Recent from talks
Nothing was collected or created yet.
Weaver ant
View on Wikipedia
| Weaver ant Temporal range: Ypresian – Recent
| |
|---|---|

| |
| Weaver ant (Oecophylla smaragdina) major worker (India). | |

| |
| Weaver ant (Oecophylla longinoda) major worker (Tanzania) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hymenoptera |
| Family: | Formicidae |
| Subfamily: | Formicinae |
| Tribe: | Oecophyllini Emery, 1895 |
| Genus: | Oecophylla Smith, 1860 |
| Type species | |
| Formica virescens (junior synonym of Oecophylla smaragdina) | |
| Diversity[1] | |
| 3 extant species 15 extinct species | |
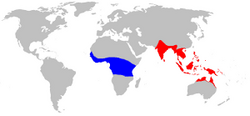
| |
| Oecophylla range map. Oecophylla longinoda in blue, Oecophylla smaragdina in red.[2] | |
Weaver ants or green ants are eusocial insects of the ant family (Formicidae) belonging to the tribe Oecophyllini. Weaver ants live in trees (they are obligately arboreal) and are known for their unique nest building behaviour where workers construct nests by weaving together leaves using larval silk.[3] Colonies can be extremely large consisting of more than a hundred nests spanning numerous trees and containing more than half a million workers. Like many other ant species, weaver ants prey on small insects and supplement their diet with carbohydrate-rich honeydew excreted by scale insects (Hemiptera). Weaver ant workers exhibit a clear bimodal size distribution, with almost no overlap between the size of the minor and major workers.[4][5] The major workers are approximately 8–10 mm (0.31–0.39 in) in length and the minors approximately half the length of the majors. Major workers forage, defend, maintain, and expand the colony whereas minor workers tend to stay within the nests where they care for the brood and 'milk' scale insects in or close to the nests.

Weaver ants vary in color from reddish to yellowish brown dependent on the species. Oecophylla smaragdina found in Australia often have bright green gasters. Weaver ants are highly territorial and workers aggressively defend their territories against intruders. Because they prey on insects harmful to their host trees, weaver ants are sometimes used by indigenous farmers, particularly in southeast Asia, as natural biocontrol agents against agricultural pests. Although weaver ants lack a functional sting they can inflict painful bites and often spray formic acid[6][7] directly at the bite wound resulting in intense discomfort.
Researchers report Weaver ants display remarkable teamwork, increasing individual effort as group size grows—unlike human teams. They build complex leaf nests using a “force ratchet” system, where some ants pull while others anchor, boosting efficiency. This coordinated labor offers insights for robotics, suggesting that mimicking ant strategies could enhance multi-agent cooperation and improve autonomous systems. Their behavior challenges long-held assumptions about group dynamics and productivity.[8]
Taxonomy
[edit]
Oecophylla (subfamily Formicinae) is one group of weaver ants containing two closely related living species: O. longinoda and O. smaragdina.[1] They are placed in a tribe of their own, Oecophyllini with the extinct genus Eoecophylla. The weaver ant genus Oecophylla is relatively old, and 15 fossil species have been described from Eocene to Miocene deposits.[2][9] The oldest members of both Oecophyllini and Oecophylla are fossils described from the mid-Ypresian Eocene Okanagan Highlands of Northwestern North America.[10] Two other genera of weaving ants, Polyrhachis and Camponotus,[11][12] also use larval silk in nest construction, but the construction and architecture of their nests are simpler than those of Oecophylla.[13]

The common features of the genus include an elongated first funicular segment, presence of propodeal lobes, helcium at midheight of abdominal segment 3 and gaster capable of reflexion over the mesosoma. Males have vestigial pretarsal claws.[14]
Genera and species
[edit]Extant species:
- Oecophylla kolhapurensis Kurane et al., 2015
- Oecophylla longinoda (Latreille, 1802)
- Oecophylla smaragdina (Fabricius, 1775)
Extinct species:
- †Oecophylla atavina Cockerell, 1915
- †Oecophylla bartoniana Cockerell, 1920
- †Oecophylla brischkei Mayr, 1868
- †Oecophylla crassinoda Wheeler, 1922
- †Oecophylla eckfeldiana Dlussky, Wappler & Wedmann, 2008
- †Oecophylla grandimandibula Riou, 1999
- †Oecophylla kraussei (Dlussky & Rasnitsyn, 1999)
- †Oecophylla leakeyi Wilson & Taylor, 1964
- †Oecophylla longiceps Dlussky, Wappler & Wedmann, 2008
- †Oecophylla megarche Cockerell, 1915
- †Oecophylla obesa (Heer, 1849)
- †Oecophylla praeclara Förster, 1891
- †Oecophylla sicula Emery, 1891
- †Oecophylla superba Théobald, 1937
- †Eoecophylla Archibald, Mathewes, & Perfilieva, 2024
Description
[edit]Oecophylla have 12-segmented antennae, a feature shared with some other ant genera. The mandibles each have 10 or more teeth, and the fourth tooth from the tip is longer than the third and fifth teeth. The palps are short, with the maxillary palps being 5-segmented and the labial palps being 4-segmented. The mesonotum is constricted and (in dorsal view) narrower than the pronotum and propodeum. The node of the petiole is low and rounded.[15]
Distribution and habitat
[edit]O. longinoda is distributed in the Afrotropics and O. smaragdina from India and Sri Lanka in southern Asia, through southeastern Asia to northern Australia and Melanesia.[16] In Australia, Oecophylla smaragdina is found in the tropical coastal areas as far south as Broome in Western Australia and across the coastal tropics of the Northern Territory down to Yeppoon in Queensland.[17]
Colony ontogeny and social organization
[edit]
Weaver ant colonies are founded by one or more mated females (queens).[18] A queen lays her first clutch of eggs on a leaf and protects and feeds the larvae until they develop into mature workers. The workers then construct leaf nests and help rear new brood laid by the queen. As the number of workers increases, more nests are constructed and colony productivity and growth increase significantly. Workers perform tasks that are essential to colony survival, including foraging, nest construction, and colony defense. The exchange of information and modulation of worker behaviour that occur during worker-worker interactions are facilitated by the use of chemical and tactile communication signals. These signals are used primarily in the contexts of foraging and colony defense. Successful foragers lay down pheromone trails that help recruit other workers to new food sources. Pheromone trails are also used by patrollers to recruit workers against territorial intruders. Along with chemical signals, workers also use tactile communication signals such as attenation and body shaking to stimulate activity in signal recipients. Multimodal communication in Oecophylla weaver ants importantly contributes to colony self-organization.[19][20] Like many other ant species, Oecophylla workers exhibit social carrying behavior as part of the recruitment process, in which one worker will carry another worker in its mandibles and transport it to a location requiring attention.[citation needed]
Nest building behaviour
[edit]
Oecophylla weaver ants are known for their cooperative behaviour used in nest construction. Possibly the first description of weaver ants' nest building behaviour was made by the English naturalist Joseph Banks, who took part in Captain James Cook's voyage to Australia in 1768. An excerpt from Joseph Banks' Journal (cited in Hölldobler and Wilson 1990) is included below:
