Recent from talks
Nothing was collected or created yet.
IUPAC nomenclature of organic chemistry
View on WikipediaThis article needs additional citations for verification. (April 2023) |
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended[1][2] by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry (informally called the Blue Book).[3] Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.[4]
To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially shorter and clearer, and so preferred. These non-systematic names are often derived from an original source of the compound. Also, very long names may be less clear than structural formulas.
Basic principles
[edit]In chemistry, a number of prefixes, suffixes and infixes are used to describe the type and position of the functional groups in the compound.
The steps for naming an organic compound are:[5]
- Identification of the most senior group. If more than one functional group, if any, is present, the one with highest group precedence should be used.
- Identification of the ring or chain with the maximum number of senior groups.
- Identification of the ring or chain with the most senior elements (In order: N, P, Si, B, O, S, C).
- Identification of the parent compound. Rings are senior to chains if composed of the same elements.
- For cyclic systems: Identification of the parent cyclic ring. The cyclic system must obey these rules, in order of precedence:
- It should have the most senior heteroatom (in order: N, O, S, P, Si, B).
- It should have the maximum number of rings.
- It should have the maximum number of atoms.
- It should have the maximum number of heteroatoms.
- It should have the maximum number of senior heteroatoms (in order: O, S, N, P, Si, B).
- For chains: Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
- It should have the maximum length.
- It should have the maximum number of heteroatoms.
- It should have the maximum number of senior heteroatoms (in order: O, S, N, P, Si, B).
- For cyclic systems and chains after previous rules:
- It should have the maximum number of multiple, then double bonds.
- It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest group precedence should be used.
- For cyclic systems: Identification of the parent cyclic ring. The cyclic system must obey these rules, in order of precedence:
- Identification of the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
- Identification of the remaining functional groups, if any, and naming them by their ionic prefixes (such as hydroxy for −OH, oxy for =O, oxyalkane for O−R, etc.).
Different side-chains and functional groups will be grouped together in alphabetical order. (The multiplier prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "dihydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in either case). When both side chains and secondary functional groups are present, they should be written mixed together in one group rather than in two separate groups. - Identification of double/triple bonds.
- Numbering of the chain. This is done by first numbering the chain in both directions (left to right and right to left), and then choosing the numbering which follows these rules, in order of precedence. Not every rule will apply to every compound, rules can be skipped if they do not apply.
- Has the lowest-numbered locant (or locants) for heteroatoms. Locants are the numbers on the carbons to which the substituent is directly attached.
- Has the lowest-numbered locants for the indicated hydrogen. The indicated hydrogen is for some unsaturated heterocyclic compounds. It refers to the hydrogen atoms not attached to atoms with double bonds in the ring system.
- Has the lowest-numbered locants for the suffix functional group.
- Has the lowest-numbered locants for multiple bonds ('ene', 'yne'), and hydro prefixes. (The locant of a multiple bond is the number of the adjacent carbon with a lower number).
- Has the lowest-numbered locants for all substituents cited by prefixes.
- Has the lowest-numbered locants for substituents in order of citation (for example: in a cyclic ring with only bromine and chlorine functional groups, alphabetically bromo- is cited before chloro- and would receive the lower locant).
- Numbering of the various substituents and bonds with their locants. If there is more than one of the same type of substituent/double bond, a prefix is added showing how many there are (di – 2, tri – 3, tetra – 4, then as for the number of carbons below with 'a' added at the end)
The numbers for that type of side chain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains with the same alpha carbon, the number will be written twice. Example: 2,2,3-trimethyl- . If there are both double bonds and triple bonds, "en" (double bond) is written before "yne" (triple bond). When the main functional group is a terminal functional group (a group which can exist only at the end of a chain, like formyl and carboxyl groups), there is no need to number it.
- Arrangement in this form: Group of side chains and secondary functional groups with numbers made in step 6 + prefix of parent hydrocarbon chain (eth, meth) + double/triple bonds with numbers (or "ane") + primary functional group suffix with numbers.
Wherever it says "with numbers", it is understood that between the word and the numbers, the prefix (di-, tri-) is used. - Adding of punctuation:
- Commas are put between numbers (2 5 5 becomes 2,5,5)
- Hyphens are put between a number and a letter (2 5 5 trimethylheptane becomes 2,5,5-trimethylheptane)
- Successive words are merged into one word (trimethyl heptane becomes trimethylheptane)
Note: IUPAC uses one-word names throughout. This is why all parts are connected.
The resulting name appears as:
- #,#-di<side chain>-#-<secondary functional group>-#-<side chain>-#,#,#-tri<secondary functional group><parent chain prefix><If all bonds are single bonds, use "ane">-#,#-di<double bonds>-#-<triple bonds>-#-<primary functional group>
where each "#" represents a number. The group secondary functional groups and side chains may not look the same as shown here, as the side chains and secondary functional groups are arranged alphabetically. The di- and tri- have been used just to show their usage. (di- after #,#, tri- after #,#,#, etc.)
Example
[edit]Here is a sample molecule with the parent carbons numbered:
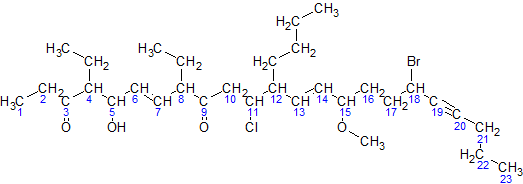
For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:

Now, following the above steps:
- The parent hydrocarbon chain has 23 carbons. It is called tricosa-.
- The functional groups with the highest precedence are the two ketone groups.
- The groups are on carbon atoms 3 and 9. As there are two, we write 3,9-dione.
- The numbering of the molecule is based on the ketone groups. When numbering from left to right, the ketone groups are numbered 3 and 9. When numbering from right to left, the ketone groups are numbered 15 and 21. 3 is less than 15, therefore the ketones are numbered 3 and 9. The smaller number is always used, not the sum of the constituents numbers.
- The side chains are: an ethyl- at carbon 4, an ethyl- at carbon 8, and a butyl- at carbon 12.
Note: the −O−CH3 at carbon atom 15 is not a side chain, but it is a methoxy functional group.- There are two ethyl- groups. They are combined to create, 4,8-diethyl.
- The side chains are grouped like this: 12-butyl-4,8-diethyl. (But this is not necessarily the final grouping, as functional groups may be added in between to ensure all groups are listed alphabetically.)
- The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, this gives 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxy.
- There are two double bonds: one between carbons 6 and 7, and one between carbons 13 and 14. They would be called "6,13-diene", but the presence of alkynes switches it to 6,13-dien. There is one triple bond between carbon atoms 19 and 20. It will be called 19-yne.
- The arrangement (with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione
- Finally, due to cis-trans isomerism, we have to specify the relative orientation of functional groups around each double bond. For this example, both double bonds are trans isomers, so we have (6E,13E)
The final name is (6E,13E)-18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione.
Hydrocarbons
[edit]Alkanes
[edit]Straight-chain alkanes take the suffix "-ane" and are prefixed depending on the number of carbon atoms in the chain, following standard rules. The first few are:
| Number of carbons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct | Non | Dec | Undec | Dodec | Tridec | Tetradec | Pentadec | Hexadec | Heptadec | Octadec | Nonadec | Icos |
For example, the simplest alkane is CH4 methane, and the nine-carbon alkane CH3(CH2)7CH3 is named nonane. The names of the first four alkanes were derived from methanol, ether, propionic acid and butyric acid, respectively. The rest are named with a Greek numeric prefix, with the exceptions of nonane, which has a Latin prefix, and undecane, which has mixed-language prefixes.
Cyclic alkanes are simply prefixed with "cyclo-": for example, C4H8 is cyclobutane (not to be confused with butene) and C6H12 is cyclohexane (not to be confused with hexene).
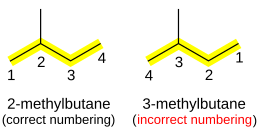
Branched alkanes are named as a straight-chain alkane with attached alkyl groups. They are prefixed with a number indicating the carbon the group is attached to, counting from the end of the alkane chain. For example, (CH3)2CHCH3, commonly known as isobutane, is treated as a propane chain with a methyl group bonded to the middle (2) carbon, and given the systematic name 2-methylpropane. However, although the name 2-methylpropane could be used, it is easier and more logical to call it simply methylpropane – the methyl group could not possibly occur on any of the other carbon atoms (that would lengthen the chain and result in butane, not propane) and therefore the use of the number "2" is unnecessary.
If there is ambiguity in the position of the substituent, depending on which end of the alkane chain is counted as "1", then numbering is chosen so that the smaller number is used. For example, (CH3)2CHCH2CH3 (isopentane) is named 2-methylbutane, not 3-methylbutane.

If there are multiple side-branches of the same size alkyl group, their positions are separated by commas and the group prefixed with multiplier prefixes depending on the number of branches. For example, C(CH3)4 (neopentane) is named 2,2-dimethylpropane. If there are different groups, they are added in alphabetical order, separated by commas or hyphens. The longest possible main alkane chain is used; therefore 3-ethyl-4-methylhexane instead of 2,3-diethylpentane, even though these describe equivalent structures. The di-, tri- etc. prefixes are ignored for the purpose of alphabetical ordering of side chains (e.g. 3-ethyl-2,4-dimethylpentane, not 2,4-dimethyl-3-ethylpentane).
Alkenes
[edit]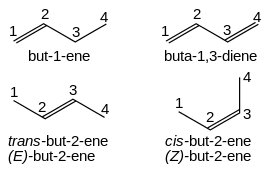
Alkenes are named for their parent alkane chain with the suffix "-ene" and a numerical root indicating the position of the carbon with the lower number for each double bond in the chain: CH2=CHCH2CH3 is but-1-ene. Multiple double bonds take the form -diene, -triene, etc., with the size prefix of the chain taking an extra "a": CH2=CHCH=CH2 is buta-1,3-diene. Simple cis and trans isomers may be indicated with a prefixed cis- or trans-: cis-but-2-ene, trans-but-2-ene. However, cis- and trans- are relative descriptors. It is IUPAC convention to describe all alkenes using absolute descriptors of Z- (same side) and E- (opposite) with the Cahn–Ingold–Prelog priority rules (see also E–Z notation).
Alkynes
[edit]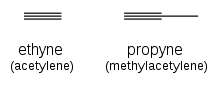
Alkynes are named using the same system, with the suffix "-yne" indicating a triple bond: ethyne (acetylene), propyne (methylacetylene).
Functional groups
[edit]Haloalkanes and haloarenes
[edit]
In haloalkanes and haloarenes (R−X), Halogen functional groups are prefixed with the bonding position and take the form of fluoro-, chloro-, bromo-, iodo-, or astato-, depending on the halogen. Multiple groups are dichloro-, trichloro-, etc., and dissimilar groups are ordered alphabetically as before. For example, CHCl3 (chloroform) is trichloromethane. The anesthetic halothane (CF3CHBrCl) is 2-bromo-2-chloro-1,1,1-trifluoroethane.
Alcohols
[edit]
Alcohols (R−OH) take the suffix "-ol" with a numerical suffix indicating the bonding position: CH3CH2CH2OH is propan-1-ol. The suffixes -diol, -triol, -tetrol, etc., are used for multiple −OH groups: Ethylene glycol CH2OHCH2OH is ethane-1,2-diol.

If higher precedence functional groups are present (see order of precedence, below), the prefix "hydroxy" is used with the bonding position: CH3CHOHCOOH is 2-hydroxypropanoic acid.
Ethers
[edit]
Ethers (R−O−R) consist of an oxygen atom between the two attached carbon chains. The shorter of the two chains becomes the first part of the name with the -ane suffix changed to -oxy, and the longer alkane chain becomes the suffix of the name of the ether. Thus, CH3OCH3 is methoxymethane, and CH3OCH2CH3 is methoxyethane (not ethoxymethane). If the oxygen is not attached to the end of the main alkane chain, then the whole shorter alkyl-plus-ether group is treated as a side-chain and prefixed with its bonding position on the main chain. Thus CH3OCH(CH3)2 is 2-methoxypropane.
Alternatively, an ether chain can be named as an alkane in which one carbon is replaced by an oxygen, a replacement denoted by the prefix "oxa". For example, CH3OCH2CH3 could also be called 2-oxabutane, and an epoxide could be called oxacyclopropane. This method is especially useful when both groups attached to the oxygen atom are complex.[6]
Aldehydes
[edit]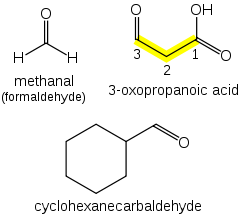
Aldehydes (R−CH=O) take the suffix "-al". If other functional groups are present, the chain is numbered such that the aldehyde carbon is in the "1" position, unless functional groups of higher precedence are present.
If a prefix form is required, "oxo-" is used (as for ketones), with the position number indicating the end of a chain: CHOCH2COOH is 3-oxopropanoic acid. If the carbon in the carbonyl group cannot be included in the attached chain (for instance in the case of cyclic aldehydes), the prefix "formyl-" or the suffix "-carbaldehyde" is used: C6H11CHO is cyclohexanecarbaldehyde. If an aldehyde is attached to a benzene and is the main functional group, the suffix becomes benzaldehyde.
Ketones
[edit]
In general ketones (R2C=O) take the suffix "-one" (pronounced own, not won) with a suffixed position number: CH3CH2CH2COCH3 is pentan-2-one. If a higher precedence suffix is in use, the prefix "oxo-" is used: CH3CH2CH2COCH2CHO is 3-oxohexanal.
