Recent from talks
Nothing was collected or created yet.
Androgen ester
View on Wikipedia
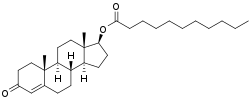
An androgen or anabolic steroid ester is an ester of an androgen/anabolic steroid (AAS) such as the natural testosterone or dihydrotestosterone (DHT) or the synthetic nandrolone (19-nortestosterone). Esterification renders AAS into metabolism-resistant prohormones of themselves, improving oral bioavailability, increasing lipophilicity, and extending the elimination half-life (which necessitates less frequent administration). In addition, with intramuscular injection, AAS esters are absorbed more slowly into the body, thus further improving the elimination half-life. Aside from differences in pharmacokinetics (e.g., duration), these esters essentially have the same effects as the parent drugs.[1] They are used in androgen replacement therapy (ART), among other indications. Examples of androgen esters include testosterone esters such as testosterone cypionate, testosterone enanthate, testosterone propionate, and testosterone undecanoate and nandrolone esters such as nandrolone decanoate and nandrolone phenylpropionate.
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–28 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–28 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21–28 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
| Testosterone ester | Form | Route | Tmax | t1/2 | MRT |
|---|---|---|---|---|---|
| Testosterone undecanoate | Oil-filled capsules | Oral | ? | 1.6 hours | 3.7 hours |
| Testosterone propionate | Oil solution | Intramuscular injection | ? | 0.8 days | 1.5 days |
| Testosterone enanthate | Castor oil solution | Intramuscular injection | 10 days | 4.5 days | 8.5 days |
| Testosterone undecanoate | Tea seed oil solution | Intramuscular injection | 13.0 days | 20.9 days | 34.9 days |
| Testosterone undecanoate | Castor oil solution | Intramuscular injection | 11.4 days | 33.9 days | 36.0 days |
| Testosterone buciclatea | Aqueous suspension | Intramuscular injection | 25.8 days | 29.5 days | 60.0 days |
| Notes: Testosterone cypionate has similar pharmacokinetics to Testosterone enanthate. Footnotes: a = Never marketed. Sources: See template. | |||||
| Androgen | Structure | Ester | Relative mol. weight |
Relative T contentb |
logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Testosterone | – | – | – | – | 1.00 | 1.00 | 3.0–3.4 | ||
| Testosterone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.19 | 0.84 | 3.7–4.9 | ||
| Testosterone isobutyrate | C17β | Isobutyric acid | Branched-chain fatty acid | – (~3) | 1.24 | 0.80 | 4.9–5.3 | ||
| Testosterone isocaproate | C17β | Isohexanoic acid | Branched-chain fatty acid | – (~5) | 1.34 | 0.75 | 4.4–6.3 | ||
| Testosterone caproate | C17β | Hexanoic acid | Straight-chain fatty acid | 6 | 1.35 | 0.75 | 5.8–6.5 | ||
| Testosterone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 5.8–6.5 | ||
| Testosterone cypionate | C17β | Cyclopentylpropanoic acid | Cyclic carboxylic acid | – (~6) | 1.43 | 0.70 | 5.1–7.0 | ||
| Testosterone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.39 | 0.72 | 3.6–7.0 | ||
| Testosterone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.53 | 0.65 | 6.3–8.6 | ||
| Testosterone undecanoate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | 6.7–9.2 | ||
| Testosterone buciclated | C17β | Bucyclic acide | Cyclic carboxylic acid | – (~9) | 1.58 | 0.63 | 7.9–8.5 | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative testosterone content by weight (i.e., relative androgenic/anabolic potency). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Never marketed. e = Bucyclic acid = trans-4-Butylcyclohexane-1-carboxylic acid. Sources: See individual articles. | |||||||||
| Anabolic steroid | Structure | Ester | Relative mol. weight |
Relative AAS contentb |
Durationc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Lengtha | ||||||
| Boldenone undecylenate | C17β | Undecylenic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | Long | ||
| Drostanolone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.18 | 0.84 | Short | ||
| Metenolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.14 | 0.88 | Short | ||
| Metenolone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.37 | 0.73 | Long | ||
| Nandrolone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.56 | 0.64 | Long | ||
| Nandrolone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6–7) | 1.48 | 0.67 | Long | ||
| Trenbolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.16 | 0.87 | Short | ||
| Trenbolone enanthated | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | Long | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative androgen/anabolic steroid content by weight (i.e., relative androgenic/anabolic potency). c = Duration by intramuscular or subcutaneous injection in oil solution. d = Never marketed. Sources: See individual articles. | |||||||||
See also
[edit]References
[edit]- ^ Richard Lawrence Miller (2002). The Encyclopedia of Addictive Drugs. Greenwood Publishing Group. pp. 416–. ISBN 978-0-313-31807-8.
