Recent from talks
Nothing was collected or created yet.
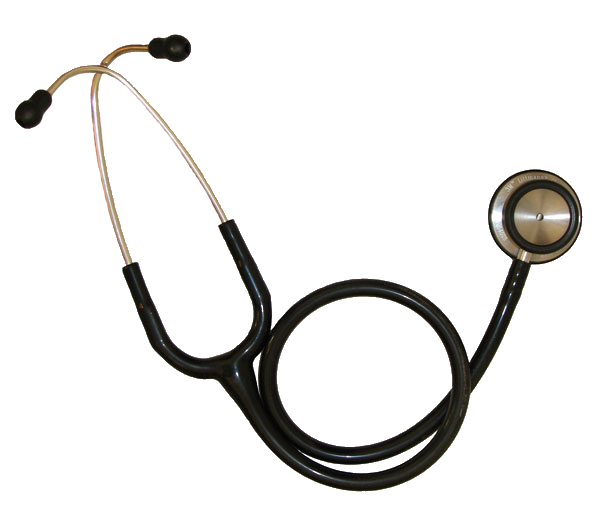
Stethoscope
View on Wikipedia| Stethoscope | |
|---|---|
 Modern stethoscope | |
| Classification | Medical device |
| Industry | Medicine |
| Application | Auscultation |
| Inventor | René Laennec in 1816; binaural version by Arthur Leared in 1851 |
| Invented | 1816 |
The stethoscope, from Ancient Greek στῆθος (stêthos), meaning "breast", and σκοπέω (skopéō), meaning "to look", is a medical device for auscultation, or listening to internal sounds of an animal or human body. It typically has a small disc-shaped resonator that is placed against the naked skin, with either one or two tubes connected to two earpieces. A stethoscope can be used to listen to the sounds made by the heart, lungs or intestines, as well as blood flow in arteries and veins. In combination with a manual sphygmomanometer, it is commonly used when measuring blood pressure. It was invented in 1816 by René Laennec and the binaural version by Arthur Leared in 1851.
A stethoscope that intensifies auscultatory sounds is called a phonendoscope.
History
[edit]



The stethoscope was invented in France in 1816 by René Laennec at the Necker-Enfants Malades Hospital in Paris.[1][2][3] It consisted of a wooden tube and was monaural. Laennec invented the stethoscope because he was not comfortable placing his ear directly onto a woman's chest in order to listen to her heart.[4][5]: 186 He observed that a rolled piece of paper, placed between the individual's chest and his ear, could amplify heart sounds without requiring physical contact.[6] Laennec's device was similar to the common ear trumpet, a historical form of hearing aid; indeed, his invention was almost indistinguishable in structure and function from the trumpet, which was commonly called a "microphone". Laennec called his device the "stethoscope"[7] (stetho- + -scope, "chest scope"), and he called its use "mediate auscultation", because it was auscultation with a tool intermediate between the individual's body and the physician's ear. (Today the word auscultation denotes all such listening, mediate or not.) The first flexible stethoscope of any sort may have been a binaural instrument with articulated joints not very clearly described in 1829.[8] In 1840, Golding Bird described a stethoscope he had been using with a flexible tube. Bird was the first to publish a description of such a stethoscope, but he noted in his paper the prior existence of an earlier design (which he thought was of little utility) which he described as the snake ear trumpet. Bird's stethoscope had a single earpiece.[9]
Binaural devices
[edit]In 1851, Irish physician Arthur Leared invented a binaural stethoscope. The following year, George Philip Cammann, a physician practicing in New York City, perfected for commercial production the design of a stethoscope that featured a plug for each ear.[10] Although improvements have been made, his design has remained essentially unchanged ever since. Cammann also wrote a major treatise on diagnosis by auscultation, which the refined binaural stethoscope made possible. By 1873, there were descriptions of a differential stethoscope that could connect to slightly different locations to create a slight stereo effect, though this did not become a standard tool in clinical practice.