Carboxylic acids
[edit]
In general, carboxylic acids (R−C(=O)OH) are named with the suffix -oic acid (etymologically a back-formation from benzoic acid). As with aldehydes, the carboxyl functional group must take the "1" position on the main chain and so the locant need not be stated. For example, CH3−CH(OH)−COOH (lactic acid) is named 2-hydroxypropanoic acid with no "1" stated. Some traditional names for common carboxylic acids (such as acetic acid) are in such widespread use that they are retained in IUPAC nomenclature,[7] though systematic names like ethanoic acid are also used. Carboxylic acids attached to a benzene ring are structural analogs of benzoic acid (Ph−COOH) and are named as one of its derivatives.

If there are multiple carboxyl groups on the same parent chain, multiplying prefixes are used: Malonic acid, CH2(COOH)2, is systematically named propanedioic acid. Alternatively, the suffix "-carboxylic acid" can be used in place of "oic acid", combined with a multiplying prefix if necessary – mellitic acid is benzenehexacarboxylic acid, for example. In the latter case, the carbon atoms in the carboxyl groups do not count as being part of the main chain, a rule that also applies to the prefix form "carboxy-". Citric acid serves as an example: it is formally named 2-hydroxypropane-1,2,3-tricarboxylic acid rather than 3-carboxy-3-hydroxypentanedioic acid.
Carboxylates
[edit]
Salts of carboxylic acids are named following the usual cation-then-anion conventions used for ionic compounds in both IUPAC and common nomenclature systems. The name of the carboxylate anion (R−C(=O)O−) is derived from that of the parent acid by replacing the "–oic acid" ending with "–oate" or "carboxylate." For example, NaC6H5CO2, the sodium salt of benzoic acid (C6H5COOH), is called sodium benzoate. Where an acid has both a systematic and a common name (like CH3COOH, for example, which is known as both acetic acid and as ethanoic acid), its salts can be named from either parent name. Thus, KCH3CO2 can be named as potassium acetate or as potassium ethanoate. The prefix form, is "carboxylato-".
Esters
[edit]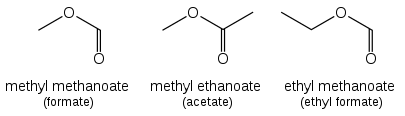
Esters (R−C(=O)O−R') are named as alkyl derivatives of carboxylic acids. The alkyl (R') group is named first. The R−C(=O)O part is then named as a separate word based on the carboxylic acid name, with the ending changed from "-oic acid" to "-oate" or "-carboxylate" For example, CH3CH2CH2CH2COOCH3 is methyl pentanoate, and (CH3)2CHCH2CH2COOCH2CH3 is ethyl 4-methylpentanoate. For esters such as ethyl acetate (CH3COOCH2CH3), ethyl formate (HCOOCH2CH3) or dimethyl phthalate that are based on common acids, IUPAC recommends use of these established names, called retained names. The "-oate" changes to "-ate." Some simple examples, named both ways, are shown in the figure above.

If the alkyl group is not attached at the end of the chain, the bond position to the ester group is suffixed before "-yl": CH3CH2CH(CH3)OOCCH2CH3 may be called butan-2-yl propanoate or butan-2-yl propionate.[citation needed]. The prefix form is "oxycarbonyl-" with the (R') group preceding.
Acyl groups
[edit]
Acyl groups are named by stripping the "-ic acid" of the corresponding carboxylic acid and replacing it with "-yl." For example, CH3CO−R is called ethanoyl-R.
Acyl halides
[edit]
Simply add the name of the attached halide to the end of the acyl group. For example, CH3COCl is ethanoyl chloride. An alternate suffix is "-carbonyl halide" as opposed to "-oyl halide". The prefix form is "halocarbonyl-".

Acid anhydrides
[edit]
Acid anhydrides (R−C(=O)−O−C(=O)−R) have two acyl groups linked by an oxygen atom. If both acyl groups are the same, then the name of the carboxylic acid with the word acid is replaced with the word anhydride and the IUPAC name consists of two words. If the acyl groups are different, then they are named in alphabetical order in the same way, with anhydride replacing acid and IUPAC name consists of three words. For example, CH3CO−O−OCCH3 is called ethanoic anhydride and CH3CO−O−OCCH2CH3 is called ethanoic propanoic anhydride.
Amines
[edit]
Amines (R−NH2) are named for the attached alkane chain with the suffix "-amine" (e.g., CH3NH2 methanamine). If necessary, the bonding position is suffixed: CH3CH2CH2NH2 propan-1-amine, CH3CHNH2CH3 propan-2-amine. The prefix form is "amino-".
For secondary amines (of the form R−NH−R), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic N: CH3NHCH2CH3 is N-methylethanamine. Tertiary amines (R−NR−R) are treated similarly: CH3CH2N(CH3)CH2CH2CH3 is N-ethyl-N-methylpropanamine. Again, the substituent groups are ordered alphabetically.
Amides
[edit]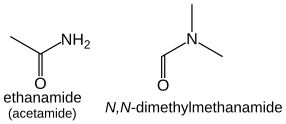
Amides (R−C(=O)NH2) take the suffix "-amide", or "-carboxamide" if the carbon in the amide group cannot be included in the main chain. The prefix form is "carbamoyl-". e.g., HCONH2 methanamide, CH3CONH2 ethanamide.
Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix N: HCON(CH3)2 is N,N-dimethylmethanamide, CH3CON(CH3)2 is N,N-dimethylethanamide.
Nitriles
[edit]
Nitriles (R−C≡N) are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the "-oic acid" of their corresponding carboxylic acids with "-carbonitrile." The prefix form is "cyano-." Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides. For example, CH3CH2CH2CH2C≡N is called pentanenitrile or butyl cyanide.
Cyclic compounds
[edit]
Cycloalkanes and aromatic compounds can be treated as the main parent chain of the compound, in which case the positions of substituents are numbered around the ring structure. For example, the three isomers of xylene CH3C6H4CH3, commonly the ortho-, meta-, and para- forms, are 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene. The cyclic structures can also be treated as functional groups themselves, in which case they take the prefix "cycloalkyl-" (e.g. "cyclohexyl-") or for benzene, "phenyl-".
The IUPAC nomenclature scheme becomes rapidly more elaborate for more complex cyclic structures, with notation for compounds containing conjoined rings, and many common names such as phenol being accepted as base names for compounds derived from them.
Order of precedence of group
[edit]When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The table below shows common groups in decreasing order of precedence. The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-en and -yn) and are used with other suffixes.
Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, not ethane-2,1-diol.) The N position indicator for amines and amides comes before "1", e.g., CH3CH(CH3)CH2NH(CH3) is N,2-dimethylpropanamine.
| Priority | Functional group | Formula | Prefix | Suffix |
|---|---|---|---|---|
| 1 | Cations
|
|
-onio-
|
-onium
|
| 2 | Carboxylic acids | −COOH
|
carboxy-
|
-oic acid*
|
| 3 | Carboxylic acid derivatives | −COOCO−
|
acyloxy-
|
-R-oic anhydride
|
| 4 | Nitriles | −CN
|
cyano-
|
-nitrile*
|
| 5 | Aldehydes | −CHO
|
formyl-
|
-al*
|
| 6 | Ketones | =O
|
oxo-
|
-one
|
| 7 | Alcohols | −OH
|
hydroxy-
|
-ol
|
| 8 | Hydroperoxides |
|
|
|
| 9 | Amines | −NH2
|
amino-
|
-amine
|
*Note: These suffixes, in which the carbon atom is counted as part of the preceding chain, are the most commonly used. See individual functional group articles for more details.
The order of remaining functional groups is only needed for substituted benzene and hence is not mentioned here.[clarification needed]
Common nomenclature – trivial names
[edit]Common nomenclature uses the older names for some organic compounds instead of using the prefixes for the carbon skeleton above. The pattern can be seen below.
| Number of carbons |
Prefix as in new system |
Common name for alcohol |
Common name for aldehyde |
Common name for acid |
Common name for ketone |
|---|---|---|---|---|---|
| 1 | Meth- | Methyl alcohol (wood alcohol) |
Formaldehyde | Formic acid | NA |
| 2 | Eth- | Ethyl alcohol (grain alcohol) |
Acetaldehyde | Acetic acid (vinegar) | NA |
| 3 | Prop- | Propyl alcohol | Propionaldehyde | Propionic acid | Acetone/dimethyl ketone |
| 4 | But- | Butyl alcohol | Butyraldehyde | Butyric acid | Methyl ethyl ketone |
| 5 | Pent- | Amyl alcohol | Valeraldehyde | Valeric acid | •Methyl propyl ketone
•Diethyl ketone |
| 6 | Hex- | Caproyl alcohol | Caproaldehyde | Caproic acid | •Butyl methyl ketone
•Ethyl propyl ketone |
| 7 | Hept- | Enanthyl alcohol | Enanthaldehyde | Enanthoic acid | •Methyl pentyl ketone
•Butyl ethyl ketone •Dipropyl ketone |
| 8 | Oct- | Capryl alcohol | Caprylaldehyde | Caprylic acid | •Hexyl methyl ketone
•Ethyl pentyl ketone •Butyl propyl ketone |
| 9 | Non- | Pelargonic alcohol | Pelargonaldehyde | Pelargonic acid | •Heptyl methyl ketone
•Ethyl hexyl ketone •Pentyl propyl ketone •Dibutyl ketone |
| 10 | Dec- | Capric alcohol | Capraldehyde | Capric acid | •Methyl octyl ketone
•Ethyl heptyl ketone •Hexyl propyl ketone •Butyl pentyl ketone |
| 11 | Undec- | - | - | - | The same pattern continues |
| 12 | Dodec- | Lauryl alcohol | Lauraldehyde | Lauric acid | |
| 13 | Tridec- | - | - | - | |
| 14 | Tetradec- | Myristyl alcohol | Myristaldehyde | Myristic acid | |
| 15 | Pentadec- | - | - | - | |
| 16 | Hexadec- | Cetyl alcohol Palmityl alcohol |
Palmitaldehyde | Palmitic acid | |
| 17 | Heptadec- | - | - | Margaric acid | |
| 18 | Octadec- | Stearyl alcohol | Stearaldehyde | Stearic acid | |
| 19 | Nonadec- | - | - | - | |
| 20 | Icos- | Arachidyl alcohol | - | Arachidic acid | |
| 21 | Henicos- | - | - | - | |
| 22 | Docos- | Behenyl alcohol | - | Behenic acid | |
| 23 | Tricos- | - | - | - | |
| 24 | Tetracos- | Lignoceryl alcohol | - | Lignoceric acid | |
| 25 | Pentacos- | - | - | - | |
| 26 | Hexacos- | Ceryl alcohol | - | Cerotic acid | |
| 27 | Heptacos- | - | - | - | |
| 28 | Octacos- | Montanyl alcohol | - | Montanic acid | |
| 29 | Nonacos- | - | - | - | |
| 30 | Triacont- | Melissyl alcohol | - | Melissic acid | |
| 31 | Hentriacont- | - | - | - | |
| 32 | Dotriacont- | Lacceryl alcohol | - | Lacceroic acid | |
| 33 | Tritriacont- | Psyllic alcohol | - | Psyllic acid | |
| 34 | Tetratriacont- | Geddyl alcohol | - | Geddic acid | |
| 35 | Pentatriacont- | - | - | Ceroplastic acid | |
| 36 | Hexatriacont- | - | - | - | |
| 37 | Heptatriacont- | - | - | - | |
| 38 | Octatriacont- | - | - | - | |
| 39 | Nonatriacont- | - | - | - | |
| 40 | Tetracont- | - | - | - |
Ketones
[edit]Common names for ketones can be derived by naming the two alkyl or aryl groups bonded to the carbonyl group as separate words followed by the word ketone.
The first three of the names shown above are still considered to be acceptable IUPAC names.
Aldehydes
[edit]The common name for an aldehyde is derived from the common name of the corresponding carboxylic acid by dropping the word acid and changing the suffix from -ic or -oic to -aldehyde.
Ions
[edit]The IUPAC nomenclature also provides rules for naming ions.
Hydron
[edit]Hydron is a generic term for hydrogen cation; protons, deuterons and tritons are all hydrons. The hydrons are not found in heavier isotopes, however.
Parent hydride cations
[edit]Simple cations formed by adding a hydron to a hydride of a halogen, chalcogen or pnictogen are named by adding the suffix "-onium" to the element's root: H4N+ is ammonium, H3O+ is oxonium, and H2F+ is fluoronium. Ammonium was adopted instead of nitronium, which commonly refers to NO+2.
If the cationic center of the hydride is not a halogen, chalcogen or pnictogen then the suffix "-ium" is added to the name of the neutral hydride after dropping any final 'e'. H5C+ is methanium, HO−(O+)H2 is dioxidanium (HO-OH is dioxidane), and H2N−(N+)H3 is diazanium (H2N−NH2 is diazane).
Cations and substitution
[edit]The above cations except for methanium are not, strictly speaking, organic, since they do not contain carbon. However, many organic cations are obtained by substituting another element or some functional group for a hydrogen.
The name of each substitution is prefixed to the hydride cation name. If many substitutions by the same functional group occur, then the number is indicated by prefixing with "di-", "tri-" as with halogenation. (CH3)3O+ is trimethyloxonium. CH3F3N+ is trifluoromethylammonium.
See also
[edit]References
[edit]- ^ The Commission on the Nomenclature of Organic Chemistry Varun kedia (1971) [1958 (A: Hydrocarbons, and B: Fundamental Heterocyclic Systems), 1965 (C: Characteristic Groups)]. Nomenclature of Organic Chemistry (3rd edition combined ed.). London: Butterworths. ISBN 0-408-70144-7.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (PDF). Henri A. Favre, Warren H. Powell, International Union of Pure and Applied Chemistry. Cambridge, England: Royal Society of Chemistry. 2014. ISBN 978-1-84973-306-9. OCLC 865143943.
