Recent from talks
Nothing was collected or created yet.
| Scar | |
|---|---|
| Other names | Cicatrix |
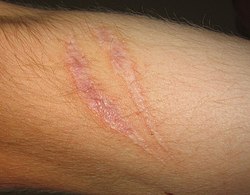 | |
| Scar tissue on an arm | |
| Specialty | Dermatology, plastic surgery |
A scar (or scar tissue) is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a natural part of the healing process. With the exception of very minor lesions, every wound (e.g., after accident, disease, or surgery) results in some degree of scarring. An exception to this are animals with complete regeneration, which regrow tissue without scar formation.
Scar tissue is composed of the same protein (collagen) as the tissue that it replaces, but the fiber composition of the protein is different; instead of a random basketweave formation of the collagen fibers found in normal tissue, in fibrosis the collagen cross-links and forms a pronounced alignment in a single direction.[1] This collagen scar tissue alignment is usually of inferior functional quality to the normal collagen randomised alignment. For example, scars in the skin are less resistant to ultraviolet radiation, and sweat glands and hair follicles do not grow back within scar tissues.[2] A myocardial infarction, commonly known as a heart attack, causes scar formation in the heart muscle, which leads to loss of muscular power and possibly heart failure. However, there are some tissues (e.g. bone) that can heal without any structural or functional deterioration.
Types
[edit]
All scarring is composed of the same collagen as the tissue it has replaced, but the composition of the scar tissue, compared to the normal tissue, is different.[1] Scar tissue also lacks elasticity[3] unlike normal tissue which distributes fiber elasticity. Scars differ in the amounts of collagen overexpressed. Labels have been applied to the differences in overexpression. Two of the most common types are hypertrophic and keloid scarring,[4] both of which experience excessive stiff collagen bundled growth overextending the tissue, blocking off regeneration of tissues. Another form is atrophic scarring (sunken scarring), which also has an overexpression of collagen blocking regeneration. This scar type is sunken, because the collagen bundles do not overextend the tissue. Stretch marks (striae) are regarded as scars by some.
High melanin levels and either African or Asian ancestry may make adverse scarring more noticeable.[5]
Hypertrophic
[edit]Hypertrophic scars occur when the body overproduces collagen, which causes the scar to be raised above the surrounding skin. Hypertrophic scars take the form of a red raised lump on the skin for lighter pigmented skin and the form of dark brown for darker pigmented skin. They usually occur within 4 to 8 weeks following wound infection or wound closure with excess tension and/or other traumatic skin injuries.[4]
Keloid
[edit]Keloid scars are a more serious form of excessive scarring, because they can grow indefinitely into large, tumorous (although benign) neoplasms.[4]
Hypertrophic scars are often distinguished from keloid scars by their lack of growth outside the original wound area, but this commonly taught distinction can lead to confusion.[6]
Keloid scars can occur on anyone, but they are most common in dark-skinned people.[7] They can be caused by surgery, cuts, accident, acne or, sometimes, body piercings. In some people, keloid scars form spontaneously. Although they can be a cosmetic problem, keloid scars are only inert masses of collagen and therefore completely harmless and not cancerous. However, they can be itchy or painful in some individuals. They tend to be most common on the shoulders and chest. Hypertrophic scars and keloids tend to be more common in wounds closed by secondary intention.[8] Surgical removal of keloid is risky and may exacerbate the condition and worsening of the keloid.
Atrophic
[edit]
An atrophic scar takes the form of a sunken recess in the skin, which has a pitted appearance. These are caused when underlying structures supporting the skin, such as fat or muscle, are lost. This type of scarring is often associated with acne,[9][10] chickenpox, other diseases (especially Staphylococcus infection), surgery, certain insect and spider bites, or accidents. It can also be caused by a genetic connective tissue disorder, such as Ehlers–Danlos syndrome.[11]
Stretch marks
[edit]Stretch marks (technically called striae) are also a form of scarring. These are caused when the skin is stretched rapidly (for instance during pregnancy,[12] significant weight gain, or adolescent growth spurts),[13] or when skin is put under tension during the healing process (usually near joints). This type of scar usually improves in appearance after a few years.[12]
Elevated corticosteroid levels are implicated in striae development.[14]
Umbilical
[edit]Humans and other placental mammals have an umbilical scar (commonly referred to as a belly button or navel) which starts to heal when the umbilical cord is cut after birth. Egg-laying animals have an umbilical scar which, depending on the species, may remain visible for life or disappear within a few days after birth.[15][16]
Pathophysiology
[edit]
A scar is the product of the body's repair mechanism after tissue injury. If a wound heals quickly within two weeks with new formation of skin, minimal collagen will be deposited and no scar will form.[17] When the extracellular matrix senses elevated mechanical stress loading, tissue will scar,[18] and scars can be limited by stress shielding wounds.[18] Small full thickness wounds under 2mm reepithelize fast and heal scar free.[19][20] Deep second-degree burns heal with scarring and hair loss.[2] Sweat glands do not form in scar tissue, which impairs the regulation of body temperature.[21] Elastic fibers are generally not detected in scar tissue younger than 3 months old.[22] In scars, rete pegs are lost;[23] through a lack of rete pegs, scars tend to shear easier than normal tissue.[23]
The endometrium, the inner lining of the uterus, is the only adult tissue to undergo rapid cyclic shedding and regeneration without scarring, shedding and restoring roughly inside a 7-day window on a monthly basis.[24] All other adult tissues, upon rapid shedding or injury, can scar.
