Recent from talks
Nothing was collected or created yet.
Leishmaniasis
View on Wikipedia
| Leishmaniasis | |
|---|---|
| Other names | Leishmaniosis |
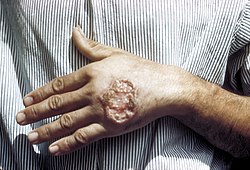 | |
| Cutaneous leishmaniasis in the hand of a Central American adult | |
| Pronunciation |
|
| Specialty | Infectious disease |
| Symptoms | Skin ulcers, fever, low red blood cells, enlarged liver[2][3] |
| Causes | Leishmania parasites spread by sandflies[2] |
| Prevention | Bug nets, insecticide[2] |
| Frequency | 4–12 million[4][5] |
| Deaths | 24,200 (2015)[6] |
Leishmaniasis is a wide array of clinical manifestations caused by protozoal parasites of the Trypanosomatida genus Leishmania.[7] It is generally spread through the bite of phlebotomine sandflies, Phlebotomus and Lutzomyia, and occurs most frequently in the tropics and sub-tropics of Africa, Asia, the Americas, and southern Europe.[2][8] The disease can present in three main ways: cutaneous, mucocutaneous, or visceral.[2] The cutaneous form presents with skin ulcers, while the mucocutaneous form presents with ulcers of the skin, mouth, and nose. The visceral form starts with skin ulcers and later presents with fever, low red blood cell count, and enlarged spleen and liver.[2][3]
Infections in humans are caused by more than 20 species of Leishmania.[8][2] Risk factors include poverty, malnutrition, deforestation, and urbanization.[2] All three types can be diagnosed by seeing the parasites under microscopy.[2] Additionally, visceral disease can be diagnosed by blood tests.[3]
Leishmaniasis can be partly prevented by sleeping under nets treated with insecticide.[2] Other measures include spraying insecticides to kill sandflies and treating people with the disease early to prevent further spread.[2] The treatment needed is determined by where the disease is acquired, the species of Leishmania, and the type of infection.[2] Recent research in leishmaniasis treatment explores combination therapies, nanotechnology-based drugs, and immunotherapy.
For cutaneous disease, paromomycin, fluconazole, or pentamidine may be effective.[9]
About 4 to 12 million people are currently infected[4][5] in some 98 countries.[3] About 2 million new cases[3] and between 20 and 50 thousand deaths occur each year.[2][10] About 200 million people in Asia, Africa, South and Central America, and southern Europe live in areas where the disease is common.[3][11] The World Health Organization has obtained discounts on some medications to treat the disease.[3] It is classified as a neglected tropical disease.[12] The disease may occur in a number of other animals, including dogs and rodents.[2]
Signs and symptoms
[edit]
The symptoms of leishmaniasis are skin sores which erupt weeks to months after the person is bitten by infected sandflies.
Leishmaniasis may be divided into the following types:[13]
- Cutaneous leishmaniasis is the most common form, which causes an open sore at each bite site, which heals in a few months to a year and a half, leaving an unpleasant-looking scar.[2][3]
- Mucocutaneous leishmaniasis causes both skin and mucosal ulcers with damage primarily of the nose and mouth.[2][3]
- Visceral leishmaniasis or kala-azar ('black fever') is the most serious form and is generally fatal if untreated.[2] Other consequences, which can occur a few months to years after infection, include fever, damage to the spleen and liver, and anemia.[2]
Leishmaniasis is considered one of the classic causes of a markedly enlarged (and therefore palpable) spleen; the organ, which is not normally felt during the examination of the abdomen, may even become larger than the liver in severe cases.[citation needed]
Cause
[edit]
Leishmaniasis is transmitted by the bite of infected female phlebotomine sandflies[2] which can transmit the protozoa Leishmania.[2] The sandflies inject the infective stage, metacyclic promastigotes, during blood meals. Metacyclic promastigotes in the puncture wound are phagocytized by macrophages, and transform into amastigotes. Amastigotes multiply in infected cells and affect different tissues, depending in part on the host, and in part on which Leishmania species is involved. These differing tissue specificities cause the differing clinical manifestations of the various forms of leishmaniasis. Sandflies become infected during blood meals on infected hosts when they ingest macrophages infected with amastigotes. In the sandfly's midgut, the parasites differentiate into promastigotes, which multiply, differentiate into metacyclic promastigotes, and migrate to the proboscis.
The genomes of three Leishmania species (L. major, L. infantum, and L. braziliensis) have been sequenced, and this has provided much information about the biology of the parasite. For example, in Leishmania, protein-coding genes are understood to be organized as large polycistronic units in a head-to-head or tail-to-tail manner; RNA polymerase II transcribes long polycistronic messages in the absence of defined RNA pol II promoters, and Leishmania has unique features concerning the regulation of gene expression in response to changes in the environment. The new knowledge from these studies may help identify new targets for urgently needed drugs and aid the development of vaccines.[14]
Vector
[edit]Although most of the literature mentions only one genus transmitting Leishmania to humans (Lutzomyia) in the New World, a 2003 study by Galati suggested a new classification for New World sand flies, elevating several subgenera to the genus level. Elsewhere in the world, the genus Phlebotomus is considered the vector of leishmaniasis.[14]
Possible non-human reservoirs
[edit]Some cases of infection of non-human animals of human-infecting species of Leishmania have been observed. In one study, L. major was identified in twelve out of ninety-one wild western lowland gorilla fecal samples[15] and in a study of fifty-two captive non-human primates under zoo captivity in a leishmaniasis endemic area, eight (all three chimpanzees, three golden lion tamarins, a tufted capuchin, and an Angolan talapoin), were found to be infected with L. infantum and capable of infecting Lutzomyia longipalpis sand flies, although "parasite loads in infected sand flies observed in this study were considered low".[16]
Organisms
[edit]Visceral disease is usually caused by Leishmania donovani, L. infantum, or L. chagasi,[3] but occasionally these species may cause other forms of disease.[3] The cutaneous form of the disease is caused by more than 15 species of Leishmania.[3]
Risk factors
[edit]Risk factors include malnutrition, deforestation, lack of sanitation, suppressed immune system, and urbanization.[2]
- Socioeconomic conditions: Poor living conditions like overcrowded housing and inadequate sanitation are associated with increased human exposure to sandflies. Poor waste management and open sewage create ideal breeding grounds for sandflies in rural and low-income urban areas. Limited access to healthcare may delay diagnosis and treatment, which can contribute to more severe disease outcomes. Poor individuals may face a financial barrier to treatment, increasing their risk of severe disease.[17]
- Malnutrition: Deficiencies in protein, iron, vitamin A, and zinc weaken the immune system, making it harder to fight Leishmania infections. This increases the risk of both cutaneous and visceral leishmaniasis, leading to more severe illness and poor treatment outcomes.[18]
- Population Mobility: Migration and displacement due to conflict, economic hardship, or environmental changes contribute to the spread of leishmaniasis, particularly when non-immune individuals enter endemic areas. Refugees and seasonal agricultural workers are at higher risk due to limited access to vector control measures. Human activity in previously uninhabited lands may increase exposure to infected sandflies and wildfire reservoirs.[19]
- Environmental and Climate Change: Temperature, humidity, and rainfall changes affect the sandfly population. Rising temperatures have been linked to higher sandfly survival and breeding rates, allowing the disease to spread into higher altitudes and previously unaffected regions, such as Southern Europe and North America. Deforestation, urbanization, and dam construction disturb sandfly habitats, creating new transmission hotspots and increasing the risk of outbreaks.[20]
Diagnosis
[edit]
Leishmaniasis is diagnosed in the hematology laboratory by direct visualization of the amastigotes (Leishman–Donovan bodies). Buffy-coat preparations of peripheral blood or aspirates from marrow, spleen, lymph nodes, or skin lesions should be spread on a slide to make a thin smear and stained with Leishman stain or Giemsa stain (pH 7.2) for 20 minutes. Amastigotes are seen within blood and spleen monocytes or, less commonly, in circulating neutrophils and in aspirated tissue macrophages. They are small, round bodies 2–4 μm in diameter with indistinct cytoplasm, a nucleus, and a small, rod-shaped kinetoplast. Occasionally, amastigotes may be seen lying free between cells.[21] However, the retrieval of tissue samples is often painful for the patient and identification of the infected cells can be difficult. So, other indirect immunological methods of diagnosis are developed, including enzyme-linked immunosorbent assay, antigen-coated dipsticks, and direct agglutination test. Although these tests are readily available, they are not the standard diagnostic tests due to their insufficient sensitivity and specificity[citation needed].
Several different polymerase chain reaction (PCR) tests are available for the detection of Leishmania DNA.[3] With this assay, a specific and sensitive diagnostic procedure is finally possible. The most sensitive PCR tests use minicircle kinetoplast DNA found in the parasite. Kinetoplast DNA contains sequences for mitochondrial proteins in its maxicircles (~25–50 per parasite), and guide RNA in its minicircles (~10,000 per parasite) of the kinetoplast. With this specific method, one can still detect Leishmania even with a very low parasite load. When needing to diagnose a specific species of Leishmania, as opposed to only detection, other PCR methods have been superior.[22]
Most forms of the disease are transmitted only from nonhuman animals, but some can be spread between humans. Infections in humans are caused by about 21 of 30 species that infect mammals;[23] the different species look the same, but they can be differentiated by isoenzyme analysis, DNA sequence analysis, or monoclonal antibodies.
Prevention
[edit]- Using insect repellent on exposed skin and under the ends of sleeves and pant legs. Follow the instructions on the label of the repellent. The most effective repellents generally are those that contain the chemical DEET (N,N-diethylmetatoluamide)[24]
- Leishmaniasis can be partly prevented by using nets treated with insecticide or insect repellent while sleeping.[2] To provide good protection against sandflies, fine mesh sizes of 0.6 mm or less are required, but a mosquito net with 1.2mm mesh will provide a limited reduction in the number of sandfly bites.[25] Finer mesh sizes have the downside of higher cost and reduced air circulation which can cause overheating. Many Phlebotomine sandfly attacks occur at sunset rather than at night, so it may also be useful to put nets over doors and windows or to use insect repellents.[24]
- Use of insecticide-impregnated dog collars and treatment or culling of infected dogs.[citation needed]
- Spraying houses and animal shelters with insecticides.[25]
- Prevention and control of leishmaniasis requires a multifaceted approach. Insecticide spraying, treated nets, and case management are commonly used strategies, while additional approaches are being explored for long-term disease control.
- Vector Control: Integrated Vector Management (IVM) approach is key to reducing sand fly populations. Some of the latest strategies include:
- Research is ongoing into genetically modifying sand flies to reduce their ability to transmit Leishmania parasites.[26]
- Attractive toxic sugar baits (ATSBs) attract and kill sand flies that feed on plant sugars.[27]
- Spatial repellents and insecticidal paint create long-term barriers against sand flies.[27]
- Reservoir Control:
- Canine control measures: domestic dogs are major reservoirs for Leishmania infantum in regions where visceral leishmaniasis is common. Instead of widespread dog culling, which has been proven ineffective and controversial, deltamethrin-impregnated dog collars have been introduced as a safer and more effective alternative.[28]
- Wildlife reservoirs: Controlling wild animal reservoirs such as rodents, marsupials, sloths, and armadillos is more challenging due to conservation concerns.[29]
Vaccination: Canine vaccinations have been developed and are now being used in some regions to reduce transmission. Human vaccinations are in development, with several candidates in clinical trials assessing their potential for long-term immunity.[30]
Treatment
[edit]
The treatment is determined by where the disease is acquired, the species of Leishmania, and the type of infection.[2] For visceral leishmaniasis in India, South America, and the Mediterranean, liposomal amphotericin B is the recommended treatment and is often used as a single dose.[3][31] Rates of cure with a single dose of amphotericin have been reported as 95%.[3] In India, almost all infections are resistant to pentavalent antimonials.[3] In Africa, a combination of pentavalent antimonials and paromomycin is recommended.[31] These, however, can have significant side effects.[3] Miltefosine, an oral medication, is effective against both visceral and cutaneous leishmaniasis.[32] Side effects are generally mild, though it can cause birth defects if taken within three months of getting pregnant.[3][32] It does not appear to work for L. major or L. braziliensis.[9] Trifluralin, a herbicide, is shown to be effective treatment as ointment, without hemolytic or cell-toxic side-effects.[33]
Recent research in leishmaniasis treatment explores combination therapies, nanotechnology-based drugs, and immunotherapy. Combination treatments, such as liposomal amphotericin B (L-AmB) with miltefosine or paromomycin, have shown high cure rates for visceral leishmaniasis while reducing treatment time and side effects.[31] The WHO recommends miltefosine-based combination therapy for specific cases of visceral leishmaniasis.[31] Nanotechnology-based treatments, including lipid and metallic nanoparticles, improve drug delivery by targeting parasites more precisely and reducing toxicity.[31] Immune-modulating therapies, such as interferon-gamma (IFN-γ), are under investigation for their potential in enhancing immune responses against Leishmania infections.[31]
The evidence around the treatment of cutaneous leishmaniasis is poor.[3] Several topical treatments may be used for cutaneous leishmaniasis. Which treatments are effective depends on the strain, with topical paromomycin effective for L. major, L. tropica, L. mexicana, L. panamensis, and L. braziliensis.[9] Pentamidine is effective for L. guyanensis.[9] Oral fluconazole or itraconazole appears effective in L. major and L. tropica.[3][9] There is limited evidence to support the use of heat therapy in cutaneous leishmaniasis as of 2015.[34]
As of 2018, no studies have determined the effect of oral nutritional supplements on visceral leishmaniasis being treated with anti-leishmanial drug therapy.[35] For the reason, it is not known if nutritional supplements are ineffective (or effective).[35] Further research including high quality randomized controlled trials are needed to determine if supplements are helpful and if so, at what dose, to help people with VL who are undergoing treatment with anti-leishmanial medications.[35]
The Institute for OneWorld Health has reintroduced the drug paromomycin for the treatment of leishmaniasis, results which led to its approval as an orphan drug. The Drugs for Neglected Diseases Initiative is also actively facilitating the search for novel therapeutics. A treatment with paromomycin will cost about US$10. The drug had originally been identified in the 1950s but had been abandoned because it would not be profitable, as the disease mostly affects poor people.[36] The Indian government approved paromomycin for sale in August 2006.[37]
By 2012 the World Health Organization had successfully negotiated with the manufacturers to achieve a reduced cost for liposomal amphotericin B, to US$18 a vial, but several vials are needed for treatment and it must be kept at a stable, cool temperature.[3]
Epidemiology
[edit]

Out of 200 countries and territories reporting to WHO, 97 countries and territories are endemic for leishmaniasis.[39] The settings in which leishmaniasis is found range from rainforests in Central and South America to deserts in western Asia and the Middle East. It affects as many as 12 million people worldwide.[40] Leishmaniasis affect an estimated 700,000 to 1 million new cases annually, with over a billion people living in endemic areas at risk of infection.[41] Visceral leishmaniasis is a fatal form with the potential for outbreak, causing, 50,000 to 90,000 cases worldwide each year. However only 25-45% are reported to the WHO.[41] Cutaneous leishmaniasis is the most common form with 600,000 to 1 million new cases each year yet only 200,000 are officially reported.[41] It is most common in Afghanistan, Algeria, Brazil, Colombia, and Iran. Mucocutaneous leishmaniasis is rarer with over 90% of cases occurring in Bolivia, Brazil, and Peru.[41] The visceral form is most common in Bangladesh, Brazil, Ethiopia, India, and Sudan.[2] In 2014, more than 90% of new cases reported to WHO occurred in six countries: Brazil, Ethiopia, India, Somalia, South Sudan and Sudan.[42] As of 2010,[update] it caused about 52,000 deaths, down from 87,000 in 1990.[10]
