Recent from talks
Nothing was collected or created yet.
Decane
View on Wikipedia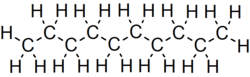 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Decane[1] | |
| Other names
Decyl hydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1696981 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.262 |
| EC Number |
|
| MeSH | decane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2247 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H22 | |
| Molar mass | 142.286 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like (in high concentrations) |
| Density | 0.730 g mL−1 |
| Melting point | −30.5 to −29.2 °C; −22.8 to −20.6 °F; 242.7 to 243.9 K |
| Boiling point | 173.8 to 174.4 °C; 344.7 to 345.8 °F; 446.9 to 447.5 K |
| log P | 5.802 |
| Vapor pressure | 195 Pa[2] |
Henry's law
constant (kH) |
2.1 nmol Pa−1 kg−1 |
| −119.74·10−6 cm3/mol | |
| Thermal conductivity | 0.1381 W m−1 K−1 (300 K)[3] |
Refractive index (nD)
|
1.411–1.412 |
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
315.46 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
425.89 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−302.1 – −299.9 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−6779.21 – −6777.45 kJ mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, moderately toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H226, H302, H304, H305 | |
| P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 46.0 °C (114.8 °F; 319.1 K) |
| 210.0 °C (410.0 °F; 483.1 K) | |
| Explosive limits | 0.8–2.6% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition.[5] These isomers are flammable liquids. Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene.[6][7] Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.[8]
Reactions
[edit]Decane undergoes combustion, just like other alkanes. In the presence of sufficient oxygen, it burns to form water and carbon dioxide.
- 2 C10H22 + 31 O2 → 20 CO2 + 22 H2O
With insufficient oxygen, carbon monoxide is also formed.
It can be manufactured in the laboratory without fossil fuels.[9]
Physical properties
[edit]It has a surface tension of 0.0238 N·m−1.[10]
See also
[edit]References
[edit]- ^ "decane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 5 January 2012.
- ^ Yaws, Carl L. (1999). Chemical Properties Handbook. New York: McGraw-Hill. pp. 159–179. ISBN 0-07-073401-1.
- ^ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- ^ Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquid n-Alkanes". Journal of Physical and Chemical Reference Data. 23 (1): 41–53. Bibcode:1994JPCRD..23...41D. doi:10.1063/1.555943. ISSN 0047-2689.
- ^ "75 Isomers of Decane". The Third Millennium Online! (in Latin). Retrieved 26 July 2021.
- ^ "Petroleum - Chemistry Encyclopedia - reaction, water, uses, elements, examples, gas, number, name". www.chemistryexplained.com. Retrieved 2016-01-28.
- ^ "n-Decane (Annotation)". Hazardous Substances Data Bank (HSDB). National Center for Biotechnology Information. Retrieved 7 July 2022.
- ^ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (15 June 2000), "Hydrocarbons", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.a13_227, ISBN 3527306730
- ^ "Method for preparing n-decane at normal pressure".
- ^ Website of Krüss Archived 2013-12-01 at the Wayback Machine (8.10.2009)
External links
[edit] Media related to Decane at Wikimedia Commons
Media related to Decane at Wikimedia Commons- Material Safety Data Sheet for Decane Archived 23 January 2011 at the Wayback Machine
- CHEMINFO Decane Archived 5 November 2006 at the Wayback Machine
Decane
View on GrokipediaNomenclature and structure
Chemical formula and structure
Decane, particularly its unbranched form known as n-decane, has the molecular formula and the condensed structural formula .[1] This represents a saturated hydrocarbon composed of ten carbon atoms and twenty-two hydrogen atoms, where the carbon chain is fully saturated with no multiple bonds.[3] n-Decane is classified as a straight-chain alkane, featuring a linear sequence of ten carbon atoms connected exclusively by single covalent bonds (C-C), with each carbon atom bonded to the appropriate number of hydrogen atoms via single C-H bonds to satisfy valence requirements.[1] This structure exemplifies the general characteristics of alkanes as saturated acyclic hydrocarbons, where the chain length of ten carbons distinguishes decane from shorter or longer homologues in the alkane series.[3] In structural representations, n-decane's bond-line notation (also known as skeletal formula) is commonly illustrated as a zigzag line depicting the nine C-C bonds connecting the ten carbon atoms, with terminal methyl groups implied and all hydrogen atoms omitted for clarity.[4] This simplified depiction highlights the unbranched, linear topology essential to its chemical identity. Decane exhibits 75 constitutional isomers in total, though the focus here remains on the straight-chain n-decane.[5]Naming conventions and isomers
