Recent from talks
Nothing was collected or created yet.
Fraxinus excelsior
View on Wikipedia
| European ash | |
|---|---|

| |
| Foliage and immature fruit | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Lamiales |
| Family: | Oleaceae |
| Genus: | Fraxinus |
| Species: | F. excelsior
|
| Binomial name | |
| Fraxinus excelsior | |
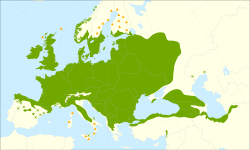
| |
| Distribution map | |
Fraxinus excelsior, known as the ash, or European ash or common ash to distinguish it from other types of ash, is a flowering plant species in the olive family Oleaceae. It is native throughout mainland Europe[2] east to the Caucasus and Alborz mountains, and west to Great Britain and Ireland, the latter determining its western boundary. The northernmost location is in the Trondheimsfjord region of Norway.[3] The species is widely cultivated and reportedly naturalised in New Zealand and in scattered locales in the United States and Canada.[4][5][6][7] The wood has many uses as it is flexible, workable, strong and lightweight.
Description
[edit]

It is a large deciduous tree growing to 12–18 m (39–59 ft) (exceptionally to 43 m or 141 ft) tall with a trunk up to 2 m (6.6 ft) (exceptionally to 3.5 m or 11 ft) diameter, with a tall, narrow crown.[2] The bark is smooth and pale grey on young trees, becoming thick and vertically fissured on old trees. The shoots are stout, greenish-grey, with jet-black buds (which distinguish it from most other ash species, which have grey or brown buds). The leaves are opposite, 20–35 cm (7.9–13.8 in) long, pinnately compound, with 7–13 leaflets with coarsely serrated margins, elliptic to narrowly elliptic, 3–12 cm (1.2–4.7 in) long and 0.8–3 cm (0.31–1.18 in) broad and sessile on the leaf rachis.[2] There are no stipules.[2] These features distinguish ash from mountain ash (Sorbus aucuparia) in which the leaves are alternate with paired stipules. The leaves are often among the last to open in spring, and the first to fall in autumn if an early frost strikes; they often fall dull green or develop a bright yellow autumn colour. The flowers are borne in short panicles, open before the leaves, and have no perianth. The female flowers are somewhat longer than the male flowers, dark purple, without petals, and are wind-pollinated. Both male and female flowers can occur on the same tree, but it is more common to find all male and all female trees. A tree that is all male one year can produce female flowers the next, and similarly a female tree can become male.[citation needed] The fruit is a samara 2.5–4.5 cm (0.98–1.77 in) long and 5–8 mm (0.20–0.31 in) broad, often hanging in bunches through the winter;[8] they are often called 'ash keys'.[3][9][10] If the fruit is gathered and planted when it is still green and not fully ripe, it will germinate straight away, however once the fruit is brown and fully ripe, it will not germinate until 18 months after sowing (i.e. not until two winters have passed).[11]
European ash rarely exceeds 250 years of age. However, there are numerous specimens estimated between 200 and 250 years old and there are a few over 250. The largest is in Clapton Court, England, and is 9 m (29.5 ft) in girth. There are several examples over 4.5 metres (14.8 ft) in Derbyshire alone.
The genome of Fraxinus excelsior has been sequenced using the self-pollinated offspring of a tree from Worcestershire, held by the Earth Trust.[12]
Distribution
[edit]Fraxinus excelsior is native to Europe from northern Spain to Russia, and from southern Fennoscandia to northern Greece.[2] It is also considered native in southwestern Asia from northern Turkey east to the Caucasus and Alborz mountains. The northernmost naturally occurring location is in the Trondheimsfjord region of Norway, though they are planted further north.[3] The species is widely cultivated and reportedly naturalized in New Zealand and in scattered locales in the United States and Canada including Nova Scotia, New Brunswick, Quebec, Massachusetts, Connecticut, New York, New Jersey, Maryland, Ontario, Ohio, Kentucky and British Columbia.[citation needed]
It is native throughout Britain and Ireland, particularly on limestone, as in northern Scotland, where the most northerly native ashwood in Britain occurs on limestone at Rassal Ashwood.[13] It is widely planted elsewhere.[14]
Ecology
[edit]Ash occurs on a wide range of soil types, but is particularly associated with basic soils on calcareous substrates. The most northerly ashwood in Britain is on limestone at Rassal, Wester Ross, latitude 57.4278 N.[15] Ash prefers moister soil types and is commonly limited by temperature and so not found at the higher colder altitudes in much of Europe, though in Iran, it may reach 2000 m asl. As a young seedling, it is shade tolerant, but older trees are light-demanding. It is an early-succession species and may well outcompete beech and oak, which are later-succession species.[16]
F. excelsior mycorrhizae are of the internal arbuscular mycorrhizal type, in which the fungus grows within the tissues of the root and forms branched, tree-like structures within the cells of the root cortex. Unlike other Fraxinus species, F. excelsior does not form ectomycorrhizae.[2]
The Biological Records Centre of the UK records 111 species of insects and mites using ash as a food plant, of which 29 are specific to ash. A further six are specific to ash and its Oleaceae relative wild privet (Ligustrum vulgare).[17] A number of Lepidoptera species use the species as a food source.[18] One example of an ash-specific feeding moth is the centre-barred sallow (Atethmia centrago). The larvae burrow into the buds when newly hatched and later feed on the flowers and leaves.[19] A common moth which causes the browning of ash leaves, as well as garden privet and lilac, is Gracillaria syringella. The usually gregarious larvae form an epidermal gallery (i.e. feed within the leaf) which leads to a brown blotch with black frass. Later, two successive cones are formed by folding the tip of a leaf downwards.[20]
In Britain, 14 galls have been recorded on ash. The British Plant Gall Society defines a gall as "... an abnormal growth produced by a plant under the influence of another organism".[21]
Ash dieback
[edit]Ash dieback is caused by the fungus Hymenoscyphus fraxineus which was previously known as Chalara fraxinea. Research into the genetics of the resistance of ash (Fraxinus excelsior) has shown that resistance does occur in European populations, but at least for the samples tested, it is neither common nor strong.[22][23][24][25] In some cases, it has been observed that the pathogen can trigger the full replacement of the species in native forests.[26] Due to the importance of F. excelsior as a host, Jönsson and Thor 2012 find that rare/threatened lichens face an unusually high (0.38) coextinction risk probability vis-a-vis the host tree in the wooded meadows of Gotland, Sweden.[27] The genomic basis of ash dieback resistance has been characterised by the sequencing of mass screening trials.[28] It was demonstrated that natural selection was acting in a natural woodland in Surrey, England, to increase resistance to ash dieback in the younger generation of ash.[29] The genomic basis of ash dieback resistance has also been investigated in continental Europe.[30]
Emerald ash borer
[edit]The emerald ash borer Agrilus planipennis has recently begun to invade the range of Fraxinus excelsior.[31] Some studies suggest that Fraxinus excelsior may be less susceptible to emerald ash borer than American species of ash are.[32] However, the effect of the interaction of ash dieback and emerald ash borer is as yet unknown.
