century, the nematode Camallanus lacustris, which infects freshwater fish, was discovered to develop in copepods. This led Russian naturalist Alexei Pavlovich Fedchenko to discover in 1870 that D. medinensis is similarly transmitted via copepod intermediate hosts.[8]
Life cycle
[edit]
D. medinensis L1 larvae are found in fresh water, where they are ingested by copepods (small crustaceans) of the genus Cyclops. Within the copepod, the D. medinensis larvae develop to an infective L3 stage within 14 days.[9] When the infected copepod is ingested by a mammalian host drinking unfiltered water, the copepod is then dissolved by stomach acid and dies and the D. medinensis larvae are released and migrate through the wall of the mammalian intestine, and enter the abdominal cavity and retro-peritoneal space, where they mature into adults. After maturing and mating within the host, the males die and females (length 60–100 cm or 24–39 in or longer[10])[11] migrate in subcutaneous tissue towards the skin's surface. Around a year after the infection, the female causes the formation of a blister on the skin's surface, generally on the lower extremities, though occasionally on the hand or scrotum. When the blister ruptures, the female slowly emerges over the course of several days or weeks.[9] This causes extreme pain and irritation to the host. During those few days to hours before the worm exits the skin, the person may develop a fever, pain, or swelling in that area. When the host—in an attempt to alleviate the excruciating burning pain—submerges the affected body part in water, the female releases thousands of larvae into the water. From here, the larvae infect copepods, continuing the life cycle.[9] After the worm exits the skin the wound caused by the emerging worm often develops a secondary bacterial infection. Permanent damage can occur if the infection goes untreated around a joint. Most cases occur in areas without access to health care facilities.[12]
Animal reservoirs
[edit]In 2020, Guinea worm was found in 1,507 domestic dogs in Chad, 15 in Ethiopia, and eight in Mali, as well as in 61 domestic cats in Chad and three in Ethiopia. Small numbers have also been found in wildcats and baboons.[13] These findings are a potential problem for the eradication program.
Epidemiology
[edit]
D. medinensis is most commonly found in the subtropic to tropical regions, especially in India, south-west Asia (Iraq, Iran, Pakistan, etc.), and rural areas of Africa, where temperatures of 25–30 °C (77–86 °F) are best for larval development.[14] The parasite relies on people accidentally consuming microcrustaceans of the genus Cyclops (copepods), that dwell in stationary bodies of water such as ponds, large, open wells (with stairs), or rain-filled cisterns.[14] The infection occurs most during times of drought or the "dry-season" in humid climates, or during or just after the rain season in the "semiarid, wet-and-dry-climates".[14] This is due to the lower surface water of the stationary bodies of water, which are prime for the growth of the infected copepods, and main source of water for many.[14]
Pathology
[edit]D. medinensis causes dracunculiasis as a result of the emergence of the female worm, non-emergence of adult worms (usually the male), and secondary bacterial infections.[15] As it emerges to the subcutaneous tissue, the female releases a toxic chemical that may result in nausea, rash at site, diarrhea, dizziness, localized edema, reddish papule, blister, and itching.[15] Arthritis or paraplegia can result from a worm that fails to reach the skin and gets calcified in or along the joint or finds its way into the central nervous tissue.[15] Aseptic abscesses and cystic swelling can also occur when worms rupture before emerging, causing an acute inflammatory response from the host's immune system.[15]
Treatment
[edit]
The female Guinea worm slowly starts to emerge from the host's skin after the blister ruptures. The most common method for removing the worm involves submerging the affected body part in water to help coax the worm out. The site is then cleaned thoroughly. Then, slight pressure is applied to the worm as it is slowly pulled out of the wound. To avoid breaking the worm, pulling should stop when resistance is met. Full extraction of the female Guinea worm usually takes several days. After each day's worth of extraction, the exposed portion of the worm is wrapped around a piece of rolled-up gauze or small stick to maintain tension.[16] This method of wrapping the worm around a stick or gauze is speculated to be the source for the Rod of Asclepius, the symbol of medicine.[17][unreliable source?] Once secure, topical antibiotics are applied to the affected region to help prevent secondary infections due to bacteria. The area is then wrapped in gauze to protect the wound. The same steps are repeated each day until the whole worm has been removed from the lesion.[16]
Control & Prevention of the Parasite
[edit]| Year | Reported cases | Countries |
|---|---|---|
| 1986 | estimated 3,500,000 | 21[19] |
| 1989 | 892,055 | 15[20] |
| 1992 | 374,202 | 15[20] |
| 1995 | 129,852 | 19[20] |
| 2000 | 75,223 | 16[20] |
| 2001 | 63,717 | 16[20] |
| 2002 | 54,638 | 14[20] |
| 2003 | 32,193 | 13[20] |
| 2004 | 16,026 | 13[20] |
| 2005 | 10,674 | 12[20] |
| 2006 | 25,217 [a] | 10 [20] |
| 2007 | 9,585 | 9[20] |
| 2008 | 4,619 | 7[20] |
| 2009 | 3,190 | 5 |
| 2010 | 1,797 | 4[22] (6[23]) |
| 2011 | 1,060 | 4[24] |
| 2012 | 542 | 4[25] |
| 2013 | 148 | 5[26] |
| 2014 | 126 | 4[27] |
| 2015 | 22 | 4[28] |
| 2016 | 25 | 3[19] |
| 2017 | 30 | 2[29] |
| 2018 | 28 | 3[30] |
| 2019 | 54 [b] | 4[31] |
| 2020 | 27 | 6[32] |
| 2021 | 15 | 4[33] |
| 2022 | 13 | 4[34] |
| 2023 | 14 | 4[35] |
| 2024 | 15 | 2[36] |
In the 1980s, the Carter Center initiated a program to eradicate the Guinea worm.[37] The campaign began in 1980 at the U.S. Centers for Disease Control and Prevention. In 1984, the CDC was designated by the World Health Organization as the "Collaborating Center for Research, Training, and Eradication of D. medinensis". More than twenty countries were affected by Guinea worms in 1986. That year, WHO started the eradication program with the Carter Center leading the effort.[38] The program included education of people in affected areas that the disease was caused by larvae in drinking water, isolation and support for affected people, and – crucially – widespread distribution of net filters and pipe filters for drinking water, and education about the importance of using them.