Somerville Scott Alison described his invention of the stethophone at the Royal Society in 1858; the stethophone had two separate bells, allowing the user to hear and compare sounds derived from two discrete locations. This was used to do definitive studies on binaural hearing and auditory processing that advanced knowledge of sound localization and eventually led to an understanding of binaural fusion.[1]
The medical historian Jacalyn Duffin has argued that the invention of the stethoscope marked a major step in the redefinition of disease from being a bundle of symptoms, to the current sense of a disease as a problem with an anatomical system even if there are no observable symptoms. This re-conceptualization occurred in part, Duffin argues, because prior to stethoscopes, there were no non-lethal instruments for exploring internal anatomy.[11]
Rappaport and Sprague designed a new stethoscope in the 1940s, which became the standard by which other stethoscopes are measured, consisting of two sides, one of which is used for the respiratory system, the other for the cardiovascular system. The Rappaport-Sprague model stethoscope was heavy and short (18–24 in (46–61 cm)) with an antiquated appearance recognizable by their two large independent latex rubber tubes connecting an exposed leaf-spring-joined pair of opposing F-shaped chrome-plated brass binaural ear tubes with a dual-head chest piece.

Several other minor refinements were made to stethoscopes until, in the early 1960s, David Littmann, a Harvard Medical School professor, created a new stethoscope that was lighter than previous models and had improved acoustics.[12][13] In the late 1970s, 3M-Littmann introduced the tunable diaphragm: a very hard (G-10) glass-epoxy resin diaphragm member with an overmolded silicone flexible acoustic surround which permitted increased excursion of the diaphragm member in a Z-axis with respect to the plane of the sound collecting area.[14] The left shift to a lower resonant frequency increases the volume of some low frequency sounds due to the longer waves propagated by the increased excursion of the hard diaphragm member suspended in the concentric accountic surround. Conversely, restricting excursion of the diaphragm by pressing the stethoscope diaphragm surface firmly against the anatomical area overlying the physiological sounds of interest, the acoustic surround could also be used to dampen excursion of the diaphragm in response to "z"-axis pressure against a concentric fret. This raises the frequency bias by shortening the wavelength to auscultate a higher range of physiological sounds.
In 1999, Richard Deslauriers patented the first external noise reducing stethoscope, the DRG Puretone. It featured two parallel lumens containing two steel coils which dissipated infiltrating noise as inaudible heat energy. The steel coil "insulation" added .30 lb to each stethoscope.
Current practice
[edit]
Stethoscopes are a symbol of healthcare professionals. Healthcare providers are often seen or depicted wearing a stethoscope around the neck. A 2012 research paper claimed that the stethoscope, when compared to other medical equipment, had the highest positive impact on the perceived trustworthiness of the practitioner seen with it.[15][16]
Prevailing opinions on the utility of the stethoscope in current clinical practice vary depending on the medical specialty. Studies have shown that auscultation skill (i.e., the ability to make a diagnosis based on what is heard through a stethoscope) has been in decline for some time, such that some medical educators are working to re-establish it.[17][18][19]
In general practice, traditional blood pressure measurement using a mechanical sphygmomanometer with inflatable cuff and stethoscope is gradually being replaced with automated blood pressure monitors.[20]
Types
[edit]Acoustic
[edit]

Acoustic stethoscopes operate on the transmission of sound from the chest piece, via air-filled hollow tubes, to the listener's ears. The chestpiece usually consists of two sides that can be placed against the patient for sensing sound: a diaphragm (plastic disc) or bell (hollow cup). If the diaphragm is placed on the patient, body sounds vibrate the diaphragm, creating acoustic pressure waves which travel up the tubing to the listener's ears. If the bell is placed on the patient, the vibrations of the skin directly produce acoustic pressure waves traveling up to the listener's ears. The bell transmits low frequency sounds, while the diaphragm transmits higher frequency sounds. To deliver the acoustic energy primarily to either the bell or diaphragm, the tube connecting into the chamber between bell and diaphragm is open on only one side and can rotate. The opening is visible when connected into the bell. Rotating the tube 180 degrees in the head connects it to the diaphragm. This two-sided stethoscope was invented by Rappaport and Sprague in the early part of the 20th century.[citation needed]
Electronic
[edit]