The ants...one green as a leaf, and living upon trees, where it built a nest, in size between that of a man's head and his fist, by bending the leaves together, and gluing them with whitish paperish substances which held them firmly together. In doing this their management was most curious: they bend down four leaves broader than a man's hand, and place them in such a direction as they choose. This requires a much larger force than these animals seem capable of; many thousands indeed are employed in the joint work. I have seen as many as could stand by one another, holding down such a leaf, each drawing down with all his might, while others within were employed to fasten the glue. How they had bent it down I had not the opportunity of seeing, but it was held down by main strength, I easily proved by disturbing a part of them, on which the leaf bursting from the rest, returned to its natural situation, and I had an opportunity of trying with my finger the strength of these little animals must have used to get it down.[13]
The weaver ants' ability to build capacious nests from living leaves has undeniably contributed to their ecological success. The first phase in nest construction involves workers surveying potential nesting leaves by pulling on the edges with their mandibles. When a few ants have successfully bent a leaf onto itself or drawn its edge toward another, other workers nearby join the effort. The probability of a worker joining the concerted effort is dependent on the size of the group, with workers showing a higher probability of joining when group size is large.[21] When the span between two leaves is beyond the reach of a single ant, workers form chains with their bodies by grasping one another's petiole (waist). Multiple intricate chains working in unison are often used to ratchet together large leaves during nest construction. Once the edges of the leaves are drawn together, other workers retrieve larvae from existing nests using their mandibles. Upon reaching a seam to be joined, these workers tap the head of the clutched larvae, which causes them to excrete silk. They can only produce so much silk, so the larva will have to pupate without a cocoon. The workers then maneuver between the leaves in a highly coordinated fashion to bind them together.[13] Weaver ants' nests are usually elliptical in shape and range in size from a single small leaf folded and bound onto itself to large nests consisting of many leaves and measure over half a meter in length. The time required to construct a nest varies depending on leaf type and eventual size, but often a large nest can be built in significantly less than 24 hours. Although weaver ants' nests are strong and impermeable to water, new nests are continually being built by workers in large colonies to replace old dying nests and those damaged by storms.[22]
Relationship with humans
[edit]In agriculture
[edit]
Large colonies of Oecophylla weaver ants consume significant amounts of food, and workers continuously kill a variety of arthropods (primarily other insects) close to their nests. Insects are not only consumed by workers, but this protein source is necessary for brood development. Because weaver ant workers hunt and kill insects that are potentially harmful plant pests, trees harboring weaver ants benefit from having decreased levels of herbivory.[23] They have traditionally been used in biological control in Chinese and Southeast Asian citrus orchards from at least 400 AD.[24][25] Many studies have shown the efficacy of using weaver ants as natural biocontrol agents against agricultural pests.[26] The use of weaver ants as biocontrol agents has especially been effective for fruit agriculture, particularly in Australia and southeast Asia.[27][28] Fruit trees harboring weaver ants produce higher quality fruits, show less leaf damage by herbivores, and require fewer applications of synthetic pesticides.[28][29] They do on the other hand protect the scale insects which they 'milk' for honeydew. In several cases the use of weaver ants has nonetheless been shown to be more efficient than applying chemical insecticides and at the same time cheaper, leaving farmers with increased net incomes and more sustainable pest control.[30]
Weaver ant husbandry is often practiced in Southeast Asia, where farmers provide shelter, food and construct ropes between trees populated with weaver ants in order to protect their colonies from potential competitors.[31]
Oecophylla colonies may not be entirely beneficial to the host plants. Studies indicate that the presence of Oecophylla colonies may also have negative effects on the performance of host plants by reducing fruit removal by mammals and birds and therefore reducing seed dispersal and by lowering the flower-visiting rate of flying insects including pollinators.[32][33] Weaver ants also have an adverse effect on tree productivity by protecting sap feeding insects such as scale insects and leafhoppers from which they collect honeydew.[33][34] By protecting these insects from predators they increase their population and increase the damage they cause to trees.[35]
As food, feed and medicine
[edit]
Weaver ants are one of the most valued types of edible insects consumed by humans (human entomophagy). In addition to being used as a biological control agent to increase plant production, weaver ants can be utilized directly as a protein and food source since the ants (especially the ant larvae) are edible for humans and high in protein and fatty acids.[36] In some countries the weaver ant is a highly prized delicacy harvested in vast amounts and in this way contribute to local socio-economics.[37] In Northeastern Thailand the price of weaver ant larvae is twice the price of good-quality beef and in a single Thai province ant larvae worth US$620,000 are harvested every year.[38][39] It has furthermore been shown that the harvest of weaver ants can be maintained while at the same time using the ants for biocontrol of pest insects in tropical plantations, since the queen larvae and pupae that are the primary target of harvest, are not vital for colony survival.[40]
The larvae of weaver ants are also collected commercially as an expensive feed for insect-eating birds in Indonesia.
In India and China, the worker ants are used in traditional medicine.[3][41]
See also
[edit]- Camponotus textor, a New World weaver ant, sometimes called the Brazilian weaver ant
- Polyrhachis, other ants that weave nests (though less complex)
- Where the Green Ants Dream, a 1984 film directed by Werner Herzog
- Myrmarachne plataleoides, a spider that mimics the weaver ant
- Nanfang Caomu Zhuang, earliest Chinese record of O. smaragdina "citrus ants" protecting orange crops
References
[edit]- ^ a b Bolton, B. (2015). "Oecophylla". AntCat. Retrieved 30 January 2015.
- ^ a b Dlussky, G.M.; Wappler, T.; Wedmann, S. (2008). "New middle Eocene formicid species from Germany and the evolution of weaver ants". Acta Palaeontologica Polonica. 53 (4): 615–626. doi:10.4202/app.2008.0406.
- ^ a b Rastogi, N (2011). "Provisioning services from ants: food and pharmaceuticals" (PDF). Asian Myrmecology. 4: 103–120.
- ^ Weber, NA (1946). "Dimorphism in the African Oecophylla worker and an anomaly (Hym.: Formicidae)" (PDF). Annals of the Entomological Society of America. 39: 7–10. doi:10.1093/aesa/39.1.7.
- ^ Wilson, Edward O. & Robert W. Taylor (1964). "A fossil ant colony: new evidence of social antiquity" (PDF). Psyche: A Journal of Entomology. 71 (2): 93–103. doi:10.1155/1964/17612. Archived from the original (PDF) on 29 August 2020. Retrieved 23 July 2008.
- ^ Bradshaw, J. W. S.; Baker, R.; Howse, P. E. (1979). "Chemical composition of the poison apparatus secretions of the African weaver ant, Oecophylla longinoda, and their role in behaviour". Physiological Entomology. 4 (1): 39–46. doi:10.1111/j.1365-3032.1979.tb00175.x. S2CID 84627128.
