Recent from talks
Nothing was collected or created yet.
Carrion's disease
View on WikipediaThis article needs more reliable medical references for verification or relies too heavily on primary sources. (July 2020) |
| Carrion's disease | |
|---|---|
| Other names | Oroya fever |
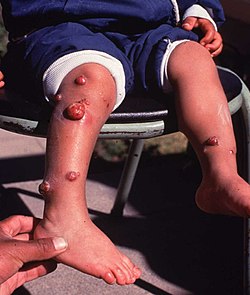 | |
| Carrion's disease chronic phase—verruga peruana (Peruvian warts) | |
| Specialty | Infectious diseases |
Carrion's disease is an infectious disease produced by Bartonella bacilliformis infection. It is named after Daniel Alcides Carrión.[1] Bacteriologist Hideyo Noguchi alongside fellow researcher Evelyn Tilden continuing his research after his death proved Carrion's disease and verruca peruana were the same species[2].
Signs and symptoms
[edit]The clinical symptoms of bartonellosis are pleomorphic and some patients from endemic areas may be asymptomatic. The two classical clinical presentations are the acute phase and the chronic phase, corresponding to the two different host cell types invaded by the bacterium (red blood cells and endothelial cells). An individual can be affected by either or both phases.[3][4]
Acute phase
[edit]The acute phase is also called the hematic phase or Oroya fever.[3] The most common findings are fever (usually sustained, but with temperature no greater than 102 °F or 39 °C), pale appearance, malaise, painless liver enlargement, jaundice, enlarged lymph nodes, and enlarged spleen. This phase is characterized by severe hemolytic anemia and transient immunosuppression. The case fatality ratios of untreated patients exceeded 40% but reach around 90% when opportunistic infection with Salmonella spp. occurs. In a recent study, the attack rate was 13.8% (123 cases) and the case-fatality rate was 0.7%.[citation needed]
Other symptoms include a headache, muscle aches, and general abdominal pain.[5] Some studies have suggested a link between Carrion's disease and heart murmurs due to the disease's impact on the circulatory system. In children, symptoms of anorexia, nausea, and vomiting have been investigated as possible symptoms of the disease.[3]
Most of the mortality of Carrion's disease occurs during the acute phase. Studies vary in their estimates of mortality. In one study, mortality has been estimated as low as just 1% in studies of hospitalized patients, to as high as 88% in untreated, unhospitalized patients.[3] In developed countries, where the disease rarely occurs, it is recommended to seek the advice of a specialist in infectious disease when diagnosed.[6] Mortality is often thought to be due to subsequent infections due to the weakened immune system and opportunistic pathogen invasion, or consequences of malnutrition due to weight loss in children.[3][7] In a study focusing on pediatric and gestational effects of the disease, mortality rates for pregnant women with the acute phase were estimated at 40% and rates of spontaneous abortion in another 40%.[3]
Chronic phase
[edit]The chronic phase is also called the eruptive phase or tissue phase, in which the patients develop a cutaneous rash produced by a proliferation of endothelial cells, known as "Peruvian warts" or "verruga peruana". Depending on the size and characteristics of the lesions, there are three types: miliary (1–4 mm), nodular or subdermic, and mular (>5mm). Miliary lesions are the most common. The lesions often ulcerate and bleed.[5]
The most common findings are bleeding of verrugas, fever, malaise, arthralgias (joint pain), anorexia, myalgias, pallor, lymphadenopathy, and liver and spleen enlargement.[citation needed]
On microscopic examination, the chronic phase and its rash are produced by angioblastic hyperplasia, or the increased rates and volume of cell growth in the tissues that form blood vessels. This results in a loss of contact between cells and a loss of normal functioning.[3][8]
The chronic phase is the more common phase. Mortality during the chronic phase is very low.[3][5]
Cause
[edit]Carrion's disease is caused by Bartonella bacilliformis.[5][8] Recent investigations show that Bartonella ancashensis may cause verruga peruana,[9][10][11] although it may not meet all of Koch's postulates.[12] There has been no experimental reproduction of the Peruvian wart in animals apart from Macaca mulatta, and there is little research on the disease's natural spread or impact in native animals.[13]
Diagnosis
[edit]
Diagnosis during the acute phase can be made by obtaining a peripheral blood smear with Giemsa stain, Columbia blood agar cultures, immunoblot, indirect immunofluorescence, and PCR. Diagnosis during the chronic phase can be made using a Warthin–Starry stain of wart biopsy, PCR, and immunoblot.