An electronic stethoscope (or stethophone) overcomes the low sound levels by electronically amplifying body sounds. However, amplification of stethoscope contact artifacts, and component cutoffs (frequency response thresholds of electronic stethoscope microphones, pre-amps, amps, and speakers) limit electronically amplified stethoscopes' overall utility by amplifying mid-range sounds, while simultaneously attenuating high- and low- frequency range sounds. Currently, a number of companies offer electronic stethoscopes. Electronic stethoscopes require conversion of acoustic sound waves to electrical signals which can then be amplified and processed for optimal listening. Unlike acoustic stethoscopes, which are all based on the same physics, transducers in electronic stethoscopes vary widely. The simplest and least effective method of sound detection is achieved by placing a microphone in the chestpiece. This method suffers from ambient noise interference and has fallen out of favor. Another method, used in Welch-Allyn's Meditron stethoscope, comprises placement of a piezoelectric crystal at the head of a metal shaft, the bottom of the shaft making contact with a diaphragm. 3M also uses a piezo-electric crystal placed within foam behind a thick rubber-like diaphragm. The Thinklabs' Rhythm 32 uses an electromagnetic diaphragm with a conductive inner surface to form a capacitive sensor. This diaphragm responds to sound waves, with changes in an electric field replacing changes in air pressure. The Eko Core enables wireless transmission of heart sounds to a smartphone or tablet. The Eko Duo can take electrocardiograms as well as echocardiograms. This enables clinicians to screen for conditions such as heart failure, which would not be possible with a traditional stethoscope.[21][22]
Because the sounds are transmitted electronically, an electronic stethoscope can be a wireless device, can be a recording device, and can provide noise reduction, signal enhancement, and both visual and audio output. Around 2001, Stethographics introduced PC-based software which enabled a phonocardiograph, graphic representation of cardiologic and pulmonologic sounds to be generated, and interpreted according to related algorithms. All of these features are helpful for purposes of telemedicine (remote diagnosis) and teaching.[citation needed]
Electronic stethoscopes are also used with computer-aided auscultation programs to analyze the recorded heart sounds pathological or innocent heart murmurs.
Recording
[edit]Some electronic stethoscopes feature direct audio output that can be used with an external recording device, such as a laptop or MP3 recorder. The same connection can be used to listen to the previously recorded auscultation through the stethoscope headphones, allowing for more detailed study for general research as well as evaluation and consultation regarding a particular patient's condition and telemedicine, or remote diagnosis.[23]
There are some smartphone apps that can use the phone as a stethoscope.[24] At least one uses the phone's own microphone to amplify sound, produce a visualization, and e-mail the results. These apps may be used for training purposes or as novelties, but have not yet gained acceptance for professional medical use.[25]
The first stethoscope that could work with a smartphone application was introduced in 2015 [26]
Fetal
[edit]
A fetal stethoscope or fetoscope is an acoustic stethoscope shaped like a listening trumpet. It is placed against the abdomen of a pregnant woman to listen to the heart sounds of the fetus.[27] The fetal stethoscope is also known as a Pinard horn after French obstetrician Adolphe Pinard (1844–1934).
Doppler
[edit]A Doppler stethoscope is an electronic device that measures the Doppler effect of ultrasound waves reflected from organs within the body. Motion is detected by the change in frequency, due to the Doppler effect, of the reflected waves. Hence the Doppler stethoscope is particularly suited to deal with moving objects such as a beating heart.[28] It was recently demonstrated that continuous Doppler enables the auscultation of valvular movements and blood flow sounds that are undetected during cardiac examination with a stethoscope in adults. The Doppler auscultation presented a sensitivity of 84% for the detection of aortic regurgitations while classic stethoscope auscultation presented a sensitivity of 58%. Moreover, Doppler auscultation was superior in the detection of impaired ventricular relaxation. Since the physics of Doppler auscultation and classic auscultation are different, it has been suggested that both methods could complement each other.[29][30] A military noise-immune Doppler based stethoscope has recently been developed for auscultation of patients in loud sound environments (up to 110 dB).
3D-printed
[edit]
A 3D-printed stethoscope is an open-source medical device meant for auscultation and manufactured via means of 3D printing.[31] The 3D stethoscope was developed by Dr. Tarek Loubani and a team of medical and technology specialists. The 3D-stethoscope was developed as part of the Glia project, and its design is open source from the outset. The stethoscope gained widespread media coverage in Summer 2015.