- ^ Peerzada, N.; Pakkiyaretnam, T.; Renaud, S.; Volatile (1990). "Oecophylla smaragdina. Agric". Biol. Chem. 54 (12): 3335–3336. doi:10.1271/bbb1961.54.3335.
- ^ The surprising ant strategy that could transform robotics sciencedaily.com - August 15, 2025
- ^ Azuma, N.; Kikuchi, T.; Ogata, K.; Higashi, S. (2002). "Molecular phylogeny among local populations of weaver ant Oecophylla smaragdina". Zoological Science. 19 (11): 1321–1328. doi:10.2108/zsj.19.1321. hdl:2115/54519. PMID 12499676. S2CID 41958256.
- ^ Archibald, S. B.; Mathewes, R. W.; Perfilieva, K. S. (2024). "Fossil weaver ants (Hymenoptera, Formicidae, Oecophyllini) of the early Eocene Okanagan Highlands of far-western North America". The Canadian Entomologist. 156. e2. doi:10.4039/tce.2023.27.
- ^ Rebecca N. Johnson, Paul-Michael Agapow & Ross H. Crozier (2003). "A tree island approach to inferring phylogeny in the ant subfamily Formicinae, with especial reference to the evolution of weaving" (PDF). Molecular Phylogenetics and Evolution. 29 (2): 317–330. doi:10.1016/S1055-7903(03)00114-3. PMID 13678687. Archived from the original (PDF) on 6 April 2012.
- ^ Vicente, Ricardo Eduardo; Ferreira-Silva, Diego; Guerreiro De Lima, Mendelson (2019). "New records of three Neotropical arboreal ant species of Camponotus, subgenus Dendromyrmex (Hymenoptera: Formicidae) for the southern Amazon, including biological information". Acta Amazonica. 49: 36–40. doi:10.1590/1809-4392201801951.
- ^ a b c Hölldober, B. & Wilson, E.O. 1990. The ants. Cambridge, Massachusetts: Harvard University Press.
- ^ Bolton, B. 2003. Synopsis and Classification of Formicidae. 370 pp. Memoirs of the American Entomological Institute, Vol. 71. Gainesville, FL.
- ^ "Key to Australian Genera of Formicinae - AntWiki". antwiki.org. Retrieved 5 April 2023.
- ^ Crozier, R.H.; Newey, P.S.; E.A., Schlüns; Robson, S.K.A. (2010). "A masterpiece of evolution – Oecophylla weaver ants (Hymenoptera: Formicidae)". Myrmecological News. 13: 57–71.
- ^ Lokkers, C (1986). "The Distribution of the Weaver Ant, Oecophylla smaragdina (Fabricius) (Hymenoptera, Formicidae) in Northern Australia". Australian Journal of Zoology. 34 (5): 683–687. doi:10.1071/ZO9860683. ISSN 0004-959X.
- ^ Peng, RK; Christian, K; Gibb, K (1998). "How many queens are there in mature colonies of the green ant, Oecophylla smaragdina (Fabricius)?". Australian Journal of Entomology. 37 (3): 249–253. doi:10.1111/j.1440-6055.1998.tb01579.x.
- ^ Hölldobler, B (1999). "Multimodal signals in ant communication". J Comp Physiol A. 184 (2): 129–141. doi:10.1007/s003590050313. S2CID 20377019.
- ^ Hölldobler, B (1983). "Territorial behavior in the green tree ant (Oecophylla smaragdina)". Biotropica. 15 (4): 241–250. doi:10.2307/2387648. JSTOR 2387648.
- ^ Deneubourg, J.L.; Lioni, A.; Detrain, C. (2002). "Dynamics of aggregation and emergence of cooperation". Biological Bulletin. 202 (3): 262–267. doi:10.2307/1543477. JSTOR 1543477. PMID 12086998. S2CID 3097108.
- ^ Offenberg, J (2014). "The use of artificial nests by weaver ants: A preliminary field observation" (PDF). Asian Myrmecology. 6: 119–128.
- ^ Offenberg, J.; Havanon, S.; Aksornkoae, S.; Macintosh, D.J.; Nielsen, M.G. (2004). "Observations on the Ecology of Weaver Ants (Oecophylla smaragdina Fabricius) in a Thai Mangrove Ecosystem and Their Effect on Herbivory of Rhizophora mucronata Lam". Biotropica. 36 (3): 344–351. doi:10.1111/j.1744-7429.2004.tb00326.x. S2CID 55160841.
- ^ Chen, S. (1991). "The oldest practice of biological control: The cultural and efficacy of Oecophylla smaragdina Fabr in orange orchards". Acta Entomologica Sinica. 11: 401–407.
- ^ Barzman, M.S.; Mills, N.J.; Thu Cuc, N.G. (1996). "Traditional knowledge and rationale for weaver ant husbandry in the Mekong delta of Vietnam". Agriculture and Human Values. 13 (4): 2–9. doi:10.1007/BF01530519. S2CID 153792425.
- ^ Van Mele, P. (2008). "A historical review of research on the weaver ant Oecophylla in biological control". Agricultural and Forest Entomology. 10 (1): 13–22. doi:10.1111/j.1461-9563.2007.00350.x.
- ^ Van Mele, P.; Cuc, N. T. T.; VanHuis, A. (2002). "Direct and indirect influences of the weaver ant Oecophylla smaragdina on citrus farmers' pest perceptions and management practices in the Mekong Delta, Vietnam". International Journal of Pest Management. 48 (3): 225–232. doi:10.1080/09670870110118713. S2CID 56250492.
- ^ a b Peng, R.; Christian, K. (2007). "The effect of the weaver ant, (Hymenoptera: Formicidae), on the mango seed weevil, (Coleoptera: Curculionidae), in mango orchards in the Northern Territory of Australia". International Journal of Pest Management. 53 (1): 15–24. doi:10.1080/09670870600968859. S2CID 83559389.
- ^ Peng, R. K.; Christian, K. (2008). "The dimpling bug, Campylomma austrina Malipatil (Hemiptera: Miridae): the damage and its relationship with ants in mango orchards in the Northern Territory of Australia". International Journal of Pest Management. 54 (2): 173–179. doi:10.1080/09670870701875243. S2CID 85159932.
- ^ Offenberg, J.; Firn, J. (2015). "Ants as tools in sustainable agriculture". Journal of Applied Ecology. 52 (5): 1197–1205. doi:10.1111/1365-2664.12496.
- ^ Van Mele, P.; Vayssières, J.F. (2007). "Weaver ants help farmers to capture organic markets". Pesticides News. 75 (6): 9–11.
- ^ Thomas, Donald W. (1988). "The influence of aggressive ants on fruit removal in the tropical tree, Ficus capensis (Moraceae)". Biotropica. 20 (1): 49–53. doi:10.2307/2388425. JSTOR 2388425.
