Recent from talks
Nothing was collected or created yet.
Palmitic acid
View on Wikipedia | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexadecanoic acid | |
| Other names
Palmitic acid
C16:0 (Lipid numbers) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.284 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H32O2 | |
| Molar mass | 256.430 g/mol |
| Appearance | White crystals |
| Density | 0.852 g/cm3 (25 °C)[2] 0.8527 g/cm3 (62 °C)[3] |
| Melting point | 62.9 °C (145.2 °F; 336.0 K)[7] |
| Boiling point | 351–352 °C (664–666 °F; 624–625 K)[8] 271.5 °C (520.7 °F; 544.6 K), 100 mmHg[2] 215 °C (419 °F; 488 K), 15 mmHg |
| 4.6 mg/L (0 °C) 7.2 mg/L (20 °C) 8.3 mg/L (30 °C) 10 mg/L (45 °C) 12 mg/L (60 °C)[4] | |
| Solubility | Soluble in amyl acetate, alcohol, CCl4,[4] C6H6 Very soluble in CHCl3[3] |
| Solubility in ethanol | 2 g/100 mL (0 °C) 2.8 g/100 mL (10 °C) 9.2 g/100 mL (20 °C) 31.9 g/100 mL (40 °C)[5] |
| Solubility in methyl acetate | 7.81 g/100 g[4] |
| Solubility in ethyl acetate | 10.7 g/100 g[4] |
| Vapor pressure | 0.051 mPa (25 °C)[3] 1.08 kPa (200 °C) 28.06 kPa (300 °C)[6] |
| Acidity (pKa) | 4.75 [3] |
| −198.6·10−6 cm3/mol | |
Refractive index (nD)
|
1.43 (70 °C)[3] |
| Viscosity | 7.8 cP (70 °C)[3] |
| Thermochemistry | |
Heat capacity (C)
|
463.36 J/(mol·K)[6] |
Std molar
entropy (S⦵298) |
452.37 J/(mol·K)[6] |
Std enthalpy of
formation (ΔfH⦵298) |
−892 kJ/mol[6] |
Std enthalpy of
combustion (ΔcH⦵298) |
10030.6 kJ/mol[3] |
| Hazards | |
| GHS labelling: | |
 [2] [2]
| |
| Warning | |
| H319[2] | |
| P305+P351+P338[2] | |
| NFPA 704 (fire diamond) | |
| Flash point | 206 °C (403 °F; 479 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Palmitic acid (hexadecanoic acid in IUPAC nomenclature) is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms.[9][10] Its chemical formula is CH3(CH2)14COOH, and its C:D ratio (the total number of carbon atoms to the number of carbon-carbon double bonds) is 16:0. It is a major component of palm oil from the fruit of Elaeis guineensis (oil palms), making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats.[11]
Palmitates are the salts and esters of palmitic acid. The palmitate anion is the observed form of palmitic acid at physiologic pH (7.4). Major sources of C16:0 are palm oil, palm kernel oil, coconut oil, and milk fat.[12]
Dietary palmitic acid intake is associated with an increased cardiovascular disease risk through raising low-density lipoprotein.[13]
Occurrence and production
[edit]Palmitic acid was discovered by saponification of palm oil, which process remains today the primary industrial route for producing the acid.[14] Triglycerides (fats) in palm oil are hydrolysed by high-temperature water and the resulting mixture is fractionally distilled.[15]
Dietary sources
[edit]Palmitic acid is produced by a wide range of plants and organisms, typically at low levels. Among common foods it is present in milk, butter, cheese, and some meats, as well as cocoa butter, olive oil, soybean oil, and sunflower oil, (see table).[16] Karukas contain 44.90% palmitic acid.[17] The cetyl ester of palmitic acid, cetyl palmitate, occurs in spermaceti.