The need for a 3D-stethoscope was borne out of a lack of stethoscopes and other vital medical equipment because of the blockade of the Gaza Strip, where Loubani, a Palestinian-Canadian, worked as an emergency physician during the 2012 conflict in Gaza. The 1960s-era Littmann Cardiology 3 stethoscope became the basis for the 3D-printed stethoscope developed by Loubani.[32]
Esophageal
[edit]Prior to the 1960s, the esophageal stethoscope was a part of the routine intraoperative monitoring.[33]
Earpieces
[edit]Stethoscopes usually have rubber earpieces, which aid comfort and create a seal with the ear, improving the acoustic function of the device. Stethoscopes can be modified by replacing the standard earpieces with moulded versions, which improve comfort and transmission of sound. Moulded earpieces can be cast by an audiologist or made by the stethoscope user from a kit.
See also
[edit]References
[edit]- ^ a b c Wade, Nicholas J.; Deutsch, Diana (July 2008). "Binaural Hearing – Before and After the Stethophone" (PDF). Acoustics Today. 4 (3): 16–27. doi:10.1121/1.2994724.
- ^ Laennec, René (1819). De l'auscultation médiate ou traité du diagnostic des maladies des poumon et du coeur. Paris: Brosson & Chaudé.
- ^ 'Laennec, R. T. H.; Forbes, John, Sir, A Treatise on the Diseases of the Chest and on Mediate Auscultation (1835). New York : Samuel Wood & Sons; Philadelphia : Desilver, Thomas & Co. .
- ^ Roguin A (September 2006). "Rene Theophile Hyacinthe Laënnec (1781–1826): The Man Behind the Stethoscope". Clin Med Res. 4 (3): 230–5. doi:10.3121/cmr.4.3.230. PMC 1570491. PMID 17048358.
- ^ Picard, Liza (2005). Victorian London: the life of a city, 1840–1870. London: Weidenfeld & Nicolson. ISBN 978-0297847335.
- ^ Risse, Guenter (1999). Mending Bodies, Saving Souls. Oxford: Oxford University Press. p. 316. ISBN 978-0-19-505523-8.
- ^ "Laennec's new system of diagnosis", The Quarterly Journal of Foreign Medicine and Surgery and of the Sciences Connected with Them, 2: 51–68, 1820.
- ^ Wilks, p. 490, cites Comins, "A flexible stethoscope", Lancet 29 August 1829.
- ^ Samuel Wilks, "Evolution of the stethoscope", Popular Science, vol. 22, no. 28, pp. 488–91, Feb 1883 ISSN 0161-7370.
Golding Bird, "Advantages presented by the employment of a stethoscope with a flexible tube", London Medical Gazette, vol. 1, pp. 440–12, 11 December 1840. - ^ Permin, Henrik; Norn, Svend (2019). "Stethoscope—Over 200 Years". Journal of Pulmonology and Respiratory Research. 3: 001–008. doi:10.29328/journal.jprr.1001010.
- ^ Duffin, Jacalyn. "Big Ideas: Jacalyn Duffin on the History of the Stethoscope". TVO. Archived from the original on 27 September 2013. Retrieved 28 November 2012.
- ^ "History of Littmann Stethoscopes at a glance". 3M.com. Retrieved 2010-01-25.
- ^ U.S. patent 3,108,652
- ^ U.S. patent 3,951,230
- ^ Jiwa, Moyez; Millett, Stephan; Meng, Xingqiong; Hewitt, Vivien M. (2012). "Impact of the Presence of Medical Equipment in Images on Viewers' Perceptions of the Trustworthiness of an Individual On-Screen". Journal of Medical Internet Research. 14 (4): e100. doi:10.2196/jmir.1986. PMC 3409609. PMID 22782078.
- ^ McLoughlin, Mario J (30 October 2012). "Cardiac auscultation: preliminary findings of a pilot study using continuous Wave Doppler and comparison with classic auscultation". International Journal of Cardiology. 167 (2): 590–591. doi:10.1016/j.ijcard.2012.09.223. PMID 23117017.