{{cite book}}: CS1 maint: others (link) - ^ "Blue Book". IUPAC | International Union of Pure and Applied Chemistry. Retrieved 19 September 2024.
- ^ "Brief Guide to Inorganic Nomenclature". iupac.qmul.ac.uk. Retrieved 19 September 2024.
- ^ Hellwich, Karl-Heinz; Hartshorn, Richard M.; Yerin, Andrey; Damhus, Ture; Hutton, Alan T. (1 March 2020). "Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report)". Pure and Applied Chemistry. 92 (3): 527–539. doi:10.1515/pac-2019-0104. ISSN 1365-3075.
- ^ "Ethers". www.chem.ucalgary.ca.
- ^ International Union of Pure and Applied Chemistry Organic Chemistry Division Commission on Nomenclature of Organic Chemistry (1995). "Table 28(a): Carboxylic acids and related group". In Panico, Robert; Powell, Warren H.; Richer, Jean-Claude (eds.). A Guide to IUPAC Nomenclature of Organic Compounds: Recommendations 1993 (including revisions, published and hitherto unpublished, to the 1979 edition of Nomenclature of Organic Chemistry) (2nd ed.). Oxford: Blackwell Scientific Publications. ISBN 9780632034888.
Bibliography
[edit]- Favre, Henri A.; Powell, Warren H. (2013). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Royal Society of Chemistry. ISBN 978-0-85404-182-4.
External links
[edit]- IUPAC Nomenclature of Organic Chemistry (online version of several older editions of the IUPAC Blue Book)
- IUPAC Recommendations on Organic & Biochemical Nomenclature, Symbols, Terminology, etc. (includes IUBMB Recommendations for biochemistry)
- Bibliography of IUPAC Recommendations on Organic Nomenclature (last updated 11 April 2003)
- ACD/Name Software for generating systematic nomenclature
- ChemAxon Name <> Structure – ChemAxon IUPAC (& traditional) name to structure and structure to IUPAC name software. As used at chemicalize.org
- chemicalize.org A free web site/service that extracts IUPAC names from web pages and annotates a 'chemicalized' version with structure images. Structures from annotated pages can also be searched.
- Eller, Gernot A. (2006). "Improving the Quality of Published Chemical Names with Nomenclature Software" (PDF). Molecules. 9 (11): 915–928. doi:10.3390/11110915. PMC 6148558. PMID 18007396.
- American Chemical Society, Committee on Nomenclature, Terminology & Symbols
- Leigh, G. J.; Favre, H. A.; Metanomski, W. V. (1998). Principles of Chemical Nomenclature. A Guide to IUPAC Recommendations (PDF). Blackwell.
IUPAC nomenclature of organic chemistry
View on GrokipediaBasic Principles
Objectives and Scope
The International Union of Pure and Applied Chemistry (IUPAC), established in 1919, plays a central role in standardizing chemical nomenclature worldwide to promote consistency and clarity in scientific communication. Efforts to systematize organic nomenclature trace back to the 1892 Geneva Congress on Organic Nomenclature, convened by the International Chemical Congress, which introduced the first international rules for naming organic compounds based on structural features rather than trivial names.[6][4] Subsequent IUPAC commissions on nomenclature, formed starting in 1921, refined these rules through iterative publications, including the comprehensive 1979 edition of Nomenclature of Organic Chemistry, the 1993 Guide to IUPAC Nomenclature of Organic Compounds, and the 2013 Nomenclature of Organic Chemistry (commonly known as the Blue Book).[1] The primary objectives of IUPAC nomenclature for organic chemistry are to generate unambiguous, unique names for chemical compounds, thereby facilitating precise communication among scientists, educators, and professionals across disciplines and borders.[1] This systematic approach ensures that each compound, including its isomers, receives a single formally accepted name, reducing confusion from historical or regional variations.[7] Additionally, it supports efficient indexing and retrieval in chemical databases, patents, and regulatory documents, enabling better organization of vast chemical knowledge and advancing research interoperability.[8][9] The scope of IUPAC organic nomenclature encompasses all compounds featuring carbon-based skeletons, primarily those involving carbon atoms bonded to hydrogen and elements from Groups 13 through 17 of the periodic table, while generally excluding purely inorganic compounds and organometallics unless explicitly addressed in specialized sections.[10] It applies to a wide range of structures, including acyclic chains, cyclic systems, and polycyclic frameworks, with rules for substitutive nomenclature that prioritize the parent hydride as the core structural unit.[11] Historically, organic nomenclature evolved from ad hoc, descriptive naming conventions—often based on natural sources or discoverers—to a fully systematic framework driven by structural logic, culminating in the 2013 Blue Book's introduction of Preferred IUPAC Names (PINs) for designating a single, globally recognized identifier per compound.[12] This progression addressed the limitations of early rules, such as those from the 1892 Geneva Congress, by incorporating modern needs for precision in complex molecules and ensuring compatibility with computational tools.[6][13]Key Terminology
In IUPAC nomenclature for organic chemistry, several foundational terms establish the framework for systematically naming compounds. These terms describe the core structural elements, modifications, and prioritization rules that ensure unambiguous identification of molecular structures. Understanding them is essential before applying specific naming conventions, as they form the basis for substitutive nomenclature, the primary method used.[10] The parent hydride refers to the unbranched or branched acyclic, cyclic, or acyclic/cyclic structure—often with a semisystematic or trivial name—to which only hydrogen atoms are attached, serving as the base from which substituents are removed or added to form the compound name. For example, methane (CH₄) acts as the parent hydride for the methyl substituent in larger alkanes. This concept anchors the naming process by identifying the principal chain or ring system before incorporating modifications.[14][15] A substituent is an atom or group that replaces one or more hydrogen atoms in the parent hydride, expressed as a prefix in the name. Common examples include the chloro group (-Cl) or alkyl groups like ethyl (-C₂H₅), which denote the specific atoms or moieties attached to the core structure. Substituents are distinguished from the principal functional group and are cited in alphabetical order to reflect their positions and types.[10][16] The functional group, also termed a characteristic group, is an atom or assembly of atoms responsible for conferring specific chemical properties and reactivity to the molecule, regardless of its context. In nomenclature, these are prioritized and expressed either as suffixes (e.g., hydroxy for -OH) or prefixes when not principal. They dictate the compound's class, such as alcohols or carboxylic acids, and influence the choice of parent structure.[17][18] A locant is a numerical (or occasionally letter-based) identifier that specifies the position of a substituent, functional group, or other structural feature within the parent hydride. For instance, the "2-" in 2-propanol indicates the carbon atom bearing the hydroxy group. Locants are assigned to achieve the lowest possible numbers, ensuring precision in describing isomerism and substitution patterns. Seniority establishes a hierarchical order among functional groups and classes to select the principal characteristic group, which receives the suffix in the name, while others are treated as prefixes. This order, detailed in IUPAC tables, prioritizes groups based on criteria like oxidation state and structural complexity (e.g., carboxylic acids over alcohols), resolving ambiguities in multifunctional compounds.[18][10] Retained names are traditional or common names preserved by IUPAC for widespread use, either as preferred IUPAC names or general alternatives to systematic ones. Examples include acetic acid for CH₃COOH and acetone for (CH₃)₂CO, allowing continuity with historical nomenclature while promoting systematic approaches for complex structures. These are limited to specific, well-established compounds to avoid confusion.[11]Types of Nomenclature
In IUPAC nomenclature for organic chemistry, several methods exist to generate systematic names for compounds, with the choice depending on the structure and context of use. The primary types include substitutive nomenclature, which is the preferred approach for most organic compounds, and functional class nomenclature, an older alternative retained for specific applications. These methods build on the concept of parent hydrides, such as alkanes or cycloalkanes, from which names are derived through substitution or classification. The 2013 IUPAC recommendations, as detailed in the Blue Book, establish a hierarchy prioritizing preferred IUPAC names (PINs) to ensure uniqueness and clarity in scientific communication.[10][19] Substitutive nomenclature is the principal IUPAC system and the main method for naming organic compounds. It treats compounds as derived from a parent hydride by replacing hydrogen atoms with functional groups or substituents, expressed through prefixes (e.g., chloro- for chlorine) or suffixes (e.g., -ol for alcohols, as in ethanol from ethane). This approach allows for the construction of names that reflect the molecular structure hierarchically, with senior functional groups cited as suffixes on the longest chain or largest ring system. Substitutive names generate the PIN for the vast majority of neutral organic compounds, promoting consistency in regulatory, patent, and general scientific contexts. Its widespread adoption stems from its flexibility in handling complex structures, including those with multiple functional groups, through rules for seniority and locants.[10][19] Functional class nomenclature, formerly known as radicofunctional nomenclature, provides an alternative by naming compounds as derivatives of functional parent compounds, treating them as combinations of substituent groups and a functional class name. For example, an ester is named as "alkyl alkanoate," such as methyl acetate, where the alkyl from the alcohol and the alkanoate from the acid are cited separately. This method originated in earlier nomenclature systems and persists in the 2013 recommendations as the preferred IUPAC name for certain classes, including esters, acid halides, and anhydrides, where substitutive names may be more cumbersome or less traditional. Radicofunctional nomenclature specifically emphasized the radical (substituent) attached to a functional group, but it has been subsumed under functional class nomenclature in modern usage to simplify terminology. While less versatile for multifunctional compounds, it remains useful for clarity in specific chemical literature and indexing.[10][20][19] The selection of nomenclature type follows the 2013 IUPAC criteria, which prioritize substitutive names for generating PINs in most cases due to their structural informativeness, except where functional class nomenclature is explicitly retained for esters, acid halides, and related derivatives to align with established practice. For acyl groups and similar entities, radicofunctional principles may still inform naming within functional class contexts. However, substitutive nomenclature has limitations and is not always applicable, particularly for ions, radicals, and certain coordination compounds, where additive nomenclature—based on assembling names from ionic or radical components—is required instead to accurately denote charge or unpaired electrons. These rules ensure adaptability across diverse organic structures while maintaining systematic rigor.[10][11][21]Hydrocarbons
Alkanes
Alkanes represent the simplest class of organic compounds, consisting of saturated hydrocarbons with only single bonds between carbon atoms. For acyclic alkanes, the general molecular formula is , where is the number of carbon atoms, reflecting their fully saturated structure with hydrogen atoms filling all available valences.[22] The IUPAC nomenclature system for alkanes establishes a systematic approach to naming these compounds, prioritizing the identification of the parent carbon chain and any attached alkyl substituents to ensure unique and unambiguous names.[1] Retained names are used for the smallest unbranched alkanes: methane for , ethane for , propane for , and butane for ; these are preferred IUPAC names and retained for general nomenclature.[11] For unbranched alkanes with five or more carbon atoms, names are constructed by combining a numerical prefix derived from Greek or Latin roots (e.g., pent- for five, hex- for six, hept- for seven) with the suffix -ane, such as pentane (), hexane (), and heptane ().[11] In branched alkanes, the parent structure is defined as the longest continuous carbon chain, which determines the root name and suffix.[23] Substituent groups, treated as alkyl derivatives of alkanes (e.g., methyl from methane, ethyl from ethane), are prefixed to the parent name with locants indicating their positions on the chain. Numbering of the parent chain begins from the end that assigns the lowest possible locants to the substituents; if ties occur, the lowest locant is given to the substituent that comes first in alphabetical order.[24] When multiple identical substituents are present, multiplicative prefixes such as di-, tri-, or tetra- are used, and the locants are listed in ascending order.[23] Different substituents are cited in alphabetical order, disregarding the multiplicative prefixes for sorting purposes (e.g., ethyl before methyl).[24] Complex branched substituents, such as the isopropyl group, are named systematically (e.g., as 1-methylethyl) when required for precision. This numbering and ordering principle ensures the lowest set of locants for structural isomers, as seen in the naming of 2,2,4-trimethylpentane, a branched isomer of octane (commonly called isooctane) with a five-carbon parent chain and three methyl groups at positions 2, 2, and 4. Similarly, a heptane parent chain with a methyl substituent at position 2 and an ethyl at position 3 is named 3-ethyl-2-methylheptane, reflecting the alphabetical precedence of ethyl over methyl and the lowest locant set.[23] The names methane, ethane, and propane are specifically retained for general use beyond their role as preferred IUPAC names, facilitating common communication in chemistry.[11]Cycloalkanes