Further reading
[edit]- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
Androgen ester
View on GrokipediaDefinition and background
Definition
Androgen esters are a class of synthetic derivatives of androgens, such as testosterone and dihydrotestosterone, formed through esterification of the 17β-hydroxyl group on the steroid's D-ring.[4] This modification converts the parent androgens into lipophilic prodrugs that are pharmacologically inactive until metabolized in the body.[5] By design, these esters enhance the compounds' suitability for therapeutic delivery, particularly via intramuscular injection or oral administration, by improving their stability and absorption properties.[6] The primary role of androgen esters is to act as prohormones, undergoing hydrolysis by endogenous esterases—primarily in blood, liver, and tissues—to liberate the active androgen and exert its physiological effects.[7] This enzymatic cleavage is essential, as the esterified forms themselves lack significant androgenic activity and resist rapid first-pass metabolism in the liver, unlike their non-esterified counterparts, which are quickly inactivated and eliminated.[8] Key characteristics include heightened lipophilicity, which facilitates formulation in oil vehicles for sustained release, and reduced susceptibility to immediate degradation, thereby extending bioavailability and therapeutic duration.[1] Androgen esters are broadly classified by the length of their ester side chain, which influences release kinetics and duration of action: short-chain variants, such as the propionate ester, yield rapid onset but shorter half-lives suitable for frequent dosing, while long-chain forms, like undecanoate, provide prolonged effects over weeks due to slower hydrolysis.[9] This classification optimizes their use in clinical settings, balancing efficacy with dosing convenience.[4]Historical development
The isolation of testosterone marked a pivotal milestone in the development of androgen therapy, occurring in 1935 when chemists Adolf Butenandt in Germany and Leopold Ruzicka in Switzerland independently synthesized the hormone from cholesterol derivatives, following its extraction from bull testes by Ernst Laqueur's team in Amsterdam.[10] This breakthrough, recognized with the 1939 Nobel Prize in Chemistry awarded to Butenandt and Ruzicka, enabled the production of pure testosterone for clinical experimentation, addressing longstanding observations of testicular effects on male physiology dating back to antiquity. Early efforts to utilize testosterone clinically revealed its short half-life and poor oral bioavailability due to rapid hepatic metabolism, prompting the development of ester derivatives to enhance duration and administration routes. In 1936, the first androgen ester, testosterone propionate, was synthesized and marketed by pharmaceutical companies Schering AG and Ciba as an intramuscular injectable, offering a half-life of 1-2 days and allowing for more practical dosing compared to unmodified testosterone.[10] Initial human clinical trials with testosterone propionate began as early as 1937, primarily targeting hypogonadism to restore secondary sexual characteristics and libido, with European institutions like Schering AG leading the pioneering work on injectable formulations.[11] The 1950s saw advancements in ester chain length to achieve extended-release profiles, exemplified by testosterone enanthate, synthesized by Karl Junkmann at Schering AG and introduced as Testoviron Depot, which provided therapeutic levels for 2-3 weeks after a single injection.[10] This shift from short-acting oral attempts—largely abandoned due to liver inactivation and inconsistent absorption—to intramuscular preparations improved patient compliance and efficacy for androgen replacement. By the 1970s, further innovations included oral testosterone undecanoate, developed to bypass first-pass metabolism via lymphatic absorption, and blend formulations like Organon's Sustanon, combining multiple esters (propionate, phenylpropionate, isocaproate, and decanoate) for sustained release.[12] Clinical applications expanded beyond hypogonadism in the 1940s to anabolic uses in the 1950s and 1960s, including muscle-wasting conditions and athletic performance enhancement, though this latter trend raised concerns over non-medical adoption.[13]Chemistry