As of 2015[update], the species has been reported to be near eradication.[37] The International Commission for the Certification of Dracunculus Eradication has certified 198 countries, territories, and other WHO represented areas. As of January 2015, eight countries were yet to be certified as Guinea worm-free: Angola, the Democratic Republic of the Congo, Kenya, Sudan, Chad, Ethiopia, Mali, and South Sudan; of these, only in Chad, Ethiopia, Mali, and South Sudan does D. medinensis remain endemic.[38]
As of 2024[update], the disease has been reduced by more than 99.99% and more than 100 million cases have been prevented.[36]
See also
[edit]Explanatory notes
[edit]References
[edit]- ^ Chaudhury, Abhijit (2022). "Dracunculiasis". Textbook of Parasitic Zoonoses. Microbial Zoonoses. pp. 427–436. doi:10.1007/978-981-16-7204-0_41. ISBN 978-981-16-7203-3.
- ^ Knopp, Stefanie; Amegbo, Ignace K.; Hamm, David M.; Schulz-Key, Hartwig; Banla, Meba; Soboslay, Peter T. (March 2008). "Antibody and cytokine responses in Dracunculus medinensis patients at distinct states of infection". Transactions of the Royal Society of Tropical Medicine and Hygiene. 102 (3): 277–283. doi:10.1016/j.trstmh.2007.12.003. PMID 18258273.
- ^ Bimi, Langbong (2007). "Potential vector species of Guinea worm (Dracunculus medinensis) in northern Ghana". Vector-Borne and Zoonotic Diseases. 7 (3): 324–329. doi:10.1089/vbz.2006.0622. PMID 17767406.
- ^ a b Schmidt, Gerald D.; Roberts, Larry S. (2009). Roberts, Larry S.; Janovy, John Jr. (eds.). Foundations of Parasitology (8th ed.). McGraw-Hill. pp. 480–484. ISBN 978-0-07-128458-5.
- ^ bin Saleem, Talha; Ahmed, Irfan (2006). "'Serpent' in the breast" (PDF). Journal of Ayub Medical College Abbottabad. 18 (4): 67–68. PMID 17591014.
- ^ Control of Neglected Tropical Diseases team (2023). World Health Organization (ed.). Criteria for the certification of dracunculiasis eradication 2023 update. World Health Organization. pp. 9, 26. ISBN 978-92-4-007333-3.
- ^ "Dracunculiasis: Historical background". who.int. World Health Organization. Archived from the original on 18 October 2014. Retrieved 27 April 2017.
- ^ Anderson, Roy C. (8 February 2000). Nematode Parasites of Vertebrates: Their development and transmission (Second ed.). CABI Publishing. p. 350. ISBN 978-0-85199-786-5. Retrieved 29 December 2024 – via Google Books.
- ^ a b c "Dracunculiasis: About Guinea-Worm Disease". who.int. World Health Organization. Archived from the original on 1 November 2006. Retrieved 20 December 2015.
- ^ Despommier DD, Griffin DO, Gwadz RW, Hotez PJ, Knirsch CA (2019). "25. Dracunculus medinensis". Parasitic Diseases (PDF) (7 ed.). Parasites Without Borders. pp. 285–290. Archived (PDF) from the original on 24 November 2021. Retrieved 26 January 2021.
- ^ "Dracunculiasis (Guinea-worm disease)". who.int. World Health Organization. 30 January 2025. Archived from the original on 21 July 2025. Retrieved 17 August 2025.
- ^ "General information – frequently asked questions (FAQs)". Guinea Worm Disease. U.S. Centers for Disease Control and Prevention (CDC). 17 September 2020. Retrieved 24 October 2020.
- ^ "Guinea worm cases fell 50% in 2020, Carter Center reports". cartercenter.org (Press release). The Carter Center. 26 January 2021.
- ^ a b c d Muller, R. (1979). "Guinea worm disease: Epidemiology, control, and treatment". Bulletin of the World Health Organization. 57 (5): 683–689. PMC 2395878. PMID 161522.
- ^ a b c d Roberts, Larry S.; Janovy, John Jr.; Nadler, Steve (27 November 2012). Gerald D. Schmidt & Larry S. Roberts' foundations of parasitology (9th ed.). New York, NY: McGraw Hill. ISBN 978-0-07-352419-1. OCLC 812614125.
- ^ a b "Management of Guinea worm disease (GWD)". Parasites – Guinea Worm. cdc.gov. U.S. Centers for Disease Control (CDC). 16 March 2018. Retrieved 22 July 2018.
- ^ Satin, Morton (2009). Death in the Pot: The impact of food poisoning on history. Amherst, NY: Prometheus Books. pp. 63–65. ISBN 978-1-61-592224-6 – via Google Books.
