Recent from talks
Nothing was collected or created yet.
Sodium thiosulfate
View on Wikipedia | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium thiosulfate
| |
| Other names
Sodium hyposulphite
Hyposulphite of soda Hypo | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.970 |
| EC Number |
|
| E number | E539 (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na2S2O3 | |
| Molar mass | 158.11 g/mol (anhydrous) 248.18 g/mol (pentahydrate) |
| Appearance | White crystals |
| Odor | Odorless |
| Density | 1.667 g/cm3 |
| Melting point | 48.3 °C (118.9 °F; 321.4 K) (pentahydrate) |
| Boiling point | 100 °C (212 °F; 373 K) (pentahydrate, - 5H2O decomposition) |
| 70.1 g/100 mL (20 °C)[1] 231 g/100 mL (100 °C) | |
| Solubility | negligible in alcohol |
Refractive index (nD)
|
1.489 |
| Structure | |
| monoclinic | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other cations
|
Thiosulfuric acid Lithium thiosulfate Potassium thiosulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula Na2S2O3·xH2O. Typically it is available as the white or colorless pentahydrate (x = 5), which is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications.[2]
Uses
[edit]Sodium thiosulfate is used predominantly in dyeing. It converts some dyes to their soluble colorless "leuco" forms. It is also used to bleach "wool, cotton, silk, soaps, glues, clay, sand, bauxite, and edible oils, edible fats, and gelatin."[2]
Medical uses
[edit]Sodium thiosulfate is used in the treatment of cyanide poisoning.[3] It is on the World Health Organization's List of Essential Medicines.[4][5] Other uses include topical treatment of ringworm and tinea versicolor,[3][6] and treating some side effects of hemodialysis[7] and chemotherapy.[8][9] In September 2022, the U.S. Food and Drug Administration (FDA) approved sodium thiosulfate under the trade name Pedmark to lessen the risk of ototoxicity and hearing loss in infant, child, and adolescent cancer patients receiving the chemotherapy medication cisplatin.[10][11]
Photographic processing
[edit]In photography, sodium thiosulfate is used in both film and photographic paper processing as a fixer, sometimes still called 'hypo' from the original chemical name, hyposulphite of soda.[12] It functions to dissolve silver halides, e.g., AgBr, components of photographic emulsions. Ammonium thiosulfate is typically preferred to sodium thiosulfate for this application.[2]
The ability of thiosulfate to dissolve silver ions is related to its ability to dissolve gold ions.
Neutralizing chlorinated water
[edit]It is used to dechlorinate tap water including lowering chlorine levels for use in aquariums, swimming pools, and spas (e.g., following superchlorination) and within water treatment plants to treat settled backwash water prior to release into rivers.[2] The reduction reaction is analogous to the iodine reduction reaction.
In pH testing of bleach substances, sodium thiosulfate neutralizes the color-removing effects of bleach and allows one to test the pH of bleach solutions with liquid indicators. The relevant reaction is akin to the iodine reaction: thiosulfate reduces the hypochlorite (the active ingredient in bleach) and in so doing becomes oxidized to sulfate. The complete reaction is:
- 4 NaClO + Na2S2O3 + 2 NaOH → 4 NaCl + 2 Na2SO4 + H2O
Similarly, sodium thiosulfate reacts with bromine, removing the free bromine from the solution. Solutions of sodium thiosulfate are commonly used as a precaution in chemistry laboratories when working with bromine and for the safe disposal of bromine, iodine, or other strong oxidizers.
Structure
[edit]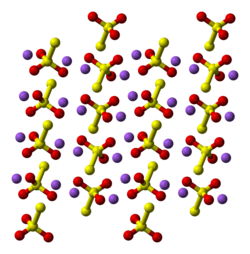
Two polymorphs are known as pentahydrate. The anhydrous salt exists in several polymorphs.[2] In the solid state, the thiosulfate anion is tetrahedral in shape and is notionally derived by replacing one of the oxygen atoms by a sulfur atom in a sulfate anion. The S-S distance indicates a single bond, implying that the terminal sulfur holds a significant negative charge and the S-O interactions have more double-bond character.
