Recent from talks
Nothing was collected or created yet.
Oxytocin (medication)
View on WikipediaThis article needs more reliable medical references for verification or relies too heavily on primary sources. (January 2024) |
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɒksɪˈtoʊsɪn/ |
| Trade names | Pitocin, Syntocinon, Viatocinon, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682685 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intranasal, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver and elsewhere (via oxytocinases) |
| Elimination half-life | 1–6 min (IV) ~2 h (intranasal)[4][5] |
| Excretion | Bile duct and kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
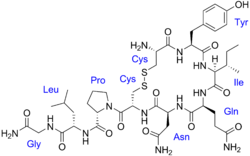
| Formula | C43H66N12O12S2 |
| Molar mass | 1007.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Synthetic oxytocin, sold under the brand name Pitocin among others, is a medication made from the peptide oxytocin.[6][7] As a medication, it is used to cause contraction of the uterus to start labor, increase the speed of labor, and to stop bleeding following delivery.[6] For this purpose, it is given by injection either into a muscle or into a vein.[6]
Oxytocin is also available in intranasal spray form for psychiatric, endocrine and weight management use as a supplement. Intranasal oxytocin works on a different pathway than injected oxytocin, primarily along the olfactory nerve crossing the blood–brain barrier to the olfactory lobe in the brain, where dense magnocellular oxytocin neurons receive the nerve impulse quickly.
The natural occurrence of oxytocin was discovered in 1906.[8][9] It is on the World Health Organization's List of Essential Medicines.[10]
Medical uses
[edit]An intravenous infusion of oxytocin is used to induce labor and to support labor in case of slow childbirth if the oxytocin challenge test fails. The physiology of labor stimulated by oxytocin administration is similar to the physiology of spontaneous labor.[11] It is associated with less tachysystole (more than five contractions in 10 minutes, averaged over a 30-minute period, which can but does not always cause fetal distress) than other induction methods and allows achievement of delivery with amniotomy to proceed faster.[12][13] Whether a high dose is better than a standard dose for labor induction is unclear. It has largely replaced ergometrine as the principal agent to increase uterine tone in acute postpartum hemorrhage. Oxytocin is also used in veterinary medicine to facilitate birth and to stimulate milk release.
The tocolytic agent atosiban (Tractocile) acts as an antagonist of oxytocin receptors. It is registered in many countries for use in suppressing premature labor between 24 and 33 weeks of gestation. It has fewer side effects than drugs previously used for this purpose (such as ritodrine, salbutamol and terbutaline).[14]
Oxytocin has not been found to be useful for improving breastfeeding success.[15]
Contraindications
[edit]Oxytocin injection (synthetic) is contraindicated in any of these conditions:[16]
- Substantial cephalopelvic disproportion
- Unfavorable fetal position or presentation (e.g., transverse lies) undeliverable without conversion before delivery
- Obstetric emergencies where maternal or fetal risk-to-benefit ratio favors surgery
- Fetal distress when delivery is not imminent
- Umbilical cord prolapse
- Uterine activity fails to progress adequately
- Hyperactive or hypertonic uterus
- Vaginal delivery is contraindicated (e.g., invasive cervical carcinoma, active genital herpes infection, total placenta previa, vasa previa, cord presentation or prolapse)
- Uterine or cervical scarring from previous cesarean section or major cervical or uterine (e.g., transfundal) surgery
- Unengaged fetal head
- History of hypersensitivity to oxytocin or any ingredient in the formulation
Side effects
[edit]Oxytocin is relatively safe when used at recommended doses, and side effects are uncommon.[17] These maternal events have been reported:[17]
- Subarachnoid hemorrhage
- Increased blood pressure when combined with other medications that raise blood pressure, particularly when used prior to administering epidural anesthesia[18]
- Cardiac arrhythmia including increased or decreased heart rate, and premature ventricular contraction
- Impaired uterine blood flow or excessive uterine contractions when combined with other medications that cause uterine contraction (carboprost, misoprostol)[18]
- Uterine rupture[18]
- Afibrinogenemia
- Anaphylaxis[6]
- Nausea and vomiting
- Changes in fetal blood flow
Many of these side effects are unable to be differentiated from the risks of normal labor versus oxytocin administration itself.[18][19]