Uses
[edit]
The resilience and rapid growth made it an important resource for smallholders and farmers. It was probably the most versatile wood in the countryside with wide-ranging uses. Until World War II, the trees were often coppiced on a 10-year cycle to provide a sustainable source of timber for fuel and poles for building and woodworking.[33] The colour of the wood ranges from creamy white to light brown, and the heart wood may be a darker olive-brown. Ash timber is hard, tough and very hard-wearing, with a coarse, open grain and a density of 710 kg/m3.[34] It lacks oak's natural resistance to decay, and is not as suitable for posts buried in the ground. Because of its high flexibility, shock resistance, and resistance to splitting, ash wood is the traditional material for bows, tool handles, especially for hammers and axes, tennis rackets, and snooker cues.[35]

Ash was extensively used in the construction of early aircraft, and is, or has been used in the construction of some motor vehicles such as Morgan[36] and Morris Traveller.[37]
Ash was commonly used green for making chair frames which would be seated with another timber or with woven rush (e.g. those made by Philip Clissett, see also The English Regional Chair[38]). The parts were turned on a pole lathe or shaped with a drawknife. The practice essentially died out in the early 20th century, but has seen a revival in recent years.
Ash is an important constituent of wood pasture, a European management system in which open woodland provided shelter and forage for grazing animals.[13] Ash was coppiced and pollarded, often in hedgerows, and evidence in the form of some huge boles with multiple trunks emerging at head height can still be seen in parts of Britain. The Glen Lyon ash is a notable example of a pollarded ash which at about 400–500 years of age achieved a girth of 6 m (20 ft).[13] In Northumberland, crab and lobster pots (traps) sometimes known as 'creeves' by local people are still made from ash sticks.[citation needed] Because of its elasticity European ash wood was commonly used for walking sticks. Poles were cut from a coppice and the ends heated in steam. The wood could then be bent in a curved vise to form the handle of the walking stick. The light colour and attractive grain of ash wood make it popular in modern furniture such as chairs, dining tables, doors, and other architectural features and wood flooring.
Ash is the only wood used for the manufacture of hurleys, referred to as hurls in parts of Leinster and known as a camán in Irish, the timber sticks used in the game of hurling in Ireland. Hurleys are manufactured from the butt log (bottom 1.5-m of the stem) and from trees ideally of a diameter at breast height around 25–30 cm (9.8–12 in). Only fast-grown, straight, and knot-free ash can be used for this purpose. Due to the lack of available ash in Ireland, over 75% of the timber needed to produce the 350,000 hurleys required for the game annually must be imported, mostly from Eastern European countries.[39] The importance of ash timber to the game of hurling is reflected in the fact that the game is referred to all over Ireland as "The Clash of the Ash".
Ash is valuable as firewood because it burns well even when 'green' (freshly cut).[40] Ash bark and leaves are used in modern herbal medicine for their astringent and laxative properties.[41]
Mythology
[edit]
In the 13th-century Edda and other writing relating to Norse mythology, the vast ash tree Yggdrasil ("the steed (gallows) of Odin"), watered by three magical springs, serves as axis mundi, sustaining the nine worlds of the cosmos in its roots and branches.[42]
Folklore
[edit]On the Isle of Bute in Scotland, lovers reportedly used to eat leaves of an ash tree known as the "Dreamin' Tree" that grew near the church of St Blane, and the pleasant dreams they then experienced revealed their actual spouses and intended fates.[43]
Cultivars
[edit]
Its many cultivars include;
- Fraxinus excelsior 'Aurea', the traditional, slow-growing golden ash ─ not to be confused with 'Jaspidea'
- Fraxinus excelsior 'Aurea Pendula' (weeping golden ash)
- Fraxinus excelsior 'Autumn Blaze'
- Fraxinus excelsior 'Autumn Purple'
- Fraxinus excelsior 'Crispa'
- Fraxinus excelsior 'Diversifolia' (one-leaved ash)
- Fraxinus excelsior 'Erosa'
- Fraxinus excelsior 'Jaspidea' a modern, vigorous golden ash
- Fraxinus excelsior 'Monophylla'
- Fraxinus excelsior 'Nana'
- Fraxinus excelsior 'Pendula' (weeping ash), one of the best-known cultivars, widely planted during the Victorian era, grows vigorously forming an attractive small to medium-sized tree with mounds of weeping branches
- Fraxinus excelsior 'Skyline'
Gallery
[edit]-
Two ash trees fused together
-
Tree 46 m tall, Château des princes de Croÿ, Le Roeulx, Belgium
-
Old tree, Belgium
-
Leaf and shoot, showing black bud
-
Weeping ash Fraxinus excelsior 'Pendula', Knightshayes Court, England
-
Buds
-
Bark
References
[edit]- ^ Khela, S.; Oldfield, S. (2018). "Fraxinus excelsior". IUCN Red List of Threatened Species. 2018 e.T203367A67807718. doi:10.2305/IUCN.UK.2018-1.RLTS.T203367A67807718.en. Retrieved 12 November 2021.
- ^ a b c d e f Thomas, Peter A. (2016). "Biological Flora of the British Isles: Fraxinus excelsior". Journal of Ecology. 104 (4): 1158–1209. Bibcode:2016JEcol.104.1158T. doi:10.1111/1365-2745.12566. S2CID 86930831.
- ^ a b c Rushforth, K. (1999). Trees of Britain and Europe. Collins ISBN 0-00-220013-9.
- ^ "Fraxinus excelsior L." Plants of the World Online. Royal Botanic Gardens, Kew. Retrieved 13 July 2023.
- ^ Kew World Checklist of Selected Plant Families, Fraxinus excelsior
- ^ Biota of North America Program, Fraxinus excelsior
- ^ Altervista Flora of the United States and Canada, Fraxinus excelsior
- ^ Kilbracken, J.1983. Larousse Easy way guide Trees. Larousse. ISBN 0-7523-0027 X
- ^ Mitchell, A. F. (1974). A Field Guide to the Trees of Britain and Northern Europe. Collins ISBN 0-00-212035-6
- ^ Mitchell, A. F. (1982). The Trees of Britain and Northern Europe. Collins ISBN 0-00-219037-0
- ^ Edlin, H. L. (1985). Broadleaves. Forestry Commission booklet 20. HMSO. p. 36. ISBN 978-0-11-710039-8.
- ^ Sollars, Elizabeth S. A.; Harper, Andrea L.; Kelly, Laura J.; Sambles, Christine M.; Ramirez-Gonzalez, Ricardo H.; Swarbreck, David; Kaithakottil, Gemy; Cooper, Endymion D.; Uauy, Cristobal; Havlickova, Lenka; Worswick, Gemma; Studholme, David J.; Zohren, Jasmin; Salmon, Deborah L.; Clavijo, Bernardo J. (January 2017). "Genome sequence and genetic diversity of European ash trees". Nature. 541 (7636): 212–216. Bibcode:2017Natur.541..212S. doi:10.1038/nature20786. hdl:11019/3605. ISSN 0028-0836. PMID 28024298.
