Recent from talks
Nothing was collected or created yet.
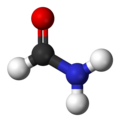
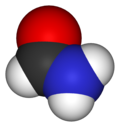
Formamide
View on Wikipedia | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formamide[1] | |||
| Systematic IUPAC name
Methanamide | |||
| Other names
Carbamaldehyde
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.766 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3NO | |||
| Molar mass | 45.04 g/mol | ||
| Appearance | Colorless, oily liquid[2] | ||
| Density | 1.133 g/cm3 | ||
| Melting point | 2 to 3 °C (36 to 37 °F; 275 to 276 K) | ||
| Boiling point | 210 °C (410 °F; 483 K) | ||
| Miscible | |||
| Vapor pressure | 0.08 mmHg at 20 °C | ||
| Acidity (pKa) | 23.5 (in DMSO)[3] | ||
| −2.19×10−5 cm3/mol | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 154 °C (309 °F; 427 K) (closed cup) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 10 ppm (15 mg/m3) [skin][2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
| Related compounds | |||
Related compounds
|
Carbamic acid Dimethylformamide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers.[4] Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.[5]
Formamides are compounds of the type RR′NCHO. One important formamide is dimethylformamide, (CH3)2NCHO.
Production
[edit]Historical production
[edit]In the past, formamide was produced by treating formic acid with ammonia, which produces ammonium formate, which in turn yields formamide upon heating:[6]
- HCOOH + NH3 → HCOO−
NH+
4 - HCOO−
NH+
4 → HCONH2 + H2O
Formamide is also generated by aminolysis of ethyl formate:[7]
- HCOOCH2CH3 + NH3 → HCONH2 + CH3CH2OH
Modern production
[edit]The current industrial process for the manufacture of formamide involves the carbonylation of ammonia:[4]
- CO + NH3 → HCONH2
An alternative two-stage process involves the ammonolysis of methyl formate, which is formed from carbon monoxide and methanol:
- CO + CH3OH → HCOOCH3
- HCO2CH3 + NH3 → HCONH2 + CH3OH
Applications
[edit]Formamide is used in the industrial production of hydrogen cyanide. It is also used as a solvent for processing various polymers such as polyacrylonitrile.[8]
It is also known that formamide is an excellent swelling agent for cellulosic fibres,[9] and is used in some specific wood-related applications.
Reactions
[edit]Formamide decomposes into carbon monoxide and ammonia when heated above 100 °C.
- HCONH2 → CO + NH3
The reaction is slow below 160 °C, but accelerates thereafter. At very high temperatures, the reaction products shift to hydrogen cyanide (HCN) and water instead:
- HC(O)NH2 → HCN + H2O
The same effect occurs in the presence of solid acid catalysts.[8]
Niche or laboratory applications
[edit]Formamide is a constituent of cryoprotectant vitrification mixtures used for cryopreservation of tissues and organs.
Formamide is also used as an RNA stabiliser in gel electrophoresis by deionizing RNA. In capillary electrophoresis, it is used for stabilizing (single) strands of denatured DNA.
Another use is to add it in sol-gel solutions in order to avoid cracking during sintering.
Formamide, in its pure state, has been used as an alternative solvent for the electrostatic self-assembly of polymer nanofilms.[10]
Formamide is used to prepare primary amines directly from ketones via their N-formyl derivatives, using the Leuckart reaction.
Biochemistry
[edit]
Formamides are intermediates in the methanogenesis cycle.
Prebiotic chemistry
[edit]Formamide has been proposed as an alternative solvent to water, perhaps with the ability to support life with alternative biochemistries to that currently found on Earth. It forms by the hydrolysis of hydrogen cyanide. With a large dipole moment, its solvation properties are similar to those of water.[12]
Formamide has been shown to convert to traces of guanine upon heating in the presence of ultraviolet light.[13]
Several prebiotic chemical reactions producing amino acid derivatives have been shown to take place in formamide.[14] Similar scenarios implicate thioformamide.[15]
Safety
[edit]Contact with skin and eyes is not recommended. With an LD50 of grams per kg, formamide is of low acute toxicity. It also has low mutagenicity.[8]
Formamide is classified as toxic to reproductive health.[16]
References
[edit]- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 841. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The traditional name 'formamide' is retained for HCO-NH2 and is the preferred IUPAC name.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0295". National Institute for Occupational Safety and Health (NIOSH).