Prolonged inflammation, as well as the fibroblast proliferation,[25] can occur. Redness that often follows an injury to the skin is not a scar and is generally not permanent (see wound healing). The time it takes for this redness to dissipate may, however, range from a few days to, in some serious and rare cases, a few years.[26][citation needed]
Scars form differently based on the location of the injury on the body and the age of the person who was injured.[citation needed]
The more severe the initial damage is, the more significant the scar will generally be. [citation needed]
Skin scars occur when the dermis (the deep, thick layer of skin) is damaged. Most skin scars are flat and leave a trace of the original injury that caused them.[citation needed]
Wounds allowed to heal secondarily tend to scar more significantly than wounds from primary closure.[8]
Collagen synthesis
[edit]An injury does not become a scar until the wound has completely healed; this can take many months, or years in the worst pathological cases, such as keloids. To begin to patch the damage, a clot is created; this clot is the beginning process that results in a provisional matrix. In the process, the first layer is a provisional matrix and is not a scar. Over time, the wounded body tissue overexpresses collagen inside the provisional matrix to create a collagen matrix. This collagen overexpression continues and crosslinks the fiber arrangement inside the collagen matrix, making the collagen dense. This densely packed collagen, morphing into an inelastic whitish collagen[25] scar wall, blocks off cell communication and regeneration; as a result, the new tissue generated will have a different texture and quality than the surrounding unwounded tissue. This prolonged collagen-producing process results in a fortuna scar.
Fibroblasts
[edit]The scarring is created by fibroblast proliferation,[25] a process that begins with a reaction to the clot.[27] To mend the damage, fibroblasts slowly form the collagen scar. The fibroblast proliferation is circular[27] and cyclically, the fibroblast proliferation lays down thick, whitish collagen[25] inside the provisional and collagen matrix, resulting in the abundant production of packed collagen on the fibers[25][27] giving scars their uneven texture. Over time, the fibroblasts continue to crawl around the matrix, adjusting more fibers and, in the process, the scarring settles and becomes stiff.[27] This fibroblast proliferation also contracts the tissue.[27] In unwounded tissue, these fibers are not overexpressed with thick collagen and do not contract.
EPF and ENF fibroblasts have been genetically traced with the Engrailed-1 genetic marker.[28] EPFs are the primary contributors to all fibrotic outcomes after wounding.[28] ENFs do not contribute to fibrotic outcomes.[28][29]
Myofibroblast
[edit]Mammalian wounds that involve the dermis of the skin heal by repair, not regeneration (except in 1st trimester inter-uterine wounds and in the regeneration of deer antlers). Full-thickness wounds heal by a combination of wound contracture and edge re-epitheliasation. Partial thickness wounds heal by edge re-epithelialisation and epidermal migration from adnexal structures (hair follicles, sweat glands and sebaceous glands). The site of keratinocyte stem cells remains unknown but stem cells are likely to reside in the basal layer of the epidermis and below the bulge area of hair follicles.
