Recent from talks
Contribute something
Nothing was collected or created yet.
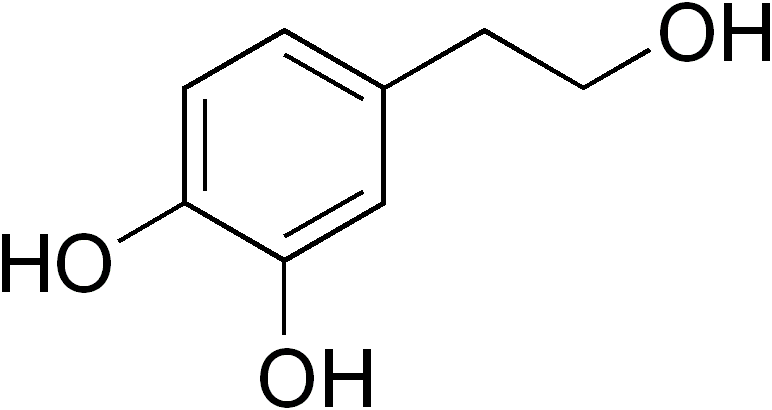
Hydroxytyrosol
View on Wikipedia
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-(2-Hydroxyethyl)benzene-1,2-diol | |
| Other names
3-Hydroxytyrosol
3,4-dihydroxyphenylethanol (DOPET) Dihydroxyphenylethanol 2-(3,4-Di-hydroxyphenyl)-ethanol (DHPE) 3,4-dihydroxyphenolethanol (3,4-DHPEA)[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.114.418 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10O3 | |
| Molar mass | 154.165 g·mol−1 |
| Appearance | colorless solid |
| 5 g/100 ml | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Causes skin irritation.
Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | [1] |
| Related compounds | |
Related alcohols
|
benzyl alcohol, tyrosol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxytyrosol is an organic compound with the formula (HO)2C6H3CH2CH2OH. It is a phenylethanoid, i.e. a relative of phenethyl alcohol. Its derivatives are found in a variety of natural sources, notably olive oils and wines. Hydroxytyrosol is a colorless solid,[3][4] although samples often turn beige during storage. It is a derivative, formally speaking, of catechol.
Hydroxytyrosol and its derivatives occur in olives and in wines.[5][6]
Occurrence
[edit]Olives
[edit]
The olives, leaves, and olive pulp contain large amounts of hydroxytyrosol derivative oleuropein, more so than olive oil.[1] Unprocessed, green (unripe) olives contain between 4.3 and 116 mg of hydroxytyrosol per 100 g of olives, while unprocessed, black (ripe) olives contain up to 413.3 mg per 100 g.[7] The ripening of an olive substantially increases the amount of hydroxytyrosol.[8] Processed olives, such as the common canned variety containing iron(II) gluconate, contain little hydroxytyrosol, as iron salts are catalysts for its oxidation.[9]
Food safety
[edit]Hydroxytyrosol is considered safe as a novel food for human consumption, with a no-observed-adverse-effect level of 50 mg/kg body weight per day, as evaluated by the European Food Safety Authority (EFSA).[10]
In the United States, hydroxytyrosol is considered to be a safe ingredient (GRAS) in processed foods at levels of 5 mg per serving.[11]
Function and production
[edit]
In nature, hydroxytyrosol is generated by the hydrolysis of oleuropein that occurs during olive ripening. Oleuropein accumulates in olive leaves and fruit as a defense mechanism against pathogens and herbivores. During olive ripening or when the olive tissue is damaged by pathogens, herbivores, or mechanical damage, the enzyme β-glucosidase catalyzes hydroxytyrosol synthesis via hydrolysis from oleuropein.[12]
Metabolism
[edit]Shortly after olive oil consumption, 98% of hydroxytyrosol in plasma and urine appears in conjugated forms (65% glucuronoconjugates), suggesting extensive first-pass metabolism and a half-life of 2.43 hours.[13]
Mediterranean diet
[edit]Mediterranean diets, characterized by regular intake of olive oil, have been shown to positively affect human health, including reduced rates of cardiovascular diseases.[5][14][15] Research on consumption of olive oil and its components includes hydroxytyrosol and oleuropein, which may inhibit oxidation of LDL cholesterol – a risk factor for atherosclerosis, heart attack or stroke.[16] The daily intake of hydroxytyrosol within the Mediterranean diet is estimated to be between 0.15 and 30 mg.[17]
Regulation
[edit]Europe
[edit]The EFSA has issued a scientific opinion on health claims in relation to dietary consumption of hydroxytyrosol and related polyphenol compounds from olive fruit and oil, and protection of blood lipids from potential oxidative damage.[18]
EFSA concluded that a cause-and-effect relationship existed between the consumption of hydroxytyrosol and related compounds from olives and olive oil and protection of blood lipids from oxidative damage,[18] providing a health claim for consumption of olive oil polyphenols containing at least 5 mg of hydroxytyrosol and its derivatives (oleuropein complex and tyrosol) per 20 g of olive oil.[18]
See also
[edit]- Echinacoside, a hydroxytyrosol-containing glycoside
- Tyrosol
- Verbascoside, another hydroxytyrosol-containing glycoside
- Resveratrol
References
[edit]- ^ a b Baldioli M, Servili M, Perretti G, Montedoro GF (1996). "Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil". Journal of the American Oil Chemists' Society. 73 (11): 1589–1593. doi:10.1007/BF02523530. S2CID 84749200.