- ^ a b c Stiven, Roland; Holl, Kate (2004). Wood Pasture. Perth, UK: Scottish Natural Heritage. ISBN 978-1-85397-386-4.
- ^ "Online atlas of the British and Irish Flora, Fraxinus excelsior (Ash)". Biological Records Centre and Botanical Society of Britain and Ireland. Archived from the original on 1 December 2021.
- ^ "Wood Pasture: Rassal Ashwood National Nature Reserve". Scottish Natural Heritage. Archived from the original on 6 September 2013.
- ^ Dorota Dobrowolska; Sebastian Hein; Anne Oosterbaan; Sven Wagner; Jo Clark; Jens Peter Skovsgaard (April 2011). "A review of European ash (Fraxinus excelsior L.): implications for silviculture Forestry". Forestry: An International Journal of Forest Research. 84 (2): 133–148. doi:10.1093/forestry/cpr001.
- ^ Rackham, Oliver (2014). The Ash Tree. Toller Fratrum, Dorset: Little Toller Books. pp. 25–6. ISBN 978-1-908213-14-3.
- ^ Alan Stubbs. "Invertebrates associated with Ash" (PDF). Archived from the original (PDF) on 3 September 2013. Retrieved 28 February 2015.
- ^ "Centre-barred Sallow Atethmia centrago". UKmoths. Retrieved 28 July 2017.
- ^ "15.014 Gracillaria syringella (Fabricius, 1794)". British leafminers. Retrieved 28 July 2017.
- ^ Redfern, Margaret; Shirley, Peter; Bloxham, Michael (2011). British Plant Galls (Second ed.). Shrewsbury: FSC Publications. ISBN 978-1-85153-284-1.
- ^ Stener LG (2012). "Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden". Scandinavian Journal of Forest Research. 28 (3): 205–216. doi:10.1080/02827581.2012.735699. S2CID 85292870.
- ^ Kjær ED, et al. (2012). "Adaptive potential of ash (Fraxinus excelsior) populations against the novel emerging pathogen Hymenoscyphus pseudoalbidus". Evolutionary Applications. 5 (3): 219–228. Bibcode:2012EvApp...5..219K. doi:10.1111/j.1752-4571.2011.00222.x. PMC 3353348. PMID 25568043.
- ^ McKinney LV, et al. (2011). "Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): an emerging infectious disease". Heredity. 106 (5): 788–797. Bibcode:2011Hered.106..788M. doi:10.1038/hdy.2010.119. PMC 3186218. PMID 20823903.
- ^ Pliūra A, Lygis V, Suchockas V, Bartkevičius E (2011). "Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chlara fraxinea". Baltic Forestry. 17 (1): 17–34. Archived from the original on 2 April 2015.
- ^ Díaz-Yáñez, Olalla; Mola-Yudego, Blas; Timmermann, Volkmar; Tollefsrud, Mari Mette; Hietala, Ari M.; Oliva, Jonàs (24 March 2020). "The invasive forest pathogen Hymenoscyphus fraxineus boosts mortality and triggers niche replacement of European ash (Fraxinus excelsior)". Scientific Reports. 10 (1): 5310. Bibcode:2020NatSR..10.5310D. doi:10.1038/s41598-020-61990-4. ISSN 2045-2322. PMC 7093550. PMID 32210276.
- ^ Oliva, Jonàs; Redondo, Miguel Ángel; Stenlid, Jan (25 August 2020). "Functional Ecology of Forest Disease". Annual Review of Phytopathology. 58 (1). Annual Reviews: 343–361. Bibcode:2020AnRvP..58..343O. doi:10.1146/annurev-phyto-080417-050028. ISSN 0066-4286. PMID 32396761. S2CID 218618105.
- ^ Stocks, Jonathan J.; Metheringham, Carey L.; Plumb, William J.; Lee, Steve J.; Kelly, Laura J.; Nichols, Richard A.; Buggs, Richard J. A. (18 November 2019). "Genomic basis of European ash tree resistance to ash dieback fungus". Nature Ecology & Evolution. 3 (12): 1686–1696. Bibcode:2019NatEE...3.1686S. doi:10.1038/s41559-019-1036-6. ISSN 2397-334X. PMC 6887550. PMID 31740845.
- ^ Metheringham, Carey L.; Plumb, William J.; Flynn, William R. M.; Stocks, Jonathan J.; Kelly, Laura J.; Nemesio Gorriz, Miguel; Grieve, Stuart W. D.; Moat, Justin; Lines, Emily R.; Buggs, Richard J. A.; Nichols, Richard A. (26 June 2025). "Rapid polygenic adaptation in a wild population of ash trees under a novel fungal epidemic". Science. 388 (6754): 1422–1425. doi:10.1126/science.adp2990. ISSN 0036-8075. PMID 40570121.
- ^ Doonan, James M.; Budde, Katharina B.; Kosawang, Chatchai; Lobo, Albin; Verbylaite, Rita; Brealey, Jaelle C.; Martin, Michael D.; Pliura, Alfas; Thomas, Kristina; Konrad, Heino; Seegmüller, Stefan; Liziniewicz, Mateusz; Cleary, Michelle; Nemesio-Gorriz, Miguel; Fussi, Barbara (May 2025). "Multiple, Single Trait GWAS and Supervised Machine Learning Reveal the Genetic Architecture of Fraxinus excelsior Tolerance to Ash Dieback in Europe". Plant, Cell & Environment. 48 (5): 3793–3809. Bibcode:2025PCEnv..48.3793D. doi:10.1111/pce.15361. ISSN 0140-7791. PMC 11963480. PMID 39822124.
- ^ Meshkova, Valentyna; Borysenko, Oleksandr; Kucheryavenko, Tetiana; Skrylnyk, Yuriy; Davydenko, Kateryna; Holusa, Jaroslav (3 April 2023). "Potential Westward Spread of Emerald Ash Borer, Agrilus planipennis Fairmaire, 1888 (Coleoptera: Buprestidae) from Eastern Ukraine". Forests. 14 (4): 736. Bibcode:2023Fore...14..736M. doi:10.3390/f14040736. ISSN 1999-4907.
- ^ Showalter, David N.; Saville, Robert J.; Orton, Elizabeth S.; Buggs, Richard J. A.; Bonello, Pierluigi; Brown, James K. M. (January 2020). "Resistance of European ash ( Fraxinus excelsior ) saplings to larval feeding by the emerald ash borer ( Agrilus planipennis )". Plants, People, Planet. 2 (1): 41–46. Bibcode:2020PlPP....2...41S. doi:10.1002/ppp3.10077. ISSN 2572-2611.
- ^ Mabey, R. (1996). Flora Britannica. Sinclair-Stevenson Ltd ISBN 1-85619-377-2.