The fibroblast involved in scarring and contraction is the myofibroblast,[30] which is a specialized contractile fibroblast.[31] These cells express α-smooth muscle actin (α-SMA).[19] The myofibroblasts are absent in the first trimester in the embryonic stage where damage heals scar-free;[19] in small incisional or excision wounds less than 2 mm that also heal without scarring;[19] and in adult unwounded tissues where the fibroblast in itself is arrested; however, the myofibroblast is found in massive numbers in adult wound healing which heals with a scar.[31]
The myofibroblasts make up a high proportion of the fibroblasts proliferating in the postembryonic wound at the onset of healing. In the rat model, for instance, myofibroblasts can constitute up to 70% of the fibroblasts,[30] and is responsible for fibrosis on tissue.[citation needed] Generally, the myofibroblasts disappear from the wound within 30 days,[32] but can remain in pathological cases in hypertrophy, such as keloids.[31][32] Myofibroblasts have plasticity and in mice can be transformed into fat cells, instead of scar tissue, via the regeneration of hair follicles.[33][34]
Mechanical stress
[edit]Wounds under 2 millimeters generally do not scar,[19][20] but larger wounds generally do scar.[19][20] In 2011, it was found that mechanical stress can stimulate scarring[18] and that stress shielding can reduce scarring in wounds.[18][35] In 2021, it was found that using chemicals to manipulate fibroblasts to not sense mechanical stress brought scar-free healing.[36] The scar-free healing also occurred when mechanical stress was placed onto a wound.[36]
Treatment
[edit]Early and effective treatment of acne scarring can prevent severe acne and the scarring that often follows.[37] In 2004, no prescription drugs for the treatment or prevention of scars were available.[38]
Chemical peels
[edit]Chemical peels are chemicals which destroy the epidermis in a controlled manner, leading to exfoliation and the alleviation of certain skin conditions, including superficial acne scars.[39] Various chemicals can be used depending upon the depth of the peel, and caution should be used, particularly for dark-skinned individuals and those individuals susceptible to keloid formation or with active infections.[40]
Filler injections
[edit]Filler injections of collagen can be used to raise atrophic scars to the level of surrounding skin.[41] Risks vary based upon the filler used, and can include further disfigurement and allergic reaction.[42]
Laser treatment
[edit]Nonablative lasers, such as the 585 nm pulsed dye laser, 1064 nm and 1320 nm Nd:YAG, or the 1540 nm Er:Glass are used as laser therapy for hypertrophic scars and keloids.[43] There is tentative evidence for burn scars that they improve the appearance.[44][45]
Ablative lasers such as the carbon dioxide laser (CO2) or Er:YAG offer the best results for atrophic and acne scars.[46] Like dermabrasion, ablative lasers work by removing the epidermis.[47][48] Healing times for ablative therapy are much longer and the risk profile is greater compared to nonablative therapy; however, nonablative therapy offers only minor improvements in cosmetic appearance of atrophic and acne scars.[43]
Radiotherapy
[edit]Low-dose, superficial radiotherapy is sometimes used to prevent recurrence of severe keloid and hypertrophic scarring. It is thought to be effective despite a lack of clinical trials, but only used in extreme cases due to the perceived risk of long-term side effects.[49]
Dressings and topical silicone
[edit]Silicone scar treatments are commonly used in preventing scar formation and improving existing scar appearance.[50] A meta-study by the Cochrane collaboration found weak evidence that silicone gel sheeting helps prevent scarring.[51] However, the studies examining it were of poor quality and susceptible to bias.[51]
Pressure dressings are commonly used in managing burn and hypertrophic scars, although supporting evidence is lacking.[52] Care providers commonly report improvements, however, and pressure therapy has been effective in treating ear keloids.[52] The general acceptance of the treatment as effective may prevent it from being further studied in clinical trials.[52]
Verapamil-containing silicone gel
[edit]Verapamil, a type of calcium channel blocker, is considered a candidate drug for the treatment of hypertrophic scars. A study conducted by the Catholic University of Korea concluded that verapamil-releasing silicone gel is effective and is a superior alternative to the conventional silicone gel where decreased median SEI, fibroblast count, and collagen density in all verapamil-added treatment groups were observed.[53]: 647–656 Gross morphologic features suggested that the combination of verapamil and silicone improves the overall quality of hypertrophic scars by reducing scar height and redness. This was verified with quantifiable histomorphometric parameters; however, oral verapamil is not a good choice because of its effect of lowering blood pressure. Intralesional injection of verapamil is also suboptimal because of the required frequency for injections. Topical silicone gel combined with verapamil does not lead to systemic hypotension, is convenient to apply, and shows enhanced results.[53]: 647–656
Steroids
[edit]A long-term course of corticosteroid injections into the scar may help flatten and soften the appearance of keloid or hypertrophic scars.[54]
Topical steroids are ineffective.[55] However, clobetasol propionate can be used as an alternative treatment for keloid scars.[56]
Topical steroid applied immediately after fractionated CO2 laser treatment is however very effective (and more efficacious than laser treatment alone) and has shown benefit in numerous clinical studies.
Surgery
[edit]
Scar revision is a process of cutting the scar tissue out. After the excision, the new wound is usually closed up to heal by primary intention, instead of secondary intention. Deeper cuts need a multilayered closure to heal optimally, otherwise depressed or dented scars can result.[57]
Surgical excision of hypertrophic or keloid scars is often associated to other methods, such as pressotherapy or silicone gel sheeting. Lone excision of keloid scars, however, shows a recurrence rate close to 45%. A clinical study is currently ongoing to assess the benefits of a treatment combining surgery and laser-assisted healing in hypertrophic or keloid scars.
