Recent from talks
Nothing was collected or created yet.
Hydrazone
View on Wikipedia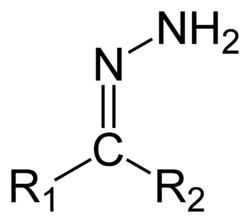
Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2.[1] They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.[2][3]
Synthesis
[edit]Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones.
Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides.[4][5] Hydrazones having 1,3-diketomoiety are also known in literature.[6]
Uses
[edit]
Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by high-performance liquid chromatography (HPLC) using a UV detector.[citation needed]
The compound carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molecular biology.
Hydrazones are the basis of bioconjugation strategies.[8][9] Hydrazone-based coupling methods are used in medical biotechnology to couple drugs to targeted antibodies (see ADC), e.g. antibodies against a certain type of cancer cell. The hydrazone-based bond is stable at neutral pH (in the blood), but is rapidly destroyed in the acidic environment of lysosomes of the cell. The drug is thereby released in the cell, where it exerts its function.[10]
Reactions
[edit]Hydrazones are susceptible to hydrolysis:
- R2C=N−NR'2 + H2O → R2C=O + H2N−NR'2
Alkyl hydrazones are 102- to 103-fold more sensitive to hydrolysis than analogous oximes.[11]
When derived from hydrazine itself, hydrazones condense with a second equivalent of a carbonyl to give azines:[12]
- R2C=N−NH2 + R2C=O → R2C=N−N=CR2 + H2O
Hydrazones are intermediates in the Wolff–Kishner reduction.
Hydrazones are reactants in hydrazone iodination, the Shapiro reaction, and the Bamford–Stevens reaction to vinyl compounds. Hydrazones can also be synthesized by the Japp–Klingemann reaction via β-keto acids or β-keto-esters and aryl diazonium salts. Hydrazones are converted to azines when used in the preparation of 3,5-disubstituted 1H-pyrazoles,[13] a reaction also well known using hydrazine hydrate.[14][15] With a transition metal catalyst, hydrazones can serve as organometallic reagent surrogates to react with various electrophiles.[16]
N,N-dialkylhydrazones
[edit]In N,N-dialkylhydrazones[17] the C=N bond can be hydrolysed, oxidised and reduced, the N–N bond can be reduced to the free amine. The carbon atom of the C=N bond can react with organometallic nucleophiles. The alpha-hydrogen atom is more acidic by 10 orders of magnitude compared to the ketone and therefore more nucleophilic. Deprotonation with for instance lithium diisopropylamide (LDA) gives an azaenolate which can be alkylated by alkyl halides.[18] The hydrazines SAMP and RAMP function as chiral auxiliary.[19][20]
Recovery of carbonyl compounds from N,N-dialkylhydrazones
[edit]Several methods are known to recover carbonyl compounds from N,N-dialkylhydrazones.[21] Procedures include oxidative, hydrolytic or reductive cleavage conditions and can be compatible with a wide range of functional groups.
Gallery
[edit]- Hydrazones
-
Benzophenone hydrazone, an illustrative hydrazone
-
Gyromitrin (acetaldehyde methylformylhydrazone), a toxin
-
Dihydralazine, an antihypertensive drug
-
X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 138 pm, N-N-C(Ar), 119 pm[22]
See also
[edit]References
[edit]- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- ^ Stork, G.; Benaim, J. (1977). "Monoalkylation of α,β-Unsaturated Ketones via Metalloenamines: 1-butyl-10-methyl-Δ1(9)-2-octalone". Organic Syntheses. 57: 69; Collected Volumes, vol. 6, p. 242.
- ^ Day, A. C.; Whiting, M. C. (1970). "Acetone hydrazone". Organic Syntheses. 50: 3; Collected Volumes, vol. 6, p. 10.
- ^ Fischer, Emil (1908). "Schmelzpunkt des Phenylhydrazins und einiger Osazone". Berichte der Deutschen Chemischen Gesellschaft. 41: 73–77. doi:10.1002/cber.19080410120.
- ^ Fischer, Emil (1894). "Ueber einige Osazone und Hydrazone der Zuckergruppe". Berichte der Deutschen Chemischen Gesellschaft. 27 (2): 2486–2492. doi:10.1002/cber.189402702249.
- ^ Singh, Raman; Halve, Anand K. (2025-06-28). "Synthesis of New Hydrazones Containing 1,3-Diketo Moiety". RSYN Chemical Sciences. 2 (1): 1–6. doi:10.70130/RCS.2025.0201001.