Production
[edit]Sodium thiosulfate is prepared by oxidation of sodium sulfite with sulfur.[2] It is also produced from waste sodium sulfide from the manufacture of sulfur dyes.[13]
This salt can also be prepared by boiling aqueous sodium hydroxide and sulfur according to the following equation.[14][15] However, this is not recommended outside of a laboratory, as exposure to hydrogen sulfide can result if improperly handled.
- 6 NaOH + 4 S → 2 Na2S + Na2S2O3 + 3 H2O
Principal reactions
[edit]Upon heating to 300 °C, it decomposes to sodium sulfate and sodium polysulfide:
- 4 Na2S2O3 → 3 Na2SO4 + Na2S5
Thiosulfate salts characteristically decompose upon treatment with acids. Initial protonation occurs at sulfur. When the protonation is conducted in diethyl ether at −78 °C, H2S2O3 (thiosulfuric acid) can be obtained. It is a somewhat strong acid with pKas of 0.6 and 1.7 for the first and second dissociations, respectively. Under normal conditions, acidification of solutions of this salt excess with even dilute acids results in complete decomposition to sulfur, sulfur dioxide, and water:[13]
- 8 Na2S2O3 + 16 HCl → 16 NaCl + S8 + 8 SO2 + 8 H2O
Coordination chemistry
[edit]Thiosulfate forms complexes with transition metal ions. One such complex is [Au(S2O3)2]3−.
Iodometry
[edit]Some analytical procedures exploit the oxidizability of thiosulfate anion by iodine. The reaction produces tetrathionate:
- 2 S2O2−3 + I2 → S4O2−6 + 2 I−
Due to the quantitative nature of this reaction, as well as because Na2S2O3·5H2O has an excellent shelf-life, it is used as a titrant in iodometry. Na2S2O3·5H2O is also a component of iodine clock experiments.
This particular use can be set up to measure the oxygen content of water through a long series of reactions in the Winkler test for dissolved oxygen. It is also used in estimating volumetrically the concentrations of certain compounds in solution (hydrogen peroxide, for instance) and in estimating the chlorine content in commercial bleaching powder and water.
Organic chemistry
[edit]Alkylation of sodium thiosulfate gives S-alkylthiosulfates, which are called Bunte salts.[16] The alkylthiosulfates are susceptible to hydrolysis, affording the thiol. This reaction is illustrated by one synthesis of thioglycolic acid:
- ClCH2CO2H + Na2S2O3 → Na[O3S2CH2CO2H] + NaCl
- Na[O3S2CH2CO2H] + H2O → HSCH2CO2H + NaHSO4
Safety
[edit]Sodium thiosulfate has low toxicity. LDLo for rabbits is 4000 mg/kg.[2]
References
[edit]- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c d e f g Barbera JJ, Metzger A, Wolf M (2012). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477. ISBN 978-3-527-30673-2.
- ^ a b Stuart MC, Kouimtzi M, Hill SR, eds. (2009). WHO Model Formulary 2008. World Health Organization. p. 66. hdl:10665/44053. ISBN 978-92-4-154765-9.
- ^ Organization, World Health (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Organization, World Health (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Sunenshine PJ, Schwartz RA, Janniger CK (2002). "Tinea versicolor". Int. J. Dermatol. 37 (9): 648–55. doi:10.1046/j.1365-4362.1998.00441.x. PMID 9762812. S2CID 75657768.
- ^ Auriemma M, Carbone A, Di Liberato L, et al. (2011). "Treatment of Cutaneous Calciphylaxis with Sodium Thiosulfate: Two Case Reports and a Review of the Literature". Am. J. Clin. Dermatol. 12 (5): 339–46. doi:10.2165/11587060-000000000-00000. PMID 21834598. S2CID 28366905.
- ^ Orgel E, Villaluna D, Krailo MD, Esbenshade A, Sung L, Freyer DR (May 2022). "Sodium thiosulfate for prevention of cisplatin-induced hearing loss: updated survival from ACCL0431". The Lancet. Oncology. 23 (5): 570–572. doi:10.1016/S1470-2045(22)00155-3. PMC 9635495. PMID 35489339.