- ^ "View Latest Worldwide Guinea Worm Case Totals". cartercenter.org. The Carter Center.
- ^ a b Martin, Jeff (11 January 2017). "Carter: Guinea worm disease reported in 3 countries in 2016". Associated Press. Archived from the original on 12 January 2017. Retrieved 11 January 2017.
- ^ a b c d e f g h i j k l
Dracunculiasis Epidemiological Data (1989-2008) (PDF). who.int (Report). World Health Organization. Retrieved 14 September 2009. - ^ Centers for Disease Control and Prevention (CDC) (17 August 2007). "Progress toward global eradication of Dracunculiasis, January 2005 – May 2007". Morbidity and Mortality Weekly Report. 56 (32). U.S. Centers for Disease Control and Prevention (CDC): 813–817. PMID 17703170. Retrieved 16 March 2011.
- ^ "Guinea worm countdown: The road to eradication". cartercenter.org. The Carter Center. Archived from the original on 6 September 2011. Retrieved 22 July 2018.
- ^ "Monthly report on dracunculiasis cases, January–December 2010" (PDF). Weekly Epidemiological Record. 86 (10). WHO: 92. 4 March 2011. Retrieved 4 March 2011.
- ^ "Wrap-up #209" (PDF). Guinea Worm Wrap-up. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis. No. 209. World Health Organization (WHO). 9 January 2012 – via The Carter Center (cartercenter.org).
- ^ "Wrap-up #218" (PDF). Guinea Worm Wrap-up. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis. No. 218. World Health Organization (WHO). 22 April 2013. Retrieved 4 November 2022 – via The Carter Center (cartercenter.org).
- ^ "Wrap-up #226" (PDF). Guinea Worm Wrap-up. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis. No. 226. World Health Organization (WHO). 9 May 2014. Retrieved 4 November 2022 – via The Carter Center (cartercenter.org).
- ^ "126 Cases of Guinea worm disease remain worldwide". cartercenter.org (Press release). The Carter Center. 11 January 2015. Retrieved 9 May 2015.
- ^ "Wrap-up #240" (PDF). Guinea Worm Wrap-up. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis. No. 240. World Health Organization (WHO). 13 May 2016. Retrieved 4 November 2022 – via The Carter Center (cartercenter.org).
- ^ "Announcement for 2017 Guinea worm disease case totals". cartercenter.org (Press release). The Carter Center. 19 January 2018. Archived from the original on 20 January 2018. Retrieved 22 July 2018.
- ^ "28 Guinea worm cases reported in 2018". cartercenter.org (Press release). Guinea worm update. The Carter Center. 6 May 2019.
- ^ a b "54* cases of Guinea worm reported in 2019". cartercenter.org (Press release). The Carter Center. January 2020.
- ^ "Guinea worm cases fell 50% in 2020, Carter Center reports". cartercenter.org (Press release). The Carter Center. 2021.
- ^ "Wrap-up #286" (PDF). Guinea Worm Wrap-up. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis. No. 286. World Health Organization (WHO). 31 March 2022. Retrieved 29 January 2023 – via The Carter Center (cartercenter.org).
- ^ "Guinea worm disease reaches all-time low: Only 13* human cases reported in 2022". The Carter Center. 24 January 2023. Retrieved 29 January 2023.
- ^ "Update: 14 Human Cases of Guinea Worm Reported in 2023". The Carter Center. 6 March 2024. Retrieved 30 December 2024.
- ^ a b
"Carter Center Reports 14 Human Guinea Worm Cases in 2024". The Carter Center. 30 January 2025. Archived from the original on 8 April 2025. Retrieved 8 April 2025.
The late U.S. President Jimmy Carter's goal to eradicate Guinea worm disease remains on track, with 14 provisional human cases reported worldwide in 2024.
- ^ a b "This species is close to extinction, and that's a good thing". Time. World Science Festival. 23 January 2015.
- ^ a b "Eradication program". Parasites – Guinea worm. U.S. Centers for Disease Control and Prevention. 2 May 2017. Retrieved 22 July 2018.
External links
[edit]- "Donors commit $240 million to fight neglected diseases". Voice of America. 2 April 2014. Archived from the original on 4 April 2014. Retrieved 4 April 2014 – via HNKC News.
- Bestall, Clifford (Spring 2014). How to Slay a Dragon (video documentary). Lifelines: The quest for global health. Al Jazeera English.
47 min.