Cycloalkanes are saturated cyclic hydrocarbons, also known as alicyclic compounds, with the general formula for monocyclic structures, where represents the number of carbon atoms in the ring.[25] This formula reflects the loss of two hydrogen atoms compared to the corresponding acyclic alkane due to the ring closure. The nomenclature for these compounds emphasizes the cyclic nature while adhering to substitutive principles, ensuring systematic and unambiguous naming for both simple and complex structures. For unsubstituted monocyclic cycloalkanes, names are formed by prefixing "cyclo-" to the name of the unbranched alkane with the same number of carbon atoms. Retained names are preferred for small rings: cyclopropane (), cyclobutane (), cyclopentane (), and cyclohexane (); these are used in general and preferred IUPAC nomenclature. For larger rings, systematic names such as cycloheptane, cyclooctane, and cyclononane are employed, though cycloheptane and cyclooctane are also retained for general use. When substituents are present, the cycloalkane ring serves as the parent structure if it has more carbon atoms than any acyclic chain; otherwise, the longest chain becomes the parent, with the ring treated as a cycloalkyl substituent. Numbering starts at a substituted carbon atom to assign the lowest possible locants to substituents; for multiple substituents, locants are chosen to give the lowest set in order of citation, with prefixes arranged alphabetically. For example, the compound with a five-membered ring bearing an ethyl group at position 1 and a methyl group at position 2 is named 1-ethyl-2-methylcyclopentane.[25] Polycyclic cycloalkanes include fused, bridged, and spiro systems, each with specific naming conventions to describe their connectivity. In ortho-fused systems, two or more rings share two adjacent atoms, and names are derived from retained parent hydrides or systematic fusion nomenclature, often using hydro prefixes to indicate saturation. A classic example is decahydronaphthalene (decalin), the fully saturated fused system of two six-membered rings, which is a retained name for general nomenclature and preferred for the trans isomer in specific contexts. Numbering follows established patterns for the parent fused system, prioritizing lowest locants for substituents or fusion sites.[26] Spiro compounds feature two or more rings linked by a single shared atom, named using the "spiro-" prefix followed by a bracketed descriptor [m.n] (where m ≤ n are the number of carbons in each branch, excluding the spiro atom) and the name of the alkane corresponding to the total number of carbons. The smaller ring is cited first, and numbering begins in the smaller ring at an atom adjacent to the spiro atom, proceeding to give lowest locants to substituents. For instance, the spiro compound with a five-membered ring and a six-membered ring sharing one carbon (total 10 carbons) is spiro[4.5]decane. This system extends to multispiro compounds with additional descriptors.[27] Bridged bicyclic and polycyclic systems, where rings are connected by three or more bridges between two bridgehead atoms, employ the von Baeyer system. The name is constructed as bicyclo[x.y.z]alkane (or polycyclo- for more rings), where x ≥ y ≥ z represent the lengths of the bridges in descending order (number of carbons linking the bridgeheads), and the alkane name reflects the total carbon count (x + y + z + 2). Bridgeheads receive locants 1 and the highest number, with the longest bridge numbered first, then the next longest, and finally the shortest; substituents get the lowest possible locants. Retained names include norbornane for bicyclo[2.2.1]heptane, a common bridged system with bridges of 2, 2, and 1 carbons. This nomenclature, revised in 1999, ensures clarity for complex structures like adamantane, which has a retained name but can also be described systematically as tricyclo[3.3.1.1^{3,7}]decane.[28]Alkenes
Alkenes, also known as olefins, are acyclic or cyclic hydrocarbons characterized by the presence of one or more carbon-carbon double bonds. In IUPAC nomenclature, the naming of alkenes follows substitutive principles, where the parent chain is derived from the corresponding alkane by replacing the terminal "-ane" suffix with "-ene" to indicate the double bond. For monoalkenes, the general molecular formula is (where ), reflecting the reduction of two hydrogen atoms compared to alkanes due to the unsaturation.[29] The position of the double bond is specified by a locant placed immediately before the "-ene" suffix, corresponding to the lower-numbered carbon atom of the double bond. The parent chain is selected as the longest continuous carbon chain that incorporates the double bond, ensuring the maximum number of non-cumulative double bonds if chains of equal length are possible. Numbering of the chain begins from the end that assigns the lowest possible locant to the double bond; if a choice remains, substituents receive the lowest set of locants. For example, the compound is named but-2-ene, as the double bond receives locant 2 rather than 3 in but-1-ene, which would not fit the structure. Retained names such as ethene and propene are used without locants for the simplest alkenes.[30][31] For compounds with multiple double bonds (polyenes), the suffix is modified to "-diene", "-triene", etc., with locants for each double bond separated by commas and placed before the suffix. The chain is numbered to provide the lowest possible set of locants for all double bonds collectively. If ties occur, the chain with the greater number of double bonds is preferred. An example is , named penta-1,3-diene, where the locants 1,3 are lower than alternatives like 1,4 for a different numbering. Conjugated or isolated double bonds are distinguished only by locant positions, with no additional descriptors in the base name.[32] Cumulated double bonds, as in allenes, are named using consecutive locants in the suffix, such as propa-1,2-diene for , the simplest member of this class. These systems are treated as dienes with adjacent double bonds sharing a central carbon atom. For exocyclic double bonds, particularly terminal methylene groups (=CH₂), the substituent prefix "methylidene" is used instead of including it in the parent chain when the double bond is not part of the principal chain. For instance, the compound with a cyclopentane ring and an exocyclic =CH₂ is named methylidenecyclopentane.[16][33] Stereochemistry in alkenes arises from restricted rotation around the double bond, leading to geometric isomerism. For alkenes with two different substituents on each carbon of the double bond, the configuration is specified using the (E)/(Z) descriptors, based on the Cahn-Ingold-Prelog (CIP) priority rules. Each substituent is assigned a priority according to atomic number (or atomic mass if tied) at the first point of difference, starting from the double-bonded carbons. The (Z) descriptor indicates higher-priority groups on the same side of the double bond (zusammen, German for "together"), while (E) denotes them on opposite sides (entgegen, "opposite"). These descriptors are placed in italics before the name, with locants if necessary. For example, in , the (E) isomer has the methyl and chloro groups trans, as chlorine has higher priority than hydrogen or carbon on their respective sides. The CIP rules ensure unambiguous assignment, extending to complex substituents by traversing the graph of attached atoms.[34]Alkynes
Alkynes are hydrocarbons that contain one or more carbon-carbon triple bonds, distinguishing them from saturated alkanes and alkenes with double bonds. For acyclic monoalkynes, the general molecular formula is , where , reflecting the removal of four hydrogen atoms compared to the corresponding alkane due to the triple bond. This formula applies to both unbranched and branched structures, with the triple bond represented as \ce{R-C#C-R'}, where R and R' can be hydrogen or alkyl groups.[35] In IUPAC substitutive nomenclature, alkynes are named by selecting the longest continuous carbon chain that includes the triple bond as the parent hydride and replacing the suffix "-ane" with "-yne". The position of the triple bond is indicated by a locant placed immediately before the suffix, referring to the lower-numbered carbon atom of the triple bond. The chain is numbered in the direction that assigns the lowest possible locant to the triple bond; if ambiguity arises, the lowest set of locants for all multiple bonds is chosen. For example, the simplest alkyne beyond ethyne is named propyne for \ce{CH3-C#CH}, where no locant is needed as the triple bond position is unambiguous, and but-1-yne for \ce{HC#C-CH2-CH3} rather than but-2-yne for the internal isomer \ce{CH3-C#C-CH3}. Multiple triple bonds are denoted by "-diyne", "-triyne", etc., with locants in ascending order.[36][37] For terminal alkynes, where the triple bond is at the end of the chain (\ce{HC#C-}), the preferred IUPAC name uses the locant "1" (e.g., pent-1-yne for \ce{HC#C-CH2-CH2-CH3}), ensuring the lowest locant for the functional group. The name acetylene is a retained preferred IUPAC name specifically for the parent compound \ce{HC#CH} (also acceptable as ethyne), but it cannot be used for substituted derivatives; instead, systematic names like ethynyl for the \ce{HC#C-} group are required. This retention preserves historical usage while prioritizing systematic nomenclature for complex structures.[16] When a chain contains both double and triple bonds (enynes), the suffix becomes "-en-yne", with endings combined and elided appropriately (e.g., no final "e" before vowels). Locants for the double and triple bonds precede their respective suffixes in numerical order, and the chain is numbered to give the lowest set of locants for all multiple bonds combined; in cases of ties, the double bond receives the lower locant. For instance, \ce{HC#C-CH=CH-CH2-CH3} is named hex-3-en-1-yne, not hex-3-en-5-yne, as the set {1,3} is lower than {3,5}, and the direction prioritizes the double bond if needed. Any double bonds in enynes may require (E) or (Z) descriptors if stereochemistry is specified, but triple bonds do not. Cumulated systems involving triple bonds are rare, but for isolated or conjugated enyne systems, the focus remains on maximizing the principal chain's unsaturation.[36][37] In branched alkynes, the parent chain is selected as the longest continuous carbon chain incorporating the triple bond, with precedence given to the chain that includes the maximum number of triple bonds (and then double bonds if present). Substituents are named as alkyl groups with the lowest possible locants, following rules analogous to those for alkenes but with the triple bond dictating chain choice and numbering priority. If multiple chains compete, the one with the greater number of multiple bonds is preferred; the triple bond takes precedence over double bonds in determining the principal chain but yields to double bonds in locant assignment during ties. For example, in a branched structure like \ce{(CH3)2CH-C#C-CH3}, the name is 4-methylpent-2-yne, selecting the five-carbon chain with the triple bond and assigning locants to minimize the triple bond's position. This ensures unambiguous and systematic naming across complex structures.[36]Arenes
Arenes, or aromatic hydrocarbons, represent a class of organic compounds characterized by their exceptional stability due to delocalized π-electrons in cyclic, planar structures satisfying specific criteria, such as Hückel's rule of 4n + 2 π-electrons for monocyclic systems. The nomenclature for arenes is primarily substitutive, with benzene serving as the foundational parent hydride. Benzene (C₆H₆) is the retained preferred IUPAC name (PIN) for the simplest arene, a six-membered ring with three alternating double bonds, though its structure is often depicted with a circle to symbolize electron delocalization. Simple derivatives of benzene are named by prefixing substituent groups to "benzene," with retained names accepted for certain unsubstituted compounds as PINs. For example, toluene (methylbenzene) is the PIN for C₆H₅CH₃, cumene (propan-2-ylbenzene) for C₆H₅CH(CH₃)₂, and styrene (ethenylbenzene) for C₆H₅CH=CH₂; these retained names are mandatory for the parent structures but systematic names are used when generating further derivatives. Other common derivatives, such as phenol or aniline, are treated as functional parents in their respective sections but follow benzene-based naming when substituted without higher-priority groups. For polysubstituted benzenes, the name is constructed by listing prefixes in alphabetical order, assigning the lowest possible locants to substituents, and choosing the direction that gives the lowest locant to the substituent that comes first in alphabetical order if ties occur. Di- and trisubstituted derivatives use special retained names like o-, m-, p- for 1,2-, 1,3-, 1,4- positions in general nomenclature, but numerical locants are required for PINs. For instance, 1-bromo-2-chlorobenzene is the PIN for the compound with Br at position 1 and Cl at 2, rather than o-bromochlorobenzene. When a principal chain is attached, the benzene ring may be expressed as a substituent using "phenyl," but if the chain has fewer than the number of carbons in the ring, benzene remains the parent. Fused polycyclic aromatic hydrocarbons employ retained names for specific ortho-fused systems, with numbering starting from a position that provides the lowest possible numbers to fusion sites and substituents. Naphthalene (C₁₀H₈) is the PIN for the two-ring system, with positions 1–8 and fusion at 4a–8a; anthracene (C₁₄H₁₀) for the linear three-ring fusion; and phenanthrene (C₁₄H₁₀) for the angular three-ring fusion, distinguished by specific fusion notation (e.g., benzoanthracene for extended systems). These retained names are used as parents for substitution, with locants chosen to give the lowest set for substituents. Partially saturated fused arenes are named as hydro derivatives of the fully aromatic parent, specifying the positions of saturation with the "hydro" prefix and locants. Tetralin, retained as a general name, is systematically 1,2,3,4-tetrahydronaphthalene, indicating saturation of one ring in the naphthalene system. Such names maintain the aromatic parent for the unsaturated portion. In contrast to true arenes, non-aromatic cyclic polyenes like annulenes are named systematically as cycloalkapolyenes without implying aromaticity. Cyclooctatetraene (1,3,5,7-cyclooctatetraene) exemplifies a [38]annulene with 8 π-electrons (4n, n=2), failing Hückel's rule and thus adopting a tub-shaped, non-planar structure rather than aromatic stabilization; the name "[38]annulene" is not recommended, with the systematic polyene name preferred. This distinction underscores that aromatic nomenclature applies only to compounds meeting electronic and structural criteria for delocalization.Functional Groups
Halogen Compounds