- ^ Dickey DT, Wu YJ, Muldoon LL, et al. (2005). "Protection against Cisplatin-Induced Toxicities by N-Acetylcysteine and Sodium Thiosulfate as Assessed at the Molecular, Cellular, and in Vivo Levels". J. Pharmacol. Exp. Ther. 314 (3): 1052–8. doi:10.1124/jpet.105.087601. PMID 15951398. S2CID 11381393.
- ^ Winstead, Edward (October 6, 2022). "Sodium Thiosulfate Reduces Hearing Loss in Kids with Cancer". National Cancer Institute. Retrieved March 9, 2023.
- ^ "FDA approves sodium thiosulfate to reduce the risk of ototoxicity associated with cisplatin in pediatric patients with localized, non-metastatic solid tumors". U.S. Food and Drug Administration. 20 September 2022. Retrieved 9 March 2023.
- ^ Gibson CR (1908). The Romance of Modern Photography, Its Discovery & Its Achievements. Seeley & Co. pp. 37.
hyposulphite-of-soda herschel fixer hypo.
- ^ a b Holleman AF, Wiberg E, Wiberg N (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Gordin HM (1913). Elementary Chemistry. Vol. 1. Inorganic Chemistry. Chicago: Medico-Dental Publishing Co. pp. 162 & 287–288.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Alonso ME, Aragona H (1978). "Sulfide Synthesis in Preparation of Unsymmetrical Dialkyl Disulfides: Sec-butyl Isopropyl Disulfide". Org. Synth. 58: 147. doi:10.15227/orgsyn.058.0147.

Sodium thiosulfate
View on GrokipediaProperties
Physical properties
Sodium thiosulfate exists in both anhydrous and hydrated forms, with the chemical formula Na₂S₂O₃ for the anhydrous compound and Na₂S₂O₃·5H₂O for the common pentahydrate.[1][5] The pentahydrate is the predominant commercial form due to its stability and ease of handling.[6] The pentahydrate appears as white or colorless efflorescent crystals or powder and is odorless.[7] It has a density of 1.69 g/cm³.[8] The pentahydrate melts incongruently at 48 °C (118 °F), losing its water of hydration to form the anhydrous salt and a saturated solution; the anhydrous form decomposes upon strong heating.[9] It does not have a defined boiling point, as it decomposes before boiling and loses its water of hydration at 100 °C.[6] Sodium thiosulfate pentahydrate exhibits high solubility in water, dissolving at a rate of 70.1 g/100 mL at 20 °C; it is slightly soluble in alcohol and insoluble in ether.[6] The thiosulfate ion (S₂O₃²⁻) is the key component responsible for this pronounced water solubility.[5] Under normal conditions, the compound is stable but effloresces in dry air, gradually losing water molecules from its crystal lattice.[10] It is hygroscopic, readily absorbing moisture from the atmosphere.[11] In acidic solutions, it decomposes to produce sulfur and sulfur dioxide.[11] The pentahydrate adopts a monoclinic crystal system.[12]Molecular structure
Sodium thiosulfate is an ionic compound consisting of two sodium cations (Na⁺) and one thiosulfate anion (S₂O₃²⁻).[1] The thiosulfate ion (S₂O₃²⁻) features a central sulfur atom bonded to three oxygen atoms and a terminal sulfur atom, resulting in a slightly distorted tetrahedral geometry around the central sulfur with approximate bond angles of 109.5°.[13] X-ray crystallographic studies indicate an S-S bond length of approximately 2.01 Å and S-O bond lengths averaging about 1.47 Å.[14] In the thiosulfate ion, the central sulfur atom has an oxidation state of +5, while the terminal sulfur atom has an oxidation state of -1, yielding an overall charge of -2 for the anion. The compound commonly exists as the pentahydrate (Na₂S₂O₃·5H₂O), in which the five water molecules occupy positions within the crystal lattice, stabilizing the structure; the anhydrous form (Na₂S₂O₃) is less stable and less commonly encountered.[5] Spectroscopic techniques confirm the bonding in the thiosulfate ion, with infrared (IR) and Raman spectroscopy revealing characteristic stretching bands: S-O stretches around 1100–1000 cm⁻¹ and S-S stretches near 470 cm⁻¹.[15] The thiosulfate ion can be viewed as a sulfur analog of the sulfate ion (SO₄²⁻), derived notionally by replacing one oxygen atom with a sulfur atom.[16]Synthesis and production
Laboratory preparation
