Community hub
0 subscribers8 pages, 0 posts
Recent from talks
All channels
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Be the first to start a discussion here.
Contribute something
Contribute something
Welcome to the community hub built to collect knowledge and have discussions related to Spiramide.
Nothing was collected or created yet.
Spiramide
Spiramide
View on Wikipediafrom Wikipedia
Chemical compound
Pharmaceutical compound
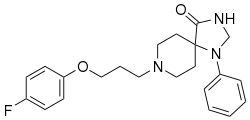 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H27FN3O2 |
| Molar mass | 384.475 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Spiramide (developmental code name AMI-193) is an experimental antipsychotic that acts as a selective 5-HT2A, 5-HT1A, and D2 receptor antagonist. It has negligible affinity for the 5-HT2C receptor.[1][2][3]
- ^ Czoty PW, Howell LL (October 2000). "Behavioral effects of AMI-193, a 5-HT(2A)- and dopamine D(2)-receptor antagonist, in the squirrel monkey". Pharmacology Biochemistry and Behavior. 67 (2): 257–64. doi:10.1016/S0091-3057(00)00321-X. PMID 11124389. S2CID 36132685.
- ^ Luparini MR, Garrone B, Pazzagli M, Pinza M, Pepeu G (November 2004). "A cortical GABA-5HT interaction in the mechanism of action of the antidepressant trazodone". Progress in Neuro-psychopharmacology & Biological Psychiatry. 28 (7): 1117–27. doi:10.1016/j.pnpbp.2004.05.046. PMID 15610924. S2CID 24076522.
- ^ Hamada K, Yoshida M, Isayama H, Yagi Y, Kanazashi S, Kashihara Y, Takeuchi K, Yamaguchi I (November 2007). "Possible involvement of endogenous 5-HT in aggravation of cerulein-induced acute pancreatitis in mice". Journal of Pharmacological Sciences. 105 (3): 240–50. doi:10.1254/jphs.FP0071049. PMID 17965538.
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |

