Recent from talks
Nothing was collected or created yet.
Propionic acid
View on Wikipedia
| |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propanoic acid | |||
| Other names
Carboxyethane
Ethanecarboxylic acid Ethylformic acid Metacetonic acid Methylacetic acid C3:0 (Lipid numbers) | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.001.070 | ||
| EC Number |
| ||
| E number | E280 (preservatives) | ||
| |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C3H6O2 | |||
| Molar mass | 74.079 g·mol−1 | ||
| Appearance | Colorless, oily liquid[1] | ||
| Odor | Pungent, rancid, unpleasant[1] | ||
| Density | 0.98797 g/cm3[2] | ||
| Melting point | −20.5 °C (−4.9 °F; 252.7 K)[8] | ||
| Boiling point | 141.15 °C (286.07 °F; 414.30 K)[8] | ||
| Sublimes at −48 °C ΔsublH | |||
| 8.19 g/g (−28.3 °C) 34.97 g/g (−23.9 °C) Miscible (≥ −19.3 °C)[4] | |||
| Solubility | Miscible in EtOH, ether, CHCl 3[5] | ||
| log P | 0.33[6] | ||
| Vapor pressure | 0.32 kPa (20 °C)[7] 0.47 kPa (25 °C)[6] 9.62 kPa (100 °C)[3] | ||
Henry's law
constant (kH) |
4.45·10−4 L·atm/mol[6] | ||
| Acidity (pKa) | 4.88[6] | ||
| −43.50·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3843[2] | ||
| Viscosity | 1.175 cP (15 °C)[2] 1.02 cP (25 °C) 0.668 cP (60 °C) 0.495 cP (90 °C)[6] | ||
| Structure | |||
| Monoclinic (−95 °C)[9] | |||
| P21/c[9] | |||
a = 4.04 Å, b = 9.06 Å, c = 11 Å[9] α = 90°, β = 91.25°, γ = 90°
| |||
| 0.63 D (22 °C)[2] | |||
| Thermochemistry | |||
Heat capacity (C)
|
152.8 J/mol·K[5][3] | ||
Std molar
entropy (S⦵298) |
191 J/mol·K[3] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−510.8 kJ/mol[3] | ||
Std enthalpy of
combustion (ΔcH⦵298) |
1527.3 kJ/mol[2][3] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive | ||
| GHS labelling:[7] | |||
  
| |||
| Danger | |||
| H314[7] | |||
| P280, P305+P351+P338, P310[7] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 54 °C (129 °F; 327 K)[7] | ||
| 512 °C (954 °F; 785 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1370 mg/kg (mouse, oral)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 10 ppm (30 mg/m3) ST 15 ppm (45 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Related compounds | |||
Related Carboxylic acids
|
Acetic acid Lactic acid 3-Hydroxypropionic acid Tartronic acid Acrylic acid Butyric acid | ||
Related compounds
|
1-Propanol Propionaldehyde Sodium propionate Propionic anhydride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
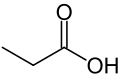
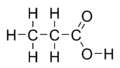
Propionic acid (/proʊpiˈɒnɪk/, from the Greek words πρῶτος : prōtos, meaning "first", and πίων : píōn, meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH
3CH
2CO
2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH
3CH
2CO−
2 as well as the salts and esters of propionic acid are known as propionates or propanoates.
About half of the world production of propionic acid is consumed as a preservative for both animal feed and food for human consumption. It is also useful as an intermediate in the production of other chemicals, especially polymers.
History
[edit]Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar.[10] Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning first, and πίων (piōn), meaning fat, because it is the smallest H(CH
2)
nCOOH acid that exhibits the properties of the other fatty acids, such as producing an oily layer when salted out of water and having a soapy potassium salt.[11]
Properties
[edit]Propionic acid has physical properties intermediate between those of the smaller carboxylic acids, formic and acetic acids, and the larger fatty acids. It is miscible with water, but can be removed from water by adding salt. As with acetic and formic acids, it consists of hydrogen bonded pairs of molecules in both the liquid and the vapor.