Sodium thiosulfate can be prepared in the laboratory primarily through the reaction of sodium sulfite with elemental sulfur in aqueous solution. The balanced equation for this process is Na₂SO₃ + S → Na₂S₂O₃.[17] This method, dating back to early 19th-century practices involving boiling sulfur in sodium sulfite solution, remains a standard for small-scale synthesis.[18] In a typical procedure, approximately 6.3 g of sodium sulfite (Na₂SO₃) is weighed and dissolved in 40 mL of distilled water within a 100 mL beaker, covered with a watch glass, and heated with constant stirring until fully dissolved. Powdered elemental sulfur (about 1.6 g, stoichiometric amount) is then added to the hot solution (maintained at 40-50 °C), and the mixture is stirred vigorously under an inert atmosphere, such as nitrogen, to minimize oxidation by air. The reaction proceeds as the sulfur dissolves and reacts, typically requiring 30-60 minutes of heating and stirring. The resulting solution is filtered to remove any unreacted sulfur, and the filtrate is concentrated by gentle evaporation before cooling to induce crystallization of the pentahydrate form, Na₂S₂O₃·5H₂O. This method yields approximately 90% based on the limiting reagent.[17][19][20] An alternative laboratory method involves sequential reaction starting from sodium hydroxide and sulfur dioxide, followed by addition of sulfur, though it generally provides a lower yield of around 70%. The process first involves bubbling SO₂ gas into aqueous NaOH to form sodium sulfite (SO₂ + 2 NaOH → Na₂SO₃ + H₂O), after which powdered sulfur is added and the mixture heated (Na₂SO₃ + S → Na₂S₂O₃). The overall simplified equation is 2 NaOH + SO₂ + S → Na₂S₂O₃ + H₂O. In practice, sulfur dioxide gas is bubbled into a dilute aqueous sodium hydroxide solution to generate sodium sulfite, after which powdered sulfur is added and the mixture heated similarly to the primary method. This approach is less common in basic lab settings due to the handling of gaseous SO₂ but is useful when sodium sulfite is unavailable.[18][21] Purification of the crude product is achieved through recrystallization from hot water, where the crystals are dissolved in minimal boiling water and then slowly cooled to room temperature, promoting the formation of pure pentahydrate crystals; acidic conditions must be avoided to prevent decomposition into sulfur and sulfur dioxide.[22][17] Laboratory preparations should be conducted in a fume hood, particularly for the alternative method involving SO₂, which is toxic and irritating; typical batch sizes range from 10-50 g to ensure safe handling and control. Protective equipment, including gloves and goggles, is essential to avoid skin and eye contact with the reagents.[23][24]Industrial production
The primary industrial route for sodium thiosulfate production involves the preparation of sodium sulfite from sodium carbonate and sulfur dioxide, followed by its reaction with elemental sulfur. Sulfur dioxide is generated by the combustion of sulfur, and the overall process proceeds as Na₂CO₃ + SO₂ → Na₂SO₃ + CO₂, then Na₂SO₃ + S → Na₂S₂O₃, typically conducted in evaporators under controlled conditions to achieve yields exceeding 95%.[25][17] The process flow employs multi-stage reactors for the sequential reactions, with sulfur dioxide bubbled into an aqueous solution of sodium carbonate to form sodium bisulfite intermediate, which is then neutralized to sodium sulfite using additional sodium carbonate. This sulfite solution is heated and reacted with sulfur powder in a boiling setup, followed by filtration to remove excess sulfur, evaporation to concentrate the solution, and cooling for crystallization of the pentahydrate form (Na₂S₂O₃·5H₂O). The product is subsequently dried to yield either solid crystals or a 60% aqueous solution for commercial distribution.[25][17] Alternative production methods include recovery from waste streams in the paper pulping industry, where sodium thiosulfate forms via oxidation of sodium sulfide present in black liquor from kraft mills.