Chemical structure
Androgen esters are derivatives of androgens such as testosterone, which possess a characteristic steroid backbone consisting of four fused rings: three six-membered cyclohexane rings (designated A, B, and C) and one five-membered cyclopentane ring (D). This gonane core, with angular methyl groups at positions C10 and C13, forms the basis of the C19 androstane skeleton in testosterone. In testosterone specifically, ring A features a Δ⁴ double bond between C4 and C5 and a ketone group at C3, while the 17β position on ring D bears a hydroxyl group essential for biological activity.[3][14] Esterification occurs at the 17β-hydroxyl group of the parent androgen, where an acyl group (R-COO⁻) is attached via the oxygen atom, yielding the general formula Androgen-17β-O-CO-R. This modification replaces the hydrogen of the hydroxyl with the acyl moiety, altering the molecule's polarity without affecting the core steroid nucleus. The resulting structure maintains the androgen's ability to bind androgen receptors upon hydrolysis but imparts prodrug properties.[1][15] Common examples illustrate variations in the R group, which determine the ester's pharmacokinetic profile through differences in chain length and branching:| Ester Name | R Group Formula | Molecular Formula | Reference |
|---|---|---|---|
| Testosterone propionate | CH₃CH₂⁻ | C₂₂H₃₂O₃ | [16] |
| Testosterone enanthate | CH₃(CH₂)₅⁻ | C₂₆H₄₀O₃ | [17] |
| Testosterone cypionate | cyclopentyl-CH₂CH₂⁻ | C₂₇H₄₀O₃ | [18] |
| Testosterone undecanoate | CH₃(CH₂)₉⁻ | C₃₀H₄₈O₃ | [19] |
Synthesis
Androgen esters are synthesized primarily through esterification of the 17β-hydroxyl group of parent androgens, such as testosterone, with a carboxylic acid derivative. The most common laboratory method involves reacting the 17β-hydroxyandrogen with a carboxylic acid chloride (R-COCl) or anhydride (R-CO)₂O in the presence of a base like pyridine to neutralize the acid produced and facilitate the reaction. This nucleophilic acyl substitution proceeds under mild conditions, typically at room temperature in an aprotic solvent such as dichloromethane or N,N-dimethylformamide. The general reaction can be represented as: For example, testosterone enanthate is prepared by treating testosterone with enanthoyl chloride (heptanoyl chloride) in pyridine, yielding the ester after workup. Similarly, testosterone undecanoate is synthesized using undecanoic anhydride with testosterone in the presence of pyridine and ethyl acetate, achieving high conversion to the pharmaceutical product. These methods ensure selectivity for the primary 17β-hydroxyl group due to its reactivity compared to any secondary hydroxyls in other androgens. Purification of the crude ester is achieved through recrystallization from organic solvents such as ethanol, acetone, or pentane to obtain pharmaceutical-grade material with purity exceeding 97%. This step removes unreacted starting materials and byproducts, enhancing stability and suitability for formulation. On an industrial scale, esterification processes have evolved from batch reactions to continuous flow systems since the 1950s, improving throughput and consistency in steroid production. Biocatalytic approaches using immobilized lipases, such as those from Candida antarctica, enable stereoselective esterification with fatty acids, offering greener alternatives with reduced solvent use and high enantiopurity for precursors like dehydroepiandrosterone. Key challenges include minimizing side reactions, such as over-acylation or hydrolysis, particularly in androgens with multiple hydroxyl groups, while optimizing yields, which typically range from 80% to 95% under controlled conditions.Pharmacology
Pharmacokinetics
Androgen esters are primarily administered via intramuscular (IM) depot injections in oil vehicles, which allow for sustained release, though oral formulations such as testosterone undecanoate in oil-filled capsules and rare transdermal applications exist.[1][22] Absorption of IM-administered androgen esters occurs slowly due to their high lipophilicity, which promotes partitioning into the oil depot at the injection site, leading to gradual hydrolysis and release of the active androgen into the bloodstream. Peak serum levels are typically achieved within 1-2 days for short-chain esters like testosterone propionate, but may take 1-2 weeks for longer-chain esters such as testosterone undecanoate. Oral testosterone undecanoate is absorbed via the lymphatic system through chylomicrons, with bioavailability enhanced by co-administration with a high-fat meal (at least 19 g of fat).[1][22][23] The duration of action for androgen esters correlates with the length of the ester side chain, as longer chains increase lipophilicity and slow the release rate from the depot. Representative half-lives for common testosterone esters administered intramuscularly are summarized below:| Ester | Chain Length (Carbons) | Approximate Elimination Half-Life (Days) | Typical Dosing Interval |
|---|---|---|---|
| Testosterone propionate | 3 | 0.8-2 | Every 1-3 days |
| Testosterone cypionate | 8 | ~8 | Every 1-2 weeks |
| Testosterone enanthate | 7 | 4.5-8 | Every 1-2 weeks |
| Testosterone undecanoate | 11 | 20-34 | Every 10-12 weeks |
Pharmacodynamics
Androgen esters function as prodrugs that are rapidly hydrolyzed by ubiquitous esterases in extracellular fluids to liberate the active parent androgen, such as testosterone from testosterone esters.[3] This hydrolysis occurs shortly after administration, releasing free testosterone into circulation to bind the androgen receptor (AR), a ligand-activated nuclear transcription factor encoded on the X chromosome.[3] Upon binding, the androgen-AR complex translocates to the nucleus, where it interacts with androgen response elements on DNA to regulate gene transcription, ultimately promoting the synthesis of proteins involved in cellular growth and differentiation.[33] In skeletal muscle, this mechanism drives anabolic effects by upregulating the expression of insulin-like growth factor-1 (IGF-1), which enhances myoblast proliferation and hypertrophy.[34] The anabolic-androgenic ratio, which quantifies the relative potency for muscle-building versus virilizing effects, is preserved from the parent androgen; testosterone esters maintain a 1:1 ratio, while nandrolone esters demonstrate greater anabolic selectivity with ratios of approximately 10:1.[35] Due to the sustained release facilitated by esterification, these compounds achieve steady plasma levels of active androgen, supporting consistent dose-response effects on target tissues compared to the rapid fluctuations of non-esterified forms.[3] Tissue selectivity of androgen actions arises from local enzymatic conversions rather than the ester moiety itself, which dissociates prior to receptor binding. In the prostate, 5α-reductase converts about 4% of testosterone to dihydrotestosterone (DHT), a more potent AR agonist that amplifies androgenic effects.[3] Conversely, testosterone predominates in muscle, exerting direct anabolic influence without significant DHT amplification. Additionally, aromatase (CYP19) metabolizes approximately 0.2% of testosterone to estradiol, enabling estrogen receptor-mediated effects in tissues like bone and brain.[3]Medical uses
Androgen replacement therapy
Androgen esters, particularly testosterone esters, are a cornerstone of androgen replacement therapy (ART) for treating male hypogonadism, which encompasses both primary (testicular failure) and secondary (pituitary or hypothalamic dysfunction) forms characterized by symptoms such as fatigue, reduced libido, erectile dysfunction, decreased muscle mass, and low serum testosterone levels below 300 ng/dL confirmed on at least two morning measurements.[36][37] This therapy aims to restore physiological testosterone levels to the mid-normal male range (typically 400-700 ng/dL) to alleviate these symptoms and prevent long-term complications like osteoporosis.[37] In adult males with confirmed hypogonadism, ART using intramuscular (IM) testosterone esters offers pharmacokinetic advantages through sustained release, enabling less frequent dosing compared to daily topical formulations, which can improve patient compliance.[37] Common regimens include IM testosterone enanthate at 150-200 mg every 2 weeks or 75-100 mg weekly, titrated based on mid-interval serum levels to avoid supraphysiological peaks.