- ^ "Hydroxytyrosol". PubChem. U.S. National Library of Medicine.
- ^ Charoenprasert S, Mitchell A (July 2012). "Factors influencing phenolic compounds in table olives (Olea europaea)". Journal of Agricultural and Food Chemistry. 60 (29): 7081–7095. Bibcode:2012JAFC...60.7081C. doi:10.1021/jf3017699. PMID 22720792.
- ^ Karković Marković A, Torić J, Barbarić M, Jakobušić Brala C (May 2019). "Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health". Molecules. 24 (10): 2001. doi:10.3390/molecules24102001. PMC 6571782. PMID 31137753.
- ^ a b Fernández-Mar MI, Mateos R, Garcia-Parrilla MC, Puertas B, Cantos-Villar E (2012-02-15). "Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review". Food Chemistry. 130 (4): 797–813. doi:10.1016/j.foodchem.2011.08.023. ISSN 0308-8146.
- ^ Hu T, He XW, Jiang JG, Xu XL (February 2014). "Hydroxytyrosol and its potential therapeutic effects". Journal of Agricultural and Food Chemistry. 62 (7): 1449–1455. Bibcode:2014JAFC...62.1449H. doi:10.1021/jf405820v. PMID 24479643.
- ^ "Showing all foods in which the polyphenol Hydroxytyrosol is found - Phenol-Explorer". phenol-explorer.eu. Retrieved 2021-07-02.
- ^ Rocha J, Borges N, Pinho O (2020). "Table olives and health: a review". Journal of Nutritional Science. 9 e57. doi:10.1017/jns.2020.50. PMC 7737178. PMID 33354328.
- ^ Marsilio V, Campestre C, Lanza B (July 2001). "Phenolic compounds change during California-style ripe olive processing". Food Chemistry. 74 (1): 55–60. doi:10.1016/S0308-8146(00)00338-1.
- ^ Turck D, Bresson JL, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, et al. (March 2017). "Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97". EFSA Journal. 15 (3): e04728. doi:10.2903/j.efsa.2017.4728. PMC 7010075. PMID 32625437.
- ^ "GRAS notice for hydroxytyrosol". US Food and Drug Administration. 13 May 2016. Retrieved 2 July 2021.
- ^ Charoenprasert S, Mitchell A (July 2012). "Factors influencing phenolic compounds in table olives (Olea europaea)". Journal of Agricultural and Food Chemistry. 60 (29): 7081–7095. Bibcode:2012JAFC...60.7081C. doi:10.1021/jf3017699. PMID 22720792.
- ^ Miro-Casas E, Covas MI, Farre M, Fito M, Ortuño J, Weinbrenner T, et al. (June 2003). "Hydroxytyrosol disposition in humans". Clinical Chemistry. 49 (6 Pt 1): 945–952. doi:10.1373/49.6.945. PMID 12765992.
- ^ Hu T, He XW, Jiang JG, Xu XL (February 2014). "Hydroxytyrosol and its potential therapeutic effects". Journal of Agricultural and Food Chemistry. 62 (7): 1449–1455. Bibcode:2014JAFC...62.1449H. doi:10.1021/jf405820v. PMID 24479643.
- ^ Martínez-González MA, Gea A, Ruiz-Canela M (March 2019). "The Mediterranean Diet and Cardiovascular Health". Circulation Research. 124 (5): 779–798. doi:10.1161/CIRCRESAHA.118.313348. PMID 30817261.
- ^ Marcelino G, Hiane PA, Freitas KC, Santana LF, Pott A, Donadon JR, Guimarães RC (August 2019). "Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota". Nutrients. 11 (8): 1826. doi:10.3390/nu11081826. PMC 6722810. PMID 31394805.
- ^ de Pablos RM, Espinosa-Oliva AM, Hornedo-Ortega R, Cano M, Arguelles S (May 2019). "Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases". Pharmacological Research. 143: 58–72. doi:10.1016/j.phrs.2019.03.005. PMID 30853597. S2CID 73726654.
- ^ a b c "Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles". European Food Safety Authority. 8 April 2011. doi:10.2903/j.efsa.2011.2033. Retrieved 2021-04-13.
From oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), "anti-inflammatory properties" (ID 1882), "contributes to the upper respiratory tract health" (ID 3468), "can help to maintain a normal function of gastrointestinal tract" (3779), and "contributes to body defences against external agents" (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006