[26] Global production of sodium thiosulfate is estimated at approximately 100,000 tons per year as of 2023, with the market valued at around USD 70 million; as of 2025, the market size is estimated at USD 120.68 million, suggesting increased production volume. Major producers include Chinese firms such as DayooChem and Shandong Aojin Chemical Technology Co., Ltd., U.S. companies like Hydrite Chemical Co., and European players such as INEOS. Bulk production costs range from $0.5 to $1 per kg, influenced by raw material prices and scale.[27][28][29][30] Quality control in industrial production ensures purity standards of ≥99% for Na₂S₂O₃ in general grades, with pharmaceutical-grade material meeting USP, BP, and Ph. Eur. specifications of 99.0-101.0% assay and stringent limits on heavy metals, such as lead below 10 ppm, to comply with regulatory requirements for medical and food applications.[31][32]Chemical reactions
Acid-base reactions
Sodium thiosulfate undergoes decomposition in acidic environments, producing elemental sulfur as a colloidal precipitate and sulfur dioxide gas. The balanced equation for the reaction with hydrochloric acid is: This process is characterized by the formation of a milky suspension due to the sulfur particles, and the rate of reaction accelerates with increasing acid concentration, as the protonation of the thiosulfate ion facilitates the breakdown.[33][34] The compound exhibits high stability in neutral to basic solutions where the pH exceeds 7, with no significant acid-base reactions occurring under alkaline conditions, enabling its application in such media for other chemical processes. In pure water, sodium thiosulfate hydrolyzes slowly, yielding sulfite ions and unstable thiosulfuric acid, though this decomposition is minimal at ambient conditions. The kinetics of the acidic decomposition are complex, following a first-order rate law with respect to thiosulfate concentration in dilute solutions. Colloidal sulfur formation is a hallmark of this reaction, and the evolution of SO₂ gas has analytical utility for detecting acidity in solution mixtures. The thiosulfate ion (O₃S–S²⁻) represents the stable structural isomer, whereas the sulfurothioate form (O₂S–SO₂²⁻) is highly unstable and not observed under typical conditions.[35][34]Redox reactions
Sodium thiosulfate serves as a versatile reducing agent in redox reactions, primarily undergoing two-electron oxidation to tetrathionate (S₄O₆²⁻) with milder oxidants or further oxidation to sulfate (SO₄²⁻) with stronger ones, depending on the reaction conditions and oxidant strength.[36] The standard reduction potential for the S₄O₆²⁻ / 2 S₂O₃²⁻ couple is +0.08 V versus the standard hydrogen electrode (SHE), indicating its moderate reducing power suitable for analytical applications.[37] A key redox reaction is the iodometric titration, where sodium thiosulfate reduces iodine to iodide, forming tetrathionate as the product. The balanced equation is: This reaction proceeds at a 1:1 molar equivalence point between thiosulfate and iodine, making it a standard method for iodine quantification in analytical chemistry.[38] With halogens like bromine and chlorine, sodium thiosulfate acts as a decolorizing agent, reducing the halogen while producing sulfate, halide, and elemental sulfur. For bromine water, the reaction is: A similar process occurs with chlorine, where thiosulfate is oxidized to sulfate, effectively neutralizing the oxidant in aqueous solutions.[39] In acidic media, sodium thiosulfate reduces hydrogen peroxide to water, forming tetrathionate. The balanced ionic equation is: This two-electron transfer highlights thiosulfate's role in peroxide decomposition under controlled pH conditions.[40] In the 2020s, sodium thiosulfate has been employed in analytical protocols for detecting trace oxidants in environmental samples, such as in modified Winkler titrations for dissolved oxygen or residual disinfectants in water, enabling precise quantification at low concentrations.Coordination chemistry
