Recent from talks
Nothing was collected or created yet.
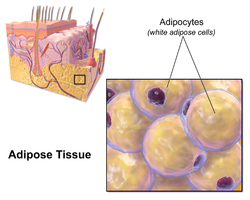
Adipocyte
View on Wikipedia| Adipocyte | |
|---|---|
 Illustration depicting white fat cells. | |
 Morphology of three different classes of adipocytes | |
| Details | |
| Identifiers | |
| Latin | adipocytus |
| MeSH | D017667 |
| TH | H2.00.03.0.01005 |
| FMA | 63880 |
| Anatomical terms of microanatomy | |
Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat.[1] Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocyte progenitors can also form osteoblasts, myocytes and other cell types.
There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which are also known as white and brown fat, respectively, and comprise two types of fat cells.
Structure
[edit]White fat cells
[edit]

White fat cells contain a single large lipid droplet surrounded by a layer of cytoplasm, and are known as unilocular. The nucleus is flattened and pushed to the periphery. A typical fat cell is 0.1 mm in diameter[2] with some being twice that size, and others half that size. However, these numerical estimates of fat cell size depend largely on the measurement method and the location of the adipose tissue.[2] The fat stored is in a semi-liquid state, and is composed primarily of triglycerides, and cholesteryl ester. White fat cells secrete many proteins acting as adipokines such as resistin, adiponectin, leptin and apelin. An average human adult has 30 billion fat cells with a weight of 30 lbs or 13.5 kg. If a child or adolescent gains sufficient excess weight, fat cells may increase in absolute number until age twenty-four.[3] If an adult (who never was obese as a child or adolescent) gains excess weight, fat cells generally increase in size, not number, though there is some inconclusive evidence suggesting that the number of fat cells might also increase if the existing fat cells become large enough (as in particularly severe levels of obesity).[3] The number of fat cells is difficult to decrease through dietary intervention, though some evidence suggests that the number of fat cells can decrease if weight loss is maintained for a sufficiently long period of time (>1 year; though it is extremely difficult for people with larger and more numerous fat cells to maintain weight loss for that long a time).[3]
A large meta-analysis has shown that white adipose tissue cell size is dependent on measurement methods, adipose tissue depots, age, and body mass index; for the same degree of obesity, increases in fat cell size were also associated with the dysregulations in glucose and lipid metabolism.[2]
Brown fat cells
[edit]Brown fat cells are polyhedral in shape. Brown fat is derived from dermatomyocyte cells. Unlike white fat cells, these cells have considerable cytoplasm, with several lipid droplets scattered throughout, and are known as multilocular cells. The nucleus is round and, although eccentrically located, it is not in the periphery of the cell. The brown color comes from the large quantity of mitochondria. Brown fat, also known as "baby fat," is used to generate heat.
Marrow fat cells
[edit]Marrow adipocytes are unilocular like white fat cells. The marrow adipose tissue depot is poorly understood in terms of its physiologic function and relevance to bone health. Marrow adipose tissue expands in states of low bone density but additionally expands in the setting of obesity.[4] Marrow adipose tissue response to exercise approximates that of white adipose tissue.[4][5][6][7] Exercise reduces both adipocyte size as well as marrow adipose tissue volume, as quantified by MRI or μCT imaging of bone stained with the lipid binder osmium.
Development
[edit]
Pre-adipocytes are undifferentiated fibroblasts that can be stimulated to form adipocytes. Studies have shed light into potential molecular mechanisms in the fate determination of pre-adipocytes although the exact lineage of adipocyte is still unclear.[8][9] The variation of body fat distribution resulting from normal growth is influenced by nutritional and hormonal status dependent on intrinsic differences in cells found in each adipose depot.[10]
Mesenchymal stem cells can differentiate into adipocytes, connective tissue, muscle or bone.[1]
The precursor of the adult cell is termed a lipoblast, and a tumor of this cell type is known as a lipoblastoma.[11]
Function
[edit]Cell turnover
[edit]Fat cells in some mice have been shown to drop in count due to fasting and other properties were observed when exposed to cold.[12]
If the adipocytes in the body reach their maximum capacity of fat, they may replicate to allow additional fat storage.