- ^ Wann, Samuel L (21 September 2022). "The Traditional Stethoscope May Be Obsolete but the Need for Human Connection Is Not". The American Journal of Cardiology. 184: 147–148. doi:10.1016/j.amjcard.2022.08.020. PMID 36153180. S2CID 252493231.
- ^ Murphy, R (2005), "The stethoscope – obsolescence or marriage?", Respir Care, 50 (5): 660–661, PMID 15912626.
- ^ Bernstein, Lenny (2016-01-02), "Heart doctors are listening for clues to the future of their stethoscopes", Washington Post.
- ^ Roerecke, M; Kaczorowski, J; Myers, MG (2019), "Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis", JAMA Intern Med, 179 (3): 351–362, doi:10.1001/jamainternmed.2018.6551, PMC 6439707, PMID 30715088.
- ^ "Smart stethoscope uses artificial intelligence to screen for heart failure". NIHR Evidence. 18 January 2023. doi:10.3310/nihrevidence_56245. S2CID 257852883.
- ^ Bachtiger, Patrik; Petri, Camille F; Scott, Francesca E; Ri Park, Se; Kelshiker, Mihir A; Sahemey, Harpreet K; Dumea, Bianca; Alquero, Regine; Padam, Pritpal S; Hatrick, Isobel R; Ali, Alfa; Ribeiro, Maria; Cheung, Wing-See; Bual, Nina; Rana, Bushra (2022-02-01). "Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study". The Lancet Digital Health. 4 (2): e117 – e125. doi:10.1016/S2589-7500(21)00256-9. PMC 8789562. PMID 34998740.
- ^ Palaniappan R, Sundaraj K, Ahamed NU, Arjunan A, Sundaraj S. Computer-based Respiratory Sound Analysis: A Systematic Review. IETE Tech Rev 2013;30:248–56
- ^ Bianca K. Chung, Brad Tritle, "The power of mobile devices and patient engagement", p. 93, chapter 8 in Jan Oldenburg (ed), Engage! Transforming Healthcare Through Digital Patient Engagement, Himss Books, 2012 ISBN 1938904397.
- ^ William Hanson, Smart Medicine: How the Changing Role of Doctors Will Revolutionize Health Care, pp. 20–22, Macmillan, 2011 ISBN 0230120938.
- ^ Matt McFarland, "Eko's stethoscope shows the potential of digital technology to reinvent health care", [1], Washington Post
- ^ Arup Kumar Majhi (2016-08-16). Bedside Clinics In Obstetrics. Academic Publishers. pp. 47–. ISBN 978-93-83420-87-2.
- ^ S. Ananthi, A Textbook of Medical Instruments, pp. 290–96, New Age International, 2006 ISBN 8122415725.
- ^ Mc Loughlin MJ and Mc Loughlin S. Cardiac auscultation: Preliminary findings of a pilot study using continuous Wave Doppler and comparison with classic auscultation Int J Cardiol. 2013 Jul 31; 167(2):5 90–91
- ^ Amazon.com: Cardiac Auscultation With Continuous Wave Doppler Stethoscope: A new method 200 years after Laennec's invention eBook: Mario Jorge Mc Loughlin, Santiago Mc Loughlin: Kindle Store. Mario J Mc Loughlin. 5 January 2013. Retrieved 24 September 2015.
- ^ Official project site at GitHub
- ^ Pauli, Darren (2015-08-14). "Gazan medico team 3D-prints world-leading stethoscope for 30c". The Register. United Kingdom. Retrieved 2015-08-17.
- ^ Brodsky, J; Lemmens, H (2007). "The history of anesthesia for thoracic surgery". Minerva Anestesiologica. 73 (10): 513–24. PMID 17380101.
External links
[edit]- The Auscultation Assistant, provides heart sounds, heart murmurs, and breath sounds in order to help medical students and others improve their physical diagnosis skills
- Demonstrations: Heart Sounds & Murmurs University of Washington School of Medicine
- "The invention of the stethoscope: A milestone in cardiology", analysis of Laennec's text (1819) on BibNum [click 'à télécharger' for English version].