Subcision is a process used to treat deep rolling scars left behind by acne or other skin diseases. It is also used to lessen the appearance of severe glabella lines, though its effectiveness in this application is debatable. Essentially the process involves separating the skin tissue in the affected area from the deeper scar tissue. This allows the blood to pool under the affected area, eventually causing the deep rolling scar to level off with the rest of the skin area. Once the skin has leveled, treatments such as laser resurfacing, microdermabrasion or chemical peels can be used to smooth out the scarred tissue.[58]
Vitamins
[edit]Research shows the use of vitamin E and onion extract (sold as Mederma) as treatments for scars is ineffective.[52] Vitamin E causes contact dermatitis in up to 33% of users and in some cases it may worsen scar appearance and could cause minor skin irritations,[55] but Vitamin C and some of its esters fade the dark pigment associated with some scars.[59]
Other
[edit]- Cosmetics; Medical makeup can temporarily conceal scars.[60] This is most commonly used for facial scars.
- Dermabrasion involves the removal of the surface of the skin with special equipment, and usually involves a local anaesthetic.
- A 2012 literature review found weak evidence that massage was efficacious in scar management. Any beneficial effect appeared to be greater in wounds created by surgical incision than for traumatic or burn wounds.[61] A 2022 scoping review covering twenty-five studies of 1515 participants reported that all studies reviewed reported favorable outcomes for scar massage, but that "while there may be benefits to scar massage in reducing pain, increasing movement and improving scar characteristics", there was a lack of "consistent research methods, intervention protocols and outcome measures".[62]
- Microneedling[63]
Society and culture
[edit]Intentional scarring
[edit]The permanence of scarring has led to its intentional use as a form of body art within some cultures and subcultures. These forms of ritual and non-ritual scarring practices can be found in many groups and cultures around the world.
Etymology
[edit]First attested in English in the late 14th century, the word scar derives from a conflation of Old French escharre, from Late Latin eschara,[64] which is the Latinisation of the Greek ἐσχάρα (eskhara), meaning "hearth, fireplace", but in medicine "scab, eschar on a wound caused by burning or otherwise",[65][66] and Middle English skar ("cut, crack, incision"), which is from Old Norse skarð ("notch, gap").[66] The conflation helped to form the English meaning. Compare the place name Scarborough for evolution of skarð to scar.
Research
[edit]Research, before 2009, focused on scar improvements with research into molecular mechanisms. Treatments involving molecular mechanisms including avotermin,[67][68] ribosomal s6 kinase (RSK),[69] and osteopontin[70][71] were investigated at the time. After successful phase I/II trials,[67] human recombinant TGF-β3 (avotermin, planned trade name Juvista) failed in Phase III trials.[72] In 2011, the scientific literature highlighted stress shielding a fresh wound through the wound healing process, brings significant scar improvement and smaller scars.[18][35]
By 2016, skin had been regenerated in vivo and in vitro. and scar-free healing had been operationalized and induced by four main regeneration techniques: by instrument, by materials, by drugs, and by in vitro 3-D printing. In 2018, a silk-derived sericin hydrogel dressing was undergoing research, the material was shown to prevent scar formation.[73] By 2021, more people were paying attention to the possibility of scar revision and new technologies.[74]
In 2021, researchers found that, verteporfin, an FDA-approved drug for eye disease, could enable scar-free healing in mice. According to the study, the drug works by blocking mechanical stress signals in fibroblast cells.[75][76][77]
See also
[edit]References
[edit]- ^ a b Sherratt, Jonathan A. (2010). "Mathematical Modelling of Scar Tissue Formation". Department of Mathematics, Heriot-Watt University. Retrieved 20 August 2010.
This is composed of the same main protein (collagen) as normal skin, but with differences in details of composition. Most crucially, the protein fibres in normal tissue have a random (basketweave) appearance, while those in scar tissue have pronounced alignment in a single direction.
- ^ a b Kraft, John; Lynde, Charles. "Giving Burns the First, Second and Third Degree - Classification of burns". skincareguide.ca. Retrieved 31 January 2012.
Formation of a thick eschar, slow healing (>1month), Obvious scarring, hair loss.
- ^ A. Bernard Ackerman, MD, Almut Böer, MD, Bruce Bennin, MD, Geoffrey J. Gottlieb, MD (January 2005). Histologic Diagnosis of Inflammatory Skin Diseases An Algorithmic Method Based on Pattern Analysis: Embryologic, Histologic, and Anatomic Aspects: Elastic Fibers (Third ed.). Ardor Scribendi. p. 522. ISBN 978-1-893357-25-9. Archived from the original on 20 June 2018. Retrieved 19 September 2017.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Gauglitz, Gerd; Korting, Hans (2011). "Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies". Molecular Medicine. 17 (1–2): 113–25. doi:10.2119/molmed.2009.00153. PMC 3022978. PMID 20927486.
- ^ Kelly, A. Paul (2009). "Update on the Management of Keloids". Seminars in Cutaneous Medicine and Surgery. 28 (2): 71–76. doi:10.1016/j.sder.2009.04.002 (inactive 1 July 2025). PMID 19608056.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Roseborough IE, Grevious MA, Lee RC (January 2004). "Prevention and treatment of excessive dermal scarring". J Natl Med Assoc. 96 (1): 108–16. PMC 2594768. PMID 14746360.
