Recent from talks
Contribute something
Nothing was collected or created yet.
Clitoral erection
View on Wikipedia

Clitoral erection (also known as clitoral tumescence or female erection)[1][2] is a physiological phenomenon where the clitoris becomes enlarged and firm.
Clitoral erection is the result of a complex interaction of psychological, neural, vascular, and endocrine factors, and is usually, though not exclusively, associated with sexual arousal. Erections should eventually subside, and the prolonged state of clitoral erection even while not aroused is a condition that could become painful.[3] This swelling and shrinking to a relaxed state seems linked to nitric oxide's effects on tissues in the clitoris, similar to its role in penile erection.[4]
Physiology
[edit]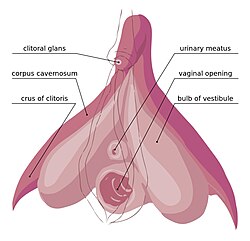
The clitoris is the homolog to the penis in the male. Similarly, the clitoris and its erection can subtly differ in size.[5]
The visible part of the clitoris, the glans clitoridis, varies in size from a few millimeters to one centimeter and is located at the front junction of the labia minora (inner lips), above the opening of the urethra. It is covered by the clitoral hood.
Any type of motion can increase blood flow to this organ and this results in increased secretions which lubricate the vagina. There are many ways to stimulate the clitoris.
Clitoral erection occurs when the corpora cavernosa, two expandable erectile structures, become engorged with blood. This may result from any of various physiological stimuli, including sexual arousal. During sexual arousal, arterial blood flow to the clitoris is increased, and trabecular smooth muscle within the clitoris relaxes allowing blood to engorge the erectile tissues. The ischiocavernosus and bulbospongiosus muscles contract to compress the dorsal vein of the clitoris to stop drainage of the clitoris, trapping the blood.[6] The erectile tissues are composed of endothelium-lined vascular spaces in a trabecular matrix, with the endothelium-lined vascular spaces surrounded by smooth muscle capable of contraction and relaxation.
During sexual arousal, arterial blood flow to the clitoris is increased, and within the clitoris, the arteries further branch to supply the erectile tissues. The trabecular smooth muscles of the erectile tissue relax increasing blood flow to fill the vascular spaces, and expanding the erectile tissues until they are fully engorged with blood.[7] The ischiocavernosus and bulbocavernosus muscles contract, compressing the dorsal vein of the clitoris. This compression of the vein restricts drainage of the erectile structures, trapping the blood.[8] This process stretches the tunica albuginea. As a result, the clitoris becomes tumescent to accommodate the increased intracavernosous pressure. The tunica albuginea of the clitoris is made up of one layer making it more elastic than the tunica albuginea of the penis, which is composed of two layers.[9] Erick Janssen (2007) elaborates on this reporting that "the corpora cavernosa of the clitoris are essentially similar to that of the penis except that there is no subalbugineal layer interposed between the tunica albuginea and the erectile tissue. In the penis, this[10] tissue engorges with blood during sexual arousal and becomes compressed against the unyielding tunica, creating penile rigidity – a true erection. The lack of this plexus in the clitoris indicates that while the organ can become tumescent or engorged, it cannot, like the penis, become stiffly erect. The clitoris thus does not become erect with sexual excitement, but engorged."[10] In addition, the tunica albuginea around the glans is thinner than around the shaft in both the clitoris and penis. This gives the glans less firmness relative to the shaft. The extrusion of the glans clitoridis and thinning of the skin enhances sensitivity to physical contact. After a female has orgasmed, the erection usually ends, but this may take time.
Medical conditions
[edit]Clitoral priapism
[edit]Priapism, while more common in males, is a condition that can also affect the clitoris.[3] Symptoms include painful engorgement, swelling, and pain in the area around the clitoris.[11]
Other animals
[edit]Among capuchin monkeys, clitoral erection is possible and makes the clitoris more visible than in its relaxed state where it is hidden by a preputial fold.[12]
See also
[edit]Notes
[edit]- ^ Kirshblum, Steven; Lin, Vernon W. (2018). Spinal Cord Medicine, Third Edition. Springer Publishing Company. p. 413. ISBN 978-0-8261-3775-3. Retrieved 3 October 2023.
- ^ Hall, John (2016). Guyton and Hall Textbook of Medical Physiology. Elsevier. p. 1052. ISBN 978-1-4557-7005-2. Retrieved 3 October 2023.
- ^ a b Medina, Carlos A (1 November 2002). "Clitoral priapism: a rare condition presenting as a cause of vulvar pain". Obstetrics & Gynecology. 100 (5, Part 2): 1089–1091. doi:10.1016/S0029-7844(02)02084-7. ISSN 0029-7844. PMID 12423816. S2CID 20764733.