Propionic acid displays the general properties of carboxylic acids: it can form amide, ester, anhydride, and chloride derivatives. It undergoes the Hell–Volhard–Zelinsky reaction that involves α-halogenation of a carboxylic acid with bromine, catalysed by phosphorus tribromide, in this case to form 2-bromopropanoic acid, CH
3CHBrCOOH.[12] This product has been used to prepare a racemic mixture of alanine by ammonolysis.[13][14]
Manufacture
[edit]Chemical
[edit]In industry, propionic acid is mainly produced by the hydrocarboxylation of ethylene using nickel carbonyl as the catalyst:[15]
It is also produced by the aerobic oxidation of propionaldehyde. In the presence of cobalt or manganese salts (manganese propionate is most commonly used), this reaction proceeds rapidly at temperatures as mild as 40–50 °C:
Large amounts of propionic acid were once produced as a byproduct of acetic acid manufacture. At the current time, the world's largest producer of propionic acid is BASF, with approximately 150 kt/a production capacity.
Biotechnological
[edit]Biotechnological production of propionic acid mainly uses Propionibacterium strains.[16] However, large scale production of propionic acid by Propionibacteria faces challenges such as severe inhibition of end-products during cell growth and the formation of by-products (acetic acid and succinic acid).[17] One approach to improve productivity and yield during fermentation is through the use of cell immobilization techniques, which also promotes easy recovery, reuse of the cell biomass and enhances microorganisms' stress tolerance.[18] In 2018, 3D printing technology was used for the first time to create a matrix for cell immobilization in fermentation. Propionic acid production by Propionibacterium acidipropionici immobilized on 3D-printed nylon beads was chosen as a model study. It was shown that those 3D-printed beads were able to promote high density cell attachment and propionic acid production, which could be adapted to other fermentation bioprocesses.[19] Other cell immobilization matrices have been tested, such as recycled-glass Poraver and fibrous-bed bioreactor.[20][21]
Alternative methods of production have been trialled, by genetically engineering strains of Escherichia coli to incorporate the necessary pathway, the Wood-Werkman cycle.[22]
Industrial uses
[edit]Propionic acid inhibits the growth of mold and some bacteria at levels between 0.1 and 1% by weight. As a result, some propionic acid produced is consumed as a preservative for both animal feed and food for human consumption. For animal feed, it is used either directly or as its ammonium salt. This application accounts for about half of the world production of propionic acid. The antibiotic monensin is added to cattle feed to favor propionibacteria over acetic acid producers in the rumen; this produces less carbon dioxide and feed conversion is better. Another major application is as a preservative in baked goods, which use the sodium and calcium salts.[15] As a food additive, it is approved for use in the EU,[23] US,[24] Australia and New Zealand.[25]
Propionic acid is also useful as an intermediate in the production of other chemicals, especially polymers. Cellulose-acetate-propionate is a useful thermoplastic. Vinyl propionate is also used. In more specialized applications, it is also used to make pesticides and pharmaceuticals. The esters of propionic acid have fruit-like odors and are sometimes used as solvents or artificial flavorings.[15]
In biogas plants, propionic acid is a common intermediate product, which is formed by fermentation with propionic acid bacteria. Its degradation in anaerobic environments (e.g. biogas plants) requires the activity of complex microbial communities.[26]
In production of the Jarlsberg cheese, a propionic acid bacterium is used to give both taste and holes.[27]
Biology
[edit]Propionic acid is produced biologically as its coenzyme A ester, propionyl-CoA, from the metabolic breakdown of fatty acids containing odd numbers of carbon atoms, and also from the breakdown of some amino acids. Bacteria of the genus Propionibacterium produce propionic acid as the end-product of their anaerobic metabolism. This class of bacteria is commonly found in the stomachs of ruminants and the sweat glands of humans, and their activity is partially responsible for the odor of Emmental cheese, American "Swiss cheese" and sweat.