Adult rats of various strains became obese when they were fed a highly palatable diet for several months. Analysis of their adipose tissue morphology revealed increases in both adipocyte size and number in most depots. Reintroduction of an ordinary chow diet[13] to such animals precipitated a period of weight loss during which only mean adipocyte size returned to normal. Adipocyte number remained at the elevated level achieved during the period of weight gain.[14]
According to some reports and textbooks, the number of adipocytes can increase in childhood and adolescence, though the amount is usually constant in adults. Individuals who become obese as adults, rather than as adolescents, have no more adipocytes than they had before.[15]
People who have been fat since childhood generally have an inflated number of fat cells. People who become fat as adults may have no more fat cells than their lean peers, but their fat cells are larger. In general, people with an excess of fat cells find it harder to lose weight and keep it off than the obese who simply have enlarged fat cells.[3]
Body fat cells have regional responses to the overfeeding that was studied in adult subjects. In the upper body, an increase of adipocyte size correlated with upper-body fat gain; however, the number of fat cells was not significantly changed. In contrast to the upper body fat cell response, the number of lower-body adipocytes did significantly increase during the course of experiment. Notably, there was no change in the size of the lower-body adipocytes.[16]
Approximately 10% of fat cells are renewed annually at all adult ages and levels of body mass index without a significant increase in the overall number of adipocytes in adulthood.[15]
Adaptation
[edit]Obesity is characterized by the expansion of fat mass, through adipocyte size increase (hypertrophy) and, to a lesser extent, cell proliferation (hyperplasia).[17][2] In the fatty tissue of obese individuals, there is increased production of metabolism modulators, such as glycerol, hormones, macrophage-stimulating chemokines, and pro-inflammatory cytokines, leading to the development of insulin resistance.[18] Production of these modulators and the resulting pathogenesis of insulin resistance are probably caused by adipocytes as well as immune system macrophages that infiltrate the tissue.[19]
Fat production in adipocytes is strongly stimulated by insulin. By controlling the activity of the pyruvate dehydrogenase and the acetyl-CoA carboxylase enzymes, insulin promotes unsaturated fatty acid synthesis. It also promotes glucose uptake and induces SREBF1, which activates the transcription of genes that stimulate lipogenesis.[20]
SREBF1 (sterol regulatory element-binding transcription factor 1) is a transcription factor synthesized as an inactive precursor protein inserted into the endoplasmic reticulum (ER) membrane by two membrane-spanning helices. Also anchored in the ER membrane is SCAP (SREBF-cleavage activating protein), which binds SREBF1. The SREBF1-SCAP complex is retained in the ER membrane by INSIG1 (insulin-induced gene 1 protein). When sterol levels are depleted, INSIG1 releases SCAP and the SREBF1-SCAP complex can be sorted into transport vesicles coated by the coatomer COPII that are exported to the Golgi apparatus. In the Golgi apparatus, SREBF1 is cleaved and released as a transcriptionally active mature protein. It is then free to translocate to the nucleus and activate the expression of its target genes.[21]

Clinical studies have repeatedly shown that even though insulin resistance is usually associated with obesity, the membrane phospholipids of the adipocytes of obese patients generally still show an increased degree of fatty acid unsaturation.[22] This seems to point to an adaptive mechanism that allows the adipocyte to maintain its functionality, despite the increased storage demands associated with obesity and insulin resistance.
A study conducted in 2013[22] found that, while INSIG1 and SREBF1 mRNA expression was decreased in the adipose tissue of obese mice and humans, the amount of active SREBF1 was increased in comparison with normal mice and non-obese patients. This downregulation of INSIG1 expression combined with the increase of mature SREBF1 was also correlated with the maintenance of SREBF1-target gene expression. Hence, it appears that, by downregulating INSIG1, there is a resetting of the INSIG1/SREBF1 loop, allowing for the maintenance of active SREBF1 levels. This seems to help compensate for the anti-lipogenic effects of insulin resistance and thus preserve adipocyte fat storage abilities and availability of appropriate levels of fatty acid unsaturation in face of the nutritional pressures of obesity.
Endocrine role
[edit]Adipocytes can synthesize estrogens from androgens,[23] potentially being the reason why being underweight or overweight are risk factors for infertility.[24] Additionally, adipocytes are responsible for the production of the hormone leptin. Leptin is important in regulation of appetite and acts as a satiety factor.[25]
See also
[edit]References
[edit]- ^ a b Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (August 2013). "Role of pericytes in skeletal muscle regeneration and fat accumulation". Stem Cells and Development. 22 (16): 2298–2314. doi:10.1089/scd.2012.0647. PMC 3730538. PMID 23517218.
- ^ a b c d Ye RZ, Richard G, Gévry N, Tchernof A, Carpentier AC (January 2022). "Fat Cell Size: Measurement Methods, Pathophysiological Origins, and Relationships With Metabolic Dysregulations". Endocrine Reviews. 43 (1): 35–60. doi:10.1210/endrev/bnab018. PMC 8755996. PMID 34100954.