Leishmaniasis is found through much of the Americas from northern Argentina to South Texas, though not in Uruguay or Chile, and has recently been shown to be spreading to North Texas and Oklahoma,[43][44] and further expansion to the north may be facilitated by climate change as more habitat becomes suitable for vector and reservoir species for leishmaniasis.[45] Leishmaniasis is also known as papalomoyo, papa lo moyo, úlcera de los chicleros, and chiclera in Latin America.[46] During 2004, an estimated 3,400 troops from the Colombian army, operating in the jungles near the south of the country (in particular around the Meta and Guaviare departments), were infected with leishmaniasis. Allegedly, a contributing factor was that many of the affected soldiers did not use the officially provided insect repellent because of its disturbing odor. Nearly 13,000 cases of the disease were recorded in all of Colombia throughout 2004, and about 360 new instances of the disease among soldiers had been reported in February 2005.[47]
The disease is found across much of Asia and in the Middle East. Within Afghanistan, leishmaniasis occurs commonly in Kabul, partly due to bad sanitation and waste left uncollected in streets, allowing parasite-spreading sand flies an environment they find favorable.[48][49] In Kabul, the number of people infected was estimated to be at least 200,000, and in three other towns (Herat, Kandahar, and Mazar-i-Sharif) about 70,000 more occurred, according to WHO figures from 2002.[50][51] Kabul is estimated as the largest center of cutaneous leishmaniasis in the world, with around 67,500 cases as of 2004.[52] Africa, in particular, the East and North,[38] is also home to cases of leishmaniasis. Leishmaniasis is considered endemic also in some parts of southern parts of western Europe and has spread towards the north in recent years.[53] For example, an outbreak of cutaneous and visceral leishmaniasis was reported from Madrid, Spain, between 2010 and 2012.[54]
Leishmaniasis is mostly a disease of the developing world and is rarely known in the developed world outside a small number of cases, mostly in instances where troops are stationed away from their home countries. Leishmaniasis has been reported by U.S. troops stationed in Saudi Arabia and Iraq since the Gulf War of 1990, including visceral leishmaniasis.[55][56][57] In September 2005, the disease was contracted by at least four Dutch marines who were stationed in Mazar-i-Sharif, Afghanistan, and subsequently repatriated for treatment.[58][59]
History
[edit]
Descriptions of conspicuous lesions similar to cutaneous leishmaniasis appear on tablets from King Ashurbanipal from the seventh century BCE, some of which may have derived from even earlier texts from 1500 to 2500 BCE. Persian physicians, including Avicenna in the 10th century CE, gave detailed descriptions of what was called balkh sore.[60] In 1756, Alexander Russell, after examining a Turkish patient, gave one of the most detailed clinical descriptions of the disease. Physicians in the Indian subcontinent would describe it as kala-azar (pronounced kālā āzār, the Urdu, Hindi, and Hindustani phrase for "black fever", kālā meaning black and āzār meaning fever or disease). In the Americas, evidence of the cutaneous form of the disease in Ecuador and Peru appears in pre-Inca pottery depicting skin lesions and deformed faces dating back to the first century CE. Some 15th- and 16th-century texts from the Inca period and from Spanish colonials mention "valley sickness", "Andean sickness", or "white leprosy", which are likely to be the cutaneous form.[61]
It remains unclear who first discovered the organism. David Douglas Cunningham, Surgeon Major of the British Indian army, may have seen it in 1885 without being able to relate it to the disease.[62][63] Peter Borovsky, a Russian military surgeon working in Tashkent, conducted research into the etiology of "oriental sore", locally known as sart sore, and in 1898 published the first accurate description of the causative agent, correctly described the parasite's relation to host tissues and correctly referred it to the protozoa. However, because his results were published in Russian in a journal with low circulation, his results were not internationally acknowledged during his lifetime.[64] In 1901, William Boog Leishman identified certain organisms in smears taken from the spleen of a patient who had died from "dum-dum fever" (Dum Dum is an area close to Calcutta) and proposed them to be trypanosomes, found for the first time in India.[65] A few months later, Captain Charles Donovan (1863–1951) confirmed the finding of what became known as Leishman-Donovan bodies in smears taken from people in Madras in southern India.[66] But it was Ronald Ross who proposed that Leishman-Donovan bodies were the intracellular stages of a new parasite, which he named Leishmania donovani.[67] The link with the disease kala-azar was first suggested by Charles Donovan, and was conclusively demonstrated by Charles Bentley's discovery of L. donovani in patients with kala-azar.[68] Transmission by the sandfly was hypothesized by Lionel Napier and Ernest Struthers at the School of Tropical Medicine at Calcutta and later proven by his colleagues.[69][70] The disease became a major problem for Allied troops fighting in Sicily during the Second World War; research by Leonard Goodwin then showed pentostam was an effective treatment.[71]
Society and culture
[edit]- Stigma and Psychological Effects: The cutaneous and mucocutaneous forms of leishmaniasis can cause visible scarring and disfigurement, leading to social stigma, discrimination, and emotional distress. In some communities, individuals with visible scarring may face social challenges, including barriers to employment, social activities, education, and marriage, due to the stigma surrounding the disease. As awareness grows, mental health support and community education programs are recognized as important disease management aspects.[72]
- Economic burden: The cost of diagnosis, treatment, and hospitalizations pose financial challenges, particularly in regions where access to free or subsidized treatment is limited. Patients may experience income loss due to illness, disability and long recovery time. In rural areas, leishmaniasis can impact livestock and working animals, contributing to economic challenges for agricultural and livestock-dependent communities.[17]
- Cultural beliefs and traditional medicine: In some endemic areas, cultural beliefs regarding the cause of leishmaniasis, including supernatural or spiritual explanations, may influence healthcare-seeking behaviors, sometimes delaying access to medical treatment.[73]
Research
[edit]
As of 2017, no leishmaniasis vaccine for humans was available.[74][75] Research to produce a human vaccine is ongoing.[75]
Currently some effective leishmaniasis vaccines for dogs exist.[76] There is also the consideration that public health practices can control or eliminate leishmaniasis without a vaccine.[75] Pyrimidine–based drugs are being explored as anti-leishmanial compounds.[77]
See also
[edit]References
[edit]- ^ "Leishmaniasis definition and meaning | Collins English Dictionary". Archived from the original on 24 December 2013. Retrieved 23 December 2013.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x "Leishmaniasis Fact sheet N°375". World Health Organization. January 2014. Archived from the original on 21 February 2014. Retrieved 17 February 2014.
- ^ a b c d e f g h i j k l m n o p q r s t u v Barrett MP, Croft SL (2012). "Management of trypanosomiasis and leishmaniasis". British Medical Bulletin. 104 (1): 175–96. doi:10.1093/bmb/lds031. PMC 3530408. PMID 23137768.
- ^ a b "Leishmaniasis Magnitude of the problem". World Health Organization. Archived from the original on 26 October 2013. Retrieved 17 February 2014.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease Injury Incidence Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ Roy M, Rawat A, Kaushik S, Jyoti A, Srivastava VK (1 August 2022). "Endogenous cysteine protease inhibitors in upmost pathogenic parasitic protozoa". Microbiological Research. 261 127061. doi:10.1016/j.micres.2022.127061. ISSN 0944-5013. PMID 35605309. S2CID 248741177.
- ^ a b Rawat A, Roy M, Jyoti A, Kaushik S, Verma K, Srivastava VK (August 2021). "Cysteine proteases: Battling pathogenic parasitic protozoans with omnipresent enzymes". Microbiological Research. 249 126784. doi:10.1016/j.micres.2021.126784. PMID 33989978. S2CID 234597200.
- ^ a b c d e Minodier P, Parola P (May 2007). "Cutaneous leishmaniasis treatment". Travel Medicine and Infectious Disease. 5 (3): 150–8. doi:10.1016/j.tmaid.2006.09.004. PMID 17448941.
- ^ a b Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253.