- ^ "Ash". Niche Timbers. Retrieved 19 August 2009.
- ^ Petrică Tudor M, Ingrid Agnes M, Szilard B. 2011. Study of Physical Characteristics, Mechanical and Technological Properties of Wood Species from the Fraxinus Genus Encountered in Romania Compared to Other Main Forestry Species. Natural Resources and Sustainable Development [1].
- ^ "Morgan reveals one-off SP1 at Salon Privé in London [w/video]". Autoblog. 3 September 2014. Retrieved 15 February 2021.
- ^ Ray Newell, Morris Minor, 60 Years on the Road, pages 106 to 118
- ^ Cotton, Bernard D. (1990). The English Regional Chair. Woodbridge, Suffolk: Antiques Collectors Club. ISBN 1-85149-023-X.
- ^ John Whiriskey; Paul McCarthy, eds. (May 2006). Ash For Hurleys (PDF) (Report). Fact Sheet No. 35. Teagasc – The Agriculture and Food Development Authority. Archived from the original (PDF) on 22 November 2007.
- ^ "The burning properties of wood" (PDF). The Scout Association. 1999. Archived from the original (PDF) on 23 December 2012. Retrieved 1 November 2010.
- ^ Pliûra, A.; Heuertz, M. (2003). Common ash – Fraxinus excelsior: Technical guidelines for genetic conservation and use (PDF) (Report). EUFORGEN.
- ^ Simek, Rudolf (2007). Dictionary of Northern Mythology. Translated by Angela Hall. D.S. Brewer. ISBN 978-0-85991-513-7.
- ^ Downie, R. Angus (1934). Bute and the Cumbraes Glasgow & London: Blackwood & Son Ltd. p. 83
External links
[edit]- Fraxinus excelsior – distribution map, genetic conservation units and related resources. European Forest Genetic Resources Programme (EUFORGEN)
- Fraxinus excelsior – Ash info, images and video at Woodland Trust
Fraxinus excelsior
View on GrokipediaTaxonomy and nomenclature
Taxonomic classification
Fraxinus excelsior is classified within the kingdom Plantae, phylum Tracheophyta, class Magnoliopsida, order Lamiales, family Oleaceae, genus Fraxinus (section Fraxinus), and species excelsior.[5][6] The species was first formally described by Carl Linnaeus in his 1753 work Species Plantarum, volume 2, page 1057, under the binomial name Fraxinus excelsior.[7] Fraxinus excelsior exhibits a subdioecious or trioecious breeding system, characterized by the presence of male, female, and hermaphroditic individuals in populations, with flowers that can vary in sex expression.[8] The genus Fraxinus comprises 40–50 species, primarily distributed in the temperate and subtropical regions of the northern hemisphere, with close European relatives including Fraxinus angustifolia.[9]Etymology and common names
The genus name Fraxinus derives from the classical Latin term for the ash tree, which also denoted spears or javelins crafted from its straight, durable wood.[10][11] The specific epithet excelsior originates from the Latin word meaning "higher" or "lofty," reflecting the species' potential to reach heights of up to 40 meters in favorable conditions.[12] In English, Fraxinus excelsior is commonly known as the European ash or common ash, names that distinguish it from other ash species.[12] Regional variations include "Esche" in German, "frêne commun" in French, and "fresno común" in Spanish, reflecting its widespread recognition across Europe.[13][14] The tree has been referenced in ancient literature under the name fraxinus, notably in Pliny the Elder's Natural History (Book XVI), where he describes its characteristics and uses, including its knot-free varieties suitable for woodworking.[15]Description
Morphology
Fraxinus excelsior is a large deciduous tree that typically reaches heights of 20-35 meters, occasionally up to 40 meters, with a trunk diameter of up to 2 meters and a broad, rounded crown featuring ascending branches that form a domed, open canopy.[13][16][17] The bark on young trees is smooth and pale grey to brown, becoming thick, fissured, and diamond-patterned with age as the tree matures.[16][18][19] Twigs are stout, greenish-grey, and glabrous.[13] The leaves are opposite and odd-pinnately compound, measuring 20-35 cm in length, with 9-13 ovate to lanceolate leaflets that are serrate-margined, dark green above and paler beneath, turning yellow in autumn.[18][16][13] Each leaflet is 3-12 cm long and 0.8-3 cm wide, with short petiolules and tufts of hair at the base of the midrib underside.[16][20] The flowers are small, petal-less, and purple-tinged, appearing in lax panicles or bunches of 100-400 before the leaves emerge in spring; they are polygamous, with male flowers producing pollen via purple anthers and female flowers featuring long styles and purple stigmas.[16][13][21] The fruits are single-seeded samaras, 3-5 cm long and 5-7 mm wide, with a broad wing extending along the entire flattened seed body, initially green and turning brown as they mature in bunches.[18][13][20]Growth habits
Fraxinus excelsior exhibits fast initial growth, with young trees increasing in height by 30-60 cm annually under favorable conditions, transitioning to more moderate rates as they mature.[22][16] This rapid early development allows saplings to establish quickly in suitable environments, reaching reproductive maturity in 30-40 years when they begin producing flowers and seeds.[23] Overall, the species develops into a large tree, typically attaining heights of 20-35 m with a broad, domed crown.[18] The lifespan of Fraxinus excelsior generally spans 200-400 years, though exceptional individuals, particularly those pollarded or in protected sites, can exceed 500 years.[24] This longevity contributes to its role as a key component in long-term forest succession, where older trees provide structural diversity. In terms of environmental tolerances, young plants are shade-tolerant, enabling establishment under canopy cover, but mature individuals become strongly light-demanding to sustain growth.[16] The species prefers neutral to alkaline soils with pH above 5.5, avoiding acidic conditions that limit nutrient availability.[23] It demonstrates robust frost hardiness, withstanding temperatures down to -30°C once established, though seedlings remain vulnerable to late spring frosts. Phenologically, leaves emerge in late spring around May, providing a delayed canopy that minimizes frost damage, and senesce early in autumn by October, often turning yellow before falling.[23] The deep root system, consisting of a taproot with wide-spreading lateral roots and vertical sinker roots, enhances anchorage and stability against wind, supporting the tree's tall stature in exposed woodlands.[23][25]Distribution and habitat
Geographic range
_Fraxinus excelsior is native to a broad expanse across Europe, extending from the United Kingdom and Norway in the north to the Mediterranean region in the south, and eastward to the Caucasus Mountains and southwestern Asia, including Asia Minor and the Alborz Mountains in Iran.[3][21][8] The species reaches its northern limit at approximately 63°40'N in Norway and its eastern extent to the Volga River basin in Russia, while it is absent from the extreme southern European territories, such as Portugal, owing to unsuitable drought-prone conditions.[21][8] The current European distribution of F. excelsior spans much of the continent's temperate zone, covering an estimated area where it occurs naturally in diverse landscapes from sea level to altitudes of up to 2200 m in parts of its range.[3][8] This range resulted from post-glacial recolonization following the Last Glacial Maximum, with populations migrating northward from refugia in southern Europe, including the Iberian Peninsula, Italian Peninsula, Alps, and Balkan Peninsula.[3][8] Outside its native range, F. excelsior has been introduced to North America, where it is cultivated in scattered locales primarily for ornamental purposes but remains uncommon and vulnerable to invasive pests such as the emerald ash borer (Agrilus planipennis).[12][19] It has also been planted in parts of Asia beyond its native southwestern extent and in Australia since 1843, mainly for timber production and as a landscape tree in parks and gardens.[25][26] As of 2025, the native geographic range of F. excelsior remains largely stable across Europe and southwestern Asia, though populations within this range are experiencing significant declines due to the widespread impact of ash dieback disease caused by the fungus Hymenoscyphus fraxineus.[3][8]Habitat requirements