Dracunculus medinensis
View on GrokipediaTaxonomy and Morphology
Taxonomy
Dracunculus medinensis is classified within the kingdom Animalia, phylum Nematoda, class Chromadorea, order Spirurida, family Dracunculidae, genus Dracunculus, and species D. medinensis.[8] This hierarchical placement reflects its position as a parasitic nematode among roundworms, distinguished by its spirurid characteristics. The species was first described by Carl Linnaeus in 1758 under the basionym Gordius medinensis in Systema Naturae, initially grouped with other thread-like worms.[9] These taxonomic adjustments highlight the evolution of nematode classification from early Linnaean systems to modern phylogenetic approaches incorporating genetic data. Within the genus Dracunculus, which comprises approximately 14 valid species, D. medinensis is the primary parasite of humans, while congeners like D. insignis infect North American mammals such as raccoons and mink, demonstrating host-specific adaptations. Other species, including those in reptiles like snakes and turtles, underscore the genus's broad host range across mammals and sauropsids. Its placement in the family Dracunculidae is justified by unique morphological traits, such as the elongated, slender body of adult females reaching 70–120 cm and males with specialized spicules and gubernaculum for reproduction, alongside molecular markers confirming phylogenetic affinity within Spirurida. These features, particularly the subcutaneous habitat and reproductive structures, differentiate Dracunculidae from related families like Camallanidae.Morphology
Dracunculus medinensis is a filiform nematode in the family Dracunculidae, notable for its extreme length and marked sexual dimorphism, with adults possessing a smooth, striated cuticle and a simple body plan adapted to a parasitic lifestyle.[10] The worm's body is cylindrical and pale white to yellow, lacking distinct external segmentation but featuring a triangular mouth opening into the esophageal lumen, which is lined with cuticle and trivoliate in cross-section.[11] The adult female represents the prominent form, reaching lengths of 70 to 120 cm and a diameter of 1 to 2 mm, forming a slender, thread-like structure that migrates subcutaneously in the host.[1][12] Gravid females are filled with first-stage larvae within their uterus, which occupies much of the body cavity, and the vulva is located near the anterior end.[13] The reproductive system includes paired ovaries extending the length of the body, connected to oviducts and uteri that produce vast numbers of larvae.[10] Internally, the esophagus is elongated, comprising a short anterior muscular portion for ingestion and a dominant glandular posterior section, while the intestine is atrophied and non-functional, with adults lacking digestive enzymes and instead absorbing nutrients osmotically from host tissues.[10][14] In contrast, the adult male is considerably smaller, measuring 12 to 40 mm in length and about 0.4 mm in diameter, with a coiled posterior end adapted for copulation.[10][15] Males possess two unequal spicules—chitinous structures used to grasp the female during mating—and a single tubular testis, highlighting the dimorphism in reproductive anatomy.[16] Microscopic identification of males relies on these spicules, the cloacal opening, and the overall reduced body size compared to females. The first-stage larvae (L1), released from gravid females, are microscopic, measuring 300 to 900 µm in length, ensheathed, and distinguished by a long, tapered tail ending in a characteristic trilobed tip that aids in identification.[10][18] These larvae exhibit chemotactic behavior, actively swimming to facilitate ingestion by copepod intermediate hosts.[19]Life Cycle and Transmission
Developmental Stages
The developmental stages of Dracunculus medinensis commence when the definitive host, typically a human, ingests untreated water containing copepods (Cyclops spp.) harboring infective third-stage larvae (L3). These larvae are released in the upper gastrointestinal tract upon digestion of the copepods, after which they penetrate the intestinal mucosa and migrate through the body cavity to subcutaneous and connective tissues.[20][1] In the human host, the L3 larvae undergo two molts over 60–70 days to develop into sexually mature adults, with males reaching 15–40 mm in length and females growing to 70–120 cm. Following maturation, the adults mate within the connective tissues; males die shortly after fertilization, while gravid females, containing up to 500,000 first-stage larvae (L1), migrate to subcutaneous sites, predominantly the lower limbs, over the subsequent 10–14 months. This migration culminates in the formation of a blister at the site, marking the pre-emergence phase approximately one year after initial infection.[21][20][1] Upon contact with water, the blister ruptures, allowing the female worm to emerge partially through the skin ulcer as a whitish filament, releasing L1 larvae in bursts each time the affected area is immersed; this process can extend up to 8 weeks until the female is fully expelled. The L1 larvae exhibit active motility in water and remain viable for up to 6 days in clean conditions, enabling ingestion by copepods to perpetuate the cycle. Similar developmental progression occurs in animal reservoirs like dogs, though with variations in migration sites.[20][16][21]Transmission Mechanisms
The transmission of Dracunculus medinensis, the causative agent of dracunculiasis (Guinea worm disease), primarily occurs through the ingestion of contaminated water containing copepods of the genus Cyclops harboring infective third-stage larvae (L3). These microscopic crustaceans serve as the essential intermediate hosts in the parasite's life cycle, facilitating the spread from infected humans to uninfected ones via environmental water sources.[4][2] Infection of copepods begins when first-stage larvae (L1), released from the gravid female worm in an infected human host, are ingested by the copepods in water bodies. The larvae penetrate the copepod's gut wall and enter the hemocoel, where they undergo two molts to develop into infective L3 larvae, a process that typically takes 12-15 days at water temperatures of 25-30°C. Development is temperature-dependent; at lower temperatures (e.g., below 20°C), larval maturation is delayed or halted, limiting transmission in cooler environments.[22][23][20] Humans acquire the infection by drinking unfiltered water from stagnant sources such as ponds, step wells, or pools, where copepod density is often high during dry seasons due to reduced water volume and lack of dilution. These conditions concentrate the intermediate hosts, increasing the likelihood of ingesting infected copepods, though no human immunity develops, allowing repeated infections.[4][2][19] The cycle completes when an infected individual with a mature female worm (approximately 70-120 cm long) contacts water, triggering the emergence of a blister on the skin—typically on the lower limbs—through which thousands of L1 larvae are actively released from the worm's uterus over 1-3 weeks. These motile larvae (250-750 μm long) swim freely in the water, seeking out and infecting nearby copepods to perpetuate transmission. Environmental factors like water temperature (optimal 24-30°C for larval survival and copepod activity), pH stability in neutral ranges (around 7-8, common in endemic stagnant waters), and copepod population density further modulate transmission efficiency, with higher densities in warm, undisturbed pools enhancing parasite dissemination.[1][20][24]Reservoirs