Oxytocin during labour is associated with a significantly higher risk of severe postpartum hemorrhage.[20]
Excessive dosage or long-term administration (over a period of 24 hours or longer) has been known to result in tetanic uterine contractions, uterine rupture, sometimes fatal. Water intoxication may be exhibited in administration through symptoms such as seizures, comas, neonatal jaundice, and potential fatality.[21] Managed fluid intake and consistent monitoring of sodium levels has been researched as crucial in the safe administration of oxytocin.[22]
The use of oxytocin during childbirth has been linked to an increased need for other medical interventions, most primarily, through the administration of an epidural anaesthetic.[23] This has been documented as creating a 'cascade effect', potentially causing detrimental impacts to the birthing process.[24][25] Oxytocin administration also, conversely, decreases the rate of cesarean sections.[26] Use of oxytocin has been found to significantly shorten labor duration.[26] Early oxytocin augmentation has also been found to increase the probability of spontaneous vaginal delivery and reduce the risk of chorioamnionitis or intrauterine infection.[27][28]
Since a landmark investigation was published in JAMA Pediatrics by researchers in 2013,[29] the potential link between oxytocin use during childbirth and increased risks of Autism Spectrum Disorder (ASD) in children's development has been a topic of debate.[30] There is no robust evidence in support of oxytocin causing ASD or other neurodevelopmental disorders.[31]
Oxytocin was added to the Institute for Safe Medication Practices's list of High Alert Medications in Acute Care Settings in 2012.[32] The list includes medications that have a high risk for harm if administered incorrectly.[32]
During pregnancy, increased uterine motility has led to decreased heart rate, cardiac arrhythmia, seizures, brain damage, and death in the fetus or neonate.[17] Increased uterine motility is a hallmark of both spontaneous labor and induced labor, therefore the risks associated with uterine motility are not specific to this medication.[33]
Use is linked to an increased risk of postpartum depression in the mother.[34]
Certain learning and memory functions are impaired by centrally administered oxytocin.[35] Also, systemic oxytocin administration can impair memory retrieval in certain aversive memory tasks.[36] However, oxytocin does seem to facilitate learning and memory specifically for social information. Healthy males administered intranasal oxytocin show improved memory for human faces, in particular happy faces.[37][38]
Pharmacodynamics
[edit]In addition to its oxytocin receptor agonism, oxytocin has been found to act as a positive allosteric modulator (PAM) of the μ- and κ-opioid receptors and this may be involved in its analgesic effects.[39][40][41][42][43][44]
Pharmacokinetics
[edit]Routes of administration
[edit]
One IU of oxytocin is the equivalent of about 1.68 μg or mcg of pure peptide.[45]
- Injection: Clinical doses of oxytocin are given by injection either into a muscle or into a vein to cause contraction of the uterus.[6] Very small amounts (< 1%) do appear to enter the central nervous system in humans when peripherally administered.[46][better source needed] The compound has a half-life of typically about 3 minutes in the blood when given intravenously. Intravenous administration requires 40 minutes to reach a steady-state concentration and achieve maximum uterine contraction response.[47]
- Buccal: Oxytocin was delivered in buccal tablets, but this is not common practice any more.[48]
- Under the tongue: Oxytocin is poorly absorbed sublingually.[49]
- Nasal administration: Oxytocin is effectively distributed to the brain when administered intranasally via a nasal spray, after which it reliably crosses the blood–brain barrier and exhibits psychoactive effects in humans.[50][51] No serious adverse effects with short-term application of oxytocin with 18~40 IU (36–80 mcg) have been recorded.[52] Intranasal oxytocin has a central duration of at least 2.25 hours and as long as 4 hours.[4][5]
- Oral: While it was originally assumed that Oxytocin administered orally would be destroyed in the gastrointestinal tract, studies have shown that Oxytocin is transported by the immunoglobulin RAGE (receptor for advanced glycation end products) across the intestinal epithelium and into the blood. Orally-administered Oxytocin has been shown to increase putamen responses to facial emotions in humans.[53] Oxytocin administered orally produces different effects on human behaviour and brain function than when given intranasally, possibly due to variations in the molecular transport and binding mechanisms.