_Fraxinus excelsior thrives on moist, fertile, base-rich soils, particularly calcareous types such as chalk, oolite, limestone, and alluvial deposits. It performs optimally on deep, well-drained soils with a pH between 6.0 and 8.0, though it can tolerate pH levels down to 4.2; below this threshold, growth is severely limited due to acidity intolerance. The species exhibits high tolerance to periodic waterlogging, including spring flooding lasting 1–3 months and intermediate tolerance to summer floods, making it suitable for clay-heavy or occasionally inundated sites, but it is sensitive to prolonged drought and anoxic conditions.[8] The tree is adapted to temperate oceanic to suboceanic climates, with mean annual temperatures around 9°C supporting optimal growth. It withstands winter extremes down to -27°C to -30°C and summer highs up to 35°C, though it is vulnerable to spring frosts below -3°C that can damage emerging leaves. Annual rainfall requirements range from a minimum of approximately 600–750 mm for adequate development, with best performance in regimes of 700–1200 mm or more, up to 2000 mm in upland areas; both regular and irregular precipitation patterns are tolerated, provided moisture deficits are avoided during the growing season.[8][25][27] Preferred sites include mixed woodlands, river valleys, hedgerows, and screes on base-rich substrates, typically at elevations from sea level up to 1500 m in Europe. It serves as an indicator of high nutrient status, with base saturation exceeding 20–30% and soil C:N ratios below 15–20, and is frequently associated with species such as Acer pseudoplatanus and Ulmus spp. in nutrient-rich forest communities like Tilio-Acerion.[8][25][28]Reproduction
Flowering and pollination
Fraxinus excelsior flowers in spring, typically from April to May, prior to leaf emergence, which facilitates wind dispersal by minimizing obstruction from foliage.[29] The inflorescences are terminal or axillary panicles measuring approximately 5-10 cm in length, bearing numerous small, inconspicuous unisexual or hermaphroditic flowers without petals or sepals.[30] The species exhibits functional dioecy, with separate male and female trees predominant, though proportions of hermaphroditic individuals vary across populations, ranging from ~9% functionally to 24-68% of trees, with many hermaphrodites exhibiting biased sex expression.[8][31] These hermaphroditic trees are rare and often unstable in sex expression, with individuals potentially shifting between male, female, or mixed states across years.[31] Pollination in F. excelsior is anemophilous, relying entirely on wind for pollen transfer, with high airborne pollen concentrations exceeding 100 grains per cubic meter sustained for 10-20 days during peak bloom.[29] Pollen dispersal distances vary, with mean effective ranges around 40-90 meters but capable of extending up to several hundred meters or more in fragmented landscapes, promoting gene flow across populations.[29] Self-compatibility is low overall; while hermaphroditic flowers can self-fertilize, their paternal contribution to seed set is significantly reduced compared to pure males, favoring outcrossing.[29] Sex ratios vary widely across populations; for example, one study found 20.5% male, 68.1% hermaphrodite, and 11.4% female trees, supporting a trioecious breeding system where hermaphrodites contribute significantly to reproduction.[8] This balance supports effective pollination in dense stands, though isolation in fragmented habitats can limit success by reducing pollen availability.[32] Environmental factors influence pollination efficacy; cold springs and spring frosts can delay flowering or damage reproductive tissues, reducing overall success rates.[8] In urban settings, air pollution correlates with altered pollen concentrations and viability, potentially exacerbating dispersal challenges in contaminated areas.[33] Ash dieback, caused by the fungus Hymenoscyphus fraxineus, reduces flower formation and pollen viability, limiting reproductive success especially in fragmented populations, though overall pollen production and seed quality remain relatively unaffected as of 2023.[34][32]Seed production and dispersal
Fraxinus excelsior produces samaras as its fruit, which develop from the syncarpous ovary containing typically one fertilized ovule out of four.[8] Trees begin fruiting at 20–25 years in open conditions or 30–40 years in forests, with production continuing until 150–220 years of age.[8] Samaras, measuring 3–4 cm in length and pale brown when ripe, fully develop by early July, with embryos maturing by August–September.[8] Viable seeds are shed from September to March, though most remain on the tree throughout winter, with prolific mast years occurring every 2–7 years and yielding up to 140,000 seeds per tree annually (approximately 10 kg).[8] Seed dispersal in F. excelsior is primarily anemochorous, facilitated by the samara's winged structure that enables autorotational flight with a vertical velocity of about 200 cm s⁻¹.[8] Mean dispersal distances range from 43.5 m to 67 m, with 95% of seeds landing within 113 m and rare long-distance events exceeding 1 km in open areas; secondary dispersal occurs via hydrochory, as samaras float for up to 12 hours (50% of seeds) or even a week.[8][35] Animals, including rodents such as the wood mouse (Apodemus sylvaticus), play a minor role, primarily through post-dispersal predation rather than directed dispersal, though caching behavior can occasionally contribute to secondary spread.[36] Germination of F. excelsior seeds is epigeal and requires breaking deep physiological dormancy through sequential stratification: an initial warm period (15–20°C for 12–16 weeks) to complete embryo growth, followed by cold stratification (2–5°C for 12–16 weeks) over winter.[8] This dormancy typically delays germination until the second spring after dispersal, with fewer than 5% of seeds germinating after one winter; viability reaches up to 95%, though field emergence varies from 1.1% to 24.5% due to predation and competition.[8] Seedlings establish readily in shaded understories, forming persistent banks that enhance survival during the early life cycle stages.[8]Ecology
Interactions with other organisms
Fraxinus excelsior serves as a host for over 200 invertebrate species in the United Kingdom, including numerous insects that rely on its foliage, buds, and wood for feeding and development.[37] For instance, the ash bud moth (Prays fraxinella) lays its eggs on ash buds, with larvae mining leaves and damaging shoots, particularly in stressed trees.[38] The tree's leaves support caterpillars of various moth species, contributing to its role in invertebrate biodiversity.[18] Seeds of F. excelsior are consumed by birds such as the Eurasian bullfinch (Pyrrhula pyrrhula), which preferentially feeds on them during winter, potentially aiding seed dispersal while reducing germination rates.[39] Mammals also interact with the tree; its twigs and foliage are browsed by deer species including roe deer (Capreolus capreolus) and red deer (Cervus elaphus), though ash is less preferred compared to other trees like oak.[8] The roots of F. excelsior form mutualistic arbuscular mycorrhizal associations primarily with fungi in the Glomeromycota phylum, including species of Glomus, which enhance nutrient uptake such as phosphorus and magnesium, particularly in base-rich soils.[40][41] These symbioses improve tree growth and stress tolerance, fostering a beneficial exchange where the fungus receives carbohydrates from the host.[8] The bark of F. excelsior supports a diverse lichen community, with over 500 species recorded in the UK, including more than 80 threatened taxa that depend on ash as a substrate for colonization.[42] These epiphytic lichens thrive on the tree's neutral to alkaline bark pH, forming symbiotic partnerships between fungi and algae that contribute to bark microhabitat diversity.[8] F. excelsior is susceptible to parasitic interactions with pathogenic fungi, notably Armillaria species such as A. mellea and A. gallica, which cause root and butt rot by invading live roots and spreading via rhizomorphs, leading to weakened stability and tree decline.[43] This root rot often synergizes with ash dieback caused by Hymenoscyphus fraxineus, where vectors like wind-dispersed ascospores facilitate infection, exacerbating mortality in infected stands.[8]Role in forest ecosystems
Fraxinus excelsior plays a significant role in forest succession as a light-demanding pioneer species that rapidly colonizes gaps in woodland canopies, facilitating the transition from open areas to more mature stands. It establishes effectively in small to medium-sized gaps through its shade-tolerant seedlings, which can achieve high densities exceeding 10,000 per hectare, competing successfully with other broadleaved trees and contributing to the structural development of mixed forests. On steeper terrains, such as slopes up to 80 degrees, its extensive adventitious root systems stabilize scree and prevent soil erosion, enhancing landscape resilience in hilly or riparian zones. Additionally, the nutrient-rich leaf litter of F. excelsior, characterized by a low C:N ratio of 14–28 and high calcium concentrations (up to 12,927 mg kg⁻¹ in leaves), promotes rapid decomposition and improves soil fertility by increasing nutrient availability, including base saturation levels above 20–30% in underlying soils.[8][8][8][8] As a keystone species in calcareous woodlands, F. excelsior supports elevated levels of biodiversity by creating heterogeneous habitats that foster diverse understory communities, including shade-tolerant herbs such as dog's mercury (Mercurialis perennis) and bluebells (Hyacinthoides non-scripta). In base-rich soils, it dominates and maintains species-rich field layers with over 15 species per square meter, while its open canopy (leaf area index of approximately 3.6 m² m⁻²) allows sufficient light penetration to sustain these assemblages. The tree also contributes to carbon sequestration, with mature stands storing around 79.5 t ha⁻¹ in mineral soils and annual uptake rates of approximately 1.5–4 t C ha⁻¹ depending on site conditions and age. Furthermore, its hydrology-regulating functions, including shallow lateral roots (0.3–0.5 m deep) that enhance water uptake and tolerate periodic flooding for 1–3 months, help mitigate flood risks in riparian ecosystems by stabilizing banks and moderating soil moisture. The canopy further influences microclimate by warming soils by about 1°C compared to denser stands, which accelerates litter breakdown and nutrient cycling.[8][8][8][44] The ongoing decline of F. excelsior due to ash dieback, caused by the fungus Hymenoscyphus fraxineus, has significantly reduced habitat heterogeneity across European forests, with continued alterations to gap dynamics and diminishing structural complexity that supports associated biodiversity. This loss threatens the persistence of understory species reliant on ash-dominated canopies and could lead to decreased overall woodland diversity, with early assessments indicating up to 38% potential loss in lichen communities alone.[45][8][46]Threats and conservation
Major diseases and pests
The primary pathological threat to Fraxinus excelsior is ash dieback, caused by the invasive ascomycete fungus Hymenoscyphus fraxineus, which was introduced from Asia to Europe in the 1990s.[47] Symptoms typically begin with wilting and browning of leaves and shoots in the canopy, progressing to necrotic lesions on bark and stems, and eventual crown dieback, often leading to tree mortality within 5–10 years of infection.[48] The fungus spreads primarily through wind-dispersed ascospores produced on pseudosclerotia in fallen petioles, facilitating rapid epidemic progression across native ranges.[49] As of 2025, ash dieback has caused mortality rates of 50–85% in affected European populations, with estimates of 50–75% in the UK and around 60% in countries affected for over 20 years.[46][50] Another significant entomological threat is the emerald ash borer (Agrilus planipennis), a beetle native to Asia that has devastated ash species in introduced ranges such as North America.[51] Larvae feed on the phloem and cambium, creating serpentine galleries under the bark that disrupt nutrient and water transport, leading to canopy thinning, branch dieback, and tree death within 2–4 years.[52] Although not yet established across much of Europe, the pest has been detected in European Russia and is approaching EU borders, posing a high invasion risk to F. excelsior due to suitable climatic conditions in central and eastern regions. As of 2025, it remains confined to Russia and Ukraine but poses an imminent threat to the EU, prompting enhanced surveillance and international workshops.[53][54][55] Additional diseases include root and collar rot caused by Phytophthora species, such as P. fraxinea, which infects roots and lower stems in wet soils, resulting in wilting, basal cankers, and predisposing trees to secondary infections.[56] Anthracnose, primarily incited by fungi like Apiognomonia errabunda, manifests as irregular leaf spots and shoot blights, causing defoliation in cool, wet springs, though it is generally less lethal than dieback.[12] Climate-exacerbated drought stress further compounds vulnerability by impairing tree defenses and water relations, intensifying dieback symptoms and mortality in water-limited sites.[57] As of 2025, resistant genotypes of F. excelsior have been identified, with 1–5% of trees showing survival and minimal crown damage after prolonged exposure, offering potential for breeding programs.[58] The disease continues to spread unchecked in regions like Ireland and the UK, with ongoing monitoring revealing near-complete infestation in unmanaged stands.[59]Conservation status and efforts
Fraxinus excelsior is assessed as Near Threatened on the global scale in the Red List of Fraxinus due to the widespread impact of ash dieback disease, which threatens significant population declines across its native range.[60] Regionally, the species faces varying levels of risk; for instance, it is classified as Critically Endangered in Sweden owing to intensified disease effects and habitat loss.[61] In the United Kingdom, it holds Least Concern status on the national Red List, though ash dieback poses a severe ongoing threat that could alter this assessment.[62] The species itself is not listed under Annex V of the EU Habitats Directive, but associated habitats such as alluvial forests (91E0) are protected under Annex I, influencing management of ash-dominated ecosystems.[63] Conservation efforts emphasize breeding programs to develop disease-resistant varieties, with notable initiatives in Ireland selecting tolerant genotypes from natural populations showing partial resistance to Hymenoscyphus fraxineus.[64] These programs, led by organizations like Teagasc, focus on propagating clones and seedlings from survivors exhibiting 1-5% tolerance rates in field assessments.[65] Monitoring relies heavily on citizen science networks, such as the Observatree project in the UK and similar European initiatives, which engage volunteers to report dieback symptoms and track disease spread across woodlands.[66] To curb epidemic progression, protocols include the systematic felling of heavily infected trees in affected areas, reducing inoculum sources and slowing local transmission.[67] Restoration strategies involve planting selected tolerant hybrids and clones in trial plots, alongside gene banking to preserve genetic diversity; EUFORGEN coordinates dynamic conservation units across Europe, targeting diverse provenances for long-term viability.[68] Recent research as of 2024 indicates that progeny from tolerant trees can show high resistance rates, with about 34% of offspring exhibiting strong tolerance to ash dieback in field assessments, informing scaled-up planting efforts.[58] Policy measures include statutory bans on the import and internal movement of ash planting material and wood in countries like the UK, implemented since 2012 to prevent further pathogen dissemination.[69] These restrictions are integrated into national forest strategies, such as the UK's Tree Health Resilience Strategy, promoting resilient silviculture and alternative species mixes in ash-dependent ecosystems.[67]Uses