| Food | % of total calories |
|---|---|
| Palm oil | 45.1% |
| Beef tallow | 26.5% |
| Butter fat | 26.2% |
| Cocoa butter | 25.8% |
| Lard | 24.8% |
| Cottonseed oil | 24.7% |
| Chicken | 23.2% |
| Corn oil | 12.2% |
| Peanut oil | 11.6% |
| Soybean oil | 11% |
| Coconut oil | 8.4% |
| Palm kernel oil | 8% |
| Rapeseed oil | 3.6% |
| Source:[18] | |
Biochemistry
[edit]Palmitic acid is the first fatty acid produced during fatty acid synthesis and is the precursor to longer fatty acids. As a consequence, palmitic acid is a major body component of animals. In humans, one analysis found it to make up 21–30% (molar) of human depot fat,[19] and it is a major, but highly variable, lipid component of human breast milk.[20] Palmitic acid comprises nearly half of total human brain saturated fatty acids.[21]
Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC), which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation.[22] Some proteins are modified by the addition of a palmitoyl group in a process known as palmitoylation. Palmitoylation is important for localisation of many membrane proteins.
Applications
[edit]Surfactant
[edit]Palmitic acid is used to produce soaps, cosmetics, and industrial mold release agents. These applications use sodium palmitate, which is commonly obtained by saponification of palm oil. To this end, palm oil, rendered from palm trees (species Elaeis guineensis), is treated with sodium hydroxide (in the form of caustic soda or lye), which causes hydrolysis of the ester groups, yielding glycerol and sodium palmitate.
Foods
[edit]Because it is inexpensive and adds texture and "mouthfeel" to processed foods (convenience food), palmitic acid and its sodium salt find wide use in foodstuffs. Sodium palmitate is permitted as a natural additive in organic products.[23]
Military
[edit]Aluminium salts of palmitic acid and naphthenic acid were the gelling agents used with volatile petrochemicals during World War II to produce napalm. The word "napalm" is derived from the words naphthenic acid and palmitic acid.[24]
Research
[edit]It is well accepted in the medical community that palmitic acid from dietary sources raises low-density lipoprotein (LDL) and total cholesterol.[18][25][26][27] The World Health Organization have stated there is convincing evidence that palmitic acid increases cardiovascular disease risk.[28] Palmitic acid intake is associated with an increased cancer risk, including prostate cancer.[29][30]
A 2021 review indicated that replacing dietary palmitic acid and other saturated fatty acids with unsaturated fatty acids, such as oleic acid, could reduce several biomarkers of cardiovascular and metabolic diseases.[31]
See also
[edit]- Retinyl palmitate
- Ascorbyl palmitate
- SN2 Palmitate
- Juniperic acid (16-hydroxypalmitic acid)
References
[edit]- ^ Merck Index, 12th Edition, 7128.
- ^ a b c d e f Sigma-Aldrich Co., Palmitic acid. Retrieved on 2014-06-02.
- ^ a b c d e f g CID 985 from PubChem
- ^ a b c d "Palmitic acid".
- ^ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. Retrieved 2014-06-02.
- ^ a b c d n-Hexadecanoic acid in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-05-11)
- ^ Beare-Rogers, J.; Dieffenbacher, A.; Holm, J.V. (2001). "Lexicon of lipid nutrition (IUPAC Technical Report)". Pure and Applied Chemistry. 73 (4): 685–744. doi:10.1351/pac200173040685. S2CID 84492006.
- ^ Palmitic acid at Inchem.org
- ^ Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra. The Lipid Handbook, 3rd ed. Boca Raton: CRC Press, 2007. ISBN 0849396883 | ISBN 978-0849396885
- ^ The most common fatty acid is the monounsaturated oleic acid. See: https://pubchem.ncbi.nlm.nih.gov/compound/965#section=Top
- ^ Gianfranca Carta; Elisabetta Murru; Sebastiano Banni; Claudia Manca (8 November 2017). "Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications". Frontiers in Physiology. 8: 902. doi:10.3389/FPHYS.2017.00902. ISSN 1664-042X. PMC 5682332. PMID 29167646. Wikidata Q46799280.