Chemistry
[edit]Peptide analogues of oxytocin with similar actions, for example carbetocin (Duratocin) and demoxytocin (Sandopart), have been developed and marketed for medical use.[54] In addition, small-molecule oxytocin receptor agonists, like TC OT 39, WAY-267464, and LIT-001 have been developed and studied.[54] However, lack of selectivity over vasopressin receptors has so far limited the potential usefulness of small-molecule oxytocin receptor agonists.[54]
History
[edit]Oxytocin's uterine-contracting properties were discovered by British pharmacologist Henry Hallett Dale in 1906.[9] Oxytocin's milk ejection property was described by Ott and Scott in 1910[55] and by Schafer and Mackenzie in 1911.[56]
Oxytocin was the first polypeptide hormone to be sequenced[57] or synthesized.[58][59] Du Vigneaud was awarded the Nobel Prize in 1955 for his work.[60]
Etymology
[edit]The word oxytocin was coined from the term oxytocic. Greek ὀξύς, oxys, and τόκος, tokos, meaning "quick birth".
Society and culture
[edit]Counterfeits
[edit]In African and Asian countries, some oxytocin products were found to be counterfeit medications.[61][62]
Other uses
[edit]The trust-inducing property of oxytocin might help those with social anxiety and depression,[63] anxiety, fear, and social dysfunctions, such as generalized anxiety disorder, post-traumatic stress disorder, and social anxiety disorder, as well as autism and schizophrenia, among others.[64][65] However, a 2013 meta-analysis only autism spectrum disorder showed a significant combined effect size.[66] A 2022 study found an indication of an effect among autistic children aged 3–5, but not among autistic children aged 5-12.[67]
People using oxytocin show improved recognition for positive social cues over threatening social cues[68][69] and improved recognition of fear.[70]
- Autism: Oxytocin may play a role in autism and may be an effective treatment for autism's repetitive and affiliative behaviors.[71]
- Relationship counseling: The use of oxytocin in relationship counseling for well-being has been suggested.[72]
- Post-traumatic stress disorder: It has been suggested that oxytocin may be a safer option than MDMA for the treatment of PTSD, although oxytocin has less evidence of efficacy.[73]
See also
[edit]References
[edit]- ^ "Syntocinon 10 IU/ml Concentrate for Solution for Infusion". (emc). 25 October 2023. Archived from the original on 14 January 2024. Retrieved 14 January 2024.
- ^ "Pitocin- oxytocin injection". DailyMed. 10 January 2008. Retrieved 14 January 2024.
- ^ "Oxytocin injection, solution". DailyMed. 16 October 2023. Retrieved 14 January 2024.
- ^ a b Weisman O, Zagoory-Sharon O, Feldman R (September 2012). "Intranasal oxytocin administration is reflected in human saliva". Psychoneuroendocrinology. 37 (9): 1582–86. doi:10.1016/j.psyneuen.2012.02.014. PMID 22436536. S2CID 25253083.
- ^ a b Huffmeijer R, Alink LR, Tops M, Grewen KM, Light KC, Bakermans-Kranenburg MJ, et al. (2012). "Salivary levels of oxytocin remain elevated for more than two hours after intranasal oxytocin administration". Neuro Endocrinology Letters. 33 (1): 21–25. PMID 22467107.
- ^ a b c d e "Oxytocin". The American Society of Health-System Pharmacists. Archived from the original on 20 May 2015. Retrieved 1 June 2015.
- ^ The Oxford Handbook of Prosocial Behavior. Oxford University Press. 2015. p. 354. ISBN 978-0-19-539981-3. Archived from the original on 1 August 2017.
- ^ Hurlemann R, Grinevich V (2018). Behavioral Pharmacology of Neuropeptides: Oxytocin. Springer. p. 37. ISBN 978-3-319-63739-6.
- ^ a b Dale HH (May 1906). "On some physiological actions of ergot". The Journal of Physiology. 34 (3): 163–206. doi:10.1113/jphysiol.1906.sp001148. PMC 1465771. PMID 16992821.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Induction of Labor". www.acog.org. Retrieved 19 April 2025.
- ^ Pennington E, Bell S, Hill JE (2023). "Should video laryngoscopy or direct laryngoscopy be used for adults undergoing endotracheal intubation in the pre-hospital setting? A critical appraisal of a systematic review". Cochrane Database of Systematic Reviews. 15 (6): 255–259. doi:10.1002/14651858. PMC 7616025. PMID 38812899.