- ^ Gragasin, F. S., Michelakis, E. D., Hogan, A., Moudgil, R., Hashimoto, K., Wu, X., ... & Archer, S. L. (2004). The neurovascular mechanism of clitoral erection: Nitric oxide and cGMP‐stimulated activation of BKCa channels. The FASEB journal, 18(12), 1382-1391.
- ^ Jackson, Lindsey A.; Hare, Adam M.; Carrick, Kelley S.; Ramirez, Denise M. O.; Hamner, Jennifer J.; Corton, Marlene M. (1 November 2019). "Anatomy, histology, and nerve density of clitoris and associated structures: clinical applications to vulvar surgery". American Journal of Obstetrics and Gynecology. 221 (5): 519.e1–519.e9. doi:10.1016/j.ajog.2019.06.048. ISSN 0002-9378. PMID 31254525. S2CID 195758555.
- ^ Dean O'Loughlin, Valerie; Stouter Bidle, Theresa; McKinley, Michael P. (2022). "Muscular System: Axial and Appendicular Muscles". Anatomy and Physiology: An Integrative Approach (Fourth ed.). McGraw Hill. p. 395. ISBN 978-1-264-26541-1.
- ^ Bono, Christopher M.; Lin, Vernon W. (14 May 2014). Spinal Cord Medicine: Principles and Practice (2nd ed.). Demos Medical Publishing. p. 1176. ISBN 978-1-935281-77-1. Archived from the original on 28 February 2023. Retrieved 17 March 2015.
- ^ Hornstein, Theresa; Schwerin, Jeri (1 January 2012). Biology Of Women (5th ed.). Cengage Learning. pp. 62–63 of 816. ISBN 978-1-285-40102-7. Archived from the original on 28 February 2023. Retrieved 17 March 2015.
- ^ Goldstein, Irwin; Meston, Cindy M.; Davis, Susan; Traish, Abdulmaged (17 November 2005). Women's Sexual Function and Dysfunction:Study, Diagnosis, and Treatment. CRC Press. p. 176. ISBN 978-1-84214-263-9. Archived from the original on 28 February 2023. Retrieved 5 November 2020.
- ^ a b Jansen, Erick (27 September 2007). The Psychophysiology of Sex. Indiana University Press. p. 41. ISBN 978-0-253-11704-5. Archived from the original on 28 February 2023. Retrieved 29 March 2015.
- ^ Yafi, Faysal A.; April, Daniel; Powers, Mary K.; Sangkum, Premsant; Hellstrom, Wayne J. G. (July 2015). "Penile Priapism, Clitoral Priapism, and Persistent Genital Arousal Disorder: A Contemporary Review". Sexual Medicine Reviews. 3 (3): 145–159. doi:10.1002/smrj.51. ISSN 2050-0521. PMID 27784607.
- ^ Carosi, M, Spani, F, Ulland, AE, Scalici, M, Suomi, SJ. Clitoral length in immature and mature captive tufted capuchin (Sapajus spp.) females: A cross-sectional study. Am J Primatol. 2020; 82:e23135. https://doi.org/10.1002/ajp.23135
References
[edit]- Akkus, E. C.; Carrier, S.; Turzan, C.; Wang, T. N.; Lue, F. (April 1995). "Duplex ultrasonography after prostaglandin E1 injection of the clitoris in a case of hyperreactio luteinalis". The Journal of Urology. 153 (4): 1237–1238. doi:10.1016/S0022-5347(01)67566-9. ISSN 0022-5347. PMID 7869513.
- Gharahbaghian, L. (1 November 2008). "Clitoral priapism with no known risk factors". The Western Journal of Emergency Medicine. 9 (4): 235–237. ISSN 1936-900X. PMC 2672283. PMID 19561754.
- Gragasin, S.; Michelakis, D.; Hogan, A.; Moudgil, R.; Hashimoto, K.; Wu, X.; Bonnet, S.; Haromy, A.; Archer, L. (September 2004). "The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels" (Free full text). The FASEB Journal. 18 (12): 1382–1391. doi:10.1096/fj.04-1978com. ISSN 0892-6638. PMID 15333581. S2CID 45447939.
- Park, K. G.; Goldstein, I.; Andry, C.; Siroky, M. B.; Krane, R. J.; Azadzoi, K. M. (March 1997). "Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency". International Journal of Impotence Research. 9 (1): 27–37. doi:10.1038/sj.ijir.3900258. ISSN 0955-9930. PMID 9138056.
- Shen, W. U.; Urosevich, Z.; Clayton, D. O. (June 1999). "Sildenafil in the treatment of female sexual dysfunction induced by selective serotonin reuptake inhibitors". The Journal of Reproductive Medicine. 44 (6): 535–542. ISSN 0024-7758. PMID 10394548.
- Toesca, A. S.; Stolfi, V. M.; Cocchia, D. (1 June 1996). "Immunohistochemical study of the corpora cavernosa of the human clitoris". Journal of Anatomy. 188 (Pt 3): 513–520. ISSN 0021-8782. PMC 1167479. PMID 8763468.