- ^ Ejazi SA, Ali N (January 2013). "Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects". Expert Review of Anti-Infective Therapy. 11 (1): 79–98. doi:10.1586/eri.12.148. PMID 23428104. S2CID 20508342.
- ^ "Neglected Tropical Diseases". cdc.gov. 6 June 2011. Archived from the original on 4 December 2014. Retrieved 28 November 2014.
- ^ James WD, Berger TG, Elston DM (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. pp. 422–428. ISBN 978-0-7216-2921-6.
- ^ a b Myler PJ, Fasel N (2008). Leishmania: After The Genome. Caister Academic Press. ISBN 978-1-904455-28-8. Archived from the original on 23 April 2008.[page needed]
- ^ Hamad I, Forestier CL, Peeters M, Delaporte E, Raoult D, Bittar F (15 January 2015). "Wild Gorillas as a Potential Reservoir of Leishmania major". J. Infect. Dis. 211 (2): 267–273. doi:10.1093/infdis/jiu380. PMC 4342692. PMID 25001460.
- ^ Ayisa Rodrigues de Oliveira, Guilherme Rafael Gomide Pinheiro, Herlandes P. Tinoco, Maria Elvira Loyola, Carlyle Mendes Coelho, Edelberto Santos Dias, Érika Michalsky Monteiro, Fabiana de Oliveira Lara e Silva, Angela Tinoco Pessanha, Andreza Geisiane Maia Souza, Nathália Cristina Lima Pereira, Nelder F. Gontijo, Ricardo T. Fujiwara, Tatiane Alves da Paixão, Renato Lima Santos (17 April 2019). "Competence of non-human primates to transmit Leishmania infantum to the invertebrate vector Lutzomyia longipalpis". PLOS Neglected Tropical Diseases. 13 (4) e0007313. doi:10.1371/journal.pntd.0007313. PMC 6488095. PMID 30995227.
- ^ a b Okwor I, Uzonna J (2 March 2016). "Social and Economic Burden of Human Leishmaniasis". The American Journal of Tropical Medicine and Hygiene. 94 (3): 489–493. doi:10.4269/ajtmh.15-0408. ISSN 0002-9637. PMC 4775878. PMID 26787156.
- ^ Sacramento LA, Lombana C, Scott P (2024). "Malnutrition disrupts adaptive immunity during visceral leishmaniasis by enhancing IL-10 production". PLOS Pathogens. 20 (11) e1012716. doi:10.1371/journal.ppat.1012716. PMC 11581394. PMID 39527629.
- ^ Wilke AB, Farina P, Ajelli M, Canale A, Dantas-Torres F, Otranto D, Benelli G (9 January 2025). "Human migrations, anthropogenic changes, and insect-borne diseases in Latin America". Parasites & Vectors. 18 (1): 4. doi:10.1186/s13071-024-06598-7. ISSN 1756-3305. PMC 11721252. PMID 39789650.
- ^ Rupasinghe R, Chomel BB, Martínez-López B (1 February 2022). "Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends". Acta Tropica. 226 106225. doi:10.1016/j.actatropica.2021.106225. ISSN 0001-706X. PMID 34758355.
- ^ Dacie JV, Bain BJ, Bates I (2006). Dacie and Lewis practical hematology. Philadelphia: Churchill Livingstone/Elsevier. ISBN 978-0-443-06660-3.[page needed]
- ^ Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL (April 2006). "Comparison of PCR assays for diagnosis of cutaneous leishmaniasis". Journal of Clinical Microbiology. 44 (4): 1435–9. doi:10.1128/JCM.44.4.1435-1439.2006. PMC 1448629. PMID 16597873.
- ^ "Parasites – Leishmaniasis". Centers for Disease Control and Prevention. January 2013.
- ^ a b Prevention CC (19 February 2020). "CDC – Leishmaniasis – Prevention & Control". cdc.gov. Retrieved 26 June 2021.
- ^ a b * Alexander B, Maroli M (March 2003). "Control of phlebotomine sandflies". Medical and Veterinary Entomology. 17 (1): 1–18. doi:10.1046/j.1365-2915.2003.00420.x. PMID 12680919. S2CID 31387956.
- ^ Kumari Y, Gunathilaka N, Amarasinghe D (27 January 2025). "A comprehensive review of biological and genetic control approaches for leishmaniasis vector sand flies; emphasis towards promoting tools for integrated vector management". PLOS Neglected Tropical Diseases. 19 (1) e0012795. doi:10.1371/journal.pntd.0012795. ISSN 1935-2735. PMC 11771870. PMID 39869587.
- ^ a b Operational Manual on Leishmaniasis Vector Control, Surveillance, Monitoring and Evaluation (1st ed.). Geneva: World Health Organization. 2023. ISBN 978-92-4-006034-0.
- ^ Halbig P, Hodjati MH, Mazloumi-Gavgani AS, Mohite H, Davies CR (2000). "Further evidence that deltamethrin-impregnated collars protect domestic dogs from sandfly bites". Medical and Veterinary Entomology. 14 (2): 223–226. doi:10.1046/j.1365-2915.2000.00229.x. ISSN 1365-2915. PMID 10872869.
- ^ Cosma C, Maia C, Khan N, Infantino M, Del Riccio M (28 October 2024). "Leishmaniasis in Humans and Animals: A One Health Approach for Surveillance, Prevention and Control in a Changing World". Tropical Medicine and Infectious Disease. 9 (11): 258. doi:10.3390/tropicalmed9110258. ISSN 2414-6366. PMC 11598728. PMID 39591264.
- ^ Coelho EA, Christodoulides M (2023), Christodoulides M (ed.), "Vaccines for Canine Leishmaniasis", Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges : Focus on Leprosy, Leishmaniasis, Melioidosis and Tuberculosis, Cham: Springer International Publishing, pp. 281–306, doi:10.1007/978-3-031-24355-4_13, ISBN 978-3-031-24355-4
- ^ a b c d e f Sundar S, Chakravarty J (January 2013). "Leishmaniasis: an update of current pharmacotherapy". Expert Opinion on Pharmacotherapy. 14 (1): 53–63. doi:10.1517/14656566.2013.755515. PMID 23256501. S2CID 207479873.
- ^ a b Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ (November 2012). "Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis". The Journal of Antimicrobial Chemotherapy. 67 (11): 2576–97. doi:10.1093/jac/dks275. PMID 22833634.
- ^ Esteves MA, Fragiadaki I, Lopes R, Scoulica E, Cruz ME (1 January 2010). "Synthesis and biological evaluation of trifluralin analogues as antileishmanial agents". Bioorganic & Medicinal Chemistry. 18 (1): 274–281. doi:10.1016/j.bmc.2009.10.059. PMID 19926293.
- ^ Von Stebut E (March 2015). "Leishmaniasis". Journal of the German Society of Dermatology. 13 (3): 191–200, quiz 201. doi:10.1111/ddg.12595. PMID 25721626. S2CID 221649492.
- ^ a b c Custodio E, López-Alcalde J, Herrero M, Bouza C, Jimenez C, Storcksdieck Genannt Bonsmann S, Mouratidou T, López-Cuadrado T, Benito A, Alvar J, et al. (Cochrane Infectious Diseases Group) (March 2018). "Nutritional supplements for patients being treated for active visceral leishmaniasis". The Cochrane Database of Systematic Reviews. 2018 (3) CD012261. doi:10.1002/14651858.CD012261.pub2. PMC 6494195. PMID 29578237.
- ^ "A Small Charity Takes the Reins in Fighting a Neglected Disease". New York Times. 31 July 2006. Archived from the original on 20 December 2016.
- ^ "Drug Program – Clinical Trial of Paromomycin". Institute for OneWorld Health. Archived from the original on 6 June 2010. Retrieved 10 February 2011.
- ^ a b Aoun K, Bouratbine A (2014). "Cutaneous leishmaniasis in North Africa: a review". Parasite. 21: 14. doi:10.1051/parasite/2014014. PMC 3952656. PMID 24626301.

- ^ "Leishmaniasis: Situation and trends". WHO Global Health Observatory. Retrieved 30 May 2018.