- ^ Hofmeyr GJ, Gülmezoglu AM, Pileggi C (October 2010). "Vaginal misoprostol for cervical ripening and induction of labour". The Cochrane Database of Systematic Reviews. 2010 (10) CD000941. doi:10.1002/14651858.CD000941.pub2. PMC 7061246. PMID 20927722.
- ^ Budden A, Chen LJ, Henry A (9 October 2014). "High-dose versus low-dose oxytocin infusion regimens for induction of labour at term". The Cochrane Database of Systematic Reviews. 10 (10) CD009701. doi:10.1002/14651858.CD009701.pub2. PMC 8932234. PMID 25300173.
- ^ "Oxytocin use while Breastfeeding". Drugs.com. Archived from the original on 15 December 2016.
- ^ "Oxytocin - FDA prescribing information, side effects and uses". Archived from the original on 21 December 2016. Retrieved 16 December 2016.
- ^ a b c "Pitocin (drug label for professionals)". Rx List. WebMD. Archived from the original on 15 April 2011. Retrieved 9 September 2010.
- ^ a b c d Osilla EV, Patel P, Sharma S (15 February 2025). "Oxytocin". StatPearls [Internet]. StatPearls Publishing. PMID 29939625. Retrieved 19 April 2025.
- ^ "Induction of Labor". www.acog.org. Retrieved 19 April 2025.
- ^ Belghiti J, Kayem G, Dupont C, Rudigoz RC, Bouvier-Colle MH, Deneux-Tharaux C (2011). "Oxytocin during labour and risk of severe postpartum haemorrhage: a population-based, cohort-nested case-control study". BMJ Open. 1 (2) e000514. doi:10.1136/bmjopen-2011-000514. PMC 3334825. PMID 22189353.
- ^ D'Souza SW, Lieberman B, Cadman J, Richards B (September 1986). "Oxytocin induction of labour: hyponatraemia and neonatal jaundice". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 22 (5–6): 309–317. doi:10.1016/0028-2243(86)90119-x. PMID 3770280.
- ^ Emre U, Kadıoğlu G, Ünal A, Atasoy HT (March 2009). "Case report: hyponatremia and generalized convulsion after intravenous oxytocin infusion". Journal of the Turkish-German Gynecological Association. 10 (1): 47–48.
- ^ Hidalgo-Lopezosa P, Hidalgo-Maestre M, Rodríguez-Borrego MA (2016). "Labor stimulation with oxytocin: effects on obstetrical and neonatal outcomes". Revista Latino-Americana de Enfermagem. 24 e2744. doi:10.1590/1518-8345.0765.2744. PMC 4982443. PMID 27463109.
- ^ Lothian JA (2014). "Healthy birth practice #4: avoid interventions unless they are medically necessary". The Journal of Perinatal Education. 23 (4): 198–206. doi:10.1891/1058-1243.23.4.198. PMC 4235054. PMID 25411540.
- ^ Hitzeman N, Chin S (August 2012). "Epidural analgesia for labor pain". American Family Physician. 86 (3): 241–242. PMID 22962986.
- ^ a b Wei S, Wo BL, Qi HP, Xu H, Luo ZC, Roy C, et al. (Cochrane Pregnancy and Childbirth Group) (August 2013). "Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care". The Cochrane Database of Systematic Reviews. 2013 (8) CD006794. doi:10.1002/14651858.CD006794.pub4. PMC 11528244. PMID 23926074.
- ^ Wei SQ, Luo ZC, Xu H, Fraser WD (September 2009). "The effect of early oxytocin augmentation in labor: a meta-analysis". Obstetrics and Gynecology. 114 (3): 641–649. doi:10.1097/AOG.0b013e3181b11cb8. PMID 19701046.
- ^ Son M, Roy A, Stetson BT, Grady NT, Vanecko MC, Bond N, et al. (June 2021). "High-Dose Compared With Standard-Dose Oxytocin Regimens to Augment Labor in Nulliparous Women: A Randomized Controlled Trial". Obstetrics and Gynecology. 137 (6): 991–998. doi:10.1097/AOG.0000000000004399. PMID 33957657.
- ^ Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML (October 2013). "Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases". JAMA Pediatrics. 167 (10): 959–966. doi:10.1001/jamapediatrics.2013.2904. PMID 23938610.