- ^ a b Tsuji, Kazuki; Ahsol Hasyim, Harlion; Koji Nakamura (2004). "Asian weaver ants, Oecophylla smaragdina, and their repelling of pollinators". Ecological Research. 19 (6): 669–673. doi:10.1111/j.1440-1703.2004.00682.x. S2CID 1039367.
- ^ Weber, Neal A. (1949). "The functional significance of dimorphism in the African ant, Oecophylla". Ecology. 30 (3): 397–400. doi:10.2307/1932624. JSTOR 1932624.
- ^ Blüthgen, N. Fiedler (2002). "Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an Australian rain forest canopy". Journal of Animal Ecology. 71 (5): 5. doi:10.1046/j.1365-2656.2002.00647.x.
- ^ Raksakantong, P; Meeso, N; Kubola, J; Siriamornpun, S (2010). "Fatty acids and proximate composition of eight Thai edible terricolous insects". Food Research International. 43 (1): 350–355. doi:10.1016/j.foodres.2009.10.014.
- ^ van Huis, Arnold; et al. (2013). Edible insects: future prospects for food and feed security (PDF). FAO Forestry Paper 171. FAO. ISBN 978-92-5-107596-8.
- ^ Sribandit, W; Wiwatwitaya, D; Suksard, S; Offenberg, J (2008). "The importance of weaver ant (Oecophylla smaragdina Fabricius) harvest to a local community in Northeastern Thailand" (PDF). Asian Myrmecology. 2: 129–138.
- ^ Offenberg, J (2011). "Oecophylla smaragdina food conversion efficiency: prospects for ant farming". Journal of Applied Entomology. 135 (8): 575–581. doi:10.1111/j.1439-0418.2010.01588.x. S2CID 83644244.
- ^ Offenberg, J; Wiwatwitaya, D (2010). "Sustainable weaver ant (Oecophylla smaragdina) farming: harvest yields and effects on worker ant density" (PDF). Asian Myrmecology. 3: 55–62.
- ^ Césard N, 2004. Harvesting and commercialisation of kroto (Oecophylla smaragdina) in the Malingpeng area, West Java, Indonesia. In: Forest products, livelihoods and conservation. Case studies of non-timber product systems (Kusters K, Belcher B, eds), Center for International Forestry Research, Bogor, 61-77
External links
[edit]- Weaver ants constructing a leaf nest on YouTube
- Weaver ant harvest in Thailand on YouTube
- Weaver Ants – National Geographic, May 2011
- AntWeb – Ants of the world
- Tree Of Life – Oecophylla
- Green tree ants
- Ants as friends. Insect Pest Management on Tree Crops with Weaver Ants by Paul Van Mele and Nguyen Thi Thu Cuc
Weaver ant
View on GrokipediaTaxonomy and evolution
Genera and species
Weaver ants are classified within the subfamily Formicinae of the ant family Formicidae, specifically in the tribe Oecophyllini, with the genus Oecophylla representing the primary taxonomic group encompassing all extant weaver ants.[5] The genus Oecophylla includes two recognized extant species: Oecophylla smaragdina (Fabricius, 1775), commonly known as the Asian green tree ant, which is distributed across tropical and subtropical regions from India through Southeast Asia, southern China, Indonesia, northern Australia, and into the Pacific islands; and O. longinoda (Latreille, 1802), the African weaver ant, which occurs throughout sub-Saharan Africa's tropical forests and savannas.[6][7][8] Phylogenetically, Oecophylla forms a sister group to the genus Gesomyrmex within Formicinae, reflecting shared arboreal adaptations among these lineages; molecular studies using mitochondrial DNA, such as the cytochrome b gene, have estimated the divergence between O. smaragdina and O. longinoda at approximately 7.5 to 15.3 million years ago during the Miocene epoch.[6] Historical taxonomy of O. smaragdina includes several synonyms, notably Oecophylla virescens (Fabricius, 1775) and Formica smaragdina (Fabricius, 1775), which were established in early descriptions but later consolidated under the current nomenclature.[9]Fossil record
The fossil record of weaver ants (genus Oecophylla) spans from the Ypresian epoch of the early Eocene, approximately 52 million years ago, to the present day, with the earliest known specimens preserved in amber deposits from the Okanagan Highlands of western North America, including recent 2024 discoveries of Oecophyllini that extend the lineage's antiquity.[10] Additional significant finds occur in Eocene Baltic amber and Miocene Dominican amber, indicating a widespread presence in ancient tropical and subtropical environments across Laurasia and Gondwana. These fossils reveal morphological features consistent with arboreal lifestyles, such as elongated legs and powerful mandibles adapted for climbing and manipulating foliage, mirroring adaptations in extant species. Over 15 extinct species of Oecophylla have been described from these deposits, including Oecophylla atavina from Eocene amber, noted for its well-preserved wing venation.[11] Another example is Oecophylla eckfeldiana from middle Eocene deposits in Germany, which exhibits body proportions and worker castes suggesting arboreal behaviors similar to modern weaver ants.[12] These extinct taxa demonstrate morphological continuity with living Oecophylla species, such as O. smaragdina and O. longinoda, but occupied more northerly latitudes during warmer Eocene climates. Paleontological evidence for advanced social organization in fossil weaver ants comes primarily from a remarkable Miocene deposit on Mfwangano Island, Kenya, where a preserved colony of Oecophylla leakeyi includes multiple castes, pupae, larvae, and worker fragments, indicating eusocial colony structure.[13] This assemblage parallels the social organization observed in contemporary species and provides direct proof of communal living in ancient lineages, though nest-weaving behaviors are inferred rather than directly preserved. The fossil record of Oecophylla underscores the deep evolutionary roots of eusociality and arboreal adaptations, originating in Eocene tropical forests and persisting through climatic shifts that restricted modern distributions. This antiquity suggests that key traits like polymorphic worker castes evolved early in the Formicinae subfamily, enabling adaptation to arboreal niches long before the Miocene radiation of angiosperm-dominated ecosystems.Physical characteristics
Body structure and castes
Weaver ants of the genus Oecophylla exhibit a polymorphic caste system typical of many advanced ant societies, with distinct morphological variations among workers, queens, and males that support their arboreal lifestyle. Workers are divided into minors and majors, reflecting size-based dimorphism that influences their physical build and capabilities. In O. smaragdina, minors measure 3-5 mm in length and possess a slender body structure optimized for tasks within the nest, such as brood care, with a reduced head width of approximately 1.10 mm and length of 1.19 mm.[14][15] Majors, in contrast, are larger at 8-10 mm long, featuring a more robust physique with an expanded head (width up to 1.60 mm and length 2.07 mm), elongated petiolar region, and narrowed thorax, adaptations suited for foraging and defense activities.[14][15] Worker sizes in O. longinoda are broadly similar, ranging 3-9 mm across castes.[16] Queens represent the reproductive caste and are the largest individuals, reaching 20-25 mm in O. smaragdina and 12-14 mm in O. longinoda, with a heavy-bodied form characterized by a well-developed thorax for flight, an expanded abdomen housing developed ovaries, and initial wings as alates during the nuptial phase.[14][17][18] Males, or drones, are smaller than queens, typically 6-7 mm, and possess wings as alates, with a lighter build focused on reproduction rather than physical labor.[17][19] Several key anatomical features distinguish weaver ants across castes and enable their canopy-dwelling existence. All castes have long, slender legs that facilitate movement and gripping on tree surfaces, as well as powerful mandibles adapted for seizing prey, manipulating leaves, and holding larvae during nest construction.[17] Notably, the larvae of final-instar workers possess specialized silk-producing glands, which adults exploit by carrying the larvae to extrude silk that binds leaves into nests, a process integral to the species' weaving behavior.[17] Sensory adaptations include large compound eyes—laterally positioned and well-developed in both minor and major workers, with majors having more ommatidia (up to 804 per eye compared to 508 in minors in O. smaragdina)—and geniculate antennae attached to the frontal sclerites, aiding in navigation, prey detection, and chemical communication within the forest canopy.[15][17][20]Coloration and adaptations