In IUPAC nomenclature, halogen compounds are named using substitutive nomenclature where halogen atoms serve as substituents on a parent hydrocarbon chain or ring, employing the prefixes fluoro-, chloro-, bromo-, or iodo-.[19] These prefixes are added to the name of the parent structure, with the position indicated by locants when necessary. For example, the simplest halogen compound, chloromethane (CH₃Cl), is named by attaching the prefix "chloro-" to the parent hydride methane. Similarly, bromoethane (CH₃CH₂Br) uses "bromo-" with the parent ethane.[19] This approach applies to both aliphatic and aromatic systems, treating halogens as non-principal characteristic groups with low precedence in the order of seniority.[19] For polyhalogenated compounds, locants are assigned to indicate the positions of each halogen atom, selecting the numbering that gives the lowest possible set of locants to the substituents as a whole.[19] When different halogens are present, the prefixes are cited in alphabetical order, disregarding multiplicative prefixes like di- or tri-, and the locants precede each prefix. For instance, the compound BrCH₂CH₂Cl is named 1-bromo-2-chloroethane, where "bromo" precedes "chloro" alphabetically, and the chain is numbered from the end that yields the lowest locant (1,2 rather than 1,2 in reverse).[19] In cases of identical halogens, such as CH₃CHCl₂, the name 1,1-dichloroethane uses geminal locants to specify both chlorines on the same carbon, without special terminology for geminal or vicinal arrangements.[19] Multiplicative prefixes (di-, tri-, etc.) are used without spaces or hyphens between the prefix and the parent name, and elided if followed by a vowel.[19] Haloarenes follow analogous rules, with benzene as the parent hydride for monosubstituted cases, such as chlorobenzene (C₆H₅Cl).[19] For disubstituted derivatives, locants and alphabetical order determine the name; for example, BrC₆H₄F (para-substituted) is 1-bromo-4-fluorobenzene, numbered to give the lowest locants (1,4) and citing prefixes alphabetically.[19] When halogens appear alongside higher-precedence functional groups, they remain as prefixes, and the numbering prioritizes the principal characteristic group.[19] Certain traditional names are retained for general use, though preferred IUPAC names (PINs) favor systematic nomenclature. For example, chloroform (HCCl₃) is a retained name acceptable in general contexts, but the PIN is trichloromethane.[19] Similarly, bromoform and iodoform are retained, corresponding to tribromomethane and triiodomethane as PINs.[19] No retained names are used as PINs for simple haloalkanes or haloarenes beyond these specified cases.[19]Hydroxy Compounds
Hydroxy compounds in which the hydroxyl group (-OH) is expressed as the principal characteristic group are named substitutively using appropriate suffixes derived from the parent hydride. For acyclic and cyclic saturated structures, alcohols are named by changing the 'e' ending of the parent hydride name to the suffix 'ol', with elision of the final 'e' before a vowel. The position of the -OH group is indicated by the lowest possible locant. For example, the compound CH₃CH₂OH is named ethanol (retained preferred IUPAC name), while CH₃CH(OH)CH₃ is propan-2-ol.[19] [P-63.1.1, P-63.1.2] The parent chain for naming alcohols is selected as the longest continuous carbon chain that includes the carbon atom attached to the -OH group. If chains of equal length are possible, the one with the maximum number of substituents cited as prefixes is chosen, but priority is given to the lowest locant for the principal -OH group. Numbering starts from the end nearest the -OH to assign it the lowest locant. For branched chains, substituents are named in alphabetical order with their locants. For instance, (CH₃)₂CHCH₂CH₂OH is named 3-methylbutan-1-ol, where the chain is numbered to give the -OH the locant 1.[19] [P-45.2.1, P-63.1.2] Polyhydroxy compounds, or polyols, are named using multiplicative suffixes such as 'diol', 'triol', or 'tetrol', with locants indicating the positions of all -OH groups, separated by commas and ordered in ascending sequence. The parent chain is chosen to include as many -OH groups as possible, with the lowest possible set of locants for them. Ethane-1,2-diol (HOCH₂CH₂OH) is a retained name for general use, while propane-1,2,3-triol is systematically named but commonly known by the retained name glycerol (preferred IUPAC name for the unsubstituted compound). For example, HOCH₂CH(OH)CH₂OH is propane-1,2,3-triol. Retained names like methanol (CH₃OH) and ethanol (CH₃CH₂OH) are preferred IUPAC names and used without locants due to their unambiguous structure.[19] [P-63.1.1, P-63.2.2.1] Phenols, where the -OH group is directly attached to a benzene ring, are named using the suffix 'ol' with the parent hydride 'benzene', resulting in the name phenol for C₆H₅OH, which is a retained preferred IUPAC name. Alternatively, substitutive names using the prefix 'hydroxy-' are allowed for general nomenclature, such as hydroxybenzene, but 'phenol' is preferred. For monosubstituted derivatives, the position is indicated relative to the -OH at position 1, e.g., 4-methylphenol for CH₃C₆H₄OH (para-cresol, retained for general use). In fused polycyclic systems, such as naphthalene, the -OH group leads to names like naphthalen-1-ol or naphthalen-2-ol, following fusion nomenclature rules with the -OH receiving the lowest possible locant consistent with the fused parent hydride orientation.[19] [P-63.1.2, P-63.1.2.2, P-25.3.2.1] For unsaturated hydroxy compounds, if the -OH group is the principal function and attached to a carbon-carbon double bond, the suffix '-enol' may be used, but preferred IUPAC names typically employ the suffix '-ol' combined with the prefix 'en-' or 'yn-' for unsaturation, forming names like alkenols or alkynols. The principal chain is selected to include both the -OH and the multiple bond(s), numbered to give the lowest locant to the -OH, then to the multiple bonds. For example, CH₂=CHOH is ethenol (vinyl alcohol), but more commonly named as the enol form in context; however, for preferred names, compounds like HOCH₂CH=CH₂ are prop-2-en-1-ol. Stereochemistry at chiral centers in hydroxy compounds is specified using the R/S system or other descriptors as per general rules for stereogenic centers.[19] [P-31.1.4.1, P-63.1.2, P-93] In cases of multiple functional groups, the -OH suffix is used only when it has seniority over other groups; otherwise, it is expressed as the prefix 'hydroxy-'. Ethers, for instance, are named using 'alkoxy-' prefixes when -OH is principal, but this section focuses on -OH as the suffix-defining group.[19] [P-63.1.4, P-41]Ethers
Ethers are organic compounds characterized by an oxygen atom bonded to two carbon atoms, typically from alkyl or aryl groups, with the general structure R–O–R', where R and R' may be the same or different. In IUPAC nomenclature, ethers are treated as substituents rather than principal characteristic groups because they have low seniority in the order of precedence for functional groups. The preferred IUPAC name (PIN) for simple ethers is generated using substitutive nomenclature, in which the senior parent structure is the longest continuous carbon chain, and the oxygen-linked group is expressed as an alkoxy or aryloxy prefix.[10][39] For unsymmetrical acyclic ethers, the parent chain is selected as the longest continuous hydrocarbon chain attached to the oxygen, and the shorter alkyl group is named as the substituent with the prefix "alkoxy-" (or "aryloxy-" if aromatic). Locants are assigned to give the lowest possible number to the carbon atom attached to the oxygen in the parent chain. For example, CH₃–O–CH₂CH₂CH₃ is named 1-methoxypropane, where propane is the parent chain and methoxy is the prefix. If the chains are of equal length, the parent is chosen based on the one that gives the simplest name or follows alphabetical order for substituents. In cases with multiple ether linkages in a chain, the structure is named as a polyalkoxyalkane, with locants assigned to provide the lowest set of numbers for the oxygen atoms.[40][41] Functional class nomenclature, while acceptable for general use, is not preferred for PINs and involves naming the two groups attached to oxygen separately in alphabetical order, followed by "ether." For instance, CH₃CH₂–O–CH₂CH₃ is diethyl ether, and CH₃–O–CH₂CH₂CH₃ is ethyl methyl ether. This method is simpler for symmetrical ethers but less systematic for complex structures. Retained names exist for certain simple ethers; anisole (C₆H₅–O–CH₃) is the only retained name for an aryl alkyl ether that serves as a PIN and is used in general nomenclature.[10][42] Cyclic ethers are named using heterocyclic nomenclature, where the oxygen is indicated by the prefix "oxa-" in a von Baeyer system for larger rings or retained names for small common rings. The three-membered ring (ethylene oxide) is named oxirane as the retained PIN. The five-membered ring (tetrahydrofuran) is oxolane, and the six-membered ring (tetrahydropyran) is oxane; both are retained for general use but substitutive names like oxolane are PINs. Substituents on cyclic ethers receive the lowest possible locants, starting from the oxygen as position 1 if applicable. For example, the compound with a methyl group on the carbon adjacent to oxygen in oxirane is 2-methyloxirane.[39][41] In more complex molecules where an ether group is subordinate to a higher-precedence functional group (such as carboxylic acids or amines), the ether is expressed solely as an alkoxy or aryloxy substituent prefix, integrated into the parent chain name without altering the principal suffix. This contrasts with hydroxy compounds, which can use the suffix "-ol" when serving as the principal function due to higher precedence. Multiple ether groups in such contexts are cited with multiplying prefixes like "di-" or "tri-" and ordered alphabetically.[10][39]Aldehydes
Aldehydes are named using substitutive nomenclature in the IUPAC system, where the principal characteristic group -CHO is expressed by the suffix '-al' attached to the name of the parent hydride chain, with elision of the final 'e' of the parent hydride name before 'al'. The carbon atom of the -CHO group is included in the parent chain, and the chain is selected as the longest continuous carbon chain containing this carbon; numbering begins at the aldehyde carbon, which receives the implied locant 1. For example, the simplest aldehyde H-C(=O)H is methanal, CH₃-C(=O)H is ethanal, and CH₃CH₂-C(=O)H is propanal.[19] Certain retained names are acceptable for general use and, in some cases, as preferred IUPAC names (PINs): formaldehyde for methanal and acetaldehyde for ethanal. For acyclic and monocyclic hydrocarbons, the suffix '-al' is used exclusively for the principal -CHO group, but when the -CHO is attached to a ring or chain where suffix attachment would be ambiguous, the suffix '-carbaldehyde' may be employed.[19][10] In the case of aromatic aldehydes, benzaldehyde is the retained preferred IUPAC name for C₆H₅-CHO, while the systematic alternatives benzenecarbaldehyde or phenylmethanal are also permitted but not preferred. For compounds containing more than one -CHO group as the principal function, multiplicative nomenclature or the suffix '-dial', '-trial', etc., is used; for dialdehydes derived from unbranched chains, the name ends in '-dial' with locants indicating the positions of the groups, and the chain is numbered to give the lowest possible locants. For example, O=CH-CH₂-CH₂-CH=O is named butanedial.[19][43] When the -CHO group is not selected as the principal characteristic group (e.g., in the presence of a higher-precedence function), it is denoted by the prefix 'formyl-'. Unlike ketones, which feature an internal >C=O group expressed as the suffix '-one', the aldehyde function requires the terminal -CHO configuration for the '-al' suffix.[19][10]Ketones
Ketones are organic compounds characterized by a carbonyl group (C=O) where the carbon atom is bonded to two other carbon atoms, distinguishing them from aldehydes where the carbonyl is terminal. In IUPAC nomenclature, ketones are named substitutively using the suffix "-one" added to the parent hydride name, with elision of the final "e" in the parent name, and the position of the carbonyl group denoted by a locant. The parent chain is selected as the longest continuous carbon chain that includes the carbonyl group; in cases of equal length, the chain receiving the lowest locant at the first point of difference for the carbonyl is preferred. Numbering starts from the end nearer the carbonyl to assign it the lowest possible locant.[19] For example, the simplest ketone, with the structure CH₃–CO–CH₃, is named propan-2-one, where the locant "2" indicates the position of the carbonyl carbon in the three-carbon chain. The retained name "acetone" is permitted for this compound in general nomenclature and is widely used. Another example is butan-2-one for CH₃–CO–CH₂–CH₃, chosen over butan-3-one to give the lowest locant to the functional group.[19] Compounds with multiple ketone groups are named using suffixes like "-dione" for two carbonyls, with locants cited in ascending order and selected to provide the lowest possible set. For instance, CH₃–CO–CH₂–CO–CH₃ is pentane-2,4-dione, as this numbering yields the set {2,4} rather than {2,4} from the opposite direction, which is equivalent but standardized. In cases involving branches or substituents, the chain is chosen and numbered to include all carbonyls while adhering to the lowest locant rule for the principal characteristic group.[19] When a ketone functional group is attached to an aromatic ring, such as in C₆H₅–CO–CH₃, the preferred IUPAC name is 1-phenylethanone, treating the chain including the carbonyl as the parent and the phenyl as a substituent. The retained name "acetophenone" is acceptable for general use. For more complex aryl alkyl ketones, the systematic name follows similar rules, prioritizing the longest chain with the carbonyl.[19] Ketones incorporating unsaturation, such as double bonds, use composite suffixes like "-enone" for one double bond and one carbonyl, with locants assigned to give the lowest number to the carbonyl group first, then to the double bond. For alpha,beta-unsaturated ketones, an example is but-3-en-2-one for CH₂=CH–CO–CH₃, where the chain is numbered to place the carbonyl at position 2 and the double bond at 3–4. Retained names and specific rules for conjugation or extended unsaturation follow the general principles of lowest set of locants for all features.[19]Carboxylic Acids