Economic and industrial uses
The wood of Fraxinus excelsior, known as European ash, is prized in the timber industry for its straight grain, toughness, elasticity, and excellent shock resistance, which enable its use in high-value products such as furniture, flooring, and tool handles. These properties stem from the tree's dense, flexible structure, allowing it to withstand bending and impact without fracturing, and it has historically formed a significant portion of Europe's broadleaf timber output. Prior to the widespread onset of ash dieback, ash timber supported a robust supply chain for these applications.[3][70][2] In the sports sector, F. excelsior wood's combination of strength and lightness— with a density of about 0.65 g/cm³—makes it ideal for equipment requiring resilience under force, including hurleys for the Irish sport of hurling, oars for rowing, and baseball bats. Around 350,000 hurleys are produced annually from ash, underscoring its cultural and commercial importance in Ireland, though alternatives are increasingly sought due to supply constraints. The wood's shock-absorbing qualities also extend its utility to other athletic gear, contributing to niche markets where performance and durability are paramount.[71][72][73][13] Beyond primary timber, ash serves in secondary industrial roles, including as fuelwood due to its high calorific value even when freshly cut, and for fencing posts, basketry materials, and coppiced rods used in hurdle-making. Coppicing practices, which involve periodic cutting to promote regrowth, have sustained these uses for centuries, providing renewable straight poles for rural industries. The European ash timber market has faced significant disruption from ash dieback (Hymenoscyphus fraxineus), which has led to widespread tree mortality estimated at 50–95% in affected areas as of 2025, prompting increased imports, shifts to substitute species, and research into disease-resistant genotypes for future timber production.[21][74][75][50][76][77]Traditional and medicinal uses
In traditional practices, the leaves of Fraxinus excelsior have been used as nutritious fodder for livestock such as sheep, goats, pigs, and horses, particularly during winter when dried, and the tree was often pollarded or coppiced to produce hedges for enclosing animals in medieval Europe.[78] The sap, tapped in spring, has been fermented to produce a wine-like beverage in rural traditions across its native range.[79] Additionally, the bark has served as a source for natural dyes, yielding yellow, green, or blue colors for textiles and leather tanning in historical applications.[80] Medicinally, the bark of F. excelsior has been employed in herbalism as a tea or decoction acting as a diuretic and laxative, attributed to compounds like fraxin, and traditionally used to alleviate rheumatism, fever, gout, and joint pain.[81][82] In medieval Welsh manuscripts such as the Meddygon Myddfai, the tree, known as "onn," was recorded for treating deafness, reflecting ethnobotanical knowledge in Celtic-influenced regions.[83] Leaves have been applied externally for wounds, ulcers, sores, and swelling, or internally for diarrhea, dysentery, and malaria in folk remedies.[84] Contemporary pharmacological research supports these uses, identifying anti-inflammatory, antioxidant, and neuroprotective effects in bark and leaf extracts, with fraxin demonstrating hepatoprotective and analgesic properties in studies.[85][86] Extracts have shown diuretic and hypotensive potential, aligning with historical applications.[87] However, the prevalence of ash dieback disease has severely limited the availability of plant material for these traditional and medicinal purposes in recent decades.[21]Cultural significance
Mythology and folklore
In Norse mythology, Fraxinus excelsior, the common ash, is prominently featured as Yggdrasil, the immense World Tree that connects the nine realms of the cosmos, supporting the heavens, earth, and underworld while serving as a central axis of existence.[88] This sacred ash was the site of Odin's self-sacrifice, where he hung from its branches for nine days and nights without food or drink to gain profound wisdom and knowledge of the runes, symbolizing themes of endurance, enlightenment, and cosmic interconnectedness.[89] Among Celtic traditions, the ash held deep reverence as one of the five guardian trees of Ireland, considered sacred to the Druids and associated with wisdom, poetic inspiration, and protective energies that bridged the mortal and spiritual worlds.[90] Druids reportedly crafted wands from ash wood to channel magical forces, direct healing, and perform enchantments, viewing the tree as a conduit for transformation and empowerment in rituals.[90] Its presence near holy wells and springs underscored beliefs in its shielding qualities against harm and its role in fostering inner vision and safeguarding communities.[89] British and Gaelic folklore attributes to the ash potent warding properties, with traditions claiming its wood and leaves repelled snakes, which were thought to avoid its vicinity due to inherent protective virtues.[89] Similarly, ash trees were believed to guard against lightning strikes, often planted near homes for this purpose, though paradoxically attracting thunderbolts as a sacrificial shield.[91] The tree's winged seeds, known as "ash keys," featured in divination practices, where their abundance or scarcity was interpreted as omens of fortune, death, or royal events, such as a failed crop foretelling calamity.[92] In rural tales, solitary ash trees were deemed fairy dwellings, portals to the otherworld inhabited by ethereal beings, and felling one invited misfortune or supernatural retribution.[91] Greek mythology links the ash to the Meliae, nymphs born from the blood of the primordial sky god Ouranos that fell upon Gaia, nurturing the Bronze Race of humanity with their honey-like sap and arming them with spears fashioned from ash wood, emphasizing the tree's martial and generative symbolism.[93] This association extends to the spear of Achilles, crafted from unyielding Pelian ash by the centaur Chiron, an invincible weapon wielded only by the hero in the Trojan War, representing unyielding strength and heroic destiny.[94] In Slavic folklore, the ash tree embodies wisdom and a bridge to the afterlife.[95]In art and literature