- ^ Martini, Frederic H. (2006). Fundamentals of Anatomy & Physiology, Seventh Edition, p. 171. Benjamin Cummings, San Francisco.
- ^ a b "Practical Plastic Surgery for Nonsurgeons - Secondary Wound Closure - Scarring" (PDF). Archived from the original (PDF) on 26 August 2016. Retrieved 11 January 2017.
Wounds that are allowed to heal secondarily tend to have larger and more noticeable scars than the scars that results from primary closure. Secondary healing also has a greater tendency for hypertrophic scar/keloid formation. (page 86)
- ^ Goodman GJ (2000). "Postacne scarring: A review of its pathophysiology and treatment". Dermatologic Surgery. 26 (9): 857–871. doi:10.1046/j.1524-4725.2000.99232.x. PMID 10971560. S2CID 25244676.
- ^ Fabbrocini G, Annunziata MC, D'Arco V, De Vita V, Lodi G, Mauriello MC, Pastore F, Monfrecola G (2010). "Acne Scars: Pathogenesis, Classification and Treatment". Dermatology Research and Practice. 2010 893080: 1–13. doi:10.1155/2010/893080. PMC 2958495. PMID 20981308.
- ^ "Clinical manifestations and diagnosis of Ehlers-Danlos syndromes". www.uptodate.com. Retrieved 15 June 2017.
- ^ a b Brennan, Miriam; Young, Gavin; Devane, Declan (14 November 2012). "Topical preparations for preventing stretch marks in pregnancy". The Cochrane Database of Systematic Reviews. 2012 (11) CD000066. doi:10.1002/14651858.CD000066.pub2. ISSN 1469-493X. PMC 10001689. PMID 23152199.
- ^ Elsaie ML, Baumann LS, Elsaaiee LT (2009). "Striae Distensae (Stretch Marks) and Different Modalities of Therapy: An Update". Dermatologic Surgery. 35 (4): 563–573. doi:10.1111/j.1524-4725.2009.01094.x. PMID 19400881. S2CID 7887237.
- ^ Hengge UR, Ruzicka T, Schwartz RA, Cork MJ (2006). "Adverse effects of topical glucocorticosteroids". Journal of the American Academy of Dermatology. 54 (1): 1–15. doi:10.1016/j.jaad.2005.01.010. PMID 16384751.
- ^ "What Types of Animals Have Been to Space and More Questions From our Readers". Smithsonian. May 2012. Retrieved 25 November 2017.
- ^ "Flat-tailed horned lizard (Phrynosoma mcallii)" (PDF). US Fish & Wildlife Service. Retrieved 25 November 2017.
- ^ "POST BURN SCAR RELATIVE TO RE-EPITHELIALIZATION" (PDF). eplasty.com. 2011. Archived from the original (PDF) on 5 October 2016. Retrieved 6 February 2016.
Healing in 2 weeks – minimal to no scar; Healing in 3 weeks – minimal to no scar except in high risk scar formers;Healing in 4 weeks or more – hypertrophic in more than 50% of patients
- ^ a b c d e Wong, Victor W.; Akaishi, Satoshi; Longaker, Michael T.; Gurtner, Geoffrey C. (2011). "Pushing Back: Wound Mechanotransduction in Repair and Regeneration". Journal of Investigative Dermatology. 131 (11): 2186–2196. doi:10.1038/jid.2011.212. PMID 21776006.
- ^ a b c d e f Wilgus, T. A. (2007). "Regenerative healing in fetal skin: A review of the literature". Ostomy/Wound Management. 53 (6): 16–31, quiz 32–3. PMID 17586870.
- ^ a b c Tam, Joshua; Wang, Ying; Vuong, Linh N.; Fisher, Jeremy M.; Farinelli, William A.; Anderson, R. Rox (2016). "Reconstitution of full-thickness skin by microcolumn grafting". Journal of Tissue Engineering and Regenerative Medicine. 11 (10): 2796–2805. doi:10.1002/term.2174. PMC 5697650. PMID 27296503.
- ^ Fu XB, Sun TZ, Li XK, Sheng ZY (February 2005). "Morphological and distribution characteristics of sweat glands in hypertrophic scar and their possible effects on sweat gland regeneration". Chinese Medical Journal. 118 (3): 186–91. PMID 15740645. Archived from the original on 29 August 2021. Retrieved 12 February 2019.
In hypertrophic scar tissue, no sweet gland and hair follicle exist usually because of the dermal and epidermal damage in extensive thermal skin injury, thus impairing regulation of body temperature
- ^ Roten SV1, Bhat S, Bhawan J. (February 1996). "Elastic fibers in scar tissue". Journal of Cutaneous Pathology. 23 (1): 37–42. doi:10.1111/j.1600-0560.1996.tb00775.x. PMID 8720985. S2CID 37823718.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b Ira D. Papel (2011). Facial Plastic and Reconstructive Surgery (Third ed.). USA: Thieme Medical Publishers. p. 7. ISBN 978-1-58890-515-4.