In IUPAC nomenclature, carboxylic acids are designated as the principal characteristic group with the highest seniority when present in a compound, expressed using the suffix "-oic acid" for the senior parent structure. The name is formed by selecting the longest continuous carbon chain that includes the carboxyl carbon atom (–COOH), which is numbered starting from the carboxyl group as position 1, ensuring the lowest possible locants for substituents and other features. For example, the compound with the formula CH₃–COOH is named ethanoic acid, where the chain has two carbons including the carboxyl.[10][19] Certain retained names for simple carboxylic acids are preferred IUPAC names and may be used in general nomenclature. These include formic acid (systematically methanoic acid, H–COOH) and acetic acid (systematically ethanoic acid, CH₃–COOH), reflecting historical usage while maintaining systematic consistency for longer chains such as propanoic acid (CH₃–CH₂–COOH). Substituents on the chain receive the lowest possible locants, as in 2-methylpropanoic acid for (CH₃)₂CH–COOH.[10][19] For aromatic carboxylic acids, the retained name benzoic acid is the preferred IUPAC name for C₆H₅–COOH, with the systematic alternative benzenecarboxylic acid used when additional functional groups require specification of the carboxyl position, such as 2-methylbenzenecarboxylic acid. In cyclic aliphatic systems, the suffix "-carboxylic acid" is employed when the –COOH group is attached to a ring, as in cyclopentanecarboxylic acid.[10][19] Dicarboxylic acids are named using the suffix "-dioic acid" for compounds with two –COOH groups attached to the same parent chain, with numbering to give the lowest set of locants to the carboxyl groups. For instance, HOOC–CH₂–CH₂–COOH is butanedioic acid, a retained name also known traditionally as succinic acid; similarly, retained names include oxalic acid (ethane-1,2-dioic acid, HOOC–COOH) and malonic acid (propanedioic acid, HOOC–CH₂–COOH). For acids with more than two carboxyl groups, all are cited as prefixes "carboxy-" if not chosen as the principal chain, or the suffix "-polycarboxylic acid" in specific cases.[19][44] Unsaturated carboxylic acids incorporate unsaturation into the parent chain name by changing the "-ane" ending to "-ene" or "-yne" before adding "-oic acid," with locants indicating both the double/triple bonds and the carboxyl position. An example is propenoic acid (traditional acrylic acid, CH₂=CH–COOH), where the chain is numbered from the carboxyl carbon.[19]Esters
Esters, which have the general structure R-COO-R', are named in IUPAC nomenclature primarily as derivatives of carboxylic acids using substitutive nomenclature, where the name consists of the alkyl group from the alcohol component followed by the name of the carboxylate from the acid component.[19] The acid part is named by replacing the "-oic acid" ending with "-oate," while the alcohol part provides the prefix (e.g., methyl, ethyl).[19] This approach is preferred for generating preferred IUPAC names (PINs), as substitutive nomenclature holds seniority over functional class nomenclature for esters.[19] For simple esters, the name is formed systematically as "alkyl alkanoate." For instance, the compound with the formula CH₃COOCH₃ is named methyl ethanoate in substitutive nomenclature.[19] However, retained names such as methyl acetate are also accepted as PINs for this compound due to its widespread use.[19] Similarly, HCOOCH₃ is methyl formate (retained PIN) or methyl methanoate (systematic).[19] In functional class nomenclature, esters are named as "alkyl alkanoates" but treated as separate words, such as methyl ethanoate, though this is used mainly for general nomenclature rather than PINs.[10] When the alkyl group from the alcohol (R') is substituted or complex, the full substitutive name of that group precedes the alkanoate name, with locants if necessary. For example, the ester (CH₃)₂CHCOOCH₂CH₃ is named ethyl 2-methylpropanoate, where "ethyl" comes from the alcohol and "2-methylpropanoate" from the acid.[19] No locant is needed for the ester linkage itself in simple cases, as it is implied at the end of the acid chain.[19] For diesters derived from dicarboxylic acids, the name uses the dianion form with two alkyl groups specified, such as dimethyl butanedioate for CH₂(COOCH₃)₂, where "butanedioate" comes from butanedioic acid.[19] Partial esters of polybasic acids are named similarly, with the unsubstituted acid groups retained as suffixes (e.g., hydrogen 4-methylpentanedioate for a monoester).[19] Retained names like acetate and formate are common in general nomenclature but not extended to PINs for complex derivatives; no other retained names for esters qualify as PINs.[19] Cyclic esters, known as lactones, are intramolecular esters of hydroxy acids and are named in three principal ways: as heterocyclic compounds using von Baeyer systems or Hantzsch-Widman names, by the suffix "-olide" added to the hydrocarbon chain name, or via retained names.[19] For example, the five-membered ring lactone from 4-hydroxybutanoic acid is named as oxolan-2-one (heterocyclic PIN), 5-oxotetrahydrofuran (retained for general use), or dihydrofuran-2(3H)-one; the traditional name γ-butyrolactone is retained only for general nomenclature.[19] Larger rings follow similar patterns, with the "-olide" suffix indicating the lactone functionality (e.g., 2-oxolanone for the parent structure).[19]Acyl Halides
Acyl halides, also known as acid halides, are compounds in which a hydroxyl group of a carboxylic acid is replaced by a halogen atom, resulting in the functional group –COX (where X = F, Cl, Br, or I). In IUPAC nomenclature, these derivatives are named using substitutive nomenclature, where the principal characteristic group is expressed by the suffix ‘oyl halide’. This approach treats the acyl halide as a high-precedence functional group derived directly from the corresponding carboxylic acid.[19] The systematic name is constructed by replacing the ending ‘oic acid’ (for acyclic or cyclic carboxylic acids) or ‘ic acid’ (for retained names of carboxylic acids) with ‘oyl’ followed by the name of the halogen (fluoride, chloride, bromide, or iodide). The parent hydride chain or ring includes the carbonyl carbon atom, ensuring the longest continuous chain or the senior ring system is selected as the parent structure. For example, the compound with the formula CH₃–CO–Cl is named ethanoyl chloride, where the two-carbon chain incorporates the carbonyl carbon. Similarly, CH₃CH₂CH₂–CO–Br is butanoyl bromide. This method prioritizes the carbonyl-inclusive chain for numbering, starting from the acyl carbon as position 1.[19][45] Certain retained names are accepted as preferred IUPAC names (PINs) for simple acyl halides, reflecting historical usage while maintaining systematic consistency. For the acetyl group (CH₃CO–), derived from the retained acid name acetic acid, the PIN is acetyl chloride rather than ethanoyl chloride, though the systematic name remains acceptable in general nomenclature. In aromatic systems, the compound C₆H₅–CO–Cl is named benzoyl chloride as the PIN, based on the retained name benzoic acid; the systematic alternative is benzenecarbonyl chloride. No other retained names are permitted for simple aliphatic acyl halides beyond acetyl, emphasizing systematic naming for longer chains.[19][46][47] For polyfunctional acyl halides derived from polycarboxylic acids, where all carboxylic groups are converted to acyl halides, the name is formed by replacing the ‘dioic acid’, ‘trioic acid’, etc., ending with ‘oyl dihalide’, ‘oyl trihalide’, and so on, using multiplicative nomenclature if necessary. If all halogens are identical, the name reflects that (e.g., ‘dichloride’); otherwise, different halogens are cited as prefixes in alphabetical order before the ‘oyl’ suffix. For instance, the derivative from succinic acid, ClCO–CH₂–CH₂–COCl, is butanedioyl dichloride. The compound ClCO–COCl, from oxalic acid, is named oxalyl dichloride (retained name) or ethanedioyl dichloride (systematic). These names ensure the parent structure is the diacid chain, with locants assigned to indicate positions if unsymmetrical.[19][48]Acid Anhydrides
Acid anhydrides are compounds consisting of two acyl groups linked by an oxygen atom, named in IUPAC nomenclature using substitutive methods that emphasize the parent carboxylic acid structures. The general formula for acyclic symmetrical anhydrides is (RCO)_2O, where the name is derived by replacing the "-oic acid" or "-ic acid" suffix of the corresponding carboxylic acid with "anhydride." This approach ensures systematic naming aligned with the seniority of carboxylic acid derivatives in functional group precedence. Symmetrical anhydrides from monocarboxylic acids follow a straightforward substitutive rule: the name of the acid is modified by substituting "anhydride" for "acid." For instance, the anhydride derived from ethanoic acid, with the structure , is named ethanoic anhydride. This naming convention applies to both aliphatic and aromatic cases; thus, the anhydride from benzoic acid, , is designated benzoic anhydride, a retained preferred IUPAC name for unsubstituted cases. Substituted symmetrical anhydrides incorporate prefixes for substituents on the parent chain, maintaining the anhydride suffix.[19] Mixed anhydrides, featuring different acyl groups (RCO-O-COR'), are named by alphabetizing the acyl portions—derived from the carboxylic acid names without the "acid" ending—and appending "anhydride." An illustrative example is ethanoyl methanoic anhydride for , where "ethanoyl" precedes "methanoyl" alphabetically. This method distinguishes mixed anhydrides from symmetrical ones and avoids ambiguity in structures involving diverse acid components.[49][19] Cyclic anhydrides formed from intramolecular condensation of dicarboxylic acids adopt names reflecting the ring system or the diacid parent. For the five-membered ring anhydride from butanedioic acid, the preferred IUPAC name is oxolane-2,5-dione, treating it as a heterocyclic ketone-like structure, while butanedioic anhydride serves as a retained name. Larger rings follow similar patterns, such as oxane-2,6-dione for the six-membered analog from hexanedioic acid, prioritizing the cyclic anhydride suffix when the ring size permits stable nomenclature. These names highlight the fused functional groups without referencing external diacid trivial names unless retained.[19][50]Amines
Amines are organic derivatives of ammonia in which one or more hydrogen atoms have been replaced by hydrocarbon groups or other substituents, classified as primary (R-NH₂), secondary (R₂NH), or tertiary (R₃N) based on the number of substituents attached to the nitrogen atom.[19] In IUPAC nomenclature, amines are named preferentially using substitutive nomenclature, where the suffix "-amine" denotes the principal characteristic group expressed as such, with the parent hydride chain selected to include the nitrogen atom.[19] This approach aligns amines with higher precedence in the order of functional groups, allowing them to serve as the parent structure when no senior functions are present.[19] For primary amines (R-NH₂), the preferred IUPAC name is formed by adding the suffix "-amine" to the name of the parent hydride with elision of the final "e" if present, treating the nitrogen as a terminal atom of the chain.[19] The parent chain is the longest continuous carbon chain that includes the carbon atom attached to the nitrogen, numbered to give the lowest locant to the nitrogen-bearing carbon. For example, CH₃NH₂ is named methanamine, and CH₃CH₂CH₂NH₂ is propan-1-amine.[19] Branched primary amines follow similar rules, with the chain chosen to include the amino group and numbered accordingly; for instance, (CH₃)₂CHNH₂ is propan-2-amine.[19] Retained names such as ethylamine and methylamine are acceptable for general use but not preferred IUPAC names except for methanamine.[19] Secondary and tertiary amines are named by identifying the longest chain attached to the nitrogen as the parent hydride, adding the suffix "-amine," and citing the other substituent groups as prefixes with the locant "N-" to indicate attachment to nitrogen.[19] For unsymmetrical secondary amines, the parent is the chain with the greatest number of skeletal atoms; for example, CH₃NHCH₂CH₃ is N-methylethanamine.[19] Tertiary amines follow the same principle, with multiple "N-" prefixes if needed; for instance, CH₃N(CH₂CH₃)₂ is named N-ethyl-N-methylethanamine, selecting ethanamine as the parent due to the longest chain, while the symmetrical (CH₃CH₂)₃N is N,N-diethylethanamine.[19] When all substituents are identical, the name uses multiplicative prefixes like "di-" or "tri-"; symmetrical cases may also employ functional class nomenclature (see below).[19] Functional class nomenclature for amines treats the compound as a derivative of ammonia, naming it as a substituted alkylamine or dialkylamine/trialkylamine, with substituents in alphabetical order enclosed in parentheses if complex.[19] This method is retained for general nomenclature but not preferred for simple cases. Examples include (CH₃)₂NH as dimethylamine and CH₃NHCH₂CH₃ as ethyl(methyl)amine.[19] It is particularly useful for complex or symmetrical amines where substitutive names become cumbersome.[19] Aromatic amines incorporate the benzene ring as the parent when the amino group is directly attached, with "aniline" retained as the preferred IUPAC name for C₆H₅NH₂ and for derivatives thereof.[19] Substituents on the ring are prefixed with locants, such as 4-methylaniline for p-toluidine. For amines where nitrogen is attached to an alkyl chain and a phenyl group, the preferred name uses the alkanamine parent with "N-phenyl-" prefix, e.g., C₆H₅NHCH₃ as N-methylaniline (retained) or N-methylbenzenamine (systematic).[19] Heterocyclic amines like pyridin-x-amine follow analogous substitutive rules.[19] Polyamines, containing two or more amino groups, are named using suffixes like "-diamine," "-triamine," etc., with locants indicating positions on the parent chain selected to include as many nitrogen atoms as possible.[19] The chain is numbered to give the lowest set of locants to the nitrogens. For example, H₂NCH₂CH₂CH₂NH₂ is propane-1,3-diamine, and H₂N(CH₂)₄NH₂ is butane-1,4-diamine (retained for general use).[19] When amino groups are substituents on a senior parent, the prefix "amino-" is used, but for principal functions, the multiplicative suffix is preferred.[19] Quaternary ammonium compounds, R₄N⁺, are named as cations under substitutive nomenclature using the suffix "-ammonium," with details provided in the section on ions and radicals.[19]Amides
Amides, as derivatives of carboxylic acids, are named substitutively by replacing the suffix '-oic acid' of the parent carboxylic acid name with '-amide'; the carbon atom of the carbonyl group is included in the parent chain. For example, the compound with the structure CH₃–C(=O)–NH₂ is named ethanamide. This nomenclature applies to both acyclic and alicyclic structures, with the suffix '-carboxamide' used when the -CONH₂ group is attached to a ring or ring system, such as cyclohexanecarboxamide for C₆H₁₁–C(=O)–NH₂. Substituents attached to the nitrogen atom are indicated by the prefix 'N-' followed by the substituent name(s), listed in alphabetical order if multiple. For instance, CH₃–C(=O)–NH–CH₃ is named N-methylethanamide, and (CH₃)₂CH–C(=O)–N(CH₃)₂ is named N,N-dimethylpropanamide. In cases of multiple identical N-substituents, multiplicative prefixes like 'di-' or 'tri-' are used, with locants omitted if unambiguous. For aromatic amides where the -CONH₂ group is directly attached to a benzene ring, the retained name benzamide is preferred, though benzenecarboxamide is also acceptable. N-Substituted derivatives follow similar rules, such as N-phenylbenzamide for C₆H₅–C(=O)–NH–C₆H₅. Compounds containing two or more amide groups are named using suffixes like '-diamide' or '-triamide', with the chain numbered to give the lowest locants to the carbonyl carbons; for example, NH₂–C(=O)–CH₂–CH₂–C(=O)–NH₂ is butanediamide. Certain simple amide names are retained for general nomenclature and may be used in preferred IUPAC names under specific conditions. These include formamide for H–C(=O)–NH₂ and acetamide for CH₃–C(=O)–NH₂, both of which are unrestricted in use. Cyclic amides, known as lactams, are named by adding the suffix '-lactam' to the name of the corresponding hydrocarbon, indicating the position of the nitrogen and the size of the ring. For example, the four-membered ring lactam is named azetidin-2-one or β-lactam, while the five-membered analog is pyrrolidin-2-one. This approach highlights the heterocyclic nature and the amide functionality within the ring structure.Nitriles