- ^ "Leishmaniasis: Magnitude of the problem". World Health Organization. Archived from the original on 26 October 2013.
- ^ a b c d "Leishmaniasis". www.who.int. Retrieved 19 March 2025.
- ^ "Epidemiological situation: Epidemiology". World Health Organization. Archived from the original on 30 June 2004. Retrieved 30 May 2018.
- ^ "Dallas News: Rare, non-fatal skin disease found in N. Texans". Archived from the original on 27 December 2009. Retrieved 2 June 2015.
- ^ Clarke CF, Bradley KK, Wright JH, Glowicz J (January 2013). "Case report: Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma". The American Journal of Tropical Medicine and Hygiene. 88 (1): 157–61. doi:10.4269/ajtmh.2012.11-0717. PMC 3541728. PMID 23185078.
- ^ González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S (January 2010). Galvani AP (ed.). "Climate change and risk of leishmaniasis in north america: predictions from ecological niche models of vector and reservoir species". PLOS Neglected Tropical Diseases. 4 (1) e585. doi:10.1371/journal.pntd.0000585. PMC 2799657. PMID 20098495.
- ^ "Papalomoyo" (PDF). Archived from the original (PDF) on 23 July 2011. Retrieved 16 August 2010.
- ^ "Informes – Informe de Fronteras Febrero 2005". Servicio Jesuita a Refugiados. Archived from the original on 10 November 2005.
- ^ "CENTRAL/S. ASIA – Kabul: A city in intensive care". Al Jazeera English. Archived from the original on 18 June 2007.
- ^ Birsel R (7 May 2007). "Disfiguring skin disease plagues Afghanistan". e-Ariana. Reuters. Archived from the original on 10 December 2015. Retrieved 8 December 2015.
- ^ Birsel R (28 June 2002). "Disfiguring epidemic hits 270,000 Afghans". e-Ariana. Reuters. Archived from the original on 10 December 2015. Retrieved 8 December 2015.
- ^ "WHO Seeking Funds to Prevent Leishmaniasis Outbreak in Afghanistan". voanews. October 2009.
- ^ "World Health Organization action in Afghanistan aims to control debilitating leishmaniasis". Archived from the original on 26 October 2010.
- ^ Medlock JM, Hansford KM, Van Bortel W, Zeller H, Alten B (June 2014). "A summary of the evidence for the change in European distribution of phlebotomine sand flies (Diptera: Psychodidae) of public health importance". Journal of Vector Ecology. 39 (1): 72–7. doi:10.1111/j.1948-7134.2014.12072.x. PMID 24820558. S2CID 20645170.
- ^ Aguado M, Espinosa P, Romero-Maté A, Tardío JC, Córdoba S, Borbujo J (May 2013). "Outbreak of cutaneous leishmaniasis in Fuenlabrada, Madrid". Actas Dermo-Sifiliograficas. 104 (4): 334–42. doi:10.1016/j.adengl.2013.03.005. PMID 23567452.
- ^ Kennedy K (30 March 2010). "VCS Advocacy in the News: VA May Designate 9 Infectious Diseases as Related to Gulf War". Veterans for Common Sense. Archived from the original on 13 February 2011. Retrieved 10 February 2011.
- ^ "Business: Company's mesh will help troops beat 'Baghdad boils'". Archived from the original on 16 March 2005.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 12 October 2007. Retrieved 17 September 2007.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Bhatia S, Goli D (2016). Leishmaniasis: Biology, Control and New Approaches for Its Treatment. CRC Press. ISBN 978-1-315-34189-7.
- ^ van Thiel PP, Leenstra T, de Vries HJ, van der Sluis A, van Gool T, Krull AC, van Vugt M, de Vries PJ, Zeegelaar JE, Bart A, van der Meide WF, Schallig HD, Faber WR, Kager PA (December 2010). "Cutaneous leishmaniasis (Leishmania major infection) in Dutch troops deployed in northern Afghanistan: epidemiology, clinical aspects, and treatment". The American Journal of Tropical Medicine and Hygiene. 83 (6): 1295–300. doi:10.4269/ajtmh.2010.10-0143. PMC 2990047. PMID 21118937.
- ^ Cox FE (October 2002). "History of human parasitology". Clinical Microbiology Reviews. 15 (4). The Wellcome Trust: 595–612. doi:10.1128/CMR.15.4.595-612.2002. ISBN 978-1-869835-86-6. OCLC 35161690. PMC 126866. PMID 12364371.
- ^ "WHO: Leishmaniasis background information – a brief history of the disease". Archived from the original on 15 March 2014.
- ^ Cunningham DD (1885). On the presence of peculiar parasitic organisms in the tissue of a specimen of Delhi boil. Scientific memoirs officers Medical Sanitary Departments Government India. Calcutta: Printed by the superintendent of government printing, India. pp. 21–31. OCLC 11826455.
- ^ Cox FE (October 2002). "History of human parasitology". Clinical Microbiology Reviews. 15 (4): 595–612. doi:10.1128/CMR.15.4.595-612.2002. PMC 126866. PMID 12364371.
- ^ Hoare CA (1938). "Early discoveries regarding the parasite of oriental sore". Transactions of the Royal Society of Tropical Medicine and Hygiene. 32 (1): 67–92. doi:10.1016/S0035-9203(38)90097-5.
- ^ Leishman WB (1903). "On the possibility of the occurrence of trypanomiasis in India". The British Medical Journal. 1 (2213): 1252–1254. doi:10.1136/bmj.1.2213.1252. PMC 2514706.
- ^ Donovan C (1903). "Memoranda: On the possibility of the occurrence of trypanosomiasis in India". The British Medical Journal.
- ^ Ross R (November 1903). "Further Notes on Leishman's Bodies". British Medical Journal. 2 (2239): 1401. doi:10.1136/bmj.2.2239.1401. PMC 2514909. PMID 20761210.
- ^ Bentley CA (24 December 1903). "Telegram to R. Ross". Ross Archives: 47/157.
- ^ "Dr. L. Everard Napier". The Indian Medical Gazette. 78 (5): 252. May 1943. PMC 5158438. PMID 29012190.
- ^ Gewurtz MS (1 January 2017). "Transnationalism in Missionary Medicine: The Case of Kala-azar in China and India, 1909–1946". Social Sciences and Missions. 30 (1–2): 30–43. doi:10.1163/18748945-03001001. ISSN 1874-8945.
- ^ "Leonard Goodwin – Telegraph". The Daily Telegraph. 14 January 2009. Archived from the original on 20 April 2009. Retrieved 18 January 2009.
- ^ Bennis I, De Brouwere V, Belrhiti Z, Sahibi H, Boelaert M (15 March 2018). "Psychosocial burden of localised cutaneous Leishmaniasis: a scoping review". BMC Public Health. 18 (1): 358. doi:10.1186/s12889-018-5260-9. ISSN 1471-2458. PMC 5855994. PMID 29544463.
- ^ Ramdas S, and van der Geest S (2 April 2020). "Not-knowing and the proliferation of assumptions: local explanations of Cutaneous Leishmaniasis in Suriname". Anthropology & Medicine. 27 (2): 144–159. doi:10.1080/13648470.2019.1627654. hdl:11245.1/95500069-4894-4615-b974-5c50c4c7d977. ISSN 1364-8470. PMID 31373516.
- ^ Srivastava S, Shankar P, Mishra J, Singh S (May 2016). "Possibilities and challenges for developing a successful vaccine for leishmaniasis". Parasites & Vectors. 9 (1) 277. doi:10.1186/s13071-016-1553-y. PMC 4866332. PMID 27175732.
- ^ a b c Ghorbani M, Farhoudi R (2018). "Leishmaniasis in humans: drug or vaccine therapy?". Drug Design, Development and Therapy. 12: 25–40. doi:10.2147/DDDT.S146521. PMC 5743117. PMID 29317800.
- ^ Moafi M, Rezvan H, Sherkat R, Taleban R (2019). "Leishmania Vaccines Entered in Clinical Trials: A Review of Literature". International Journal of Preventive Medicine. 10: 95. doi:10.4103/ijpvm.IJPVM_116_18. PMC 6592111. PMID 31360342.