- ^ a b c d Pool R (2001). Fat: fighting the obesity epidemic. Oxford [Oxfordshire]: Oxford University Press. pp. 68. ISBN 978-0-19-511853-7.
- ^ a b Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. (August 2017). "Exercise Decreases Marrow Adipose Tissue Through β-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research. 32 (8): 1692–1702. doi:10.1002/jbmr.3159. PMC 5550355. PMID 28436105.
- ^ Pagnotti GM, Styner M (2016). "Exercise Regulation of Marrow Adipose Tissue". Frontiers in Endocrinology. 7: 94. doi:10.3389/fendo.2016.00094. PMC 4943947. PMID 27471493.
- ^ Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. (August 2015). "Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice". Endocrinology. 156 (8): 2753–2761. doi:10.1210/en.2015-1213. PMC 4511140. PMID 26052898.
- ^ Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. (July 2014). "Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise". Bone. 64: 39–46. doi:10.1016/j.bone.2014.03.044. PMC 4041821. PMID 24709686.
- ^ Coskun H, Summerfield TL, Kniss DA, Friedman A (July 2010). "Mathematical modeling of preadipocyte fate determination". Journal of Theoretical Biology. 265 (1): 87–94. Bibcode:2010JThBi.265...87C. doi:10.1016/j.jtbi.2010.03.047. PMID 20385145.
- Lay summary in: "Scientists closer to finding what causes the birth of a fat cell". ScienceDaily. August 18, 2010.
- ^ Coskun H, Summerfield TL, Kniss DA, Friedman A (July 2010). "Mathematical modeling of preadipocyte fate determination". Journal of Theoretical Biology. 265 (1): 87–94. Bibcode:2010JThBi.265...87C. doi:10.1016/j.jtbi.2010.03.047. PMID 20385145.
- ^ Fried SK, Lee MJ, Karastergiou K (July 2015). "Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology". Obesity (Review). 23 (7): 1345–1352. doi:10.1002/oby.21133. PMC 4687449. PMID 26054752.
- ^ Hong R, Choi DY, Do NY, Lim SC (July 2008). "Fine-needle aspiration cytology of a lipoblastoma: a case report". Diagnostic Cytopathology. 36 (7): 508–511. doi:10.1002/dc.20826. PMID 18528880. S2CID 22668394.
- ^ Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, et al. (May 2016). "Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16". Nature Communications. 7 11533. Bibcode:2016NatCo...711533D. doi:10.1038/ncomms11533. PMC 4895052. PMID 27240637.
- ^ Warden CH, Fisler JS (April 2008). "Comparisons of diets used in animal models of high-fat feeding". Cell Metabolism. 7 (4): 277. doi:10.1016/j.cmet.2008.03.014. PMC 2394560. PMID 18396128.
Regular chow is composed of agricultural byproducts, such as ground wheat, corn, or oats, alfalfa and soybean meals, a protein source such as fish, and vegetable oil and is supplemented with minerals and vitamins. Thus, chow is a high fiber diet containing complex carbohydrates, with fats from a variety of vegetable sources. Chow is inexpensive to manufacture and is palatable to rodents.
- ^ Faust IM, Johnson PR, Stern JS, Hirsch J (September 1978). "Diet-induced adipocyte number increase in adult rats: a new model of obesity". The American Journal of Physiology. 235 (3): E279 – E286. doi:10.1152/ajpendo.1978.235.3.E279. PMID 696822. S2CID 7744250.
- ^ a b Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. (June 2008). "Dynamics of fat cell turnover in humans". Nature. 453 (7196): 783–787. Bibcode:2008Natur.453..783S. doi:10.1038/nature06902. PMID 18454136. S2CID 4431237.
- ^ Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD (October 2010). "Regional differences in cellular mechanisms of adipose tissue gain with overfeeding". Proceedings of the National Academy of Sciences of the United States of America. 107 (42): 18226–18231. doi:10.1073/pnas.1005259107. PMC 2964201. PMID 20921416.
- ^ Blüher M (June 2009). "Adipose tissue dysfunction in obesity". Experimental and Clinical Endocrinology & Diabetes. 117 (6): 241–250. doi:10.1055/s-0029-1192044. PMID 19358089.
- ^ Kahn SE, Hull RL, Utzschneider KM (December 2006). "Mechanisms linking obesity to insulin resistance and type 2 diabetes". Nature. 444 (7121): 840–846. Bibcode:2006Natur.444..840K. doi:10.1038/nature05482. PMID 17167471. S2CID 120626.
- ^ Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. (March 2006). "Recent advances in the relationship between obesity, inflammation, and insulin resistance". European Cytokine Network. 17 (1): 4–12. PMID 16613757.