- ^ Loften, J.R.; Linn, J.G.; Drackley, J.K.; Jenkins, T.C.; Soderholm, C.G.; Kertz, A.F. (August 2014). "Invited review: Palmitic and stearic acid metabolism in lactating dairy cows". Journal of Dairy Science. 97 (8): 4661–4674. doi:10.3168/jds.2014-7919. ISSN 0022-0302. PMID 24913651.
- ^ Annevelink CE, Sapp PA, Petersen KS, Shearer GC, Kris-Etherton PM (2023). "Diet-derived and diet-related endogenously produced palmitic acid: Effects on metabolic regulation and cardiovascular disease risk". J Clin Lipidol. 17 (5): 577–586. doi:10.1016/j.jacl.2023.07.005. PMC 10822025. PMID 37666689.
- ^ Frémy, E. (1842). "Memoire sur les produits de la saponification de l'huile de palme". Journal de Pharmacie et de Chimie. XII: 757.
- ^ Anneken, David J.; Both, Sabine; Christoph, Ralf; Fieg, Georg; Steinberner, Udo; Westfechtel, Alfred (2006). "Fatty Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_245.pub2. ISBN 978-3527306732.
- ^ "Chemical Characteristics". Olive Oil Source. Archived from the original on February 18, 2010. Retrieved November 11, 2021.
- ^ Purwanto, Y.; Munawaroh, Esti (2010). "Etnobotani Jenis-Jenis Pandanaceae Sebagai Bahan Pangan di Indonesia" [Ethnobotany Types of Pandanaceae as Foodstuffs in Indonesia] (PDF). Berkala Penelitian Hayati (in Indonesian). 5A: 97–108. ISSN 2337-389X. OCLC 981032990. Retrieved 10 November 2021.
- ^ a b Nelson, Gary J. (1991). Health Effects of Dietary Fatty Acids. American Oil Chemists' Society. pp. 84-86. ISBN 978-0935315318
- ^ Kingsbury, K. J.; Paul, S.; Crossley, A.; Morgan, D. M. (1961). "The fatty acid composition of human depot fat". Biochemical Journal. 78 (3): 541–550. doi:10.1042/bj0780541. PMC 1205373. PMID 13756126.
- ^ Jensen, RG; Hagerty, MM; McMahon, KE (June 1978). "Lipids of human milk and infant formulas: a review". Am. J. Clin. Nutr. 31 (6): 990–1016. doi:10.1093/ajcn/31.6.990. PMID 352132.
- ^ Smith ME, Bazinet RP (2024). "Unraveling brain palmitic acid: Origin, levels and metabolic fate". Prog Lipid Res. 96 101300. doi:10.1016/j.plipres.2024.101300. PMID 39222711.
- ^ "Fatty acid biosynthesis - Reference pathway". KEGG. Archived from the original on 2024-07-15. Retrieved 2023-05-18. Pathway Map 00061
- ^ US Soil Association standard 50.5.3
- ^ Mysels, Karol J. (1949). "Napalm. Mixture of Aluminum Disoaps". Industrial & Engineering Chemistry. 41 (7): 1435–1438. doi:10.1021/ie50475a033.
- ^ Mensink RP, Zock PL, Kester AD, Katan MB (2003). "Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials". Am J Clin Nutr. 77 (5): 1146–1155. doi:10.1093/ajcn/77.5.1146. PMID 12716665.
- ^ Mensink, Ronald P. (2016). "Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis". World Health Organization. Retrieved 14 March 2023.
- ^ Rao, Gundu HR. (2020). Clinical Handbook of Coronary Artery Disease. Jaypee Brothers Medical Publishers. pp. 186-187. ISBN 978-9389188301
- ^ "Diet, Nutrition and the Prevention of Chronic Diseases". World Health Organization. p. 82. Retrieved 16 March 2023.
- ^ He JW, Huang ZX, Su YF, Mo YQ (2025). "Dietary saturated fatty acids and prostate cancer: insights into NF-κB pathway and lipid metabolism mechanisms". Discov Oncol. 16 (1) 1166. doi:10.1007/s12672-025-03005-0. PMC 12181547. PMID 40542303.