Nitriles are organic compounds characterized by the presence of the functional group -C≡N, known as the cyano group when functioning as a substituent. In IUPAC nomenclature, nitriles are recognized as a senior functional group and are expressed as the principal characteristic group when selected as the suffix for the parent structure. The carbon atom of the -C≡N group is incorporated into the parent hydride chain, and the suffix "-nitrile" is added to the name of the corresponding alkane or other parent hydride. This approach ensures that the name reflects the total carbon count, including the nitrile carbon, which is assigned the locant 1 in unbranched chains. For simple acyclic nitriles, the naming follows the substitutive nomenclature method, where the alkane name is modified by replacing the final "-e" with "-nitrile." For instance, the compound with the formula CH₃-C≡N is named ethanenitrile, as the chain consists of two carbons, one from the methyl group and one from the nitrile. The retained name acetonitrile is accepted for general use but not as a preferred IUPAC name. In cases where the nitrile group is not the principal function—such as when a higher-precedence group like a carboxylic acid is present—it is cited as the prefix "cyano-." Aromatic nitriles, where the -C≡N group is directly attached to a benzene ring or similar aromatic system, are named using the suffix "-carbonitrile" attached to the parent hydride name, such as benzonitrile for C₆H₅-C≡N. The retained name benzonitrile is the preferred IUPAC name. For polycyclic aromatic systems, the numbering follows the rules for the parent hydrocarbon, with the carbonitrile group receiving the lowest possible locant. Compounds containing multiple nitrile groups are named using multiplicative suffixes like "-dinitrile" or "-trinitrile," with locants indicating the positions of the nitrile carbons, chosen to yield the lowest possible set of locants. For example, the symmetric dinitrile NC-CH₂-CH₂-C≡N is named butanedinitrile, with the chain numbered from one nitrile carbon to the other. The retained name succinonitrile is permitted for general nomenclature. In cyclic compounds, such as those where nitrile groups are attached to rings, the parent hydride is the cycle, and locants are assigned to give the lowest numbers to the nitrile groups.Rules for Parent Structure Selection
Order of Precedence for Functional Groups
In IUPAC nomenclature for organic compounds, the order of precedence, also known as the seniority order of classes, determines the principal characteristic group that receives the suffix in the name of a multifunctional compound, while subordinate groups are expressed as prefixes. This hierarchy ensures unambiguous naming by prioritizing the highest-ranking functional group as the basis for the parent structure. The rules are outlined in Section P-41 of the IUPAC Recommendations 2013.[18] The general principle is that only the senior functional group is cited as the suffix, with all others denoted by appropriate prefixes; for instance, a compound containing both a carboxylic acid and a hydroxy group is named as a hydroxy-substituted carboxylic acid (e.g., 2-hydroxypropanoic acid), where "-oic acid" is the suffix and "hydroxy-" is the prefix.[18] Within the same class, further seniority criteria apply, such as the order of acids (carboxylic > sulfonic > sulfinic) or derivatives (anhydrides > esters > acyl halides > amides).[18] The 2013 IUPAC recommendations introduced specific rankings for radicals and ions, placing them at the top of the hierarchy to reflect their importance in naming species like free radicals and charged compounds, superseding earlier orders where acids were highest.[18] Radical classes precede anionic and cationic species, with radicals ranked first overall.[51] The full order of precedence is presented in Table 4.1 of the IUPAC Blue Book, categorizing classes from 1 (highest seniority) to 20 (lowest among suffix-expressed groups), followed by prefix-only classes like hydrocarbons. Exceptions occur with retained names for certain compounds (e.g., acetic acid), which may override strict seniority in preferred IUPAC names but are limited to avoid conflicts.[18]| Class Number | Seniority Class | Examples of Functional Groups |
|---|---|---|
| 1 | Radicals | -yl (e.g., methyl) |
| 2 | Radical anions | -idyl (e.g., methanidyl) |
| 3 | Radical cations | -iumyl (e.g., methyliumyl) |
| 4 | Anions | -ide, -olate (e.g., acetate) |
| 5 | Zwitterionic compounds | Betaines (e.g., sulfonium zwitterions) |
| 6 | Cations | -ium (e.g., ammonium) |
| 7 | Acids | Carboxylic acids (-oic acid), sulfonic acids (-sulfonic acid) |
| 8 | Anhydrides | -oic anhydride |
| 9 | Esters | -oate (e.g., methyl acetate) |
| 10 | Acyl halides | -oyl halide (e.g., acetyl chloride) |
| 11 | Amides | -amide |
| 12 | Hydrazides | -hydrazide |
| 13 | Imides | -imide |
| 14 | Nitriles | -nitrile |
| 15 | Aldehydes | -al |
| 16 | Ketones | -one |
| 17 | Alcohols and phenols | -ol |
| 18 | Hydroperoxides, peroxols, etc. | -peroxol |
| 19 | Amines | -amine |
| 20 | Imines | -imine |
| (Prefix only) | Ethers, alkenes, alkynes, halides, hydrocarbons | alkoxy-, -ene, -yne, halo-, alkane |
Criteria for Choosing Parent Hydrides
After the principal characteristic group has been identified according to the order of precedence for functional groups, the parent hydride is selected from possible chains or rings that incorporate this group, using a hierarchical set of criteria to ensure systematic and unambiguous naming. These rules, outlined in the IUPAC Recommendations, prioritize structural features that maximize the representation of the compound's core skeleton while minimizing complexity in substitution.[19] The primary criterion is to choose the parent hydride that includes the maximum number of occurrences of the principal characteristic group, allowing the suffix to express as many instances as possible. For diols, for example, a chain or ring encompassing both hydroxy groups is preferred over one that treats the second as a prefix like 'hydroxy'. This approach applies similarly to other multiplicative suffixes, such as 'dione' for ketones.[19][10] If this does not distinguish options, the parent hydride with the greater number of skeletal atoms is selected, corresponding to the longest continuous carbon chain for acyclic structures or the largest ring (or ring system) for cyclic ones. In an acyclic alcohol, a five-carbon chain including the -OH group would supersede a four-carbon alternative, even if the latter has more branches. For ring-chain choices, a six-membered ring is senior to a five-carbon chain of equal functional group count.[19][10] The next criteria emphasize unsaturation: the parent hydride with the maximum number of multiple bonds; if this does not distinguish, the one with the maximum number of double bonds. A chain with two double bonds is thus preferred over one with one triple bond when lengths and functional groups match.[19] For parent hydrides containing heteroatoms, such as in heterocyclic rings or chains, the structure with the heteroatom of highest seniority is chosen, following the order O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl (based on periodic table groups and rows). Oxygen-containing parents, like furan over pyrrole for equivalent structures, take precedence over nitrogen-containing ones. In cases of multiple heteroatoms, the one with the maximum number or lowest locants for senior heteroatoms is selected.[19] When these structural criteria do not resolve the choice, tie-breakers include selecting the parent hydride with the lowest set of locants for the principal characteristic group, followed by the lowest locants for substituents or multiple bonds. Numbering begins from the end affording the lowest number to the principal group; for a symmetric diol like HO-CH₂-CH₂-CH₂-OH, locants 1,3 are inherent to the propane chain. Retained names may apply if they satisfy these rules without conflict.[19][10]| Criterion | Description | Example |
|---|---|---|
| Maximum principal groups | Includes most instances of the suffix-expressible group | Pentane-1,5-diol over 4-(hydroxymethyl)butan-1-ol (both have two -OH, but former uses diol suffix twice) |
| Greatest skeletal atoms | Longest chain or largest ring | Hexan-1-ol over a branched pentane with -OH |
| Maximum multiple bonds | Most multiple bonds; if equal, most double bonds | Hexa-2,4-dien-1-ol over hex-5-yn-1-ol |
| Senior heteroatom | O > S > N order | Tetrahydropyran over piperidine for six-membered rings |
| Lowest locants | Lowest numbers for principal group, then substituents | Butane-1,4-diol (locants 1,4) over alternative numbering |
Common and Retained Names
Retained Names for Hydrocarbons
In IUPAC nomenclature, retained names for hydrocarbons are traditional designations that have been preserved due to their widespread use and historical significance, with some designated as preferred IUPAC names (PINs) for unambiguous identification in scientific and regulatory contexts, while others are acceptable only in general nomenclature. These names apply specifically to parent hydrocarbon structures and are not substitutable unless explicitly allowed. The 2013 IUPAC Recommendations outline these retained names to balance systematic naming principles with practical utility. For alkanes, the names methane (CH₄), ethane (CH₃CH₃), propane (CH₃CH₂CH₃), and butane (CH₃CH₂CH₂CH₃) are retained as PINs and serve as the basis for naming longer unbranched chains via systematic extension. In contrast, the traditional name isobutane for (CH₃)₂CHCH₃ is retained only for general use, with 2-methylpropane established as the PIN to reflect its branched structure. This distinction ensures consistency in substitutive nomenclature for derivatives.[19] Cycloalkanes from cyclopropane (C₃H₆) through cyclohexane (C₆H₁₂) have their names retained as PINs, allowing direct use for these common monocyclic saturated hydrocarbons without systematic alternatives like trimethylene or tetramethylene, which are obsolete. Larger rings, such as cycloheptane, follow systematic naming, but these retained names facilitate recognition in fused and bridged systems.[10] Among alkenes, ethene (H₂C=CH₂) and propene (H₂C=CHCH₃) are retained as PINs, replacing older systematic forms like ethylethene, while ethylene and propylene remain acceptable in general contexts but not as PINs. These names are used for the parent structures in naming unsaturated chains and rings. For alkynes, ethyne (HC≡CH) is the PIN, with acetylene retained solely for the unsubstituted parent in general nomenclature; no substitution is permitted under the retained name. For arenes, benzene (C₆H₆) is retained as the PIN for the parent monocyclic aromatic hydrocarbon. Toluene (C₆H₅CH₃) and the xylene isomers—o-xylene (1,2-dimethylbenzene), m-xylene (1,3-dimethylbenzene), and p-xylene (1,4-dimethylbenzene)—are also retained as PINs, though limited substitution is allowed only at specified positions to maintain specificity. These names underpin nomenclature for polysubstituted benzenoids.[19] Fused polycyclic hydrocarbons include retained PINs such as naphthalene (C₁₀H₈) for the ortho-fused benzene dimer and anthracene (C₁₄H₁₀) for the linearly fused trimer, both fully substitutable. These names are preferred over von Baeyer systems for these specific structures, ensuring compatibility with stereochemical and functional extensions in complex molecules.| Hydrocarbon Class | Retained PINs | Retained for General Use Only | Source |
|---|---|---|---|
| Alkanes | Methane, ethane, propane, butane | Isobutane | IUPAC Blue Book 2013 |
| Cycloalkanes | Cyclopropane, cyclobutane, cyclopentane, cyclohexane | None specified | IUPAC Brief Guide 2021 |
| Alkenes | Ethene, propene | Ethylene, propylene | IUPAC Blue Book 2013 |
| Alkynes | Ethyne | Acetylene | IUPAC Blue Book 2013 |
| Arenes | Benzene, toluene, o-xylene, m-xylene, p-xylene | None specified | IUPAC Blue Book 2013 |
| Fused Systems | Naphthalene, anthracene | None specified | IUPAC Blue Book 2013 |
Trivial Names for Functional Compounds
In the IUPAC nomenclature of organic chemistry, trivial names, also known as retained names, for functional compounds are traditional designations that have been preserved for their widespread use and historical significance, despite the preference for systematic names in many contexts. These names are particularly common for simple molecules containing key functional groups such as carboxylic acids, aldehydes, ketones, alcohols, and amines. The 2013 IUPAC recommendations specify which trivial names serve as preferred IUPAC names (PINs) and which are acceptable only for general nomenclature, ensuring consistency while allowing flexibility for established terminology. Retained names must not be used for generating more complex names unless explicitly permitted, and their application is limited to unsubstituted or specifically allowed substituted structures. For carboxylic acids, the trivial name acetic acid (CH₃COOH) is a retained preferred IUPAC name, alongside formic acid (HCOOH) and benzoic acid (C₆H₅COOH). Other common trivial names, such as propionic acid (CH₃CH₂COOH), butyric acid (CH₃(CH₂)₂COOH), valeric acid (CH₃(CH₂)₃COOH), caproic acid (CH₃(CH₂)₄COOH), caprylic acid (CH₃(CH₂)₆COOH), pelargonic acid (CH₃(CH₂)₇COOH), capric acid (CH₃(CH₂)₈COOH), lauric acid (CH₃(CH₂)₁₀COOH), myristic acid (CH₃(CH₂)₁₂COOH), palmitic acid (CH₃(CH₂)₁₄COOH), and stearic acid (CH₃(CH₂)₁₆COOH), are retained for general use but not as PINs; systematic names like propanoic acid and octadecanoic acid are preferred for regulatory and indexing purposes. These retained names for unbranched chain acids up to C18 reflect their importance in natural products and industrial applications, but substitution is generally not allowed on the parent structure except for specific cases like acrylic acid (propenoic acid). Aldehydes feature prominent retained trivial names, with formaldehyde (HCHO) designated as a preferred IUPAC name due to its fundamental role in chemistry. Acetaldehyde (CH₃CHO) is the preferred IUPAC name (PIN), with ethanal as the systematic alternative. These names are limited to the unsubstituted compounds, and more complex aldehydes require systematic nomenclature to avoid ambiguity.[19] In the case of ketones, acetone (CH₃COCH₃) is a retained preferred IUPAC name for propan-2-one, widely used in solvents and synthesis. Methyl ethyl ketone (CH₃COCH₂CH₃), or butan-2-one systematically, is retained for general use but not as a PIN. The 2013 rules restrict retained ketone names to these simple examples, emphasizing systematic names like pentan-3-one for higher homologs to maintain clarity in structural descriptions. Alcohols have several retained trivial names that are preferred IUPAC names, including methanol (CH₃OH) and ethanol (CH₃CH₂OH), which are essential in nomenclature for derivatives and are unrestricted for substitution in many contexts. Glycerol (HOCH₂CH(OH)CH₂OH), or propane-1,2,3-triol systematically, is retained for general nomenclature and preferred in biochemical contexts, though its use is confined to the unsubstituted triol or specific esters. These names highlight the historical precedence of common alcohols in everyday and industrial chemistry. For amines, aniline (C₆H₅NH₂) is a retained preferred IUPAC name, reflecting its significance as a parent for aromatic amine nomenclature, with substitution allowed on the ring. The toluidines—o-toluidine (2-methylaniline), m-toluidine (3-methylaniline), and p-toluidine (4-methylaniline)—are retained for general use, providing concise alternatives to systematic names while adhering to the limitations that prevent their extension to more complex structures. The 2013 IUPAC guidelines underscore that such trivial names for functional compounds should not supplant systematic nomenclature in formal scientific communication, promoting global standardization.Ions and Radicals