Weaver ant workers exhibit varied coloration: bright green in some Australian O. smaragdina populations, reddish-brown in Asian O. smaragdina ones, and orange-brown to dark brown, with reddish to bright red hues in some East African populations including Uganda, in O. longinoda, enabling effective camouflage against the foliage and branches of their host trees in tropical environments. [21][22][23] Queens are characteristically darker, often blackish or dark brown, which may aid in concealing them during vulnerable colony-founding phases. [24] This visual adaptation supports their arboreal lifestyle by reducing visibility to predators. A key chemical defense in weaver ants involves the production and spraying of formic acid from their anal glands, which irritates the skin and eyes of predators and intruders, deterring attacks effectively. [25] Major workers possess higher concentrations of formic acid, approximately 9.7 mg/g of tissue in O. smaragdina, enhancing their role in colony protection. [24] This venomous secretion acts synergistically with hydrocarbons like n-undecane to provoke aggressive responses from nestmates. [17] Physiologically, weaver ants demonstrate a high metabolic rate that sustains their intense foraging activity across expansive territories in hot, humid tropics. [26] They exhibit notable tolerance to elevated temperatures and humidity, allowing sustained activity in environments where other species falter, though recovery from heat stress varies with ramping rates. [27] Sexual dimorphism is evident in weaver ants, particularly in males, who possess reduced mandibles compared to workers, reflecting their primary focus on reproduction rather than foraging or defense tasks. This morphological specialization aligns with the division of labor in the colony, where males contribute solely to mating and dispersal.Distribution and habitat
Geographic range
The genus Oecophylla comprises two extant species with markedly disjunct distributions across the tropical regions of the Old World, reflecting ancient biogeographic patterns. Oecophylla longinoda is confined to sub-Saharan Africa, while O. smaragdina occupies Asia, Australasia, and parts of the western Pacific, with no overlap between the ranges of the two species.[1][8][28] This separation is linked to the genus's deep evolutionary history, evidenced by Eocene fossils from Europe indicating a formerly broader distribution that contracted over time.[29] Oecophylla longinoda, the African weaver ant, ranges across humid tropical and subtropical zones of sub-Saharan Africa, documented in over 500 sites from 34 countries including Senegal, South Africa, Kenya, Uganda, and Ethiopia. Its northern limit reaches approximately 15°N near Niayes, Senegal, and the southern extent is about 28.4°S at St Lucia Estuary, South Africa, though it is absent from major desert areas such as the Sahara and parts of the Horn of Africa. The species predominates in Köppen-Geiger climate groups A (tropical rainforests, monsoons, and savannas), with rarer occurrences in semi-arid and subtropical zones.[8] In contrast, Oecophylla smaragdina spans a vast area from India and southern China through Southeast Asia (including Indonesia, Malaysia, Thailand, and Vietnam) to northern Australia (Northern Territory and Queensland), New Guinea, and the Solomon Islands in Melanesia. This species thrives mainly in tropical climates but extends into some subtropical areas, with records broadly distributed across these regions. Its range crosses major biogeographic barriers, such as Wallace's Line between the Asian and Australasian realms, facilitated by the species's winged queens enabling long-distance dispersal. Accidental human-mediated introductions have contributed to its presence on certain Pacific islands beyond its core native range.[28][30][31][32]Environmental preferences
Weaver ants, particularly the Asian species Oecophylla smaragdina, inhabit tropical and subtropical forests, showing a strong preference for lowland rainforests with dense canopies that provide ample foliage for nesting and foraging.[33] These environments offer the structural complexity necessary for their arboreal colonies, which span multiple interconnected nests within tree crowns.[25] The species avoids open or fragmented landscapes, favoring undisturbed or semi-disturbed forest canopies where vegetation density supports territorial dominance.[30] Abiotic conditions play a critical role in their distribution and activity. Weaver ants thrive in temperatures between 20°C and 30°C, with optimal foraging occurring around 28°C, while extreme highs above 35°C or lows below 20°C reduce colony efficiency.[34] High relative humidity exceeding 70%, typically ranging from 79% to 87% in their native habitats, is essential for larval silk production and overall colony health, leading to avoidance of arid zones where moisture levels drop significantly.[35] They are generally most abundant at lowland elevations but have been recorded up to approximately 2,000 m, where cooler temperatures and lower humidity may limit proliferation.[36] Their strictly arboreal lifestyle underscores reliance on elevated tree structures, minimizing ground contact to evade predators and desiccation. Colonies construct nests exclusively in the canopy, using live leaves bound by larval silk, which further ties their survival to forested environments with suitable host trees.[4] Climate change and associated habitat alterations pose emerging threats. Warming temperatures may drive range shifts, with modeling predicting expansions into new subtropical areas by 2050 and 2070, potentially benefiting agricultural biocontrol but straining native ecosystems.[36] However, other recent modeling suggests potential constriction of suitable habitat for O. smaragdina in regions like the Indian Peninsula under high-emission scenarios by 2070.[37] Concurrently, deforestation for monoculture plantations, such as rubber, has led to habitat loss in Southeast Asian tropics, reducing canopy availability and disrupting mutualistic interactions, as observed in studies showing altered ant community assembly in converted landscapes. These pressures, including increased fragmentation, could exacerbate local population declines despite overall range potential.[38]Social organization
Colony founding and growth
Colonies of the weaver ant Oecophylla smaragdina are primarily founded claustrally by a single queen following her nuptial flight, although pleometrotic founding with multiple queens (2–4) can occur and significantly boosts initial brood production. The queen selects a suitable leaf and forms a small chamber, which she seals using silk produced by her first-generation larvae once they develop, and relies entirely on her metabolic reserves to rear the first brood without foraging. She lays an initial clutch of eggs, which hatch into larvae after about 7–10 days; the queen nourishes these with regurgitated secretions from her salivary glands. Larval development proceeds rapidly, with pupation occurring around 17–23 days post-egg laying, and the first workers typically emerge after 24–30 days under optimal temperatures (24–30°C). These early workers are intermediate in size and number approximately 10 individuals, enabling the queen to shift focus to sustained egg production while they initiate foraging and nest expansion.