- ^ Mei J, Qian M, Hou Y, Liang M, Chen Y, Wang C, Zhang J (2024). "Association of saturated fatty acids with cancer risk: a systematic review and meta-analysis". Lipids in Health and Disease. 23 (1) 32. doi:10.1186/s12944-024-02025-z. PMC 10826095. PMID 38291432.
- ^ Sellem, Laury; Flourakis, Matthieu; Jackson, Kim G; Joris, Peter J; Lumley, James; Lohner, Szimonetta; Mensink, Ronald P; Soedamah-Muthu, Sabita S; Lovegrove, Julie A (2021-11-25). "Impact of Replacement of Individual Dietary SFAs on Circulating Lipids and Other Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials in Humans". Advances in Nutrition. 13 (4): 1200–1225. doi:10.1093/advances/nmab143. ISSN 2161-8313. PMC 9340975. PMID 34849532.
External links
[edit] Media related to Palmitic acid at Wikimedia Commons
Media related to Palmitic acid at Wikimedia Commons- . Encyclopædia Britannica (11th ed.). 1911.
Palmitic acid
View on GrokipediaChemical properties
Structure and formula
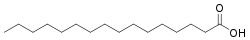
Palmitic acid, whose systematic IUPAC name is hexadecanoic acid, has the molecular formula C₁₆H₃₂O₂.[4] The common name "palmitic acid" originates from its initial isolation through saponification of palm oil in the 19th century.[2] As a saturated fatty acid, it features a straight hydrocarbon chain of 16 carbon atoms with no carbon-carbon double bonds, terminating in a carboxylic acid functional group (-COOH) that imparts its acidic properties.[4] The condensed structural formula of palmitic acid is CH₃(CH₂)₁₄COOH, illustrating the unbranched chain of 14 methylene (-CH₂-) groups between a terminal methyl (-CH₃) group and the carboxyl (-COOH) group.[4] In visual representations, the molecule is depicted as a linear zigzag chain to reflect the tetrahedral geometry around each carbon atom, with the carboxyl group often shown in expanded form as -C(=O)OH to highlight the carbonyl and hydroxyl components.[5] In biological contexts, palmitic acid refers specifically to the n- (straight-chain) isomer, which predominates in natural lipids.[4] Although branched-chain C16 fatty acids, such as iso- and anteiso-hexadecanoic acids, occur naturally in microbial membranes and ruminant tissues, these variants differ chemically from the straight-chain palmitic acid and are classified separately.[6]Physical characteristics
Palmitic acid appears as a white, waxy solid at room temperature.[4] This form is characteristic of its saturated hydrocarbon chain, which contributes to its solidity under standard conditions.[7] It melts at 62.9 °C and boils at 351 °C.[8] The molecular weight is 256.42 g/mol, and the density of the liquid phase is 0.848 g/cm³.[4] In its pure form, palmitic acid is odorless and tasteless.[9] Palmitic acid has very low solubility in water (0.05 mg/L at 20 °C), reflecting its hydrophobic nature.[8] However, it is readily soluble in various organic solvents, including ethanol, diethyl ether, and chloroform.[8]Reactivity
Palmitic acid, a saturated carboxylic acid, displays characteristic acidic behavior with a pKa value of 4.95, enabling it to readily donate its carboxyl proton in aqueous solutions and form corresponding salts, such as sodium palmitate, upon reaction with bases like sodium hydroxide.[4][10] This salt formation is a neutralization reaction where the carboxylate anion pairs with the metal cation, producing soaps used in various applications.[11] A prominent reactivity of palmitic acid is esterification, typically achieved through acid-catalyzed reactions with alcohols, as exemplified by the Fischer esterification with methanol to yield methyl palmitate. The balanced equation for this reversible process is: This reaction proceeds via a nucleophilic acyl substitution mechanism, where the alcohol attacks the protonated carbonyl carbon, and is influenced by factors such as catalyst loading, temperature, and reactant ratios to achieve high conversions, often exceeding 80% under optimized conditions.