- ^ Ramesh D, Sarkar D, Joji A, Singh M, Mohanty AK, G Vijayakumar B, Chatterjee M, Sriram D, Muthuvel SK, Kannan T (April 2022). "First-in-class pyrido[2,3- d ]pyrimidine-2,4(1 H ,3 H )-diones against leishmaniasis and tuberculosis: Rationale, in vitro, ex vivo studies and mechanistic insights". Archiv der Pharmazie. 355 (4) 2100440. doi:10.1002/ardp.202100440. ISSN 0365-6233. PMID 35106845. S2CID 246474821.
External links
[edit]Leishmaniasis
View on GrokipediaLeishmaniasis encompasses a group of vector-borne parasitic diseases caused by over 20 species of intracellular protozoan parasites belonging to the genus Leishmania, which are transmitted primarily through the bites of infected female phlebotomine sandflies of genera such as Phlebotomus and Lutzomyia.[1][2] The parasites exhibit a digenetic life cycle, alternating between promastigote forms in the sandfly vector and amastigote forms within mammalian hosts, including humans and various reservoir animals like rodents and canines.[1][3] Clinically, leishmaniasis manifests in three principal forms: cutaneous leishmaniasis, characterized by localized skin ulcers that typically self-heal but can cause disfigurement; mucocutaneous leishmaniasis, involving destructive lesions of mucous membranes in the nose, mouth, and throat; and visceral leishmaniasis (also known as kala-azar), the most severe form that disseminates to internal organs such as the spleen, liver, and bone marrow, leading to fever, weight loss, anemia, and high mortality rates exceeding 95% if untreated.[4][5] Globally, leishmaniasis affects populations in over 90 countries across tropical and subtropical regions of Africa, Asia, the Americas, and the Mediterranean, with an estimated 700,000 to 1 million new cases annually, predominantly cutaneous, alongside 50,000 to 90,000 visceral cases, many underreported due to diagnostic challenges in resource-limited settings.[1][2] Classified as a neglected tropical disease, it imposes a substantial burden on impoverished communities, exacerbated by factors like poor housing, malnutrition, and conflict, with visceral leishmaniasis responsible for 20,000 to 30,000 deaths yearly despite available treatments like antimonials and amphotericin B.[1][6]
Clinical Manifestations
Cutaneous Leishmaniasis
Cutaneous leishmaniasis (CL) represents the predominant clinical manifestation of leishmaniasis, resulting from infection by dermotropic species of the protozoan genus Leishmania transmitted via phlebotomine sandfly bites. It primarily affects exposed skin areas such as the face, arms, and legs, with lesions developing at the inoculation site. The incubation period typically spans 2 weeks to several months, though it can extend longer in some cases. Globally, CL accounts for the majority of the estimated 600,000 to 1 million annual leishmaniasis cases, predominantly in endemic regions of the tropics and subtropics.[7][8][9] Initial lesions emerge as small, erythematous papules or nodules that progressively enlarge and ulcerate over weeks to months, forming characteristic open sores with raised, indurated borders and a central crater often covered by a hemorrhagic crust or scab. These ulcers, sometimes described as "volcano-like," are generally painless unless secondarily infected, though regional lymphadenopathy may occur. Lesion size varies from millimeters to several centimeters, and multiple lesions can arise from repeated bites. Without intervention, ulcers persist for months to years, eventually healing spontaneously with atrophic, hypopigmented scars that may cause cosmetic disfigurement.[8][7][9] Clinical variants include localized cutaneous leishmaniasis (LCL), the most common form featuring solitary or few self-limiting ulcers; diffuse cutaneous leishmaniasis (DCL), a rare disseminated variant in immunocompromised individuals or with species like L. aethiopica, presenting as widespread nodules and plaques without ulceration; and leishmaniasis recidiva, a chronic relapsing form with persistent papules and scarring resembling lupus erythematosus. Manifestations differ by Leishmania species and geographic origin: Old World CL (e.g., L. major, L. tropica in Africa, Asia, Mediterranean) tends toward dry, self-resolving ulcers, while New World CL (e.g., L. mexicana, L. braziliensis in the Americas) often produces larger, wetter lesions with potential for satellite dissemination or secondary bacterial infection. Systemic symptoms are absent in uncomplicated CL, distinguishing it from visceral forms.[7][9][8]
Mucocutaneous Leishmaniasis
Mucocutaneous leishmaniasis (MCL), also known as espundia, represents a severe, progressive form of New World leishmaniasis characterized by destructive inflammation and ulceration of the mucous membranes, particularly in the nasopharynx, oral cavity, and larynx. It is primarily caused by protozoan parasites of the species Leishmania (Viannia) braziliensis, with less frequent causation by L. guyanensis or L. panamensis.[10][11] The disease arises from hematogenous or lymphatic dissemination of parasites from an initial cutaneous infection site, typically manifesting months to years after the primary skin lesion heals.[5][12] Endemic to Latin America, MCL accounts for an estimated 90% of global cases, with the highest incidence in Bolivia (up to 10,000 cases annually as of 2023 data), followed by Brazil and Peru, where environmental factors such as deforestation and proximity to sylvatic reservoirs exacerbate transmission via Lutzomyia sandfly vectors.[1] Cases outside the Americas are exceptional, limited to sporadic Old World reports involving L. aethiopica or L. tropica, though these rarely progress to mucosal destruction.[12] Risk factors include male sex, rural occupation, and poor nutritional status, which correlate with delayed immune responses permitting parasite persistence.[13] Clinically, MCL begins insidiously with nasal symptoms such as persistent congestion, epistaxis, and serosanguinous discharge, mimicking chronic rhinosinusitis.[8] Over time, granulomatous inflammation evolves into confluent ulcers that erode soft tissues, perforate the nasal septum in up to 70% of untreated cases, and invade the hard palate or buccal mucosa, causing fetid odor, dysphagia, and nutritional impairment.[10] Laryngeal extension occurs in approximately 20-30% of advanced cases, resulting in hoarseness, stridor, and potential asphyxiation if untreated.[5] Skin involvement may coexist or precede mucosal lesions, but isolated primary mucosal disease is rare, affecting less than 5% of patients.[11] Histopathologically, lesions show amastigote-laden macrophages amid mixed inflammatory infiltrates, with Th1-mediated immunity often inadequate to clear the parasite, leading to chronic suppuration and fibrosis.[12] Complications include secondary bacterial superinfections, extensive scarring, and social stigma from facial disfigurement, which can necessitate reconstructive surgery post-treatment.[1] Without intervention, mortality approaches 10% due to respiratory obstruction or sepsis, underscoring the need for early recognition of at-risk cutaneous cases in endemic zones.[13][10]Visceral Leishmaniasis
Visceral leishmaniasis (VL), also known as kala-azar, is the most severe clinical form of leishmaniasis, characterized by systemic infection of the reticuloendothelial system, particularly the spleen, liver, bone marrow, and lymph nodes. Initial symptoms often emerge after an incubation period of 2 to 6 months, though it can extend to years, with insidious onset including prolonged irregular fever in bouts that may follow a quotidian, double-quotidian, or tertian pattern, accompanied by chills and rigors. Night sweats and malaise are common early features, progressing to marked weight loss, anorexia, and generalized weakness as parasitization impairs hematopoiesis and organ function.[8][14] Physical examination reveals progressive splenomegaly, often massive and extending beyond the umbilicus, with hepatomegaly in approximately 50-60% of cases; abdominal discomfort from organ enlargement is frequent. Anemia leads to pallor and fatigue, while thrombocytopenia may cause petechiae or easy bruising; leukopenia increases susceptibility to infections. Hyperpigmentation of the skin, especially on the face, hands, knuckles, and abdomen—lending the disease its name "kala-azar" (black fever in Hindi)—occurs due to adrenal involvement or melanin deposition. Laboratory abnormalities include pancytopenia, hypoalbuminemia, and polyclonal hypergammaglobulinemia, reflecting chronic inflammation and immune dysregulation.[8][1][14] Untreated VL leads to cachexia, secondary bacterial infections (e.g., pneumonia, diarrhea, or tuberculosis), and hemorrhagic complications, with mortality approaching 100% from multiorgan failure or overwhelming sepsis. In immunocompromised individuals, such as those with HIV co-infection, progression is accelerated, symptoms more atypical, and visceral burden higher, exacerbating pancytopenia and relapse risk. Post-kala-azar dermal leishmaniasis (PKDL) manifests in 5-10% of cases as hypopigmented or erythematous skin lesions during or after apparent cure, serving as a reservoir for transmission. Annual global incidence estimates range from 50,000 to 90,000 cases, predominantly in South Asia, East Africa, and Brazil, with higher lethality in malnourished children under 5 years.[1][3][14]Etiology and Pathogenesis