The metabolism of propionic acid begins with its conversion to propionyl coenzyme A, the usual first step in the metabolism of carboxylic acids. Since propionic acid has three carbons, propionyl-CoA cannot directly enter either beta oxidation or the citric acid cycles. In most vertebrates, propionyl-CoA is carboxylated to D-methylmalonyl-CoA, which is isomerised to L-methylmalonyl-CoA. A vitamin B12-dependent enzyme catalyzes rearrangement of L-methylmalonyl-CoA to succinyl-CoA, which is an intermediate of the citric acid cycle and can be readily incorporated there.[28]
Propionic acid serves as a substrate for hepatic gluconeogenesis via conversion to succinyl-CoA.[29][30] Additionally, exogenous propionic acid administration results in more endogenous glucose production than can be accounted for by gluconeogenic conversion alone.[31] Exogenous propionic acid may upregulate endogenous glucose production via increases in norepinephrine and glucagon, suggesting that chronic ingestion of propionic acid may have adverse metabolic consequences.[32]
In propionic acidemia, a rare inherited genetic disorder, propionate acts as a metabolic toxin in liver cells by accumulating in mitochondria as propionyl-CoA and its derivative, methylcitrate, two tricarboxylic acid cycle inhibitors. Propanoate is metabolized oxidatively by glia, which suggests astrocytic vulnerability in propionic acidemia when intramitochondrial propionyl-CoA may accumulate. Propionic acidemia may alter both neuronal and glial gene expression by affecting histone acetylation.[33][34] When propionic acid is infused directly into rodents' brains, it produces reversible behavior (e.g., hyperactivity, dystonia, social impairment, perseveration) and brain changes (e.g., innate neuroinflammation, glutathione depletion) that may be used as a means to model autism in rats.[33]
Human occurrence
[edit]The human skin is host of several species of Propionibacteria. The most notable one is the Cutibacterium acnes (formerly known as Propionibacterium acnes), which lives mainly in the sebaceous glands of the skin and is one of the principal causes of acne.[35] Propionate is observed to be among the most common short-chain fatty acids produced in the large intestine of humans by gut microbiota in response to indigestible carbohydrates (dietary fiber) in the diet.[36][37] The role of the gut microbiota and their metabolites, including propionate, in mediating brain function has been reviewed.[38]
A study in mice suggests that propionate is produced by the bacteria of the genus Bacteroides in the gut, and that it offers some protection against Salmonella there.[39] Another study finds that fatty acid propionate can calm the immune cells that drive up blood pressure, thereby protecting the body from damaging effects of high blood pressure.[40]
Bacteriology
[edit]The Bacteria species Coprothermobacter platensis produces propionate when fermenting gelatin.[41] Prevotella brevis and Prevotella ruminicola also generate propionate when fermenting glucose.[42]
Propionate salts and esters
[edit]The propionate /ˈproʊpiəneɪt/, or propanoate, ion is C
2H
5COO−
, the conjugate base of propionic acid. It is the form found in biological systems at physiological pH. A propionic, or propanoic, compound is a carboxylate salt or ester of propionic acid. In these compounds, propionate is often written in shorthand, as CH
3CH
2CO
2 or simply EtCO
2.
Propionates should not be confused with propenoates (commonly known as acrylates), the ions/salts/esters of propenoic acid (also known as 2-propenoic acid or acrylic acid).
Examples
[edit]Salts
[edit]- Sodium propionate NaC
2H
5CO
2 - Potassium propionate KC
2H
5CO
2 - Calcium propionate Ca(C
2H
5CO
2)
2 - Zirconium propionate Zr(C
2H
5CO
2)
4
Esters
[edit]- Methyl propionate C
2H
5(CO)OCH
3 - Ethyl propionate C
2H
5(CO)OC
2H
5 - Propyl propionate C
2H
5(CO)OC
3H
7 - Pentyl propionate C
2H
5(CO)OC
5H
11 - Fluticasone propionate C
25H
31F
3O
5S
See also
[edit]References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0529". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d e Lagowski, J.J., ed. (2012). The Chemistry of Nonaqueous Solvents. Vol. III. Elsevier. p. 362. ISBN 978-0-323-15103-0.
- ^ a b c d e f Propanoic acid in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 13 June 2014)
- ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). D. Van Nostrand Company. p. 569.
- ^ a b c "chemister.ru (archived copy)". Archived from the original on 9 October 2016. Retrieved 13 June 2014.
- ^ a b c d e CID 1032 from PubChem
- ^ a b c d e Sigma-Aldrich Co., Propionic acid. Retrieved on 13 June 2014.
- ^ a b Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b c Strieter, F. J.; Templeton, D. H.; Scheuerman, R. F.; Sass, R. L. (1962). "The crystal structure of propionic acid". Acta Crystallographica. 15 (12): 1233–1239. Bibcode:1962AcCry..15.1233S. doi:10.1107/S0365110X62003278.
