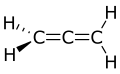Recent from talks
Nothing was collected or created yet.
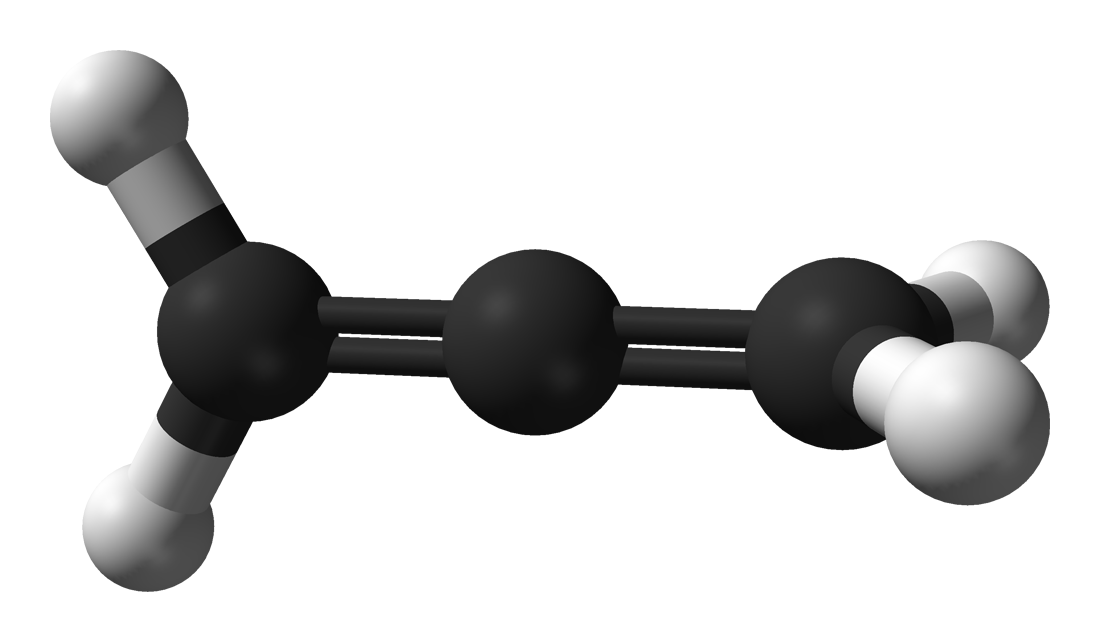
Propadiene
View on Wikipedia
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propadiene[1] | |||
| Other names
Allene[1]
Propadiene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730774 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.670 | ||
| EC Number |
| ||
| 860 | |||
| MeSH | Propadiene | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2200 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4 | |||
| Molar mass | 40.065 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Melting point | −136 °C (−213 °F; 137 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| log P | 1.45 | ||
| Hazards | |||
| GHS labelling: | |||
 [2] [2]
| |||
| Danger | |||
| H220[2] | |||
| P210, P377, P381, P410+P403[2] | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 13% | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propadiene (/proʊpəˈdaɪiːn/) or allene (/ˈæliːn/) is the organic compound with the formula H2C=C=CH2. It is the simplest allene, i.e. a compound with two adjacent carbon double bonds.[3] As a constituent of MAPP gas, it has been used as a fuel for specialized welding.
Production and equilibrium with methylacetylene
[edit]Propadiene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for methylacetylene-propadiene:
- H3C−C≡CH ⇌ H2C=C=CH2
for which Keq = 0.22 at 270 °C or 0.1 at 5 °C.
MAPD is produced as a side product, often an undesirable one, of dehydrogenation of propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene.[4]
Occurrence in Space
[edit]In 2019 it was announced that propadiene had been detected in the atmosphere of Saturn's moon Titan using the NASA Infrared Telescope Facility.[5] This was the first time that propadiene had been detected in space, and the second structural isomeric pair (paired with propyne) detected in Titan's atmosphere, after HCN-HNC.[6][7]
References
[edit]- ^ a b Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 375. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
The name allene, for CH2=C=CH2, is retained for general nomenclature only; substitution is allowed, but not by alkyl or any other group that extends the carbon chain, nor characteristic groups expressed by suffixes. The systematic name, propadiene, is the preferred IUPAC name.
- ^ a b c Record of Allene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 17 November 2020.
- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "allenes". doi:10.1351/goldbook.A00238
- ^ Klaus Buckl, Andreas Meiswinkel "Propyne" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.m22_m01
- ^ Lombardo, Nicholas A; Nixon, Conor A; Greathouse, Thomas K; Bézard, Bruno; Jolly, Antoine; Vinatier, Sandrine; Teanby, Nicholas A; Richter, Matthew J; G Irwin, Patrick J; Coustenis, Athena; Flasar, F Michael (2019-08-20). "Detection of Propadiene on Titan". The Astrophysical Journal Letters. 881 (2): L33. arXiv:1908.07424. doi:10.3847/2041-8213/ab3860. ISSN 2041-8205.
- ^ Moreno, R.; Lellouch, E.; Lara, L. M.; Courtin, R.; Bockelée-Morvan, D.; Hartogh, P.; Rengel, M.; Biver, N.; Banaszkiewicz, M.; González, A. (December 2011). "First detection of hydrogen isocyanide (HNC) in Titan's atmosphere". Astronomy & Astrophysics. 536: L12. doi:10.1051/0004-6361/201118189. ISSN 0004-6361.
- ^ Hébrard, E.; Dobrijevic, M.; Loison, J. C.; Bergeat, A.; Hickson, K. M. (May 2012). "Neutral production of hydrogen isocyanide (HNC) and hydrogen cyanide (HCN) in Titan's upper atmosphere". Astronomy & Astrophysics. 541: A21. doi:10.1051/0004-6361/201218837. ISSN 0004-6361.