- ^ Oberg AS, D'Onofrio BM, Rickert ME, Hernandez-Diaz S, Ecker JL, Almqvist C, et al. (September 2016). "Association of Labor Induction With Offspring Risk of Autism Spectrum Disorders". JAMA Pediatrics. 170 (9): e160965. doi:10.1001/jamapediatrics.2016.0965. hdl:10616/45629. PMC 5297393. PMID 27454803.
- ^ Lønfeldt NN, Verhulst FC, Strandberg-Larsen K, Plessen KJ, Lebowitz ER (April 2019). "Assessing risk of neurodevelopmental disorders after birth with oxytocin: a systematic review and meta-analysis". Psychological Medicine. 49 (6): 881–890. doi:10.1017/S0033291718003021. PMID 30444210.
- ^ a b "High-Alert Medications in Acute Care Settings". Institute For Safe Medication Practices. 16 November 2017. Retrieved 6 May 2019.
- ^ "Induction of Labor". www.acog.org. Retrieved 19 April 2025.
- ^ Kroll-Desrosiers AR, Nephew BC, Babb JA, Guilarte-Walker Y, Moore Simas TA, Deligiannidis KM (February 2017). "Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year". Depression and Anxiety. 34 (2): 137–146. doi:10.1002/da.22599. PMC 5310833. PMID 28133901.
- ^ Gimpl G, Fahrenholz F (April 2001). "The oxytocin receptor system: structure, function, and regulation". Physiological Reviews. 81 (2): 629–83. doi:10.1152/physrev.2001.81.2.629. PMID 11274341. S2CID 13265083.
- ^ de Oliveira LF, Camboim C, Diehl F, Consiglio AR, Quillfeldt JA (January 2007). "Glucocorticoid-mediated effects of systemic oxytocin upon memory retrieval". Neurobiology of Learning and Memory. 87 (1): 67–71. doi:10.1016/j.nlm.2006.05.006. PMID 16997585. S2CID 25371427.
- ^ Guastella AJ, Mitchell PB, Mathews F (August 2008). "Oxytocin enhances the encoding of positive social memories in humans". Biological Psychiatry. 64 (3): 256–58. doi:10.1016/j.biopsych.2008.02.008. PMID 18343353. S2CID 38681820.
- ^ Rimmele U, Hediger K, Heinrichs M, Klaver P (January 2009). "Oxytocin makes a face in memory familiar". The Journal of Neuroscience. 29 (1): 38–42. doi:10.1523/JNEUROSCI.4260-08.2009. PMC 6664913. PMID 19129382.
- ^ Drakopoulos A, Moianos D, Prifti GM, Zoidis G, Decker M (July 2022). "Opioid Ligands Addressing Unconventional Binding Sites and More Than One Opioid Receptor Subtype". ChemMedChem. 17 (14) e202200169. doi:10.1002/cmdc.202200169. PMID 35560796.
- ^ Kaczyńska K, Wojciechowski P (December 2021). "Non-Opioid Peptides Targeting Opioid Effects". Int J Mol Sci. 22 (24) 13619. doi:10.3390/ijms222413619. PMC 8709238. PMID 34948415.
- ^ Carter CS, Kingsbury MA (August 2022). "Oxytocin and oxygen: the evolution of a solution to the 'stress of life'". Philos Trans R Soc Lond B Biol Sci. 377 (1858) 20210054. doi:10.1098/rstb.2021.0054. PMC 9272143. PMID 35856299.
- ^ Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, Ogino T, et al. (May 2018). "Neuropeptide oxytocin enhances μ opioid receptor signaling as a positive allosteric modulator". J Pharmacol Sci. 137 (1): 67–75. doi:10.1016/j.jphs.2018.04.002. PMID 29716811.
- ^ Miyano K, Yoshida Y, Hirayama S, Takahashi H, Ono H, Meguro Y, et al. (October 2021). "Oxytocin Is a Positive Allosteric Modulator of κ-Opioid Receptors but Not δ-Opioid Receptors in the G Protein Signaling Pathway". Cells. 10 (10): 2651. doi:10.3390/cells10102651. PMC 8534029. PMID 34685631.
- ^ Mizuguchi T, Miyano K, Yamauchi R, Yoshida Y, Takahashi H, Yamazaki A, et al. (January 2023). "The first structure-activity relationship study of oxytocin as a positive allosteric modulator for the µ opioid receptor". Peptides. 159 170901. doi:10.1016/j.peptides.2022.170901. PMID 36347314.