Dracunculus medinensis, the causative agent of dracunculiasis (Guinea worm disease), has definitive animal reservoirs primarily in dogs (Canis familiaris) and cats (Felis catus), with dogs being the most common in endemic regions of sub-Saharan Africa. These animals serve as alternative hosts to humans, harboring the adult worms that emerge through the skin to release larvae into water sources. Infections in dogs and cats were first systematically reported in the early 2010s, notably in Chad starting in 2012, where they were identified as a significant barrier to eradication efforts. The life cycle in these animal reservoirs mirrors that in humans, involving ingestion of copepods harboring third-stage larvae, internal development of the parasite over approximately 10–14 months, and subsequent emergence of gravid females from cutaneous ulcers, typically in the lower limbs.00209-3/fulltext)[25] Frogs and certain fish species in endemic areas act as paratenic hosts, potentially facilitating transmission by harboring infective larvae after consuming infected copepods, which can then be passed to definitive hosts like dogs or humans upon predation. Experimental studies have demonstrated that tadpoles and fish, such as tilapia, can maintain viable D. medinensis larvae for weeks, supporting a secondary pathway distinct from the classic waterborne route. However, dogs and cats remain the dominant reservoirs, as they complete the full reproductive cycle and directly contaminate aquatic environments with larvae.[26][27] In 2024, a provisional total of 664 animal infections were reported globally, compared to just 15 human cases, underscoring the shift toward zoonotic transmission. Chad accounted for the highest burden, with 281 animal cases (234 in dogs and 47 in cats) across 184 villages, followed by Cameroon with 310 infections (primarily in dogs). These reservoirs pose zoonotic risks by releasing larvae into shared water sources used by both animals and humans, thereby sustaining low-level transmission cycles in the absence of widespread human infections.[28])Epidemiology
Historical Distribution
_Dracunculus medinensis, the causative agent of dracunculiasis (commonly known as Guinea worm disease), has a long history of endemicity in regions of Africa, the Middle East, India, and the Arabian Peninsula. Archaeological evidence indicates its presence in ancient Egypt, with remnants of the parasite identified in mummies of nobles dated to approximately 1450 BCE from Thebes, suggesting infection through contaminated water sources prevalent in the Nile Valley and surrounding areas.[29] Biblical references to the "fiery serpent" afflicting the Israelites near the Red Sea may also describe symptoms consistent with dracunculiasis, pointing to its persistence in the Middle East and North Africa for millennia.[30] By historical accounts, the disease was well-documented in medical texts from ancient Persia and Arabia, where it was associated with rural water-dependent communities.[31] In the 20th century, prior to widespread intervention efforts, dracunculiasis affected vast swaths of sub-Saharan Africa, parts of Asia, and the Middle East, with an estimated 3.5 million cases occurring annually in the mid-1980s across 20 countries, including 17 in Africa.[2] The parasite's distribution was tied to tropical and semi-tropical climates where stagnant water bodies served as transmission foci, enabling its spread from West Africa (such as Senegal) eastward to Ethiopia and southward into Yemen and Saudi Arabia on the Arabian Peninsula.[16] In Asia, endemic transmission persisted in India and Pakistan until mid-century control measures began reducing cases, though the disease remained a significant burden in rural populations reliant on unprotected ponds and step-wells.[32] Regional hotspots in sub-Saharan Africa included countries like Nigeria and Ghana, where the disease imposed heavy socioeconomic burdens in the 1980s, with Nigeria reporting the highest global caseload at the time due to dense rural populations and seasonal water scarcity.[33] In these areas, prevalence rates exceeded 50% in some villages, exacerbating agricultural disruptions during peak emergence seasons.[34] Similarly, Yemen and parts of the Arabian Peninsula served as foci in the Middle East, where nomadic and agrarian lifestyles facilitated intermittent outbreaks linked to shared water sources.[16] The spread of Dracunculus medinensis was profoundly influenced by socio-environmental factors, particularly rural poverty and the absence of safe drinking water infrastructure, which forced dependence on contaminated surface water harboring the intermediate copepod host.[2] Human migration, including seasonal labor movements and conflict-driven displacement, further disseminated the parasite by introducing infected individuals to new water bodies, perpetuating cycles in underserved communities.[35] These conditions were most acute in isolated villages lacking sanitation, where poverty limited access to filtration or boiling methods that could interrupt transmission.[3] Prior to the global eradication initiative launched in 1986, localized control programs achieved partial declines in several regions. In southern Iran, systematic case containment and water source protection eliminated transmission by 1972, reducing endemic foci that had persisted for centuries.[3] In Pakistan, early 1950s efforts involving community education and temephos application to water sources curbed prevalence in Balochistan and Sindh provinces, setting the stage for full eradication later in the century.[36] These pre-1986 interventions highlighted the feasibility of breaking the parasite's life cycle through targeted environmental modifications, though broader impacts were limited without coordinated international support.[37]Current Status
As of 2024, dracunculiasis persists at historically low levels, with 15 confirmed human cases reported globally—nine in Chad and six in South Sudan—marking a slight increase from the 14 cases in 2023.[38] These figures represent final data, with all 2024 specimens confirmed.[39] The disease remains endemic in Chad and South Sudan for human transmission, while Angola, Ethiopia, Mali, and Sudan are considered provisionally endemic or in pre-certification due to recent animal infections or historical reports, though no human cases were confirmed in these countries in 2024.[5] Animal infections, primarily in dogs but also cats and other species, pose a significant challenge to eradication, with 664 cases reported in 2024 across 251 communities, a 25% decrease from 887 in 2023 and concentrated mostly in Chad.[40] These non-human reservoirs complicate human case interruption by serving as alternative hosts in contaminated water sources.[41] As of September 18, 2025, provisional data indicate 4 human cases reported worldwide, a decline from 2024, primarily in Chad and South Sudan. Provisional animal infections totaled 98 (83 dogs and 15 cats) from January to July 2025, with most contained through interventions.[42] Progress toward global eradication continues, with the World Health Organization (WHO) having certified 200 countries, territories, and areas free of transmission since surveillance began, encompassing 188 Member States.[2] The eradication target, originally set for 2020, has been extended to 2030 to address ongoing hurdles such as insecurity in conflict-affected regions like South Sudan and Chad, gaps in surveillance coverage, and the rise in animal cases.[43] Six countries—Angola, Chad, Ethiopia, Mali, South Sudan, and Sudan—still await certification after three consecutive years of zero human and animal cases under active monitoring.[5]Clinical Aspects
Pathogenesis
Upon ingestion of water containing infected copepods, the larvae of Dracunculus medinensis are released in the stomach and actively penetrate the intestinal mucosa, burrowing through the wall into the peritoneal cavity.[1] From there, the larvae migrate via the body cavity to subcutaneous connective tissues, where they develop into immature adults over approximately 10 to 14 months.[20] This initial migration occurs without significant immediate tissue damage, allowing the parasite to establish infection asymptomatically during the early developmental stages, as detailed in the life cycle timeline.[2] As the worms mature, male and female adults (with females reaching up to 1 meter in length) pair and mate in the subcutaneous tissues, after which males die and females migrate further through connective tissues to coil in deeper subcutaneous sites.[20] The presence of these coiled adult worms triggers a host immune response, characterized by localized inflammation due to eosinophil and lymphocyte infiltration around the parasite, leading to granuloma formation and tissue irritation.[20] This immune-mediated reaction helps contain the parasite but contributes to chronic low-level tissue damage without rapid host death, enabling the worm's long-term survival.[12] When gravid, the female worm migrates to the skin, typically in the lower extremities, and secretes substances that induce the formation of a painful blister or vesicle at the site.[20] Exposure to cold water stimulates the blister to rupture, allowing the worm to emerge gradually over 1-3 weeks, during which larvae are released externally while the ulcerated site becomes susceptible to secondary bacterial infections, which exacerbate local inflammation through additional immune activation and pus formation.[1] These infections are common due to the open wound and poor hygiene in endemic areas, often leading to cellulitis or abscesses.[20] Systemic effects arise primarily from aberrant worm migration or complications, such as larval or adult movement into joint spaces causing synovitis and infectious arthritis through inflammatory responses and secondary bacterial invasion.[44] In rare cases, untreated secondary infections can progress to bacteremia and sepsis, potentially fatal but uncommon given the localized nature of the primary pathology.[20] The host-parasite interaction involves partial immune modulation, where the worm evades complete eradication by downregulating acute responses, permitting persistence until larval release without overwhelming the host.[45]Symptoms and Complications
Infection with Dracunculus medinensis is typically asymptomatic for approximately one year following ingestion of contaminated water containing infective larvae, during which the parasites mature within the human host.[2] As the adult female worm migrates subcutaneously toward the skin surface, patients may experience nonspecific systemic symptoms, including mild fever, nausea, vomiting, diarrhea, urticarial rash, and intense pruritus.[1] These pre-emergence signs are often mild and transient, affecting a subset of cases before the characteristic blister forms.[46] The emergence phase begins when a painful blister develops, most commonly on the lower limbs in about 80-90% of cases, accompanied by intense burning pain and localized swelling.[2] Upon contact with water, the blister ruptures, allowing the female worm—up to 1 meter long—to emerge gradually over 1-3 weeks, releasing larvae through an ulcerated lesion.[1] This process causes severe discomfort and inflammation, peaking as the worm emerges, and may be exacerbated by an allergic reaction to materials released by the parasite, leading to additional symptoms such as joint pain and debilitating arthritis.[47] To fully extract the intact worm without breakage, it is wound slowly around a small stick at approximately 1 cm per day, often requiring 2-6 weeks.[48] Complications arise primarily from secondary bacterial infections at the emergence site, resulting in cellulitis, abscess formation, and, in severe cases, systemic sepsis or tetanus.[3] Untreated infections can lead to pyogenic arthritis, permanent joint damage, and disability lasting several months, severely limiting work and daily activities.[49] Although dracunculiasis itself is rarely fatal, secondary sepsis can contribute to death in a small percentage (estimated at less than 1%) of untreated cases, particularly in remote areas with limited healthcare access.[2] In rare instances, the worm may emerge from ectopic sites such as the eyes, causing inflammation and potential blindness, or the genitals, leading to sterility or severe local complications.[50] [51] These atypical presentations, occurring in less than 10% of cases, heighten the risk of long-term sequelae due to the sensitivity of affected tissues.[46]Diagnosis and Treatment