- ^ "Endometrial repair". princehenrys.org. 18 September 2012. Archived from the original on 14 September 2009. Retrieved 30 June 2013.
Importantly, the endometrium is the only adult tissue to undergo rapid cyclic repair without scarring.
- ^ a b c d e "Facts about fibroblast: scar tissue formation". Britannica.com. Retrieved 19 April 2010.
As part of the healing process, specialized cells called fibroblasts in adjacent areas of skin produce a fibrous connective tissue made up of collagen. The bundles formed by these whitish, rather inelastic fibres make up the bulk of the scar tissue...
- ^ Bayat A, McGrouther DA, Barton JJ, Ferguson MW (11 January 2003). "Skin scarring". BMJ (Clinical Research Ed.). 326 (7380). The BMJ: 88–92. doi:10.1136/bmj.326.7380.88. PMC 5398751. PMID 12521975.
- ^ a b c d e Wipff, Pierre-Jean; Rifkin, Daniel B.; Meister, Jean-Jacques; Hinz, Boris (2007). "Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix". The Journal of Cell Biology. 179 (6): 1311–1323. doi:10.1083/jcb.200704042. PMC 2140013. PMID 18086923.
- ^ a b c Jiang, D; Rinkevich, Y (2020), "Scars or Regeneration?—Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses", International Journal of Molecular Sciences, 21 (2): 617, doi:10.3390/ijms21020617, PMC 7014275, PMID 31963533
- ^ Rinkevich, Y (2015), "Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential", Science, 348 (6232) aaa2151, doi:10.1126/science.aaa2151, PMC 5088503, PMID 25883361
- ^ a b Linge, Claire (Harrow, GB), Mackie, Ian Paul (Sheffield, GB) (10 March 2005). "Method of preventing or reducing scarring of human skin". freepatentsonline.com. Retrieved 26 March 2010.
myofibroblasts become differentiated from other cells in the wound within a few days after the onset of healing, and in the rat model can reach a peak where about 70% of the fibroblastic cells present are of the myofibroblast phenotype.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ a b c Linge, Claire (Harrow, GB), Mackie, Ian Paul (Sheffield, GB) (10 March 2005). "Method of preventing or reducing scarring of human skin". freepatentsonline.com. Retrieved 26 March 2010.
These cells, which differentiate from the unwounded tissue cell type (fibroblasts), are responsible for laying down scar tissue. Indeed, myofibroblasts remain present in hypertrophic scars up to four years after the original wounding event. An in vitro assay was accordingly developed to identify actives which prevent or reduce myofibroblast formation and thus identify actives which are effective in reducing and/or preventing scar tissue formation.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ a b Linge, Claire (Harrow, GB), Mackie, Ian Paul (Sheffield, GB) (10 March 2005). "Method of preventing or reducing scarring of human skin". freepatentsonline.com. Retrieved 26 March 2010.
the number of myofibroblasts present in the forming scar tissue begins to reduce via apoptosis, until by about 30 days no myofibroblasts are obvious within the scar.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Plikus; et al. (5 January 2017). "Regeneration of fat cells from myofibroblasts during wound healing". Science. 355 (6326): 748–752. Bibcode:2017Sci...355..748P. doi:10.1126/science.aai8792. PMC 5464786. PMID 28059714.
- ^ Horsley, Watt (6 April 2017). "Repeal and Replace: Adipocyte Regeneration in Wound Repair". Cell Stem Cell (Submitted manuscript). 20 (4): 424–426. doi:10.1016/j.stem.2017.03.015. PMID 28388424.
- ^ a b Monstrey, Stan (1 August 2014). "Updated Scar Management Practical Guidelines: Non-invasive and invasive measures". Journal of Plastic, Reconstructive & Aesthetic Surgery. 67 (8): 1017–25. doi:10.1016/j.bjps.2014.04.011. hdl:11577/2834337. PMID 24888226.
- ^ a b Molteni, Megan (22 April 2021). "In mouse experiments, scientists unlock the key to scar-free skin healing". statnews.com. Retrieved 1 May 2021.
- ^ "What is a Scar". American Academy of Dermatology. 2011. Archived from the original on 12 February 2011. Retrieved 25 August 2011.
Early and effective acne treatment can prevent severe acne and the scarring that often follows
- ^ Ferguson MW, O'Kane S (May 2004). "Scar-free healing: from embryonic mechanisms to adult therapeutic intervention". Philos. Trans. R. Soc. Lond. B Biol. Sci. 359 (1445): 839–50. doi:10.1098/rstb.2004.1475. PMC 1693363. PMID 15293811.