[37] For longer-acting options, IM testosterone undecanoate (e.g., Aveed) is administered as 750 mg initially, followed by 750 mg at 4 weeks, and then every 10 weeks thereafter, providing stable levels with dosing intervals up to 14 weeks in some protocols.[38][37] These ester formulations are recommended by the Endocrine Society for symptomatic hypogonadism, with a preference for IM injections over gels in scenarios prioritizing adherence due to their extended duration of action.[37] In transgender men undergoing masculinizing hormone replacement therapy, similar regimens are employed to achieve male-range testosterone levels, inducing secondary sex characteristics like increased muscle mass and voice deepening while suppressing estrogen effects; recent guidelines (as of 2023) emphasize monitoring for cardiovascular risks and fertility options.[39][40] Therapy benefits include restoration of libido and erectile function, increased lean muscle mass and strength, improved bone mineral density to prevent osteoporosis, and modest enhancements in mood and energy.[37] These outcomes are supported by meta-analyses showing small to moderate effect sizes for sexual function (standardized mean difference [SMD] 0.17 for libido) and significant gains in vertebral and femoral bone strength.[37] Monitoring is essential to ensure efficacy and safety, involving serum testosterone measurement (mid-interval for short-acting esters, trough for long-acting), hematocrit (to detect polycythemia, with discontinuation if >54%), and prostate-specific antigen (PSA) levels at baseline, 3-12 months, and annually thereafter, with adjustments for age or elevated baseline values.[37] The Endocrine Society's 2018 guidelines emphasize individualized dosing and regular follow-up to optimize benefits while minimizing risks like erythrocytosis.[37]Other indications
Androgen esters have been employed in the treatment of delayed puberty in adolescents, particularly through short-term administration of nandrolone decanoate to stimulate linear growth and secondary sexual characteristics in cases of constitutional delay or hypogonadism.[35] This approach leverages the anabolic properties of nandrolone to promote height velocity without long-term suppression of endogenous hormone production, though its use is typically limited to monitored periods to avoid premature epiphyseal closure.[41] In the context of HIV/AIDS-associated wasting syndrome during the 1990s and 2000s, nandrolone decanoate provided anabolic support by increasing lean body mass and weight, with studies demonstrating significant gains in fat-free mass (approximately 3.5 kg over 12 weeks) and improvements in quality of life among patients with moderate weight loss.[42] These benefits were attributed to nandrolone's promotion of protein synthesis and nitrogen retention, making it a valuable adjunctive therapy in resource-limited settings before widespread antiretroviral availability. Testosterone esters, such as enanthate or cypionate, have been utilized to treat anemia by stimulating erythropoiesis, particularly in hypogonadal or chronic kidney disease patients, where they elevate hemoglobin levels through enhanced erythropoietin production and red blood cell maturation.[43] Clinical evidence indicates that such therapy can correct normocytic anemia in up to 50% of responsive cases, establishing its role in conditions refractory to other interventions.[44] Limited applications of androgen therapy extend to women, including combinations with estrogen for alleviating menopausal symptoms like fatigue and diminished libido, as well as supporting bone density in osteoporosis prevention, though efficacy remains debated due to variable responses and potential virilizing effects; ester formulations are not typically used due to administration challenges. For instance, estrogen-androgen regimens have shown additive benefits in preserving vertebral bone mineral density over estrogen alone in postmenopausal cohorts (as of 2022 guidelines).[45] Emerging investigations explore androgen esters for managing cachexia in cancer and chronic illnesses, where agents like nandrolone or testosterone esters aim to counteract muscle wasting by enhancing anabolic signaling pathways and reducing inflammation, with preliminary trials reporting modest improvements in body composition among advanced cancer patients.[46] In veterinary medicine, boldenone undecylenate serves as an anabolic agent in horses to treat debilitation, promoting weight gain, appetite, and overall condition in non-breeding animals through intramuscular administration at doses of 0.5-1 mg/kg every 3-4 weeks.[47][48]Adverse effects