Several factors derived not only from adipocytes but also from infiltrated macrophages probably contribute to the pathogenesis of insulin resistance.
- ^ Kahn BB, Flier JS (August 2000). "Obesity and insulin resistance". The Journal of Clinical Investigation. 106 (4): 473–481. doi:10.1172/JCI10842. PMC 380258. PMID 10953022.
- ^ Rawson RB (August 2003). "The SREBP pathway--insights from Insigs and insects". Nature Reviews. Molecular Cell Biology. 4 (8): 631–640. doi:10.1038/nrm1174. PMID 12923525. S2CID 20818196.
- ^ a b Carobbio S, Hagen RM, Lelliott CJ, Slawik M, Medina-Gomez G, Tan CY, et al. (November 2013). "Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity". Diabetes. 62 (11): 3697–3708. doi:10.2337/db12-1748. PMC 3806615. PMID 23919961.
- ^ Nelson LR, Bulun SE (September 2001). "Estrogen production and action". Journal of the American Academy of Dermatology. 45 (3 Suppl): S116 – S124. doi:10.1067/mjd.2001.117432. PMID 11511861.
- ^ "FERTILITY FACT: Female Risks". American Society for Reproductive Medicine (ASRM). Archived from the original on 22 September 2007.
- ^ Klok MD, Jakobsdottir S, Drent ML (January 2007). "The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review". Obesity Reviews. 8 (1): 21–34. doi:10.1111/j.1467-789X.2006.00270.x. PMID 17212793. S2CID 24266123.
External links
[edit]- Histology image: 08201loa – Histology Learning System at Boston University – "Connective Tissue: unilocular (white) adipocytes "
- Histology image: 04901lob – Histology Learning System at Boston University – "Connective Tissue: multilocular (brown) adipocytes"
Adipocyte
View on GrokipediaOverview and Classification
Definition and Role in the Body
Adipocytes, also known as fat cells, are specialized cells of connective tissue that primarily function to store energy in the form of lipids, particularly triglycerides, while also providing insulation and mechanical cushioning to the body.[1] These cells originate from mesenchymal precursor cells within the stromal vascular fraction of adipose tissue, undergoing a differentiation process known as adipogenesis to become mature lipid-laden cells.[1] In humans, the total number of adipocytes is established during childhood and adolescence, with adults typically possessing 20–60 billion such cells on average, though this can vary based on factors like body mass index and obesity status.[5] Adipocytes are distributed across various depots in the body, including subcutaneous adipose tissue beneath the skin, visceral adipose tissue surrounding internal organs such as the liver and intestines, and intra-organ depots like epicardial fat around the heart.[1] These locations allow adipocytes to serve as a dynamic energy reservoir, storing excess caloric intake during periods of abundance and mobilizing fatty acids through lipolysis when energy demands increase, such as during fasting or exercise.[6] Beyond energy homeostasis, adipocytes contribute to thermal insulation by reducing heat loss from the body and offer mechanical protection by cushioning vital organs against physical trauma.[1] From an evolutionary perspective, adipocytes play a crucial role in maintaining energy balance, enabling survival during periods of food scarcity or famine by providing a readily accessible reserve of calories that can sustain vital functions.[6] This adaptation underscores the importance of adipose tissue as a metabolic buffer in fluctuating nutritional environments. Historically, adipocytes were first recognized as distinct cellular entities in the 19th century by anatomists studying connective tissues, marking the beginning of systematic investigations into their structure and function.[7] While adipocytes are broadly classified into types such as white, brown, and beige based on their metabolic properties, their core role remains centered on lipid management across all variants.Types of Adipocytes
White adipocytes represent the predominant type of fat cells in adults, characterized by a unilocular morphology with a single large lipid droplet that occupies most of the cell volume, enabling efficient long-term energy storage primarily as triglycerides. These cells are distributed across subcutaneous depots beneath the skin and visceral depots surrounding internal organs, collectively accounting for the majority (~90%) of total body fat storage.[8] In contrast, brown adipocytes are multilocular cells containing multiple small lipid droplets and a dense concentration of mitochondria, adaptations that support their primary function of thermogenesis through uncoupled respiration. These cells are concentrated in specific depots, such as the interscapular region in infants for non-shivering heat production, and persist into adulthood in areas like the neck and along the spine.[9][10] Beige adipocytes constitute an inducible subtype of multilocular cells that arise within white adipose depots in response to environmental or hormonal stimuli, including cold exposure and β-adrenergic signaling, thereby exhibiting thermogenic capabilities that bridge white and brown adipocyte functions. This subtype was characterized in research emerging in the early 2000s, highlighting their role as an adaptive thermogenic reserve.[11][12] Specialized variants include marrow adipocytes, which populate bone marrow and regulate hematopoiesis as a distinct white adipocyte subtype, and pink adipocytes, which transiently form in mammary glands during lactation to support milk lipid secretion and are also derived from white adipocyte lineages.[13][14] Adipocyte distribution evolves postnatally, with white adipose tissue expanding to accommodate increasing energy storage demands, brown adipose tissue regressing after infancy while retaining adult depots for metabolic flexibility, and beige adipocytes emerging as an inducible response to physiological stressors.[15]Cellular Structure and Morphology