- ^ "WHO International Standard OXYTOCIN 4th International Standard NIBSC code: 76/575 Instructions for use (Version 4.0, Dated 30/04/2013)" (PDF). nibsc.org.
- ^ Baribeau DA, Anagnostou E (2015). "Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits". Frontiers in Neuroscience. 9: 335. doi:10.3389/fnins.2015.00335. PMC 4585313. PMID 26441508.
- ^ Seitchik J, Castillo M (December 1982). "Oxytocin augmentation of dysfunctional labor. I. Clinical data". American Journal of Obstetrics and Gynecology. 144 (8): 899–905. doi:10.1097/00006254-198307000-00010. PMID 7148921.
- ^ Mehta AC (1986). "Buccal and oral drugs: induction of labour". Acta Chirurgica Hungarica. 27 (3): 157–63. PMID 3469841.
- ^ De Groot AN, Vree TB, Hekster YA, Pesman GJ, Sweep FC, Van Dongen PJ, et al. (1995). "Bioavailability and pharmacokinetics of sublingual oxytocin in male volunteers" (PDF). The Journal of Pharmacy and Pharmacology. 47 (7): 571–75. doi:10.1111/j.2042-7158.1995.tb06716.x. hdl:2066/21581. PMID 8568623. S2CID 8615529.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 7: Neuropeptides". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-148127-4.
Oxytocin can be delivered to humans via nasal spray following which it crosses the blood–brain barrier. ... In a double-blind experiment, oxytocin spray increased trusting behavior compared to a placebo spray in a monetary game with real money at stake.
- ^ McGregor IS, Callaghan PD, Hunt GE (May 2008). "From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use?". British Journal of Pharmacology. 154 (2): 358–68. doi:10.1038/bjp.2008.132. PMC 2442436. PMID 18475254.
Recent studies also highlight remarkable anxiolytic and prosocial effects of intranasally administered OT in humans, including increased 'trust', decreased amygdala activation towards fear-inducing stimuli, improved recognition of social cues and increased gaze directed towards the eye regions of others (Kirsch et al., 2005; Kosfeld et al., 2005; Domes et al., 2006; Guastella et al., 2008).
- ^ Lee SY, Lee AR, Hwangbo R, Han J, Hong M, Bahn GH (2015). "Is Oxytocin Application for Autism Spectrum Disorder Evidence-Based?". Experimental Neurobiology. 24 (4): 312–24. doi:10.5607/en.2015.24.4.312. PMC 4688331. PMID 26713079.
- ^ Kou J, Lan C, Zhang Y, Wang Q, Zhou F, Zhao Z, et al. (February 2021). "In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing". Translational Psychiatry. 11 (1) 94. doi:10.1038/s41398-021-01241-w. PMC 7862637. PMID 33542175.
- ^ a b c Nashar PE, Whitfield AA, Mikusek J, Reekie TA (2022). "The Current Status of Drug Discovery for the Oxytocin Receptor". Oxytocin. Methods Mol Biol. Vol. 2384. New York, NY: Springer. pp. 153–174. doi:10.1007/978-1-0716-1759-5_10. ISBN 978-1-0716-1758-8. PMID 34550574. S2CID 239090096.
- ^ Ott I, Scott JC (1910). "The Action of Infundibulum upon Mammary Secretion". Proceedings of the Society for Experimental Biology and Medicine. 8 (2): 48–49. doi:10.3181/00379727-8-27. S2CID 87519246.
- ^ Schafer EA, Mackenzie K (July 1911). "The Action of Animal Extracts on Milk Secretion". Proceedings of the Royal Society B. 84 (568): 16–22. Bibcode:1911RSPSB..84...16S. doi:10.1098/rspb.1911.0042. S2CID 93718970.
- ^ Du Vigneaud V, Ressler C, Trippett S (December 1953). "The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin". The Journal of Biological Chemistry. 205 (2): 949–57. doi:10.1016/S0021-9258(18)49238-1. PMID 13129273.
- ^ du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG, Gordon S (1953). "The synthesis of an octapeptide amide with the hormonal activity of oxytocin". J. Am. Chem. Soc. 75 (19): 4879–80. Bibcode:1953JAChS..75.4879V. doi:10.1021/ja01115a553.