General androgenic effects
Androgen esters, upon hydrolysis to their active androgen forms, exert a range of androgenic effects that can manifest as adverse outcomes, particularly at supraphysiological doses. These effects arise from the binding of androgens to androgen receptors, influencing various tissues and physiological processes. While therapeutic use in hypogonadism aims to restore physiological levels, excesses can amplify risks, which are generally dose-dependent and more pronounced in women due to their lower baseline androgen exposure.[3] Virilization represents a primary androgenic adverse effect, especially in females, where androgens promote masculinizing changes. Common manifestations include acne due to increased sebaceous gland activity, hirsutism from enhanced hair follicle stimulation, and voice deepening resulting from laryngeal hypertrophy; the latter is often irreversible in women. In males, acne and oily skin may also occur but are typically less severe. These effects stem directly from androgen receptor activation in skin and hair tissues.[35][49] Reproductive system disruptions are another key consequence of androgen ester use. Exogenous androgens suppress gonadotropin-releasing hormone and luteinizing hormone, leading to impaired spermatogenesis and azoospermia in males. In both sexes, aromatization of excess androgens to estrogens can induce gynecomastia, characterized by breast tissue enlargement due to estrogen receptor stimulation in mammary glands. These changes are reversible upon discontinuation but may persist with prolonged exposure.[50][51] Cardiovascular risks associated with androgenic activity include elevated hematocrit levels, resulting in polycythemia from stimulated erythropoiesis in the bone marrow. This increases blood viscosity and thrombotic potential. Additionally, androgens can contribute to hypertension through fluid retention and vascular effects; in February 2025, the U.S. Food and Drug Administration (FDA) issued class-wide labeling changes for testosterone products, adding a warning about the risk of increased blood pressure, which may further elevate cardiovascular risks.[52] Recent research as of 2025 has also associated longer-term testosterone therapy (beyond one year) with an increased risk of major adverse cardiovascular events, such as heart attack and stroke, particularly in men aged 51 years and older.[53] Furthermore, androgens can contribute to dyslipidemia by altering lipid metabolism, often raising low-density lipoprotein cholesterol while lowering high-density lipoprotein. These effects heighten overall cardiovascular strain, particularly at higher doses.[54][55] Hepatic effects from androgen esters are generally milder compared to oral 17α-alkylated androgens, as esters undergo first-pass metabolism avoidance via injection. Mild elevations in transaminase levels may occur but are typically transient and less pronounced than with oral forms. Rare but serious complications include peliosis hepatis, a vascular lesion involving blood-filled hepatic cysts, which can lead to rupture if untreated.[56] Psychological impacts involve alterations in mood and behavior mediated by androgen influences on the central nervous system. Users may experience mood swings, increased irritability, and heightened aggression, though the popularized notion of "roid rage"—implying uncontrollable violent outbursts—is a non-scientific exaggeration and not consistently supported by evidence. Instead, symptoms more commonly include anxiety or depressive episodes, which resolve post-cessation. These risks escalate with supraphysiological dosing.[49][57] In clinical settings, these androgenic effects can be mitigated through regular monitoring of hormone levels, hematocrit, lipids, and liver function to maintain therapeutic dosing and promptly address deviations.[56]Ester-specific considerations