[39][40][41] Once the first workers emerge, colony growth accelerates through a series of phases tied to seasonal cues, particularly peaking during the wet season (November–May) when temperature and rainfall support high activity. Expansion occurs via budding, a gradual process where workers transport brood and eggs to nearby leaves or trees to establish satellite nests, fostering a polydomous structure of interconnected nests distributed across 45–100+ trees spanning up to 500 m². This polydomy enhances resource access and defense, with individual nests housing up to 50,000 ants but decentralizing during peak growth to optimize foraging trails. Mature colonies can attain 100,000–500,000 workers, with reproduction and larval production maximized during the wet season; early growth can be further hastened by transplanting 30–60 pupae from donor colonies, yielding 110–200% more brood in the first 3 months compared to unassisted founding. Worker roles in budding and transport briefly support this expansion before specializing further. Similar patterns occur in O. longinoda, though with potentially higher polygyny in some populations.[40][41][3] Queens exhibit remarkable longevity, sustaining colonies for 8–10 years on average, with one laboratory-reared individual surviving at least 13 months and field colonies lasting up to 8 years before decline. In contrast, workers have shorter lifespans of 1–2 months, influenced by caste and task allocation; minor workers average 74 days, while majors live about 48 days due to higher energetic demands in defense. Individual nests endure 7–10.8 weeks before relocation or abandonment. Growth is constrained by predation—fledgling colonies face near-100% mortality from predators like birds, other ants (e.g., Iridomyrmex purpureus), and arthropods—and resource scarcity, including low rainfall (<500 mm), unsuitable temperatures (<17°C or >35°C), and limited tree density or flowering for nectar and honeydew. Polydomy mitigates these risks by dispersing the population and brood, reducing vulnerability to localized threats.[40][42]Roles within the colony
Weaver ant colonies exhibit a clear division of labor among castes, with queens primarily responsible for reproduction, laying eggs to sustain colony growth. Workers are divided into minor and major castes based on body size polymorphism; minor workers, being smaller, focus on brood care, nest maintenance, and cleaning tasks within the colony, while major workers, larger in size, specialize in foraging, territory defense, and guarding against intruders.[43] This caste-based specialization enhances colony efficiency by assigning tasks according to physical capabilities.[44] Queen-worker dimorphism is pronounced, with queens larger and more fecund than workers, adapted solely for egg production in mature colonies that often become oligynous, hosting multiple queens to boost reproductive output. Worker reproduction is largely suppressed through queen-derived pheromones that inhibit ovarian development in workers, maintaining reproductive monopoly by the queen or queens; while worker policing—where workers destroy eggs laid by other workers—occurs in some social insects, in weaver ants it is secondary to chemical suppression.[17][45] Communication within the colony relies heavily on pheromones for coordination, including trail pheromones from the rectal gland to guide foraging and recruitment to food sources or new nest sites. Tandem running, where a knowledgeable worker physically leads a follower to a resource or location, serves as a key recruitment mechanism, particularly for nest relocation or discovering high-value food patches, allowing efficient mobilization without mass chemical trails.[46][47] Age polyethism further refines task allocation, with young workers tending to internal duties like nursing brood and nest hygiene, transitioning to external roles such as foraging and defense as they age and move to the colony periphery. Recent studies highlight that while morphological caste strongly dictates specialization in weaver ants, age-dependent shifts provide flexibility in task distribution, especially in response to colony needs, with models showing age polyethism advantageous when task mortality risks vary. In a 2025 analysis of Asian weaver ant sub-castes, age influenced the progression from minor internal roles to intermediate and major peripheral activities, underscoring dynamic allocation beyond strict caste boundaries.[17][48][49]Nest construction
Building process
Weaver ants (Oecophylla spp.) initiate nest construction by selecting suitable leaves on host trees, typically gripping the edges or tips with their mandibles to begin pulling them into position. Workers identify non-random sites, such as leaf tips, where initial bites occur preferentially to facilitate manipulation (Bochynek & Robson, 2014)[50]. This pulling action involves individual ants walking backward while clamped to the leaf, exerting force to bend or draw it toward adjacent foliage, often forming living bridges between leaves or branches to align them for binding (Bochynek & Robson, 2014)[50]. The process is similar in both O. smaragdina and O. longinoda. Cooperative pulling escalates as additional workers join the effort, grasping the bodies of preceding ants to form extensible chains that amplify collective force. These chains can involve up to dozens of ants, with workers self-assembling through positive feedback mechanisms that cluster activity spatially and temporally at pulling sites (Stewardson et al., 2025)[51]; (Bochynek & Robson, 2014)[50]. The process operates via a "force ratchet" mechanism, where active pullers at the chain's end generate incremental force, stored elastically and released through frictional resistance among passive chain members, enabling superefficient teamwork that counters typical coordination losses in larger groups (Stewardson et al., 2025)[51]. Experimental measurements show force output per ant nearly doubles as team size increases, allowing effective deformation of stiff leaves (Stewardson et al., 2025)[51]. Once leaves are approximated, workers apply silk by holding mature larvae—whose silk glands produce the binding material—as "living glue" dispensers, shuttling them between leaf edges to weave threads into a cohesive mesh (Bochynek & Robson, 2014)[50]; (Stewardson et al., 2025)[51]. This larval silk, detailed in studies of caste-specific adaptations, solidifies rapidly to secure the structure without additional materials in initial phases (Bochynek & Robson, 2014)[50]. A single nest is typically completed within 1-2 days through these coordinated steps, though large colonies may construct multiple nests concurrently over extended periods.Nest design and function
Weaver ant nests are typically ovoid and multi-chambered structures, measuring 10-30 cm in height and constructed by weaving together living leaves using silk produced by the larvae. These nests are built in the crotches of tree branches within the canopy, often at heights averaging 3.2 m, to optimize access to sunlight and resources while minimizing ground-based threats. Small circular entrances, reinforced with silk sheets, facilitate worker movement and provide ventilation to regulate internal humidity and airflow, preventing overheating or stagnation within the chambers.[52][40][53] Colonies of Oecophylla smaragdina are polydomous, comprising interconnected networks of up to 151 sub-nests distributed across multiple trees, supporting populations of hundreds of thousands to half a million ants. These sub-nests are linked by arboreal trails marked with persistent pheromones from the ants' rectal glands, which guide foraging and recruitment, supplemented by silk strands for structural stability in some connections. This decentralized arrangement enhances colony efficiency by decentralizing brood distribution and resource access, with each sub-nest housing up to 50,000 individuals.[40][52][54] The primary functions of these nests include brood protection and thermoregulation, achieved through a stable internal microclimate maintained at 20-34°C via ant clustering to generate metabolic heat during cooler periods and nest positioning for solar insolation. Nests shield brood and adults from heavy rain by enclosing them in waterproof leaf enclosures and deter intruders through vigilant patrolling at entrances, reducing vulnerability to predators and environmental stressors. Major workers reinforce external structures, while minors manage internal chambers for pupal development under optimal conditions of 24-30°C and high humidity.[40] Nests are relocated or rebuilt seasonally, typically occupied for 7-18 weeks before abandonment due to leaf senescence, colony expansion, or disturbances, with rebuilding peaking in the wet season (November-March) to coincide with new foliage growth. This adaptability confers resilience to storms, as colonies can repair or relocate post-cyclone damage, with surviving fledgling groups demonstrating rapid recovery in tropical environments. Relocations often occur at dusk, involving coordinated transport of brood along pheromone trails to new sites.[40]Behavior