Diagnosis of Dracunculus medinensis infection primarily relies on clinical observation of the characteristic emergence of a mature female worm from a painful blister or ulcer on the skin, most commonly on the lower extremities, approximately one year after ingestion of contaminated water.[4] This presentation, often preceded by localized swelling, intense pain, and sometimes fever, is distinctive enough that laboratory confirmation is typically unnecessary in endemic areas.[1] Microscopic examination of fluid expressed from the blister can reveal rhabditiform larvae, providing supportive evidence of infection.[1] Advanced laboratory methods include polymerase chain reaction (PCR) assays targeting the parasite's mitochondrial cytochrome b gene, which enable detection of D. medinensis DNA in tissue or fluid samples with high sensitivity (limit of detection at 10 copies per reaction) and specificity, useful for early or atypical cases.[52] Serological tests for antibodies against D. medinensis antigens exist but are limited by significant cross-reactivity with other filarial parasites, such as Onchocerca volvulus, reducing their diagnostic utility.[53] No antiparasitic medications are effective against D. medinensis, and there is no vaccine available.[4] The cornerstone of treatment is manual extraction of the intact female worm once it emerges from the skin lesion, achieved by slowly winding it around a small stick or matchstick—a few centimeters per day initially, but approximately 1 cm per day to prevent breakage, which could lead to severe inflammatory complications.[47] This process, which may take several weeks for the worm's full length (up to 1 meter) to be removed, requires careful wound care to minimize secondary bacterial infections.[48] Supportive care includes daily cleaning of the wound with antiseptic solutions, application of antibiotic ointments to prevent secondary infections, and administration of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen to alleviate pain and reduce swelling.[4] Surgical removal is contraindicated in most cases, as it can provoke intense allergic reactions and further tissue damage; manual extraction remains the preferred method.[13] To prevent transmission, the affected area must be kept dry and away from water sources during extraction, ensuring larvae are not released into drinking water.[48]Prevention and Control
Strategies
The primary strategies for preventing and controlling Dracunculus medinensis infection focus on interrupting the transmission cycle by targeting the copepod intermediate host, preventing water contamination by emerging worms, and ensuring access to uncontaminated drinking water. These approaches, implemented through community-based interventions, have been central to global eradication efforts since the 1980s.[2][4] Water filtration remains a cornerstone method, utilizing cloth or monofilament nylon filters with pore sizes smaller than 150 µm to effectively remove copepods from drinking water sources. These filters, often distributed to households in endemic areas, capture the Cyclops species that harbor the infective larvae, thereby preventing ingestion by humans or animals. Studies have demonstrated that filters with 100 µm pores are particularly efficient at retaining even early developmental stages of copepods, outperforming coarser 200 µm variants in field trials.[2][54][40] Community education programs emphasize behavioral changes to avoid contaminated water and manage emerging infections. Residents are taught to filter all drinking water and to contain emerging worms by wrapping the affected limb in clean cloth or seeking care at containment centers, preventing the release of larvae into water bodies. These initiatives, often led by trained volunteers, also promote hygiene practices and the recognition of early symptoms to facilitate prompt isolation.[2][4][55] Providing safe water infrastructure addresses the root cause of transmission in villages reliant on surface water. The installation of boreholes equipped with handpumps or piped water systems delivers uncontaminated sources, reducing dependence on copepod-infested ponds and streams. Such interventions not only target dracunculiasis but also mitigate other waterborne diseases, with community involvement ensuring long-term maintenance.[2][48] Case containment involves immediate reporting of infections through surveillance networks, followed by isolation of affected individuals or animals to avert water contamination. Infected persons receive wound care at designated centers, where worms are slowly extracted onto sticks over days or weeks, while domestic animals like dogs are tethered away from water sources during emergence. This proactive measure has been crucial in high-risk areas, minimizing secondary transmission.[2][4][55] Vector control supplements other methods by applying temephos (Abate®), an organophosphate larvicide, to stagnant water bodies to kill copepods harboring larvae. Treatments are targeted and infrequent—typically monthly during peak seasons—to preserve efficacy and mitigate emerging resistance observed in some copepod populations after prolonged use. This chemical intervention is used judiciously, prioritizing filtration and safe water provision to avoid over-reliance.[2][4][55]Eradication Efforts
The international campaign to eradicate Dracunculus medinensis, the causative agent of dracunculiasis or Guinea worm disease, began in 1980 under the leadership of the U.S. Centers for Disease Control and Prevention (CDC). This effort was formalized and intensified in 1986 when The Carter Center, founded by former U.S. President Jimmy Carter, launched the Guinea Worm Eradication Program in collaboration with the World Health Organization (WHO), the United Nations Children's Fund (UNICEF), the CDC, and ministries of health in endemic countries.[41][40] Jimmy Carter personally championed the initiative, leveraging his post-presidency influence to secure funding, diplomatic support, and technical expertise, which transformed the program into a model of global public health coordination.[56][40] Significant milestones underscore the program's success. In 1986, an estimated 3.5 million human cases occurred annually across 21 countries; by the end of 2024, this had plummeted to just 15 human cases, representing a 99.99% reduction and averting over 100 million cases.[40][41] As of August 2025, 3 provisional human cases were reported worldwide. The WHO has certified 200 countries and territories as free of transmission since 1995, with the disease now endemic in only five countries: Angola, Chad, Ethiopia, Mali, and South Sudan.