Injection site reactions are a common adverse effect associated with intramuscular administration of androgen esters, particularly due to the oil-based vehicles used for solubility. For instance, testosterone cypionate, formulated in cottonseed oil, frequently causes local pain, inflammation, erythema, and abscess formation at the injection site, with reported incidences of injection site erythema up to 26% and general reactions around 4%. These reactions arise from the irritant properties of the oil depot and the mechanical trauma of injection, potentially leading to sterile abscesses if not managed properly. Allergic responses to excipients in androgen ester formulations can manifest as hypersensitivity reactions, including skin rashes, urticaria, or anaphylactoid events. Benzyl alcohol, a preservative commonly used in short-acting esters like testosterone propionate (typically in sesame oil with benzyl alcohol or benzoate), has been linked to such allergies, though severe cases are rare in adults; however, it poses risks in neonates due to potential toxicity. These excipient-related issues highlight the need for patch testing or alternative formulations in susceptible individuals. Short-acting androgen esters, such as testosterone propionate, produce pronounced peaks and troughs in serum testosterone levels following administration, leading to fluctuations in mood, energy, and overall well-being. This "roller coaster" effect can exacerbate emotional instability or fatigue during trough periods, contrasting with more stable profiles from longer-acting esters. Patients often report mood swings and variable energy levels tied to these pharmacokinetic oscillations. Long-term use of depot androgen esters via frequent intramuscular injections may rarely result in fibrosis or scar tissue formation at injection sites, potentially complicating future administrations or causing chronic pain. Such issues stem from repeated tissue trauma and oil accumulation, though they occur infrequently with proper rotation of injection sites. Oral androgen esters, like testosterone undecanoate capsules, carry risks of gastrointestinal upset, including nausea, diarrhea, and burping, which are typically mild and transient but can affect compliance. Absorption of these formulations is highly variable, relying on lymphatic uptake and requiring co-administration with fatty meals to optimize bioavailability; inconsistent intake can lead to erratic serum levels and suboptimal therapeutic effects.Examples
Testosterone esters
Testosterone esters are synthetic derivatives of testosterone in which the 17β-hydroxyl group is esterified with a carboxylic acid to prolong the duration of action by slowing release from intramuscular or subcutaneous depots. These modifications allow for less frequent dosing compared to unmodified testosterone, which has a very short half-life of about 10 minutes following injection.[22] The most common testosterone esters used clinically include propionate, enanthate, cypionate, and undecanoate, each varying in ester chain length, pharmacokinetics, and administration regimens. These esters are primarily employed in androgen replacement therapy for hypogonadism to restore physiological testosterone levels.[12] Testosterone propionate, the first testosterone ester introduced for clinical use in 1937, features a short propionic acid ester chain (three carbons), resulting in a brief elimination half-life of approximately 2 days.[24] Due to its rapid clearance, it requires frequent dosing, typically 25-50 mg administered intramuscularly every other day, to maintain stable serum levels.[58] Testosterone enanthate, with a seven-carbon enanthic acid ester chain, has an intermediate elimination half-life of 7-9 days, enabling biweekly administration.[59] Common dosing for testosterone replacement therapy is 200-250 mg intramuscularly every two weeks, making it one of the most widely adopted formulations due to its balance of efficacy and convenience.[60][58] Testosterone cypionate, featuring an eight-carbon cyclopentylpropionic acid ester chain, exhibits pharmacokinetics similar to enanthate, with an elimination half-life of about 8 days.