- ^ Christie, R.; Hill, J.; Rosair, G. (2006). "The crystal structure of CI Pigment Yellow 97, a superior performance Hansa yellow pigment". Dyes and Pigments. 71 (3): 194–198. doi:10.1016/j.dyepig.2005.07.001.
- ^ Kölmel, Dominik K.; Kool, Eric T. (2017). "Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis". Chemical Reviews. 117 (15): 10358–10376. doi:10.1021/acs.chemrev.7b00090. PMC 5580355. PMID 28640998.
- ^ Algar, W. Russ; Prasuhn, Duane E.; Stewart, Michael H.; Jennings, Travis L.; Blanco-Canosa, Juan B.; Dawson, Philip E.; Medintz, Igor L. (2011). "The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry". Bioconjugate Chemistry. 22 (5): 825–858. doi:10.1021/bc200065z. PMID 21585205.
- ^ Wu, Anna M.; Senter, Peter D. (7 September 2005). "Arming antibodies: prospects and challenges for immunoconjugates". Nature Biotechnology. 23 (9): 1137–46. doi:10.1038/nbt1141. PMID 16151407. S2CID 27226728.
- ^ Kalia, J.; Raines, R. T. (2008). "Hydrolytic stability of hydrazones and oximes". Angew. Chem. Int. Ed. 47 (39): 7523–6. doi:10.1002/anie.200802651. PMC 2743602. PMID 18712739.
- ^ Day, A. C.; Whiting, M. C. (1970). "Acetone Hydrazone". Organic Syntheses. 50: 3. doi:10.15227/orgsyn.050.0003.
- ^ Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry, Section B. 57B (3): 362–373.
- ^ Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry. 45 (2): 503–505. doi:10.1002/jhet.5570450231.
- ^ Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-pot synthesis of 3,5-diphenyl-1H-pyrazoles from chalcones and hydrazine under mechanochemical ball milling". Heterocycles. 89 (1): 103–112. doi:10.3987/COM-13-12867.
- ^ Wang, H; Dai, X.-J.; Li, C.-J. (2017). "Aldehydes as alkyl carbanion equivalents for additions to carbonyl compounds". Nature Chemistry. 9 (4): 374–378. doi:10.1038/nchem.2677. PMID 28338683. S2CID 11653420.
- ^ Lazny, R.; Nodzewska, A. (2010). "N,N-dialkylhydrazones in organic synthesis. From simple N,N-dimethylhydrazones to supported chiral auxiliaries". Chemical Reviews. 110 (3): 1386–1434. doi:10.1021/cr900067y. PMID 20000672.
- ^ Enders, Dieter; Reinhold, Ulrich (1997). "Asymmetric synthesis of amines by nucleophilic 1,2-addition of organometallic reagents to the CN-double bond". Tetrahedron: Asymmetry. 8 (12): 1895–1946. doi:10.1016/S0957-4166(97)00208-5.
- ^ Enders, Dieter; Fey, Peter; Kipphardt, Helmut (1987). "(S)-(−)-1-Amino-2-methoxymethylpyrrolidine (SAMP) and (R)-(+)-1-amino-2-methoxymethylpyrrolidine (RAMP), Versatile Chiral Auxiliaries". Organic Syntheses. 65: 173. doi:10.15227/orgsyn.065.0173. S2CID 260330996.
- ^ Enders, Dieter; Kipphardt, Helmut; Fey, Peter (1987). "Asymmetric Syntheses Using the SAMP-/RAMP-Hydrazone Method: (S)-(+)-4-methyl-3-heptanone". Organic Syntheses. 65: 183. doi:10.15227/orgsyn.065.0183.
- ^ Enders, Dieter; Wortmann, Lars; Peters, René (2000). "Recovery of Carbonyl Compounds from N,N-Dialkylhydrazones". Accounts of Chemical Research. 33 (3): 157–169. doi:10.1021/ar990062y. PMID 10727205.
- ^ Tameem, Abdassalam Abdelhafiz; Salhin, Abdussalam; Saad, Bahruddin; Rahman, Ismail Ab.; Saleh, Muhammad Idiris; Ng, Shea-Lin; Fun, Hoong-Kun (2006). "Benzophenone 2,4-dinitrophenylhydrazone". Acta Crystallographica Section E. 62 (12): o5686–o5688. doi:10.1107/S1600536806048112.








![X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 138 pm, N-N-C(Ar), 119 pm[22]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/39/NERYOZ.png/120px-NERYOZ.png)