Cations
Organic cations in IUPAC nomenclature are positively charged species derived from parent hydrides, characteristic groups, or their derivatives, primarily named using substitutive methods that incorporate the suffix '-ium' to denote the positive charge. The position of the charged atom is indicated by a locant when necessary, and the structure is enclosed in square brackets if the charge is delocalized. These names follow principles outlined in the IUPAC recommendations for radicals, ions, and related species, ensuring unambiguous identification based on the parent structure and the nature of the charge formation, either by hydron (H⁺) addition or hydride (H⁻) loss.[52] Cations formed by the addition of a hydron to a parent hydride are named by replacing the final 'e' of the hydride name with '-ium'. For instance, the protonation of methane (CH₄) yields methanium (CH₅⁺), and protonation of ammonia (NH₃) produces ammonium (NH₄⁺). In cases involving nitrogen, substituted ammonium ions from protonated amines use the parent amine name followed by '-ium', such as trimethylammonium for (CH₃)₃NH⁺ derived from trimethylamine. Quaternary ammonium cations, which do not require further protonation, are named as substituted alkanaminium ions; the preferred IUPAC name for (CH₃)₄N⁺ is N,N,N-trimethylmethanaminium, where the substituents are listed in alphabetical order with locants if needed. Alkylium cations, where the positive charge is localized on a carbon atom (often called carbenium ions in older literature), are named by replacing the 'ane' ending of the corresponding alkane with 'ylium' and specifying the locant of the charged carbon. These are formally derived from the parent hydride by loss of a hydride ion. Examples include methylium (CH₃⁺) from methane and ethylium (CH₃CH₂⁺) from ethane. For branched structures, the parent chain is selected according to standard rules for hydrocarbons, yielding names like 1-methylethylium (isopropylium, (CH₃)₂CH⁺) or 2-methylpropan-2-ylium for the tert-butyl cation ((CH₃)₃C⁺). When substituents are present on the charged carbon or elsewhere, they are prefixed with appropriate locants, ensuring the lowest possible numbers for the charge and substituents.[21] Parent hydride cations beyond simple protonation, such as those involving heteroatoms, follow similar conventions, with the '-ium' suffix applied to the hydride name. Substitution in these cations requires locants to specify both substituent positions and the site of the charge; for polycations, multiple '-ium' suffixes or 'diium', 'triium', etc., are used with elision rules, and charges are indicated explicitly if delocalized (e.g., [C₆H₆]²⁺ as benzenediium). This approach parallels but contrasts with anion nomenclature, which employs the suffix '-ide' for negatively charged species.Anions
In IUPAC nomenclature, organic anions are named using substitutive methods that reflect the parent structure and the site of deprotonation or charge, often employing suffixes such as -ide, -oate, -olate, or -amide to indicate the anionic nature.[19] These rules, outlined in the 2013 recommendations, prioritize the selection of the parent hydride and adjust for the loss of a proton, ensuring unambiguous identification of the charged species.[19] For simple cases, the anion is derived directly from the neutral parent by replacing a hydrogen with the appropriate suffix, while more complex anions incorporate functional group priorities. Carbanions, formed by deprotonation at a carbon atom, are named by adding the suffix -ide to the name of the parent hydride, with elision of the final 'e' if present. For example, the anion CH₃⁻ is named methanide, derived from methane.[19] This general -ide suffix applies to monoanions; for multiple deprotonations, prefixes like di-, tri- are used, yielding -diide, -triide, etc., as in ethanediide for ⁻CH₂CH₂⁻.[19] The 2013 IUPAC rules specify that such names are generated from the parent hydride name, ensuring systematic construction for acyclic, cyclic, or polycyclic structures.[19] Carboxylate anions, derived from carboxylic acids by deprotonation, use the suffix -oate in place of -oic acid, with the parent chain numbered to include the carboxyl carbon as position 1. For instance, the anion from acetic acid (CH₃COOH) is acetate or systematically ethanoate (CH₃COO⁻).[19] When the carboxylate is attached to a ring, the name becomes benzoate for C₆H₅COO⁻ or more generally arenecarboxylate.[19] Retained names like acetate are allowed for general use, but systematic names are preferred for complex molecules under the 2013 guidelines.[19] Alkoxide anions, resulting from deprotonation of alcohols, are named using the suffix -olate added to the parent hydride name, often retaining common names for simplicity. The methoxide ion (CH₃O⁻) is a classic example, systematically methanolate but commonly methoxide.[19] For longer chains, such as ethanol deprotonated to ⁻OCH₂CH₃, the name is ethoxide or ethanolate.[19] The 2013 rules permit both systematic and retained names, with -olate emphasizing the oxygen-centered charge.[19] Amide anions, formed by deprotonation of primary amides (R-CONH₂), are named using substitutive nomenclature that reflects the anionic nitrogen, such as acylazanide for the preferred IUPAC name. For example, the anion from acetamide (CH₃CONH₂) is acetylazanide (CH₃CONH⁻).[21] This follows the general pattern for nitrogen-centered anions in the 2013 recommendations, where the acyl group is cited as a substituent to the azanide parent. Retained or common names like acetamidate may be used in general nomenclature.[21] Delocalized anions, such as those from dicarboxylic acids, often use retained names for brevity and historical precedence. The dianion of phthalic acid (1,2-benzenedicarboxylic acid) is named phthalate, reflecting its resonance-stabilized structure.[19] Under the 2013 IUPAC rules, such names are accepted as preferred for common compounds, while systematic alternatives like benzene-1,2-dicarboxylate are available for precision.[19]Radicals
In IUPAC nomenclature, neutral radicals, which are odd-electron species, are named by modifying the parent hydride to indicate the removal of one or more hydrogen atoms, using suffixes such as '-yl' for monovalent radicals.[21] This approach ensures systematic naming while allowing for retained traditional names in specific cases. The 2013 recommendations emphasize that radicals differ from ions by being uncharged, focusing on subtractive operations from parent hydrides without implying charge. Monovalent radicals are formed by removing one hydrogen atom from a parent hydride and are named using the suffix '-yl', with elision of the final 'e' in the parent name if present. For example, the radical derived from methane (CH₄) is named methyl (•CH₃), and from ethane (CH₃-CH₃) is ethyl (CH₃-CH₂•).[21] In chains, locants specify the position of the free valence; for instance, the radical from propane at the terminal carbon is propyl (CH₃-CH₂-CH₂•), while at the central carbon it is 1-methylethyl or propan-2-yl ((CH₃)₂CH•). Aromatic monovalent radicals use retained names like phenyl for C₆H₅•, derived from benzene.[21] Acyl radicals, such as acetyl (CH₃C(O)•) from acetic acid, are also retained for common use.[21] For polyvalent radicals, including biradicals, the nomenclature employs multiplicative suffixes like '-diyl' to indicate multiple free valences, signaling the removal of two hydrogen atoms from the parent hydride. For example, the biradical •CH₂-CH₂• is named ethane-1,2-diyl.[21] Locants are assigned to give the lowest possible numbers to the free valences, and the suffixes are cited in order of increasing complexity (e.g., '-yl' before '-diyl'). Retained names for polyvalent radicals are limited, but systematic construction is preferred for complex structures to maintain clarity.[21] The 2013 Blue Book distinguishes these neutral species from charged analogs like cations and anions by avoiding charge-indicating suffixes such as '-ium' or '-ide'.Stereochemistry
Configurational Nomenclature
Configurational nomenclature specifies the absolute three-dimensional arrangement of atoms in a molecule, particularly around tetrahedral stereogenic centers, using the Cahn-Ingold-Prelog (CIP) priority rules to assign unambiguous descriptors. Developed in the mid-20th century, this system ensures consistent naming of enantiomers and other stereoisomers by ranking substituents based on atomic composition and connectivity. The rules prioritize substituents by comparing the atomic numbers of directly attached atoms, with higher numbers receiving higher priority; ties are resolved by moving outward along the bonds to compare subsequent atoms, treating multiple bonds as if the atoms are replicated (phantom atoms) to account for their higher electron density. This hierarchical approach allows for the precise determination of configuration at each chiral center.[53] To assign the descriptors, the molecule is oriented such that the substituent with the lowest priority is directed away from the viewer. The configuration is then designated as R (from Latin rectus, right) if the remaining three substituents decrease in priority in a clockwise manner, or S (from Latin sinister, left) if counterclockwise. These italicized descriptors are enclosed in parentheses and prefixed to the systematic name, with locants for multiple centers, as in (R)-lactic acid for (2R)-2-hydroxypropanoic acid or (2R,3S)-2,3-butanediol. For compounds with more than one stereogenic center, all are specified in order of their locants, enabling distinction between diastereomers. Racemic mixtures, consisting of equal amounts of enantiomers, are named using the prefix rac- before the name of one enantiomer (e.g., rac-lactic acid), preferred over the older (±)- notation, to indicate the absence of optical activity without specifying individual configurations. In contrast, meso compounds possess two or more chiral centers but are achiral overall due to an internal plane of symmetry that makes them superimposable on their mirror images; such forms, like meso-tartaric acid ((2R,3S)-2,3-dihydroxybutanedioic acid), are named without stereodescriptors or with relative configuration indicators like rel-(2R,3S)- to denote the fixed relative arrangement. Absolute configuration provides the true handedness at each center, while relative configuration describes inter-center relationships without absolute assignment, using lowercase r/s descriptors in some contexts.[54]Stereodescriptors for Double Bonds and Rings
In IUPAC nomenclature, stereodescriptors for double bonds specify the configuration around the C=C unit using the (E)/(Z) system, which is based on the Cahn-Ingold-Prelog (CIP) priority rules to assign priorities to substituents on each end of the double bond.[34] The descriptor (Z) indicates that the two higher-priority substituents are on the same side (zusammen), while (E) indicates they are on opposite sides (entgegen); these are placed in front of the name with locants if necessary, such as (E)-but-2-ene for the trans isomer of butene where the methyl groups have higher priority than hydrogen. This system applies to alkenes and other double bonds, including those with heteroatoms, and is mandatory for preferred IUPAC names when ambiguity exists in cis/trans notation. For cumulated double bonds like allenes, axial chirality arises from perpendicular planes, designated as (Ra) or (Sa) [or (P) or (M)] using the CIP helicity rule, where the configuration is determined by viewing along the axis and assigning based on the arrangement of substituents; for example, (Ra)-penta-2,3-diene. For cyclic compounds, stereodescriptors describe relative configurations of substituents on rings, primarily using cis/trans for disubstituted cycloalkanes where the ring size allows clear distinction, limited to small rings like cyclopropane, cyclobutane, and cyclopentane to avoid ambiguity in larger systems. In cis-1,2-dimethylcyclopropane, both methyl groups are on the same face of the ring, while in the trans isomer, they are on opposite faces; these descriptors are placed before the name with locants, and for preferred IUPAC names in small rings (three to seven members), they supersede R/S for relative configuration unless absolute chirality is specified. For larger rings or more complex cases, reference configurations using 'r' (reference), 'c' (cis), and 't' (trans) may be used relative to a defined stereogenic center, but cis/trans remains acceptable for simple disubstituted cases. Atropisomers, arising from restricted rotation about a single bond in systems like biaryls, exhibit axial chirality and are designated using (P) or (M) descriptors based on the helicity rule, where (P) corresponds to a right-handed screw sense and (M) to left-handed when viewing along the axis with the lowest-numbered substituent in front.[55] For example, in 2,2'-dimethyl-1,1'-biphenyl, the stable atropisomers are named as (P)-(2,2'-dimethyl[1,1'-biphenyl]) and (M)-(2,2'-dimethyl[1,1'-biphenyl]), applicable only when the barrier to rotation allows isolation of enantiomers. This notation extends to other axially chiral systems but is distinct from point chirality descriptors like (R)/(S). Conformational stereodescriptors are not included in systematic IUPAC names for most organic compounds, as conformers interconvert rapidly at room temperature, but specific notations like δ (delta) for twist-boat or lowercase letters (e.g., sc for synclinal) may be used in descriptive contexts for rings such as cyclohexane's chair or boat forms.[56] For persistent conformers in atropisomers, however, the (P)/(M) descriptors capture the fixed conformation as a stereogenic unit.References
- Systematic nomenclature began in 1892 with the establishment of the Geneva Rules by a committee appointed by the International Chemical Congress of 1889. The ...