- ^ F. G. Bordwell; J. E. Bartmess; J. A. Hautala (1978). "Alkyl effects on equilibrium acidities of carbon acids in protic and dipolar aprotic media and the gas phase". J. Org. Chem. 43 (16): 3095–3101. doi:10.1021/jo00410a001.
- ^ a b Hohn, A. (1999). "Formamide". In Kroschwitz, Jacqueline I. (ed.). Kirk-Othmer Concise Encyclopedia of Chemical Technology (4th ed.). New York: John Wiley & Sons, Inc. pp. 943–944. ISBN 978-0471419617.
- ^ "How to improve the search for aliens". The Economist.
- ^ Lorin, M. (1864). "Preparation of Formamide by means of Formiates and Oxalates". The Chemical News and Journal of Physical Science. IX: 291. Retrieved 14 June 2014.
- ^ Phelps, I. K.; Deming, C. D. (1908). "The Preparation of Formamide from Ethyl Formate and Ammonium Hydroxide". The Chemical News and Journal of Physical Science. 97: 86–87. Retrieved 14 June 2014.
- ^ a b c Bipp, H.; Kieczka, H. (2012). "Formamides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_001.pub2. ISBN 978-3-527-30673-2.
- ^ Mantanis, G. I.; Young, R. A.; Rowell, R. M. (1995). "Swelling of compressed cellulose fiber webs in organic liquids". Cellulose. 2 (1): 1–22. doi:10.1007/BF00812768. ISSN 0969-0239.
- ^ Vimal K. Kamineni; Yuri M. Lvov; Tabbetha A. Dobbins (2007). "Layer-by-Layer Nanoassembly of Polyelectrolytes Using Formamide as the Working Medium". Langmuir. 23 (14): 7423–7427. doi:10.1021/la700465n. PMID 17536845.
- ^ Thauer, R. K. (1998). "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson". Microbiology. 144: 2377–2406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- ^ Committee On The Limits Of Organic Life In Planetary Systems (2007). The Limits of Organic Life in Planetary Systems. Washington, DC: The National Academies Press. p. 74. ISBN 978-0-309-66906-1. Retrieved 2012-08-29.
- ^ "Origin of Life: Adding UV Light Helps Form 'Missing G' of RNA Building Blocks". Science Daily. June 14, 2010.
- ^ Green, N. J.; Russell, D. A.; Tanner, S. H.; Sutherland, J. D. (2023). "Prebiotic Synthesis of N-Formylaminonitriles and Derivatives in Formamide". Journal of the American Chemical Society. 145 (19): 10533–10541. Bibcode:2023JAChS.14510533G. doi:10.1021/jacs.2c13306. PMC 10197134. PMID 37146260.
- ^ Patel, Bhavesh H.; Percivalle, Claudia; Ritson, Dougal J.; Duffy, Colm D.; Sutherland, John D. (2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature Chemistry. 7 (4): 301–307. Bibcode:2015NatCh...7..301P. doi:10.1038/nchem.2202. PMC 4568310. PMID 25803468.
- ^ "Support document for identification of formamide as a substance of very high concern because of its cmr1 properties". European Chemicals Agency.
Formamide
View on GrokipediaProperties
Physical properties
Formamide has the molecular formula HCONH₂ and a molecular weight of 45.04 g/mol.[1] It is a colorless, hygroscopic liquid with a faint ammonia-like odor.[1][6] Key physical constants of formamide are summarized in the following table:| Property | Value | Conditions |
|---|---|---|
| Melting point | 2.55 °C | - |
| Boiling point | 210 °C | 760 mmHg |
| Density | 1.134 g/cm³ | 20 °C |
| Viscosity | 3.76 mPa·s | 20 °C (dynamic) |