Recent from talks
Nothing was collected or created yet.
Glipizide
View on Wikipedia
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucotrol, Glucotrol XL, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684060 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sulfonylurea |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (regular formulation) 90% (extended release) |
| Protein binding | 98 to 99% |
| Metabolism | Liver hydroxylation |
| Elimination half-life | 2 to 5 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.919 |
| Chemical and physical data | |
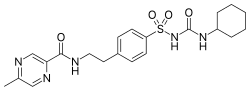
| Formula | C21H27N5O4S |
| Molar mass | 445.54 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 209 °C (406 to 408 °F) |
| |
| |
| | |
Glipizide, sold under the brand name Glucotrol among others, is an anti-diabetic medication of the sulfonylurea class used to treat type 2 diabetes.[1][2] It is used together with a diabetic diet and exercise.[1][2] It is not indicated for use by itself in type 1 diabetes.[1][2] It is taken by mouth.[1][2] Effects generally begin within half an hour and can last for up to a day.[1]
Common side effects include nausea, diarrhea, low blood sugar, and headache.[1] Other side effects include sleepiness, skin rash, and shakiness.[3] The dose may need to be adjusted in those with liver or kidney disease.[1] Use during pregnancy or breastfeeding is not recommended.[3] It works by stimulating the pancreas to release insulin and increases tissue sensitivity to insulin.[1]
Glipizide was approved for medical use in the United States in 1984.[1] It is available as a generic medication.[1] In 2023, it was the 42nd most commonly prescribed medication in the United States, with more than 15 million prescriptions.[4][5]
Mechanism of action
[edit]Glipizide sensitizes the beta cells of pancreatic islets of Langerhans insulin response, meaning that more insulin is released in response to glucose than would be without glipizide ingestion.[2] Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes, which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells.[6]
History
[edit]It was patented in 1969, and approved for medical use in 1971.[7] Glipizide was approved for medical use in the United States in 1984.[1]
Synthesis
[edit]Synthesis of glipizide:[8][9] Alternative:[10] Cmp#3:[11] Related article:[12]

5-Methylpyrazine-2-carboxylic acid [5521-55-1] (1) is converted to its acid chloride. A Schotten-Baumann reaction is then performed with 4-(2-aminoethyl)benzene sulfonamide [35303-76-5] (2) to give the corresponding amide PC9883549 [33288-71-0] (3). This forms glipizide on reaction with cyclohexylisocyanide and base in acetone.
References
[edit]- ^ a b c d e f g h i j k "Glipizide Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 13 January 2020. Retrieved 24 December 2018.
- ^ a b c d e "Glucotrol XL- glipizide tablet, extended release". DailyMed. 17 August 2018. Archived from the original on 16 February 2017. Retrieved 31 July 2020.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 693. ISBN 9780857113382.
- ^ "Top 300 of 2023". ClinCalc. Archived from the original on 12 August 2025. Retrieved 12 August 2025.
- ^ "Glipizide Drug Usage Statistics, United States, 2013 - 2023". ClinCalc. Retrieved 18 August 2025.
- ^ Bösenberg LH, Van Zyl DG (December 2008). "The mechanism of action of oral antidiabetic drugs: a review of recent literature". Journal of Endocrinology, Metabolism and Diabetes of South Africa. 13 (3): 80–8. doi:10.1080/22201009.2008.10872177. hdl:2263/10139.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495. Archived from the original on 10 January 2023. Retrieved 31 July 2020.
- ^ Ambrogi et al., Arzneim.-Forsch. 21, 200 (1971).
- ^ DE2012138 idem Vittorio Bresso Mailand Ambrogi, Willy Mailand Logemann, US3669966 (1978 to Carlo Erba S.P.A., Mailand (Italien)).
- ^ Mikael Dahlström & Yngve Malmen, EP0149592 (1985 to LAKEMEDELSFABRIKEN-MEDICA AB).
- ^ R. K. Sarma & P. L. Kamat, US5516906 (1995 to USV Pvt Ltd).
- ^ Ambrogi, V.; Bloch, K.; Daturi, S.; Logemann, W.; Parenti, M.A. (1972). "Synthesis of Pyrazine Derivatives as Potential Hypoglycemic Agents". Journal of Pharmaceutical Sciences. 61 (9): 1483–1486. doi:10.1002/jps.2600610933.