- ^ Khunger N (January 2008). "Standard guidelines of care for acne surgery". Indian Journal of Dermatology, Venereology and Leprology. 74 Suppl: S28–36. PMID 18688101.
- ^ Khunger N (January 2008). "Standard guidelines of care for chemical peels". Indian Journal of Dermatology, Venereology and Leprology. 74 Suppl: S5–12. PMID 18688104.
- ^ Cooper JS, Lee BT (December 2009). "Treatment of facial scarring: lasers, filler, and nonoperative techniques". Facial Plastic Surgery. 25 (5): 311–5. doi:10.1055/s-0029-1243079. PMID 20024872. S2CID 260136591.
- ^ Lemperle G, Rullan PP, Gauthier-Hazan N (September 2006). "Avoiding and treating dermal filler complications". Plastic and Reconstructive Surgery. 118 (3 Suppl): 92S – 107S. doi:10.1097/01.prs.0000234672.69287.77. PMID 16936549. S2CID 32471639.
- ^ a b Elsaie ML, Choudhary S (November 2010). "Lasers for scars: A review and evidence-based appraisal". Journal of Drugs in Dermatology. 9 (11): 1355–62. PMID 21061757.
- ^ Willows, B.M.; Ilyas, M.; Sharma, A. (4 August 2017). "Laser in the management of burn scars". Burns. 43 (7): 1379–1389. doi:10.1016/j.burns.2017.07.001. PMID 28784339.
- ^ Friedstat, JS; Hultman, CS (2014). "Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials". Annals of Plastic Surgery. 72 (6): S198–201. doi:10.1097/SAP.0000000000000103. PMID 24835874. S2CID 47573176.
- ^ Khatri KA, Mahoney DL, McCartney MJ (April 2011). "Laser scar revision: A review". Journal of Cosmetic and Laser Therapy. 13 (2): 54–62. doi:10.3109/14764172.2011.564625. PMID 21401378. S2CID 11520661.
- ^ Verma, Neil; Yumeen, Sara; Raggio, Blake S. (2022), "Ablative Laser Resurfacing", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32491406, retrieved 21 July 2022
- ^ "Laser resurfacing". Mayo Clinic. Retrieved 21 July 2022.
- ^ Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T (October 2009). "Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis". Plastic and Reconstructive Surgery. 124 (4): 1196–201. doi:10.1097/PRS.0b013e3181b5a3ae. PMID 19935303. S2CID 25294698.
- ^ Stavrou D, Weissman O, Winkler E, Yankelson L, Millet E, Mushin OP, Liran A, Haik J (October 2010). "Silicone-based scar therapy: a review of the literature". Aesthetic Plastic Surgery. 34 (5): 646–51. doi:10.1007/s00266-010-9496-8. PMID 20354695. S2CID 43820233.
- ^ a b O'Brien, L; Jones, DJ (12 September 2013). "Silicone gel sheeting for preventing and treating hypertrophic and keloid scars". The Cochrane Database of Systematic Reviews. 9 (9) CD003826. doi:10.1002/14651858.CD003826.pub3. PMC 7156908. PMID 24030657.
- ^ a b c d Shih R, Waltzman J, Evans GR (March 2007). "Review of over-the-counter topical scar treatment products". Plastic and Reconstructive Surgery. 119 (3): 1091–5. doi:10.1097/01.prs.0000255814.75012.35. PMID 17312518. S2CID 2756632.
- ^ a b Choi, Jangyoun; Han, Yu Na; Rha, Eun Young; Kang, Hwi Ju; Kim, Ki Joo; Park, Il Kyu; Kim, Hyun-Jung; Rhie, Jong Won (17 March 2021). "Verapamil-containing silicone gel reduces scar hypertrophy". International Wound Journal. 18 (5): 647–656. doi:10.1111/iwj.13566. ISSN 1742-4801. PMC 8450805. PMID 33733593.
- ^ Roques C, Téot L (2008). "The Use of Corticosteroids to Treat Keloids: A Review". The International Journal of Lower Extremity Wounds. 7 (3): 137–145. doi:10.1177/1534734608320786. PMID 18611924. S2CID 9577277.
- ^ a b Jenkins M, Alexander JW, MacMillan BG, Waymack JP, Kopcha R. Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J Burn Care Rehabil. 1986 Jul–Aug;7(4):309–312.
- ^ Nor, N. M.; Ismail, R.; Jamil, A.; Shah, S. A.; Imran, F. H. (25 November 2016). "A Randomized, Single-Blind Trial of Clobetasol Propionate 0.05% Cream Under Silicone Dressing Occlusion Versus Intra-Lesional Triamcinolone for Treatment of Keloid". Clinical Drug Investigation. 37 (3): 295–301. doi:10.1007/s40261-016-0484-x. PMID 27888448. S2CID 33614354.
- ^ "Scar revisions". Archived from the original on 19 January 2012. Retrieved 16 October 2010.