- ^ Johann Gottlieb (1844) "Ueber die Einwirkung von schmelzendem Kalihydrat auf Rohrzucker, Gummi, Stärkmehl und Mannit" (On the effect of molten potassium hydroxide on raw sugar, rubber, starch powder, and mannitol), Annalen der Chemie und Pharmacie, 52 : 121–130. After combining raw sugar with an excess of potassium hydroxide and distilling the result, Gottlieb obtained a product that he called "Metacetonsäure" (meta-acetone acid) on p. 122: "Das Destillat ist stark sauer und enthält Ameisensäure, Essigsäure und eine neue Säure, welche ich, aus unten anzuführenden Gründen, Metacetonsäure nenne." (The distillate is strongly acidic and contains formic acid, acetic acid, and a new acid, which for reasons to be presented below I call "meta-acetone acid".)
- ^ Dumas, Malaguti, and F. Leblanc (1847) "Sur l'identité des acides métacétonique et butyro-acétique" [On the identity of metacetonic acid and butyro-acetic acid], Comptes rendus, 25 : 781–784. Propionic acid is named on p. 783: "Ces caractères nous ont conduits à désigner cet acide sous le nom d'acide propionique, nom qui rappelle sa place dans la séries des acides gras: il en est le premier." (These characteristics led us to designate this acid by the name of propionic acid, a name that recalls its place in the series of fatty acids: it is the first of them.)
- ^ Marvel, C. S.; du Vigneaud, V. (1931). "α-Bromoisovaleric acid". Organic Syntheses. 11: 20. doi:10.15227/orgsyn.011.0020; Collected Volumes, vol. 2, p. 93.
- ^ Tobie, Walter C.; Ayres, Gilbert B. (1937). "Synthesis of d,l-Alanine in Improved Yield from α-Bromopropionic Acid and Aqueous Ammonia". Journal of the American Chemical Society. 59 (5): 950. Bibcode:1937JAChS..59..950T. doi:10.1021/ja01284a510.
- ^ Tobie, Walter C.; Ayres, Gilbert B. (1941). "dl-Alanine". Organic Syntheses. doi:10.15227/orgsyn.009.0004; Collected Volumes, vol. 1, p. 21.
- ^ a b c Samel, Ulf-Rainer; Kohler, Walter; Gamer, Armin Otto; Keuser, Ullrich; Yang, Shang-Tian; Jin, Ying; Lin, Meng; Wang, Zhongqiang; Teles, Joaquim Henrique (2018). "Propionic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_223.pub4. ISBN 978-3-527-30673-2.
- ^ Piwowarek, Kamil; Lipińska, Edyta; Hać-Szymańczuk, Elżbieta; Kieliszek, Marek; Ścibisz, Iwona (2018). "Propionibacterium SPP.—source of propionic acid, vitamin B12, and other metabolites important for the industry". Applied Microbiology and Biotechnology. 102 (2): 515–538. doi:10.1007/s00253-017-8616-7. PMC 5756557. PMID 29167919. S2CID 23599974.
- ^ Liu, Long; Zhu, Yunfeng; Li, Jianghua; Wang, Miao; Lee, Pengsoon; Du, Guocheng; Chen, Jian (2012). "Microbial production of propionic acid from propionibacteria: Current state, challenges and perspectives". Critical Reviews in Biotechnology. 32 (4): 374–381. doi:10.3109/07388551.2011.651428. PMID 22299651. S2CID 25823025.
- ^ Alonso, Saúl; Rendueles, Manuel; Díaz, Mario (2015). "A novel approach to monitor stress-induced physiological responses in immobilized microorganisms". Applied Microbiology and Biotechnology. 99 (8): 3573–3583. doi:10.1007/s00253-015-6517-1. hdl:10651/31795. PMID 25776062. S2CID 860853.
- ^ Belgrano, Fabricio dos Santos; Diegel, Olaf; Pereira, Nei; Hatti-Kaul, Rajni (2018). "Cell immobilization on 3D-printed matrices: A model study on propionic acid fermentation". Bioresource Technology. 249: 777–782. Bibcode:2018BiTec.249..777B. doi:10.1016/j.biortech.2017.10.087. PMID 29136932.