Foraging and diet
Weaver ants (Oecophylla spp.) maintain a primarily carnivorous diet, preying on small arthropods including flies, caterpillars, and other insects such as bagworms and silkworms.[55][56] Workers employ coordinated group ambushes to capture prey, swarming the target en masse to subdue it rapidly, often within minutes for early larval stages.[56] This collective predation enables the colony to exploit resources beyond the capacity of solitary foragers, with major workers leading these efforts.[56] A significant portion of their nutrition comes from trophobiosis, where ants harvest honeydew—a carbohydrate-rich excretion—from hemipteran insects such as scale insects.[57] Weaver ants actively protect these hemipterans by incorporating them into nests or guarding them on foliage, deterring predators and parasitoids to sustain the honeydew supply.[57] This mutualistic relationship complements their protein intake from arthropod prey, balancing the colony's nutritional needs.[58] Foraging occurs along established arboreal trails extending considerable distances from the nest, primarily during daylight hours with bimodal peaks at dawn and dusk driven by favorable temperature and humidity conditions.[59] Activity is diurnal overall, relying on visual cues for prey detection, though intensity varies with brood hunger levels and environmental factors.[60] Once collected, food is distributed colony-wide via trophallaxis, in which foragers regurgitate liquefied portions to nestmates, while solid prey is preferentially fed to larvae serving as the primary protein reservoir for adult consumption.[61][4]Defense and territoriality
Weaver ants (Oecophylla spp.) maintain exclusive arboreal territories through aggressive defense strategies that involve coordinated patrols and rapid responses to intrusions. Territories can extend up to 1500 m² in area, with colonies establishing barrack nests at boundaries to station guard forces of major workers, which actively patrol to monitor and repel potential threats.[62] These patrols are primarily conducted by larger major workers, who exhibit heightened aggression in defending against conspecific rivals or other ant species encroaching on their domain.[44] A key component of territorial defense is the formation of living barriers by major workers, who link together using their mandibles to create chain-like formations that block access to nest areas and spray chemical secretions at intruders.[62] These workers bite with powerful mandibles and eject irritant compounds from the poison gland, primarily hydrocarbons such as undecane, which deter attackers by causing physical and chemical irritation.[63] Although hydrocarbons dominate the poison gland content, formic acid is also present in worker tissues at concentrations around 9.7 mg/g in majors, contributing to the defensive spray that enhances the painful effect of bites.[24] This chemical defense is particularly effective against floaters or neighboring colonies seeking to expand, as it inflicts immediate harm and signals colony strength.[64] Swarm raids represent a collective offensive strategy, where alarm pheromones trigger mass mobilization of workers to overwhelm large prey or rival colonies. Upon detecting a threat, a scout releases pheromones from the mandibular gland, prompting nearby workers to join in biting and spraying attacks that can decimate intruders.[63] These raids often target competing ant species, enforcing interspecific territoriality through selective aggression that results in a mosaic distribution of colonies in shared habitats.[62] Territorial boundaries are reinforced through persistent pheromone deposition along patrol routes, which marks and communicates colony limits while deterring competitors. Weaver ants distribute these pheromones across host trees, creating a chemical landscape that maintains exclusivity and facilitates ongoing conflicts with other ants, such as excluding non-nesting species from foraging areas.[54] This system ensures that costs of defense, including energy expenditure on patrols, are balanced by the benefits of resource control in tropical ecosystems.[64]Cooperative mechanisms
Weaver ants (Oecophylla spp.) exhibit sophisticated cooperative mechanisms that enable decentralized group coordination without centralized control, allowing colonies to tackle complex tasks such as resource acquisition and navigation. These behaviors rely on local interactions among individuals, integrating chemical, tactile, and visual cues to achieve collective outcomes that surpass individual capabilities. Studies as of 2025 highlight how these mechanisms demonstrate emergent efficiency in decision-making and force application, providing insights into biological teamwork.[51] Recruitment in weaver ants involves multiple decentralized strategies for task allocation, primarily tandem running and pheromone trails, which facilitate the mobilization of workers to food sources, new nest sites, or defense sites without a hierarchical leader. In tandem running, an experienced forager physically guides a naïve nestmate to a resource by maintaining close contact, allowing information transfer through tactile and chemical signals, a process observed during colony relocation and initial foraging discovery. Complementing this, workers lay pheromone trails from hindgut secretions to mark paths, attracting additional nestmates in a mass recruitment fashion that amplifies group response to high-value targets. These systems, numbering at least five in O. longinoda, enable rapid scaling of effort based on need, ensuring efficient resource exploitation across the colony's arboreal territory.[65] During cooperative transport of heavy prey, weaver ants employ leaderless consensus decision-making, where groups pool directional preferences through physical interactions to resolve navigation conflicts and select optimal paths. This "wisdom of the crowd" approach allows small teams of 4–10 ants to transport loads at efficiencies exceeding individual efforts by integrating individual opinions via tugging and alignment, achieving consensus in seconds without a dominant leader. Experimental observations using tethered objects show that ants adjust pulling directions based on collective feedback, minimizing deviations and maximizing progress even on uneven surfaces.[66] A striking example of advanced coordination is chain formation, where workers interlink their bodies to bridge gaps or collectively pull objects like leaves for nest construction, inverting the Ringelmann effect observed in human groups by increasing per capita force output as team size grows. In experiments as of 2025, chains generated forces scaling superlinearly with size due to a division of labor where "pullers" at the front exert effort while "anchors" at the rear provide passive stability via friction hairs and adhesive pads. This superefficiency arises from adaptive load-sharing and enhanced grip, enabling the colony to manipulate objects far beyond solitary capabilities. Findings from these mechanisms have inspired robotics research, suggesting algorithms for multi-robot systems to achieve similar scalable teamwork in tasks like object manipulation or swarm navigation.[51]Ecological interactions
Relationships with other species