The preferred IUPAC name for the unbranched chain isomer of C₁₀H₂₂ is decane, reflecting the systematic nomenclature for alkanes where the root "dec-" indicates ten carbon atoms and the suffix "-ane" denotes a saturated hydrocarbon.[1] This name applies specifically to n-decane, the straight-chain structure, while branched variants receive names based on the longest carbon chain with substituents indicated by prefixes such as "methyl-" or "ethyl-".[6] Historically, n-decane has been referred to as decyl hydride, an older term emphasizing its composition as a hydride of the decyl radical.[1] The term "decane" in scientific and industrial contexts typically refers to n-decane unless a specific isomer is indicated, distinguishing it from the broader set of compounds sharing the formula C₁₀H₂₂.[1] These compounds exhibit constitutional isomerism, where isomers differ in the bonding sequence of atoms, leading to varied chain lengths and branching patterns. In contrast, stereoisomerism—arising from different spatial arrangements of atoms—is absent in n-decane and most simple alkane isomers due to the lack of chiral centers or geometric constraints in acyclic structures; however, certain highly branched isomers may possess optical stereoisomers if they contain asymmetric carbons.[7] The total number of constitutional isomers for C₁₀H₂₂ is 75, encompassing unbranched, mono-branched, and multi-branched forms. Key examples of branched constitutional isomers include 2-methylnonane, which features a methyl group on the second carbon of a nine-carbon chain, and 2,2-dimethyloctane, with two methyl groups on the second carbon of an eight-carbon chain; these illustrate how branching reduces the longest chain length while maintaining the total carbon count.[8] Such nomenclature follows IUPAC rules prioritizing the longest continuous chain as the parent structure, with substituents numbered to yield the lowest possible locants.[6]Physical properties
Appearance and phase behavior
Decane is a colorless liquid at room temperature and standard pressure, exhibiting a characteristic gasoline-like odor.[9] Under standard conditions, decane exists in the liquid phase, with a melting point ranging from -30.5 °C to -29.2 °C and a boiling point between 173.8 °C and 174.4 °C. This phase behavior reflects its nonpolar molecular structure, which limits intermolecular forces and results in relatively low transition temperatures compared to more complex hydrocarbons.[10] The density of decane is 0.730 g/mL at 20 °C, contributing to its lower density than water and thus its tendency to float on aqueous surfaces. Its surface tension measures 0.0238 N/m, indicative of weak cohesive forces typical of nonpolar liquids.[10] Decane is insoluble in water due to its hydrophobic nature but readily soluble in organic solvents such as ethanol and ether.[1]Thermodynamic and spectroscopic properties
The standard molar entropy of liquid n-decane at 298 K is 364.6 J·K⁻¹·mol⁻¹.[11] The standard enthalpy of formation for the liquid phase is -300.9 kJ/mol at 298 K.[12] The enthalpy of vaporization is 51.42 kJ/mol at 25 °C.[1] The standard enthalpy of combustion for the liquid is -6778.33 ± 0.88 kJ/mol at 298 K.[12] Infrared spectroscopy of n-decane reveals characteristic absorption bands for aliphatic C-H stretching vibrations in the 2850–3000 cm⁻¹ region, including asymmetric CH₂ stretch near 2925 cm⁻¹ and symmetric CH₂ stretch near 2850 cm⁻¹, along with weaker C-C stretching modes around 800–1000 cm⁻¹.[13] These features are typical of long-chain alkanes and aid in structural confirmation. Proton NMR spectroscopy of n-decane in CDCl₃ displays distinct signals: a triplet at approximately 0.88 ppm (3H, terminal -CH₃ groups) and a multiplet at around 1.26 ppm (16H, -CH₂- groups), reflecting the symmetric chain structure with equivalent methylene environments in the interior.[14] The refractive index of n-decane is 1.4102 at 20 °C.[1] Its dynamic viscosity is 0.838 mPa·s at 25 °C, decreasing to 0.359 mPa·s at 100 °C, indicative of typical alkane flow behavior.[1]| Property | Value | Conditions | Source |
|---|---|---|---|
| Standard enthalpy of formation (liquid) | -300.9 kJ/mol | 298 K | NIST |
| Enthalpy of vaporization | 51.42 kJ/mol | 25 °C | PubChem |
| Enthalpy of combustion (liquid) | -6778 kJ/mol | 298 K | NIST |
| Refractive index | 1.4102 | 20 °C | PubChem |
| Viscosity | 0.838 mPa·s | 25 °C | PubChem |