[41][5] Following the emergence of animal reservoirs around 2012, eradication efforts shifted post-2015 to address zoonotic transmission, particularly in dogs, which accounted for 664 infections in 2024—a 25% decline from the previous year; provisional data for January–July 2025 shows 83 dog and 15 cat infections, continuing the downward trend.[40][57] Interventions include proactive tethering of dogs to restrict access to contaminated water sources and community-based meat inspection of fish viscera, as raw fish innards have been identified as a potential foodborne transmission route for animals.[41][58] In 2023, the WHO updated its eradication roadmap to emphasize zoonotic control, enhanced surveillance, and cross-border collaboration, setting a new target of global eradication by 2030 after previous deadlines were missed due to animal cases and logistical hurdles. In May 2025, the World Health Assembly adopted resolutions to accelerate neglected tropical disease eradication efforts, including dracunculiasis.[59][60][61] Despite progress, challenges persist, including civil unrest and insecurity in Chad and South Sudan that limit program access and surveillance, as well as the need for sustained annual funding of approximately $50 million to maintain operations through 2030.[41][40][62]History
Discovery and Etymology
The parasite Dracunculus medinensis, commonly known as the guinea worm, has been recognized in historical records for millennia, with possible allusions in ancient texts. One interpretation links it to the "fiery serpents" described in the Old Testament Book of Numbers (21:6–9), where the affliction of the Israelites in the wilderness is suggested to reflect the burning pain caused by emerging worms, a view proposed by parasitologist Friedrich Küchenmeister in 1855.[63] Early medical descriptions appear in ancient Greco-Roman literature, where the condition was noted for painful ulcers on the legs from which thread-like worms emerged. Hippocrates (c. 460–375 BCE) referenced similar subcutaneous worms and associated leg ulcers in the Corpus Hippocraticum, marking one of the earliest documented observations of helminthic infections in humans. By the Roman era, physicians like Rufus of Ephesus (1st–2nd century CE) provided more detailed accounts of the worm's emergence from the skin, distinguishing it from other parasites.[63][31] The scientific study of D. medinensis began in the 17th century with Georg Hieronymus Velschius, who in 1674 published Exercitatio de Vena Medinensis, the first illustrated treatise on the parasite, depicting its extraction and linking it to ancient icons. Formal taxonomic naming occurred in 1758 when Carl Linnaeus classified it as Gordius medinensis in Systema Naturae, later emended to Dracunculus medinensis to reflect its serpentine form; the genus Dracunculus derives from Latin for "little dragon," alluding to the worm's painful emergence, while medinensis refers to Medina in Arabia, a historical epicenter of the disease based on medieval Arabic texts like those of Avicenna.[63][64][65] Significant advances in understanding the parasite's biology followed in the 19th century. In 1836, surgeon John Forbes observed larvae in water sources, hinting at an aquatic transmission route. The life cycle was elucidated in 1870 by Russian naturalist Alexei Pavlovich Fedchenko, who demonstrated that copepod crustaceans (Cyclops spp.) serve as intermediate hosts, ingesting larvae released from infected humans and transmitting them when water is consumed; this discovery was later confirmed by Patrick Manson in 1894. In 1849, French zoologist Émile Blanchard established the family Dracunculidae, solidifying its classification among filarial nematodes.[31][66][24]Historical Impact
Dracunculus medinensis, the causative agent of dracunculiasis or guinea worm disease, has been documented since ancient times, with one of the earliest references appearing in the Ebers Papyrus around 1550 BCE in Egypt, describing symptoms consistent with the parasite's emergence from the skin.[31] Paleopathological evidence, including a worm-like calcified structure found in a mummy from the Roman period (circa 70–130 CE), confirms its presence in ancient Egyptian and Arabian populations, where it likely spread through contaminated water sources like open wells in arid regions.[31] During the Roman Imperial era, physicians such as Rufus of Ephesus and Galen detailed the disease as "drakontia" or "dracontiasis," noting its painful ulceration and extraction methods, though its impact remained confined to Egypt and the Arabian Peninsula without significant spread to Europe.[31] In the Middle Ages, the disease featured in Byzantine and Arabic medical texts, including those by Paul of Aegina and Avicenna, who described treatment by winding the worm around a stick—a practice echoed in ancient accounts—but it was often conflated with veterinary parasites in European contexts, limiting broader recognition.[31] By the modern period starting in the 16th century, European travelers like Jan Huyghen van Linschoten reported cases in Iran and India, while 18th-century explorers such as James Bruce documented its prevalence in Ethiopia and Sudan.[31] The transatlantic slave trade (1501–1888) played a pivotal role in disseminating the parasite to the Americas, introducing it to enslaved Africans from endemic West African regions like Ghana, leading to outbreaks in Caribbean plantations such as Barbados and Grenada, where poor water conditions enabled local transmission.[67] This association reinforced perceptions of dracunculiasis as an "African disease" by the mid-18th century, as noted by physicians like Hans Sloane and Richard Towne, and it caused debilitating blisters that reduced enslaved individuals' labor capacity, straining plantation economies.[67] Historically, the disease has imposed substantial socio-economic burdens in endemic rural communities, earning the moniker "disease of empty granaries" for its role in disrupting agriculture and education through temporary or permanent disability.[31] In agricultural societies of sub-Saharan Africa and Asia, infections peaked during dry seasons, sidelining workers for weeks or months and contributing to chronic complications like arthritis and joint contractures that further diminished productivity.[31] For instance, 19th-century British military records from India reported high incidence among troops, such as 84 cases in Aden between 1858 and 1859, impairing operational efficiency in colonial forces.[67] Economic analyses from endemic areas, such as a study in Benin, estimated annual losses per case at around $20–$40 in medical costs and foregone income, scaling to community-wide impacts in millions when prevalence was high, perpetuating poverty cycles in resource-limited settings.[3]References
- https://www.[jstor](/page/JSTOR).org/stable/3272072