The thiosulfate ion (S₂O₃²⁻) functions as a versatile ambidentate ligand in coordination chemistry, capable of monodentate coordination through either the terminal sulfur atom (S-bound) or an oxygen atom (O-bound), or bidentate coordination bridging sulfur and oxygen atoms. This flexibility arises from the ion's asymmetric structure, with the central sulfur atom bonded to three oxygens and a terminal sulfur, allowing multiple donor sites. Binding preferences align with the hard-soft acid-base (HSAB) theory: soft metals like Ag⁺ and Hg²⁺ favor the soft S-donor, while harder metals such as Co³⁺ may prefer the harder O-donor, though S-binding predominates in many cases due to stronger metal-sulfur interactions.[41][42] Notable examples include the silver(I) complex [Ag(S₂O₃)₂]³⁻, in which two thiosulfate ligands coordinate monodentately via sulfur atoms to the linear Ag⁺ center, enhancing solubility of silver salts. Similarly, the mercury(II) complex [Hg(S₂O₃)₂]²⁻ features two S-bound thiosulfate ligands, reflecting mercury's soft acid character. For platinum(II), the square-planar complex [Pt(S₂O₃)₄]⁶⁻ incorporates four monodentate S-bound thiosulfates, as confirmed by ¹⁹⁵Pt NMR spectroscopy. These complexes highlight thiosulfate's utility in stabilizing low-valent soft metals through sulfur donation.[43] The stability of these coordination compounds varies with the metal and ligand stoichiometry, often determined by stepwise formation equilibria. For the silver(I) system, complexation proceeds via Ag⁺ + S₂O₃²⁻ ⇌ [Ag(S₂O₃)]⁻ (log K₁ ≈ 8.8) followed by [Ag(S₂O₃)]⁻ + S₂O₃²⁻ ⇌ [Ag(S₂O₃)₂]³⁻ (log K₂ ≈ 4.6), yielding an overall formation constant log β₂ ≈ 13.4 at 25°C and low ionic strength; these values underscore the complex's robustness against dissociation. Synthetic routes typically involve direct reaction of the metal salt (e.g., AgNO₃ or HgCl₂) with Na₂S₂O₃ in aqueous media, often at neutral pH to avoid decomposition. Spectroscopic techniques provide evidence for thiosulfate's coordination modes and electronic effects in these complexes. UV-Vis spectroscopy reveals ligand-to-metal charge-transfer (LMCT) bands in the 250–350 nm range for S-bound thiosulfates, with bathochromic shifts relative to free ligand absorptions indicating sulfur-metal σ-donation. In cases of bidentate coordination, such as certain Pd(II) or Ni(II) complexes, additional vibrational modes in IR spectra (e.g., S–O stretches at ~1100 cm⁻¹) confirm O-involvement. NMR and EPR studies further elucidate site-specific binding, showing distinct chemical shifts for S₂O₃²⁻ protons or electrons in paramagnetic systems.[44][45]Applications
Medical uses
Sodium thiosulfate is primarily used as an antidote for acute cyanide poisoning, where it is administered intravenously as part of a combination therapy with sodium nitrite. The standard dosage is 250 mg/kg (approximately 12.5 g for a 50 kg adult) of a 25% or 30% solution infused over 10 minutes, following sodium nitrite administration to enhance efficacy. This treatment works by donating sulfur to the enzyme rhodanese, which converts toxic cyanide ions to the less harmful thiocyanate, for renal excretion.[46][47][48] Recent studies as of 2024 have confirmed its otoprotective efficacy in adult patients with cancer treated with platinum compounds, reducing cisplatin-induced hearing loss beyond pediatric applications. Additionally, as of January 2025, research demonstrates its chemoprotective role against cisplatin-induced nephrotoxicity through hydrogen sulfide donation, supporting further investigation for renal protection during chemotherapy. A November 2025 case report also highlights its use in treating nephrogenic systemic fibrosis by addressing metastatic pulmonary calcification.[49][50][51] In patients with end-stage renal disease, sodium thiosulfate is commonly used off-label for the treatment of calciphylaxis (calcific uremic arteriolopathy), a rare and painful condition involving vascular calcification and skin necrosis. Typical dosing involves a 25% solution administered intravenously at 25 g three times per week, often during hemodialysis sessions, which has been associated with reduced pain and lesion improvement in observational studies. Although not specifically FDA-approved for this indication, its use is supported by its safety profile and potential mechanisms, including calcium chelation and antioxidant effects.