- ^ du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG (June 1954). "The synthesis of oxytocin". J. Am. Chem. Soc. 76 (12): 3115–21. Bibcode:1954JAChS..76.3115D. doi:10.1021/ja01641a004.
- ^ Du Vigneaud V (June 1956). "Trail of sulfur research: from insulin to oxytocin". Science. 123 (3205): 967–74. Bibcode:1956Sci...123..967D. doi:10.1126/science.123.3205.967. PMID 13324123.
- ^ Torloni MR, Gomes Freitas C, Kartoglu UH, Metin Gülmezoglu A, Widmer M (December 2016). "Quality of oxytocin available in low- and middle-income countries: a systematic review of the literature". BJOG: An International Journal of Obstetrics and Gynaecology. 123 (13): 2076–86. doi:10.1111/1471-0528.13998. PMID 27006180.
- ^ Stanton C, Koski A, Cofie P, Mirzabagi E, Grady BL, Brooke S (2012). "Uterotonic drug quality: an assessment of the potency of injectable uterotonic drugs purchased by simulated clients in three districts in Ghana". BMJ Open. 2 (3) e000431. doi:10.1136/bmjopen-2011-000431. PMC 3346944. PMID 22556159.
- ^ Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. (April 2010). "Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans". The Journal of Neuroscience. 30 (14): 4999–5007. doi:10.1523/JNEUROSCI.5538-09.2010. PMC 6632777. PMID 20371820.
- ^ Cochran DM, Fallon D, Hill M, Frazier JA (2013). "The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings". Harvard Review of Psychiatry. 21 (5): 219–47. doi:10.1097/HRP.0b013e3182a75b7d. PMC 4120070. PMID 24651556.
- ^ Neumann ID, Slattery DA (2016). "Oxytocin in General Anxiety and Social Fear: A Translational Approach". Biological Psychiatry. 79 (3): 213–21. doi:10.1016/j.biopsych.2015.06.004. PMID 26208744.
- ^ Bakermans-Kranenburg MJ, van I Jzendoorn MH (2013). "Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy". Translational Psychiatry. 3 (5): e258. doi:10.1038/tp.2013.34. PMC 3669921. PMID 23695233.
- ^ Guastella AJ, Boulton KA, Whitehouse AJ, Song YJ, Thapa R, Gregory SG, et al. (February 2023). "The effect of oxytocin nasal spray on social interaction in young children with autism: a randomized clinical trial". Molecular Psychiatry. 28 (2): 834–842. doi:10.1038/s41380-022-01845-8. PMC 9607840. PMID 36302965.
- ^ Unkelbach C, Guastella AJ, Forgas JP (November 2008). "Oxytocin selectively facilitates recognition of positive sex and relationship words". Psychological Science. 19 (11): 1092–94. doi:10.1111/j.1467-9280.2008.02206.x. PMID 19076479. S2CID 19670817.
- ^ Marsh AA, Yu HH, Pine DS, Blair RJ (April 2010). "Oxytocin improves specific recognition of positive facial expressions". Psychopharmacology. 209 (3): 225–32. doi:10.1007/s00213-010-1780-4. PMID 20186397. S2CID 4820244.
- ^ Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y (2010). "The effect of intranasal administration of oxytocin on fear recognition". Neuropsychologia. 48 (1): 179–84. doi:10.1016/j.neuropsychologia.2009.09.003. PMID 19747930. S2CID 34778485.
- ^ Bartz JA, Hollander E (2008). "Oxytocin and experimental therapeutics in autism spectrum disorders". Advances in Vasopressin and Oxytocin – from Genes to Behaviour to Disease. Progress in Brain Research. Vol. 170. Elsevier. pp. 451–62. doi:10.1016/S0079-6123(08)00435-4. ISBN 978-0-444-53201-5. PMID 18655901.
- ^ Wudarczyk OA, Earp BD, Guastella A, Savulescu J (2013). "Could intranasal oxytocin be used to enhance relationships? Research imperatives, clinical policy, and ethical considerations". Current Opinion in Psychiatry. 26 (5): 474–84. doi:10.1097/YCO.0b013e3283642e10. PMC 3935449. PMID 23880593.
- ^ Parrott AC (2014). "The potential dangers of using MDMA for psychotherapy". Journal of Psychoactive Drugs. 46 (1): 37–43. doi:10.1080/02791072.2014.873690. PMID 24830184.