Treatment
[edit]Because Carrion's disease is often comorbid with Salmonella infections, chloramphenicol has historically been the treatment of choice.[6]
Fluoroquinolones (such as ciprofloxacin) or chloramphenicol in adults and chloramphenicol plus beta-lactams in children are the antibiotic regimens of choice during the acute phase of Carrion's disease.[6] Chloramphenicol-resistant B. bacilliformis has been observed.[3][6]
During the eruptive phase, in which chloramphenicol is not useful, azithromycin, erythromycin, and ciprofloxacin have been used successfully for treatment. Rifampin or macrolides are also used to treat both adults and children.[3][6]
Because of the high rates of comorbid infections and conditions, multiple treatments are often required. These have included the use of corticosteroids for respiratory distress, red blood cell transfusions for anemia, pericardiectomies for pericardial tamponades, and other standard treatments.[3][14]
Society and culture
[edit]The disease was featured in an episode of The WB supernatural drama Charmed that aired on February 3, 2000. In the episode Piper Halliwell becomes infected when a sandfly bites her while she is importing a crate of Kiwano for her club, P3. Piper slowly begins to die of the condition as her sisters Prue and Phoebe rush to find a magical way to save her.
References
[edit]- ^ synd/3112 at Whonamedit?
- ^ Renaud; Freney, Francois; Jean (2011). Pioneers of Bacteriology. Eska Publishing. p. 164.
- ^ a b c d e f g h i j k Huarcaya, Erick; Maguiña, Ciro; Torres, Rita; Rupay, Joan; Fuentes, Luis (2004-10-01). "Bartonelosis (Carrion's Disease) in the pediatric population of Peru: an overview and update". Brazilian Journal of Infectious Diseases. 8 (5): 331–339. doi:10.1590/S1413-86702004000500001. ISSN 1413-8670. PMID 15798808.
- ^ "Carrion's disease - RightDiagnosis.com". www.rightdiagnosis.com. Retrieved 2016-11-02.
- ^ a b c d "Bartonella Infection (Cat Scratch Disease, Trench Fever, and Carrión's Disease)". www.cdc.gov. Retrieved 2016-10-17.
- ^ a b c d e "Bartonellosis - NORD (National Organization for Rare Disorders)". NORD (National Organization for Rare Disorders). Retrieved 2016-10-17.
- ^ Maguina C, Garcia PJ, Gotuzzo E, Cordero L, Spach DH (September 2001). "Bartonellosis (Carrión's disease) in the modern era". Clin. Infect. Dis. 33 (6): 772–779. doi:10.1086/322614. PMID 11512081. S2CID 16680459.
- ^ a b Maco V, Maguiña C, Tirado A, Maco V, Vidal JE (2004). "Carrion's disease (Bartonellosis bacilliformis) confirmed by histopathology in the High Forest of Peru". Rev. Inst. Med. Trop. Sao Paulo. 46 (3): 171–174. doi:10.1590/S0036-46652004000300010. PMID 15286824.
- ^ Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Maguiña C, Jarman RG, Blazes DL, Richards AL (2013). "Molecular Typing of "Candidatus Bartonella ancashi," a New Human Pathogen Causing Verruga Peruana". Journal of Clinical Microbiology. 51 (11): 3865–3868. doi:10.1128/JCM.01226-13. PMC 3889784. PMID 23985925.
- ^ Blazes DL, Mullins K, Smoak BL, Jiang J, Canal E, Solorzano N, et al. (2013). "Novel Bartonella agent as cause of verruga peruana". Emerging Infectious Diseases. 19 (7): 1111–1114. doi:10.3201/eid1907.121718. PMC 3713980. PMID 23764047.
- ^ Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Ventosilla P, Maguiña C, Jarman RG, Blazes D, Richards AL (2015). "Description of Bartonella ancashensis sp. nov., isolated from blood of two patients with verruga peruana". International Journal of Systematic and Evolutionary Microbiology. 65 (10): 3339–3343. doi:10.1099/ijsem.0.000416. PMID 26296673.
- ^ Salinas-Flores, D (2014). "The new Bartonella ancashi to cause the Peruvian wart: Does Koch's postulates?". Acta Medica Peruana (in Spanish). 31 (1): 34–36. Retrieved 24 February 2023.
- ^ Garcilla-Quintanilla M, Dichter AA, Guerra H, Kempf VA (2019). "Carrion's disease: more than a neglected disease". Parasites & Vectors. 12 (1): 141. doi:10.1186/s13071-019-3390-2. PMC 6434794. PMID 30909982. 141.
- ^ Camacho, Cesar Henriquez (7 December 2002). "Human Bartonellosis Caused By Bartonella Bacilliformis". University of Pittsburgh. Retrieved 2 November 2016.