[52][53][54] Sodium thiosulfate is FDA-approved for reducing the risk of ototoxicity associated with cisplatin chemotherapy in pediatric patients aged 1 month and older with localized, non-metastatic solid tumors. The recommended dose is 12.5 g/m² administered by intravenous infusion over 15 minutes, starting 6 hours after each cisplatin dose. Clinical trials demonstrated a significant reduction in hearing loss, with incidence rates of 39% in the sodium thiosulfate group compared to 68% in the cisplatin-only group, representing approximately a 43% relative risk reduction.[55][56][57] As an antidote for chemotherapy extravasation, particularly with alkylating agents like mechlorethamine or cisplatin, sodium thiosulfate is injected subcutaneously around the site at a concentration of 1/6 M (approximately 2 mL per site) to neutralize the vesicant and limit tissue damage. Topically, a 20-25% solution is applied twice daily for the treatment of pityriasis versicolor, a superficial fungal infection, with clinical resolution often requiring weeks of therapy.[58][59] Pharmacokinetically, sodium thiosulfate exhibits rapid distribution following intravenous administration, with a plasma half-life of approximately 20-50 minutes and total clearance of about 2.2 mL/min/kg in pediatric patients. Approximately 20-50% is eliminated unchanged in the urine, while the remainder is metabolized or oxidized to sulfate; in the context of cyanide detoxification, the produced thiocyanate is primarily excreted renally.[60][61][57]Photographic processing
Sodium thiosulfate serves as a key fixing agent in traditional photographic processing, where it dissolves unexposed silver halide crystals from the emulsion after development, stabilizing the image by forming a soluble coordination complex that prevents further reaction to light.[62] The primary reaction involves silver bromide, a common halide in black-and-white emulsions:This process removes the unexposed AgBr as the sodium silver thiosulfate complex, Na₃[Ag(S₂O₃)₂], which is water-soluble and can be washed away, leaving only the developed metallic silver image intact.[63] In practice, the fixing solution, commonly known as "hypo," is prepared as a 10-20% aqueous solution of sodium thiosulfate, typically around 160 g/L for standard use.[64] Film or paper is immersed in this solution with agitation for 5-10 minutes, depending on the material thickness and emulsion type, to ensure complete removal of halides; a two-bath method—5 minutes in each fresh bath—is often recommended for archival processing to extend solution life and thoroughness.[65] These formulations are usually buffered to a neutral pH of 6-7 using additives like sodium sulfite to maintain stability and prevent emulsion hardening, though rapid variants incorporate ammonium chloride or sulfate to accelerate fixing by partially converting to ammonium thiosulfate in situ.[66] The use of sodium thiosulfate in photography dates to its discovery in 1819 by Sir John Herschel, who identified its ability to dissolve silver halides, providing the first reliable fixer for permanent images; he shared this finding with pioneers like William Henry Fox Talbot and Louis Daguerre in 1839, making it indispensable for early processes such as daguerreotypes and later gelatin silver emulsions.[67] Compared to earlier fixers like sodium chloride or toxic cyanide salts, sodium thiosulfate offered a non-toxic, effective alternative that acted rapidly without excessively hardening the gelatin emulsion, enabling safer and more consistent results in both amateur and professional workflows.[68] While sodium thiosulfate remains favored in archival black-and-white processing for its stability and compatibility with traditional emulsions, its role has declined in modern rapid workflows, where ammonium thiosulfate fixers—twice as fast and better suited to high-iodide films—have largely replaced it for commercial labs and color processing.[69]