Deep cuts need multi-layered closure to heal optimally; otherwise, depressed or dented scars can result
- ^ "Chemical peels vs laser peels vs microdermabrasion: which one is right for you?". Beautiful Canadian Laser and Skincare Clinic. 17 September 2014. Retrieved 24 June 2021.
- ^ Farris PK. Topical vitamin C: a useful agent for treating photoaging and other dermatologic conditions. Although many people claim that vitamin therapy does in fact help. Dermatol Surg 2005;31:814-818.
- ^ Mee, Donna; Wong, Brian (1 October 2012). "Medical Makeup for Concealing Facial Scars" (PDF). Facial Plastic Surgery. 28 (5): 536–540. doi:10.1055/s-0032-1325647. PMID 23027221. S2CID 23900892. Archived from the original (PDF) on 5 October 2016. Retrieved 30 January 2016.
- ^ Shin, Thuzar M.; Bordeaux, Jeremy S. (2012). "The Role of Massage in Scar Management: A Literature Review". Dermatologic Surgery. 38 (3): 414–423. doi:10.1111/j.1524-4725.2011.02201.x. ISSN 1076-0512. PMID 22093081. S2CID 1018590.
- ^ Scott, Helen C.; Stockdale, Claire; Robinson, Andrea; Robinson, Luke S; Brown, Ted (2022). "Is massage an effective intervention in the management of post-operative scarring? A scoping review". Journal of Hand Therapy. 35 (2): 186–199. doi:10.1016/j.jht.2022.01.004. PMID 35227556.
- ^ Cohen, BE; Elbuluk, N (February 2016). "Microneedling in skin of color: A review of uses and efficacy". Journal of the American Academy of Dermatology. 74 (2): 348–55. doi:10.1016/j.jaad.2015.09.024. PMID 26549251.
- ^ eschara, Charlton T. Lewis, Charles Short, A Latin Dictionary, on Perseus
- ^ ἐσχάρα, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on perseus
- ^ a b Online Etymology Dictionary
- ^ a b Ferguson, Mark WJ; Duncan, Jonathan; Bond, Jeremy; Bush, James; Durani, Piyush; So, Karen; Taylor, Lisa; Chantrey, Jonquille; Mason, Tracey; James, Gaynor; Laverty, Hugh; Occleston, Nick L.; Sattar, Abdul; Ludlow, Anna; O'Kane, Sharon (2009). "Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies". The Lancet. 373 (9671): 1264–1274. doi:10.1016/S0140-6736(09)60322-6. PMID 19362676. S2CID 35671002.
- ^ Tredget, Edward E.; Ding, Jie (2009). "Wound healing: From embryos to adults and back again". The Lancet. 373 (9671): 1226–1228. doi:10.1016/S0140-6736(09)60705-4. PMID 19362658. S2CID 10236414.
- ^ "Liver damage 'could be reversed'". BBC News. 27 December 2007. Retrieved 1 January 2008.
- ^ 'Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring'
- ^ "Gel 'to speed up wound healing'". BBC News. 22 January 2008. Retrieved 23 May 2010.
- ^ Renovo shares plummet 75% as scar revision product Juvista fails to meet study endpoints, 14 February 2011
- ^ Dey, Becky (29 October 2018). "Silk route to scar-free skin". Chemistry World. Retrieved 18 June 2019.
- ^ Tang, Xuwen (1 December 2021). "Micro-compound tissue grafting for repairing linear scars". Chinese Journal of Plastic and Reconstructive Surgery. 3 (4). China: 202–203. doi:10.1016/j.cjprs.2021.11.002. ISSN 2096-6911. S2CID 244827273.
- ^ Vaughan, Christopher (22 April 2021). "Researchers find drug that enables healing without scarring". Stanford Medicine. Retrieved 13 September 2024.
- ^ Mascharak, Shamik; desJardins-Park, Heather E.; Davitt, Michael F.; Griffin, Michelle; Borrelli, Mimi R.; Moore, Alessandra L.; Chen, Kellen; Duoto, Bryan; Chinta, Malini; Foster, Deshka S.; Shen, Abra H.; Januszyk, Michael; Kwon, Sun Hyung; Wernig, Gerlinde; Wan, Derrick C. (23 April 2021). "Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring". Science. 372 (6540) eaba2374. doi:10.1126/science.aba2374. ISSN 0036-8075. PMC 9008875. PMID 33888614.
- ^ Kolata, Gina (22 April 2021). "Imagine, Surgery Without a Scar". The New York Times. Archived from the original on 9 December 2023. Retrieved 13 September 2024.
External links
[edit]- WebMd.com: Skin Scars Directory (archived 21 September 2017)
- American Academy of Dermatology: What is a scar? Archived 21 November 2018 at the Wayback Machine
.jpg/250px-Scar_(xndr).jpg)
.jpg)