[12] The reverse of esterification, saponification, involves the alkaline hydrolysis of palmitic acid-derived esters, cleaving the ester linkage to regenerate the carboxylate salt and release the alcohol component, such as glycerol from triglyceride esters. This base-promoted reaction, commonly using sodium hydroxide, follows a mechanism involving nucleophilic attack by hydroxide on the carbonyl, leading to tetrahedral intermediate formation and subsequent expulsion of the alkoxide.[13] Owing to its saturated aliphatic chain devoid of double bonds, palmitic acid exhibits high resistance to auto-oxidation and peroxidation, contrasting with unsaturated fatty acids that are vulnerable at alkene sites.[14] Chemically, this stability arises from the absence of reactive sites for radical initiation in oxidative processes. In analogy to beta-oxidation, palmitic acid can undergo stepwise degradation by cleaving two-carbon acetyl units through dehydrogenation and hydration steps, though this is primarily enzymatic in nature.[15] Furthermore, being fully saturated, palmitic acid remains unchanged under standard hydrogenation conditions, as no carbon-carbon multiple bonds are present to accept hydrogen.[16]Sources and occurrence
Natural distribution
Palmitic acid is the most common saturated fatty acid found in nature, accounting for approximately 20–30% of total fatty acids in many organisms across biological kingdoms.[17] This prevalence stems from its role as a fundamental building block in lipid structures, enabling efficient energy storage and membrane formation in diverse species.[18] In plants, palmitic acid is highly concentrated in tropical oils, where it comprises about 44% of the fatty acids in palm oil and 8–10% in coconut oil, alongside presence in other tropical plant oils.[19] It also occurs in seeds and leaves of various plant species, contributing to cuticular waxes and structural lipids that protect against environmental stress.[20] Animal sources feature palmitic acid prominently in fats such as lard (25–30%), beef tallow (around 26%), and butter (25–30%), as well as in mammalian adipose tissue, where it often represents 20–30% of total fatty acids.[19] In microbial and algal systems, it is a key component of lipids produced by bacteria and algae; for instance, it can constitute up to 45% of total fatty acids in species like Chlorella vulgaris, supporting applications in microbial lipid production for biofuels.[21][22] Environmentally, palmitic acid is detected in soils and aquatic sediments as a biomarker of organic matter decomposition, and it forms part of natural waxes on plant surfaces and in bee products.[23][20]Dietary sources
Palmitic acid is a prevalent saturated fatty acid in many everyday foods, particularly those rich in animal fats and tropical plant oils. It constitutes a significant portion of the total fat content in these sources, contributing to overall dietary saturated fat intake. Major dietary contributors include palm oil-based products, such as margarine, baked goods, and shortenings, where palm oil typically contains 41–44% palmitic acid by weight of total fatty acids.[24][25] Dairy products like milk, cheese, and butter are also key sources; for instance, unsalted butter provides about 24 g of palmitic acid per 100 g serving.[24] Meats, especially beef fat (tallow), contain approximately 19–25% palmitic acid, making red meat a notable contributor in omnivorous diets.[24] Chocolate derives its content primarily from cocoa butter, which is composed of roughly 25–26% palmitic acid.[26] In processed foods, palmitic acid is commonly incorporated through shortenings, emulsifiers, and frying oils derived from palm or animal fats; for example, vegetable shortening can contain up to 26 g per 100 g.[24] The following table summarizes palmitic acid levels in select common foods (per 100 g edible portion, based on USDA-derived data):| Food Item | Palmitic Acid (g/100 g) |
|---|---|
| Palm oil | 41.0 |
| Butter (unsalted) | 24.0 |
| Beef fat (tallow) | 24.0 |
| Cocoa butter | 26.0 |
| Cheddar cheese | 9.0 |
| Vegetable shortening | 26.0 |