- ^ Dishisha, Tarek; Alvarez, Maria Teresa; Hatti-Kaul, Rajni (2012). "Batch- and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici" (PDF). Bioresource Technology. 118: 553–562. Bibcode:2012BiTec.118..553D. doi:10.1016/j.biortech.2012.05.079. PMID 22728152. S2CID 29658955.
- ^ Suwannakham, Supaporn; Yang, Shang-Tian (2005). "Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor". Biotechnology and Bioengineering. 91 (3): 325–337. Bibcode:2005BiotB..91..325S. doi:10.1002/bit.20473. PMID 15977254.
- ^ Gonzalez-Garcia, Ricardo A.; McCubbin, Timothy; Turner, Mark S.; Nielsen, Lars K.; Marcellin, Esteban (2020). "Engineering Escherichia coli for propionic acid production through the Wood–Werkman cycle". Biotechnology and Bioengineering. 117 (1): 167–183. Bibcode:2020BiotB.117..167G. doi:10.1002/bit.27182. PMID 31556457. S2CID 203438727.
- ^ "Current EU approved additives and their E Numbers". UK Food Standards Agency. Retrieved 27 October 2011.
- ^ "Listing of Food Additives Status Part II". US Food and Drug Administration. Archived from the original on 8 January 2010. Retrieved 27 October 2011.
- ^ "Standard 1.2.4 – Labelling of ingredients". Australia New Zealand Food Standards Code. Comlaw.au. 8 September 2011. Retrieved 27 October 2011.
- ^ Ahlert, Stephan; Zimmermann, Rita; Ebling, Johannes; König, Helmut (2016). "Analysis of propionate-degrading consortia from agricultural biogas plants". MicrobiologyOpen. 5 (6): 1027–1037. doi:10.1002/mbo3.386. PMC 5221444. PMID 27364538.
- ^ www.jarlsberg.com quote: " In the production of Jarlsberg®, propionic acid bacteria (the Secret Recipe!) is used to give the cheese its characteristic taste and holes."
- ^ Lehninger, Albert L. (2005). Lehninger principles of biochemistry. Nelson, David L. (David Lee), 1942-, Cox, Michael M. (Fourth ed.). New York: W.H. Freeman. ISBN 0-7167-4339-6. OCLC 55476414.
- ^ Aschenbach, JR; Kristensen, NB; Donkin, SS; Hammon, HM; Penner, GB (December 2010). "Gluconeogenesis in dairy cows: the secret of making sweet milk from sour dough". IUBMB Life. 62 (12): 869–77. doi:10.1002/iub.400. PMID 21171012. S2CID 21117076.
- ^ Perry, Rachel J.; Borders, Candace B.; Cline, Gary W.; Zhang, Xian-Man; Alves, Tiago C.; Petersen, Kitt Falk; Rothman, Douglas L.; Kibbey, Richard G.; Shulman, Gerald I. (21 March 2016). "Propionate Increases Hepatic Pyruvate Cycling and Anaplerosis and Alters Mitochondrial Metabolism". Journal of Biological Chemistry. 291 (23): 12161–12170. doi:10.1074/jbc.m116.720631. ISSN 0021-9258. PMC 4933266. PMID 27002151.
- ^ Ringer, A. I. (2 August 1912). "The Quantitative Conversion of Propionic Acid into Glucose". Journal of Biological Chemistry. 12: 511–515. doi:10.1016/S0021-9258(18)88686-0.
- ^ Tirosh, Amir; Calay, Ediz S.; Tuncman, Gurol; Claiborn, Kathryn C.; Inouye, Karen E.; Eguchi, Kosei; Alcala, Michael; Rathaus, Moran; Hollander, Kenneth S.; Ron, Idit; Livne, Rinat (24 April 2019). "The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans". Science Translational Medicine. 11 (489) eaav0120. doi:10.1126/scitranslmed.aav0120. ISSN 1946-6234. PMID 31019023.
- ^ a b D. F. MacFabe; D. P. Cain; K. Rodriguez-Capote; A. E. Franklin; J. E. Hoffman; F. Boon; A. R. Taylor; M. Kavaliers; K.-P. Ossenkopp (2007). "Neurobiological effects of intraventricular propionic acid in rats: Possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders". Behavioural Brain Research. 176 (1): 149–169. doi:10.1016/j.bbr.2006.07.025. PMID 16950524. S2CID 3054752.