General Features
Adipocytes are defined by their unique cellular architecture, featuring a prominent central lipid droplet that occupies up to 90% of the cell volume and primarily stores triglycerides. This droplet is enveloped by a phospholipid monolayer that interfaces with the surrounding cytoplasm, maintaining structural integrity while allowing metabolic interactions. The dominance of the lipid droplet compresses the nucleus and other organelles to the cell periphery, creating a thin cytoplasmic rim that encases the storage core. Within this peripheral cytoplasm, essential organelles support cellular maintenance and lipid handling. The endoplasmic reticulum plays a key role in de novo lipid synthesis, facilitating the assembly of triglycerides from fatty acids and glycerol. The Golgi apparatus handles protein processing, glycosylation, and packaging for secretion or membrane integration, while lysosomes contribute to the degradation of cellular waste and damaged components through hydrolytic enzymes. The plasma membrane of adipocytes is adapted for tissue integration and responsiveness, incorporating integrins that anchor the cell to the extracellular matrix via adhesion to collagen and laminin. Caveolae, flask-shaped invaginations rich in caveolin proteins, cluster signaling molecules and regulate mechanosensing and lipid transport at the membrane surface. Adipocyte size varies typically from 50 to 200 μm in diameter, allowing flexibility in lipid storage capacity while the lipid droplet consistently dominates the intracellular volume. Electron microscopy provides high-resolution views of the lipid droplet's monolayer and peripheral organelle arrangement, revealing fine structural details not visible by light microscopy. In histological preparations, Oil Red O staining specifically targets neutral lipids, imparting a red coloration to the droplets for clear visualization in frozen tissue sections. While these general features are shared across adipocyte types, subtle variations exist in organelle distribution and droplet characteristics.Type-Specific Variations
White adipocytes are characterized by a single large unilocular lipid droplet that occupies most of the cell volume, accompanied by few mitochondria and sparse cytoplasm, which optimizes the cell for efficient lipid storage.[16] This morphology results in a flattened nucleus pushed to the cell periphery and minimal intracellular space for other organelles.[17] In contrast, brown adipocytes feature multiple small lipid droplets distributed throughout the cytoplasm, creating a multilocular appearance, along with abundant mitochondria rich in uncoupling protein 1 (UCP1) that supports uncoupled respiration.[9] These cells also exhibit a dense capillary network for enhanced nutrient and oxygen delivery, and their characteristic brown pigmentation arises from iron-containing cytochromes in the mitochondria.[18] The nucleus is typically centrally located amid the lipid droplets and organelles.[17] Beige adipocytes display a transitional multilocular morphology with inducible UCP1 expression in their mitochondria, featuring an intermediate density of these organelles compared to white and brown types.[19] They exhibit heterogeneity across depots, with some cells showing clustered small lipid droplets and others retaining larger ones, and their nucleus often shifts from a peripheral to a more central position upon activation.[17] Vascularization in beige adipocytes is generally less dense than in brown but more prominent than in white.[9] Pink adipocytes, which appear transiently in the mammary gland adipose tissue during late pregnancy and lactation in rodents and other mammals, undergo dedifferentiation from white adipocytes, resulting in smaller cells with reduced and fragmented lipid droplets, enhanced secretory machinery, and an intermediate morphology between adipocytes and milk-producing alveoli to facilitate lipid transfer for milk production.[20] Upon weaning, they revert to white adipocyte morphology.[14]| Feature | White Adipocytes | Brown Adipocytes | Beige Adipocytes | Pink Adipocytes |
|---|---|---|---|---|
| Lipid Droplet Number | Single (unilocular) | Multiple (multilocular) | Multiple (transitional multilocular) | Reduced/fragmented (transient) |
| Mitochondrial Density | Low | High (UCP1-rich) | Intermediate (inducible UCP1) | Low (similar to white) |
| Vascularization | Sparse | Dense capillary network | Moderate, variable by depot | Enhanced in mammary gland context |
| Pigmentation | None (pale) | Brown (iron in cytochromes) | Pale to light brown | None (pale) |