[61] It is typically dosed at 200 mg intramuscularly every two weeks and is particularly preferred in the United States for its enhanced chemical stability in oil-based formulations.[62][58] Testosterone undecanoate, esterified with an 11-carbon undecanoic acid chain, provides the longest duration among these esters, with an elimination half-life of approximately 34 days when formulated in castor oil.[19] This extended release supports infrequent dosing, such as 1000 mg intramuscularly every 10 weeks (as in the Nebido formulation) or 40-80 mg two to three times daily with meals (total 80-240 mg/day) in lipid vehicles.[38][63] The castor oil vehicle allows for a reduced injection volume of 4 mL per 1000 mg dose compared to earlier formulations, improving patient tolerability.[64]| Ester | Ester Chain Length (Carbons) | Elimination Half-Life (Days) | Typical Dose | Administration Route |
|---|---|---|---|---|
| Testosterone propionate | 3 | ~2 | 25-50 mg every other day | Intramuscular |
| Testosterone enanthate | 7 | 7-9 | 200-250 mg biweekly | Intramuscular |
| Testosterone cypionate | 8 | ~8 | 200 mg biweekly | Intramuscular |
| Testosterone undecanoate | 11 | ~34 | 1000 mg every 10 weeks (IM); 40-80 mg two to three times daily (total 80-240 mg/day) (oral) | Intramuscular or oral |
Other androgen esters
Other androgen esters encompass a range of compounds derived from non-testosterone androgens, often featuring modifications to enhance anabolic properties relative to androgenic effects. These esters are typically used in medical or veterinary contexts for conditions involving tissue wasting or growth promotion, though many have limited or historical human applications due to side effect profiles and regulatory status.[65] Nandrolone decanoate, marketed as Deca-Durabolin, is a 19-nortestosterone derivative with a terminal half-life of 7 to 12 days following intramuscular injection, allowing for dosing intervals of 200 to 400 mg monthly. It is indicated for treating anemia associated with chronic renal failure and muscle wasting in conditions like HIV/AIDS or dialysis-related sarcopenia, where it promotes erythropoiesis and lean body mass gains.[66][65][67] Nandrolone phenylpropionate, a shorter-acting ester of the same parent androgen, exhibits a half-life of approximately 2.7 days following intramuscular injection, necessitating more frequent dosing such as every other day. It shares similar indications for anemia and cachexia but is less commonly used today due to the availability of longer-acting alternatives like nandrolone decanoate.[68] Drostanolone propionate, derived from dihydrotestosterone (DHT) via 2α-methylation, has a half-life of about 2 days and was historically employed in the palliative treatment of advanced breast cancer in postmenopausal women, leveraging its anti-estrogenic properties to inhibit tumor growth. Its use has declined with the advent of safer hormonal therapies.[69][70] Boldenone undecylenate, known as Equipoise, is primarily a veterinary anabolic agent with a half-life of 14 days, administered to horses for improving appetite and muscle condition. Human use is rare and not approved, occurring occasionally off-label despite its structural similarity to testosterone esters.[71][72] Trenbolone esters, including the acetate (half-life ~1 to 3 days) and enanthate (half-life ~7 to 10 days) forms, are highly potent 19-nortestosterone derivatives used mainly as veterinary growth promoters in livestock to enhance feed efficiency and muscle accretion. Medical applications in humans are limited, with most documented use in non-therapeutic contexts due to its exceptional anabolic potency.[73][74]| Parent Androgen | Ester Type | Primary Use | Status |
|---|---|---|---|
| Nandrolone | Decanoate | Anemia, muscle wasting | Medical (human) |
| [Nandrolone | Phenylpropionate](/page/Nandrolone_phenylpropionate) | Anemia, cachexia | Medical (human, less common) |
| [Drostanolone | Propionate](/page/Drostanolone_propionate) | Breast cancer palliation | Medical (historical) |
| [Boldenone | Undecylenate](/page/Boldenone_undecylenate) | Appetite/muscle enhancement | Veterinary |
| [Trenbolone | Acetate/Enanthate](/page/Trenbolone) | Growth promotion | Veterinary |