Causative Protozoa
Leishmaniasis is caused by obligate intracellular protozoan parasites belonging to the genus Leishmania in the family Trypanosomatidae.[3] [6] More than 20 Leishmania species are pathogenic to humans, with approximately 21 of the 30 known mammal-infecting species capable of causing disease.[1] [3] These kinetoplastid parasites exhibit a dimorphic life cycle, alternating between flagellated promastigote forms in the insect vector and non-flagellated amastigote forms within mammalian host cells, primarily macrophages of the reticuloendothelial system.[3] [6] The taxonomy of Leishmania divides the genus into subgenera based on the site of promastigote development in the sand fly gut: primarily Leishmania (Leishmania) for species maturing in the hindgut and foregut, and Viannia for those restricted to the hindgut.[6] A newer subgenus, Mundinia, includes emerging human-pathogenic species like L. (Mundinia) martiniquensis, but most human infections involve species from the Leishmania and Viannia subgenera.[6] Pathogenicity varies by species due to differences in virulence factors, such as surface glycoproteins and enzymes that enable immune evasion and intracellular survival, though host factors also influence disease outcome.[6] Key Leishmania species and their primary associations with clinical forms are summarized below; note that some species can cause multiple syndromes, and distribution overlaps Old World (Africa, Asia, Europe) and New World (Americas) regions.[3] [6]| Complex/Species | Subgenus | Primary Disease Form(s) | Notes |
|---|---|---|---|
| L. donovani complex (L. donovani, L. infantum, L. chagasi) | Leishmania | Visceral leishmaniasis (kala-azar) | L. infantum also causes cutaneous forms; zoonotic in dogs.[3] |
| L. tropica, L. major, L. aethiopica | Leishmania | Cutaneous leishmaniasis | L. tropica anthroponotic; L. major zoonotic in rodents.[3] |
| L. mexicana complex (L. mexicana, L. amazonensis, L. venezuelensis) | Leishmania | Cutaneous leishmaniasis (diffuse or localized) | New World; rodents as reservoirs.[3] |
| L. (V.) braziliensis complex (L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) panamensis, L. (V.) peruviana) | Viannia | Mucocutaneous and cutaneous leishmaniasis | High risk of mucosal destruction; New World.[3] |
Vectors and Reservoirs
Leishmaniasis is transmitted exclusively by the bite of female phlebotomine sand flies, belonging to the genera Phlebotomus in the Eastern Hemisphere and Lutzomyia (previously Lutzomyia and Psychodopygus) in the Western Hemisphere.[3] Over 90 sand fly species are known to transmit various Leishmania species, with transmission occurring when promastigotes in the sand fly's proboscis are deposited into the skin during a blood meal.[1] Certain vectors demonstrate specificity; for example, Phlebotomus papatasi primarily transmits L. major in the Old World, while P. argentipes is a key vector for visceral leishmaniasis caused by L. donovani in India.[15] In the Americas, Lutzomyia longipalpis serves as the principal vector for L. infantum.[16] Reservoir hosts differ by Leishmania species, clinical form, and endemic region, sustaining the parasite in zoonotic cycles while humans often act as incidental hosts or primary reservoirs in anthroponotic transmission. For cutaneous leishmaniasis due to L. major, synanthropic rodents such as Meriones spp. (gerbils) and Rhombomys opimus (great gerbil) function as main reservoirs in Central Asia and the Middle East.[17] In visceral leishmaniasis endemic to the Mediterranean, Middle East, and Latin America, domestic dogs (Canis familiaris) are the primary reservoir for L. infantum, with infection rates in dogs reaching 67–80% in some vector-transmitted foci via serology or PCR detection.[18] Emerging evidence from Bihar, India, indicates dogs as potential reservoirs for L. donovani, traditionally considered anthroponotic, with DNA detected in village dogs alongside goats and cows.[18] Wild mammals contribute as sylvatic reservoirs, including foxes (Vulpes spp.), jackals, and rodents in various cycles, while cats may serve as secondary hosts for L. infantum, facilitating transmission to humans and dogs.[19] In the Americas, diverse synanthropic and wild hosts such as opossums, armadillos, sloths, and rodents harbor L. braziliensis and other species causing mucocutaneous forms.[17] In anthroponotic visceral leishmaniasis foci like parts of India and Sudan, humans maintain the cycle without evident animal reservoirs, though recent studies challenge this by identifying peridomestic mammals.[20] Reservoir competence varies, with some species like dogs exhibiting high parasitemia and infectivity to vectors, underscoring their epidemiological significance.[21]Transmission and Immune Evasion
Leishmania parasites are transmitted to humans and other mammals primarily via the bite of infected female phlebotomine sandflies, which inject metacyclic promastigotes—the infective stage—into the host's skin during blood meals.[1][22] These vectors, belonging to over 70 species across genera such as Phlebotomus (Old World) and Lutzomyia (New World), acquire the parasite during feeding on infected hosts and support its development in their midgut over 4–25 days before transmission.[1][23] Transmission efficiency depends on factors like sandfly microbiota, which promotes parasite survival and vector competence through inflammasome-mediated IL-1β production, and the parasites' ability to evade digestion in the fly's gut.[24] Zoonotic cycles involve reservoirs such as rodents (e.g., Rhombomys opimus for cutaneous forms) and canids (e.g., dogs for L. infantum), while anthroponotic cycles rely on human reservoirs, particularly in urban visceral leishmaniasis foci.[25][26] Non-vector routes, including blood transfusions, shared needles, congenital transfer, and rarely sexual contact, account for fewer than 1% of cases but pose risks in endemic areas with poor screening.[7][27] Following inoculation, promastigotes are rapidly phagocytosed by host macrophages and dendritic cells but evade intracellular killing through multiple strategies that subvert phagolysosome maturation and oxidative bursts.[28] Key surface glycoproteins like GP63 cleave complement components and inhibit reactive oxygen species (ROS) production, while lipophosphoglycan (LPG) blocks lysosomal enzyme recruitment and fusion, allowing transformation into replicative amastigotes within parasitophorous vacuoles.[29][30] Parasites further manipulate host signaling by activating protein tyrosine phosphatase SHP-1, which dephosphorylates kinases like JAK2 and MAPK, thereby suppressing NF-κB activation and pro-inflammatory cytokine release (e.g., IL-12, TNF-α) essential for Th1 immunity.[30] Leishmania enzymes, such as arginase and superoxide dismutase, deplete host L-arginine and neutralize ROS, respectively, promoting parasite persistence and shifting immune responses toward Th2 dominance with elevated IL-10 and TGF-β.[31][32] In adaptive immunity, Leishmania impairs antigen presentation by downregulating MHC class II expression on infected macrophages and inducing regulatory T cells (Tregs) that suppress CD4+ T cell proliferation via IL-10 and CTLA-4 pathways.[33] Parasite-derived exosomes and secreted proteins further disseminate immunosuppressive signals, inhibiting dendritic cell maturation and promoting exhaustion of effector T cells.[28] These mechanisms collectively enable chronic infection, with species-specific variations—such as L. donovani's enhanced A2 protein-mediated amastigote survival in visceral sites—contributing to diverse clinical outcomes.[34] Despite robust innate responses, evasion of apoptosis in host cells via modulation of Bcl-2 family proteins prolongs the intracellular niche, underscoring the parasite's evolutionary adaptations to macrophage defenses.[29][35]