- ^ N. H. T. Nguyen; C. Morland; S. Villa Gonzalez; F. Rise; J. Storm-Mathisen; V. Gundersen; B. Hassel (2007). "Propionate increases neuronal histone acetylation, but is metabolized oxidatively by glia. Relevance for propionic acidemia". Journal of Neurochemistry. 101 (3): 806–814. doi:10.1111/j.1471-4159.2006.04397.x. PMID 17286595. S2CID 514557.
- ^ Bojar, Richard A.; Holland, Keith T. (2004). "Acne and propionibacterium acnes". Clinics in Dermatology. 22 (5): 375–379. doi:10.1016/j.clindermatol.2004.03.005. PMID 15556721.
- ^ Cani, Patrice D.; Knauf, Claude (27 May 2016). "How gut microbes talk to organs: The role of endocrine and nervous routes". Molecular Metabolism. 5 (9): 743–752. doi:10.1016/j.molmet.2016.05.011. PMC 5004142. PMID 27617197.
- ^ den Besten, G; van Eunen, K; Groen, AK; Venema, K; Reijngoud, DJ; Bakker, BM (September 2013). "The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism". Journal of Lipid Research. 54 (9): 2325–40. doi:10.1194/jlr.R036012. PMC 3735932. PMID 23821742.
- ^ Rogers, G. B.; Keating, D. J.; Young, R. L.; Wong, M-L; Licinio, J.; Wesselingh, S. (2016). "From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways". Molecular Psychiatry. 21 (6): 738–748. doi:10.1038/mp.2016.50. PMC 4879184. PMID 27090305. S2CID 18589882.
- ^ Jacobson, Amanda; Lam, Lilian; Rajendram, Manohary; Tamburini, Fiona; Honeycutt, Jared; Pham, Trung; Van Treuren, Will; Pruss, Kali; Stabler, Stephen Russell; Lugo, Kyler; Bouley, Donna M.; Vilches-Moure, Jose G.; Smith, Mark; Sonnenburg, Justin L.; Bhatt, Ami S.; Huang, Kerwyn Casey; Monack, Denise (2018). "A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection". Cell Host & Microbe. 24 (2): 296–307.e7. doi:10.1016/j.chom.2018.07.002. PMC 6223613. PMID 30057174.
- ^ Bartolomaeus, Hendrik; Balogh, András; Yakoub, Mina; Homann, Susanne; Markó, Lajos; Höges, Sascha; Tsvetkov, Dmitry; Krannich, Alexander; Wundersitz, Sebastian; Avery, Ellen G.; Haase, Nadine; Kräker, Kristin; Hering, Lydia; Maase, Martina; Kusche-Vihrog, Kristina; Grandoch, Maria; Fielitz, Jens; Kempa, Stefan; Gollasch, Maik; Zhumadilov, Zhaxybay; Kozhakhmetov, Samat; Kushugulova, Almagul; Eckardt, Kai-Uwe; Dechend, Ralf; Rump, Lars Christian; Forslund, Sofia K.; Müller, Dominik N.; Stegbauer, Johannes; Wilck, Nicola (2019). "Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage". Circulation. 139 (11): 1407–1421. doi:10.1161/CIRCULATIONAHA.118.036652. PMC 6416008. PMID 30586752.
- ^ Etchebehere, C.; Pavan, M. E.; Zorzópulos, J.; Soubes, M.; Muxí, L. (October 1998). "Coprothermobacter platensis sp. nov., a new anaerobic proteolytic thermophilic bacterium isolated from an anaerobic mesophilic sludge". International Journal of Systematic Bacteriology. 48 Pt 4 (4): 1297–1304. doi:10.1099/00207713-48-4-1297. ISSN 0020-7713. PMID 9828430.
- ^ Zhang, Bo; Lingga, Christopher; De Groot, Hannah; Hackmann, Timothy J (30 September 2023). "The oxidoreductase activity of Rnf balances redox cofactors during fermentation of glucose to propionate in Prevotella". Research Gate. Retrieved 26 September 2024.