The ash tree (Fraxinus excelsior) has been a recurring motif in English literature, often symbolizing endurance and the passage of time.[96] In Irish literature, the ash features prominently in poetry celebrating its cultural role, particularly in the sport of hurling, where its flexible wood crafts hurleys; early works like those referenced in medieval texts highlight the tree's strength as a metaphor for national vigor and communal bonds.[73] J.R.R. Tolkien's depiction of ents in The Lord of the Rings draws inspiration from ancient British trees, including venerable ashes, portraying them as guardians of the forest embodying slow, steadfast wisdom and the vitality of nature against industrialization.[97] In visual arts, the Romantic painter John Constable captured the ash's majestic form in works like Study of Ash Trees (c. 1821–1822), where the tree's branching structure and vibrant foliage emphasize its organic harmony and seasonal renewal, serving as a study in natural light and texture. Similarly, in Salisbury Cathedral from the Meadows (1831), a prominent ash frames the cathedral, symbolizing resilience amid transient weather, with the tree's enduring presence contrasting human architecture to underscore themes of stability in the Romantic ideal of sublime nature.[98] In contemporary art and literature, the ash's decline due to dieback disease has inspired works addressing environmental fragility. Eco-fiction and reports from 2025 highlight the tree's loss as a narrative of ecological grief, with authors weaving stories of infected woodlands to explore climate-induced collapse and human intervention. Photographers such as Daniel James Greenwood document dying ash groves through series like those in Unlocking Landscapes, using stark imagery of blackened trunks and skeletal canopies to evoke the tree's fading strength, contrasting its historical symbolism of renewal with modern motifs of vulnerability in climate art.[99] These representations often juxtapose the ash's traditional attributes of fortitude—rooted in brief mythical echoes of world trees—with its current portrayal as a casualty of global change, urging reflection on biodiversity loss. As of November 2025, conservation efforts have incorporated folklore elements, such as reviving Yggdrasil motifs in public awareness campaigns to emphasize the tree's cultural heritage amid ecological threats.[91]Varieties and cultivation
Cultivars
Fraxinus excelsior has numerous cultivated varieties, with approximately 20 registered cultivars primarily selected for ornamental qualities such as distinctive foliage, growth habit, and branch structure, as documented in early checklists of European ash variants. These selections were largely developed before the widespread impact of ash dieback disease (caused by Hymenoscyphus fraxineus), focusing on aesthetic appeal for gardens, parks, and urban landscapes rather than timber production, though a few emphasize wood quality.[100] One prominent cultivar is 'Aurea', characterized by golden-yellow leaves that emerge pale yellow in spring, turn pale green in summer, and deepen to gold in autumn, with slower growth forming a broadly conical or rounded canopy up to 12 m tall, making it suitable for ornamental use in parks and gardens.[101][26] Another is 'Pendula', a strong-growing deciduous tree reaching about 15 m in height with an umbrella-shaped crown of pendulous branches that arch to the ground, featuring dark green pinnate leaves that may turn yellow in autumn, often planted in parks for its weeping form.[102][100] 'Jaspidea' stands out for its vigorous growth and golden-yellow young shoots with yellowish longitudinal streaking on stems and branches, which remain conspicuous in winter, complemented by pinnate leaves that are yellow when young and in autumn, forming a conical crown up to 12 m tall and valued for year-round ornamental interest.[103][104] 'Diversifolia', also known as the one-leaved ash, features simple rather than pinnate leaves that are entire or occasionally 3-lobed, dark green and up to 18 cm long, with a narrow-oval to pyramidal crown growing 15-20 m tall, selected for its unusual foliage variation in landscape settings.[105][106][100] Other notable cultivars include 'Asplenifolia' with narrow, linear, pendent leaflets for a feathery appearance; 'Crispa' displaying dark green, crisped and pleated leaflets; and 'Doorenbos', chosen specifically for superior wood quality and straight growth from selections made in the 1940s.[100] Variegated forms like 'Argentea' with white-marbled foliage and 'Punctata' featuring yellow-dotted leaflets further enhance aesthetic options, while weeping variants such as 'Heterophylla Pendula' combine variable leaves with cascading branches.[100] Cultivar selection historically prioritized form and foliage prior to the ash dieback epidemic, but post-2020 breeding efforts have increasingly targeted tolerance to the disease through genomic prediction and evaluation of resistant genotypes, though few named cultivars with verified resistance have been registered to date.[100][107] These tolerant selections aim to support sustainable cultivation amid ongoing threats, with propagation typically involving grafting onto rootstocks for true-to-type reproduction.[107]Hybrids and propagation
Fraxinus excelsior commonly hybridizes with the closely related F. angustifolia, forming the interspecific hybrid F. × excelsior-angustifolia in zones of sympatry across Europe from northern Spain to Turkey.[108] These hybrids exhibit intermediate leaf morphology, including leaflet number and size that overlap but fall between the broader leaves of F. excelsior and the narrower ones of F. angustifolia.[109] Inheritance of traits in reciprocal F1 hybrids is asymmetric, influenced by maternal and paternal effects, such as variations in leaf mass, area, and margin dentition depending on the direction of the cross.[108] Hybridization efforts leverage the greater resistance of F. angustifolia to ash dieback (Hymenoscyphus fraxineus), with some F. excelsior × F. angustifolia hybrids showing vigorous growth and no disease symptoms in inoculated trials, supporting their use in breeding programs for tolerant stock.[110][111] Propagation of F. excelsior primarily occurs through seeds, which require cold stratification to break dormancy; seeds are typically stratified at 1–7°C for 8–16 weeks following an optional warm phase at 15–20°C, achieving germination rates of 60–80% under controlled conditions.[112][113] Vegetative methods include semi-hardwood cuttings taken in July from 1–3-year-old juvenile stock, treated with 1–2% indole-3-butyric acid (IBA) in mist units using peat or coir substrates, yielding rooting success up to 91% after 16 months, though rates drop to 0% for mature trees over 10 years old.[114] Hardwood cuttings collected in February from young trees, also with 2% IBA, root at about 54% after 9 months.[114] Grafting onto seedling rootstocks, using techniques like whip-and-tongue or cleft grafts, provides high viability of 85–97% for diverse genotypes, enabling rapid deployment of selected material while avoiding infection risks from seed-raised rootstocks.[115] Tissue culture techniques, including in vitro shoot multiplication from epicotyls or meristems on media with cytokinins like benzyladenine, followed by rooting on auxin-supplemented agar (e.g., IBA and naphthaleneacetic acid), support clonal propagation of resistant clones with rooting rates up to 97% in juvenile explants.[116] For cultivation aimed at timber production, F. excelsior is planted at spacings of 2–2.5 m initially (2000–2500 plants/ha), thinning to 3–5 m to promote straight boles and quality wood, on sites prepared with herbicide control of weeds and grasses to favor moisture retention in fertile, well-drained soils.[25] As of 2025, emphasis has shifted to propagating resistant stock through hybrids and selected clones to counter ash dieback, which diminishes propagule viability by infecting seeds and cuttings, reducing overall success rates and necessitating rejuvenation techniques like coppicing for mature material.[114][111]References
- https://en.wiktionary.org/wiki/fraxinus