Diagnosis
Clinical Evaluation
Clinical evaluation of leishmaniasis relies on a detailed patient history and comprehensive physical examination to identify features suggestive of cutaneous, mucocutaneous, or visceral disease, particularly in individuals with exposure history.[5] A key element is assessing for residence or travel to endemic regions, such as parts of Asia, Africa, the Middle East, Central and South America, or the Mediterranean basin, where sandfly vectors transmit Leishmania species.[1] Immunosuppression, including HIV coinfection, increases risk and alters presentation, often leading to atypical or disseminated forms.[7] For cutaneous leishmaniasis, history typically reveals a slowly progressing lesion starting as a pruritic papule at the sandfly bite site, evolving over 2-8 weeks into a nodule and then a painless ulcer with indurated, raised borders; lesions commonly appear on exposed areas like the face, arms, or legs and may self-resolve over 3-18 months but leave scars.[36] Physical examination confirms volcano-like ulcers with central crusting and satellite lesions in some cases, though multiple lesions suggest dissemination in immunocompromised patients.[8] Differential considerations include bacterial or fungal infections, sporotrichosis, cutaneous tuberculosis, and squamous cell carcinoma, necessitating exclusion via biopsy or culture in ambiguous cases.[37] Mucocutaneous leishmaniasis evaluation focuses on persistent nasal symptoms such as stuffiness, epistaxis, or congestion emerging months to years after initial cutaneous infection, primarily from L. braziliensis in the Americas.[5] Examination may reveal mucosal erythema, nodules, or destructive ulcers in the nasal septum, palate, or pharynx, progressing to perforation or disfigurement if untreated.[38] Oral or laryngeal involvement can cause hoarseness or dysphagia, with differentials encompassing syphilis, leprosy, yaws, and midline granulomas.[7] Visceral leishmaniasis presents with insidious onset of irregular fever (often bi-phasic, peaking in evenings), profound fatigue, anorexia, and significant weight loss over weeks to months, accompanied by abdominal pain from organomegaly.[8] Physical findings include marked splenomegaly (extending >10 cm below the costal margin in advanced cases), moderate hepatomegaly, and generalized lymphadenopathy, alongside signs of anemia, thrombocytopenia, and hypergammaglobulinemia evident in pallor, petechiae, or bleeding.[36] In endemic areas, differentials include malaria, typhoid fever, brucellosis, lymphoma, and tuberculosis, requiring careful history to distinguish based on epidemiology and absence of response to antimicrobials.[1] Post-kala-azar dermal leishmaniasis may follow resolved visceral infection, manifesting as hypopigmented macules or nodules on the face and trunk.[7] While clinical features guide suspicion, definitive diagnosis demands parasitological confirmation due to overlapping syndromes.[37]Laboratory Confirmation
Laboratory confirmation of leishmaniasis primarily relies on direct parasitological methods, which detect the intracellular amastigote form of Leishmania parasites in clinical specimens.[3] For cutaneous leishmaniasis, specimens include lesion scrapings, punch biopsies, or aspirates stained with Giemsa or Wright's stain to visualize amastigotes within macrophages; sensitivity ranges from 50% to 80%, depending on lesion chronicity and parasite load.[39] In visceral leishmaniasis, bone marrow aspirates or splenic punctures yield higher detection rates, up to 95% for bone marrow microscopy, though splenic sampling is riskier due to potential hemorrhage.[40] Histopathological examination of biopsies reveals amastigotes alongside inflammatory responses like granulomas, but requires expertise to distinguish from other intracellular pathogens.[41] Culture isolation remains a reference standard for viable parasite confirmation and species identification, using media such as Novy-MacNeal-Nicolle (NNN) or Schneider's Drosophila medium incubated at 26–28°C for 4–21 days.[42] Success rates vary: 70–90% for fresh cutaneous specimens but lower for older lesions or frozen samples, with limitations including slow growth, overgrowth by contaminants, and biosafety level 2 requirements.[39] Molecular methods, particularly polymerase chain reaction (PCR) targeting kinetoplast DNA or ITS regions, offer superior sensitivity (90–100%) and specificity (>95%), enabling detection in low-parasite-load cases and rapid species typing via sequencing or probes.[3] Real-time PCR assays, validated for tissue, blood, and buffy coat, reduce contamination risks and provide quantitative data on parasite burden.[42] Serological tests, such as direct agglutination test (DAT) or rK39 rapid diagnostic tests, support visceral leishmaniasis diagnosis with sensitivities of 85–95% in endemic areas but are less reliable for cutaneous forms due to inconsistent antibody responses.[1] Cross-reactivity with other infections like Chagas disease limits specificity in co-endemic regions, necessitating parasitological corroboration.[41] Emerging techniques like loop-mediated isothermal amplification (LAMP) provide field-applicable alternatives to PCR, with comparable sensitivity in resource-limited settings.[39] Overall, combining microscopy with PCR enhances diagnostic accuracy, particularly for atypical presentations, though access to specialized labs remains a barrier in endemic areas.[38]Diagnostic Challenges
Diagnosis of leishmaniasis is complicated by its nonspecific clinical features, which frequently overlap with bacterial, fungal, or other parasitic infections, resulting in presumptive diagnoses that delay confirmatory testing.[43] [44] In resource-limited endemic areas, where the disease burdens over 90% of cases, shortages of trained microscopists and laboratory infrastructure further impede accurate detection.[45] [46] Parasitological confirmation via microscopy of lesion aspirates or biopsies remains the frontline method but yields sensitivities of 50-90% for cutaneous leishmaniasis, dropping below 70% in chronic lesions or visceral forms due to sparse parasite loads and operator-dependent visualization of amastigotes.[42] [47] This approach also fails to differentiate Leishmania species, essential for tailoring antimonial versus miltefosine therapy in regions with varying drug responsiveness.[48] Culture enhances specificity but demands specialized media like Novy-MacNeal-Nicolle, with growth taking 1-6 weeks and overall sensitivity under 60-70%, rendering it impractical for rapid diagnosis.[42] Serological tests, including indirect immunofluorescence or ELISA for anti-Leishmania antibodies, achieve 80-95% sensitivity in visceral leishmaniasis but perform poorly (60-80%) in cutaneous forms and exhibit cross-reactivity with Trypanosoma cruzi or other pathogens, complicating interpretation in co-endemic zones.[49] [50] These assays cannot distinguish active infection from resolved or subclinical cases, limiting their value for treatment monitoring or epidemiological surveys.[50] Molecular diagnostics, particularly real-time PCR targeting ITS1 or kDNA minicircles, offer superior sensitivity (>95%) and species identification but require thermocyclers, trained technicians, and DNA extraction kits, with costs prohibitive for peripheral health centers in low-income countries.[51] [52] Field-deployable loop-mediated isothermal amplification shows promise for point-of-care use yet lacks widespread validation and standardization across Leishmania taxa.[53] In visceral leishmaniasis, gold-standard parasitological diagnosis necessitates invasive splenic or bone marrow aspirations, associated with complication risks up to 0.5-5%, particularly in pediatric or HIV-co-infected patients where parasite burdens vary.[42] Immunosuppression, atypical presentations, and co-infections further erode test accuracies, contributing to diagnostic delays averaging 4.5 years from symptom onset in systematic reviews of imported cases.[54] [44]| Diagnostic Method | Typical Sensitivity Range | Key Limitations |
|---|---|---|
| Microscopy | 50-90% (cutaneous); <70% (visceral) | Operator-dependent; low yield in low-parasite cases; no speciation[42] |
| Culture | 50-70% | Slow (weeks); requires expertise and media; contamination risk[42] |
| Serology | 70-95% | Cross-reactivity; cannot confirm active disease[49] [50] |
| PCR | >95% | Infrastructure needs; cost; limited field access[51] |