Weaver ants (Oecophylla spp.) engage in mutualistic relationships with honeydew-producing hemipterans, such as aphids and scale insects, where the ants provide protection from predators and parasites in exchange for the sugary honeydew excretion.[67] These interactions are widespread in tropical ecosystems, with O. smaragdina dominating collections of honeydew from multiple hemipteran genera, accounting for over half of observed ant-hemipteran contacts in some fire-prone habitats.[68] Similar mutualisms occur with O. longinoda in African systems, tending species like mealybugs.[69] By tending these trophobionts on host plants, weaver ants enhance hemipteran survival and reproduction while securing a reliable carbohydrate source, though excessive tending can lead to plant damage from hemipteran feeding.[57] Predators of weaver ants include various vertebrates and invertebrates that target workers, larvae, and pupae. Birds such as drongos and lizards prey on foraging workers, exploiting the ants' arboreal lifestyle despite their aggressive defenses.[70] Myrmecophilous arthropods, including certain beetles from the subfamily Paussinae, inhabit weaver ant nests, feeding on brood while evading host aggression through chemical mimicry or behavioral adaptations.[71] In competitive interactions, weaver ants aggressively exclude other ant species from their territories using physical attacks and chemical defenses, particularly formic acid sprays that deter intruders.[72] This territorial dominance forms "ant mosaics" in canopies, reducing overlap with subordinate species and limiting their access to resources.[73] Occasional social parasitism occurs, with rare incursions by slave-making ants that raid nests to capture brood, though such events are infrequent due to the weaver ants' robust colony defenses.[74] Recent studies indicate climate-driven shifts are altering weaver ant interactions, with projected range expansions under warming scenarios potentially intensifying competitive exclusion of native ants and altering mutualisms in newly invaded areas.[36] In regions like Southeast Asia and sub-Saharan Africa, invasive spread facilitated by habitat changes has led to heightened conflicts with local fauna, including increased predation pressure on hemipteran partners in altered ecosystems.[75]Role in ecosystems
Weaver ants (Oecophylla spp.) serve as key predators in tropical ecosystems, significantly contributing to pest control by reducing populations of herbivorous arthropods that damage foliage and fruits. This predation indirectly enhances plant diversity by alleviating pressure on vegetation, allowing a broader range of tree species to thrive in forested habitats. Studies have documented their effectiveness in suppressing pests across diverse tropical environments, where their aggressive foraging behavior maintains ecological balance without relying on chemical interventions.[33][76] In terms of nutrient cycling, weaver ants facilitate the exchange of essential macronutrients, such as carbon and nitrogen, between themselves and host plants through trophobiosis and waste deposition. Their foraging activities deposit organic waste from captured prey onto the forest floor, enriching soil fertility and supporting microbial activity. Additionally, debris from their leaf-woven nests provides microhabitats for decomposer organisms, accelerating the breakdown of organic matter and promoting nutrient return to the ecosystem. This process underscores their role as ecosystem engineers in tropical canopies.[77][78][79] As biodiversity indicators, the presence and abundance of weaver ants signal the health of tropical forest ecosystems, reflecting intact canopy structures and minimal disturbance. Their populations decline in fragmented habitats, where edge effects and reduced connectivity disrupt foraging and nesting, serving as an early warning for broader environmental degradation. Recent 2025 research highlights their resilience to climate change, predicting potential range expansions that could bolster ecosystem stability in warming tropics.[80][81][36][82]Human relationships
Agricultural uses
Weaver ants (Oecophylla spp.) have a long history of use in agricultural pest management, particularly as biological control agents against arthropod pests in citrus orchards. In China, farmers deployed O. smaragdina to combat citrus pests as early as the fourth century AD, marking one of the earliest documented applications of biological control in agriculture.[83] This practice involved introducing ant colonies to trees, leveraging their predatory behavior to reduce pest populations such as aphids, scale insects, and caterpillars, which can significantly damage fruit quality and yield. Studies have shown that O. smaragdina predation can increase fruit yields by up to 49% in treated plots compared to untreated ones, primarily through direct consumption of pests and indirect deterrence via territorial aggression.[84] Modern methods for integrating weaver ants into farming systems focus on nest transplantation and habitat enhancement to establish stable colonies in orchards. Nests are collected from natural areas and relocated to crop trees, often with the addition of bridges or ropes to facilitate ant movement between plants and supplemental feeding to support colony growth. This approach is commonly applied in integrated pest management (IPM) programs for mango and coconut plantations across Southeast Asia using O. smaragdina and in Africa using O. longinoda, where ants effectively suppress key pests like fruit borers and coreid bugs while minimizing reliance on chemical pesticides.[85] Their territorial behaviors further aid in pest exclusion by patrolling foliage and attacking intruders.[33] The benefits of weaver ant biocontrol include providing a non-toxic, environmentally friendly alternative to synthetic pesticides, reducing chemical residues on produce and supporting biodiversity in agroecosystems.[86] Economically, adoption in regions like Thailand has led to net income increases of over 70% for farmers through higher yields and lower input costs, contributing significantly to sustainable farming in Asia and Africa.[84] However, challenges persist, including the labor-intensive nature of colony transplantation, which can induce stress and high mortality in relocated nests if not managed carefully.[84] Post-2020 field trials continue to affirm efficacy, such as demonstrations of ant-released volatiles repelling pests in orchards, though ongoing research addresses variability in colony establishment under changing climates.[87]Culinary and medicinal applications
Weaver ant larvae and pupae are harvested as a nutritious food source in regions such as Thailand and India, where they provide high protein content ranging from approximately 46% to 60% on a dry weight basis, making them a valuable edible insect option.[88] In northeastern Thailand, particularly in Nakhon Ratchasima province, the annual market value of harvested weaver ants exceeds $620,000, supporting local economies through wild collection and small-scale farming.[89] Culinary applications of weaver ants emphasize their larvae, pupae, and adults, which are often fried or roasted to enhance flavor and texture, or incorporated into salads and soups for added protein.[90] The ants impart a distinctive citrusy tang derived from formic acid in their bodies, serving as a natural flavoring agent in dishes across Southeast Asia.[91] Additionally, processed weaver ant brood is used as feed for fish and poultry, providing a high-protein supplement that improves growth rates in aquaculture and livestock farming.[92][93] In traditional medicine, weaver ant extracts have been employed as a folk remedy for arthritis in parts of Africa and Asia, where the bioactive compounds are believed to reduce inflammation and joint pain.[94] Modern studies have explored weaver ant extracts for their antibacterial properties, demonstrating inhibition of pathogens such as Staphylococcus aureus and Monilinia fructigena.[95][96] Sustainability concerns arise from overharvesting, which can deplete wild populations and disrupt local ecosystems, prompting recommendations for regulated farming to balance commercial demand with conservation.[97] While nutritional analyses confirm the ants' value, further research on commercialization could mitigate risks and expand safe harvesting practices.[89]References
- https://www.antwiki.org/wiki/Oecophylla
- https://www.antwiki.org/wiki/Oecophylla_smaragdina
- https://antwiki.org/wiki/Lake_Victoria,_Kenya_Fossil
- https://www.antwiki.org/wiki/The_Ants_Chapter_7

