Recent from talks
Nothing was collected or created yet.
Indigo carmine
View on Wikipedia
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Disodium [2(2′)E]-3,3′-dioxo-1,1′,3,3′-tetrahydro[2,2′-biindolylidene]-5,5′-disulfonate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.011.572 |
| EC Number |
|
| E number | E132 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H8N2Na2O8S2 | |
| Molar mass | 466.36 g/mol |
| Appearance | purple solid |
| Melting point | >300 °C (572 °F) |
| 10 g/L (25 °C (77 °F)) | |
| Hazards | |
| GHS labelling: | |
 [1] [1]
| |
| Warning | |
| H302[1] | |
| NFPA 704 (fire diamond) | |
| Pharmacology | |
| V04CH02 (WHO) | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
 Container of indigotindisulfonate sodium for medical use | |
| Clinical data | |
|---|---|
| Trade names | Bludigo |
| License data | |
| Identifiers | |
| E number | E132 (colours) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.572 |
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by aromatic sulfonation, which renders the compound soluble in water. Like indigo, it produces a blue color, and is used in food and other consumables, cosmetics, and as a medical contrast agent and staining agent; it also acts as a pH indicator. It is approved for human consumption in the United States and European Union.[3][4] It has the E number E132, and is named Blue No. 2 by the US Federal Food, Drug, and Cosmetic Act.[5]
Uses
[edit]| Indigo Carmine (pH indicator) | ||
| below pH 11.4 | above pH 13.0 | |
| 11.4 | ⇌ | 13.0 |

Indigo carmine in a 0.2% aqueous solution is blue at pH 11.4 and yellow at 13.0. Indigo carmine is also a redox indicator, turning yellow upon reduction. Another use is as a dissolved ozone indicator[6] through the conversion to isatin-5-sulfonic acid.[6] This reaction has been shown not to be specific to ozone: it also detects superoxide, an important distinction in cell physiology.[7] It is also used as a dye in the manufacturing of pharmaceutical capsules.
Medical uses
[edit]Indigotindisulfonate sodium, sold under the brand name Bludigo, is used as a contrast agent during surgical procedures.[2] It is indicated for use in cystoscopy in adults following urological and gynecological procedures.[2][8] It was approved for medical use in the United States in July 2022.[specify][2][8]
In obstetric surgery, it may be used to detect amniotic fluid leaks. In urologic surgery, intravenous indigo carmine can be used to highlight portions of the urinary tract. The dye is filtered rapidly by the kidneys from the blood, and colors the urine blue. However, the dye can cause a potentially dangerous acute increase in blood pressure in some cases.[9]
Indigo carmine stain is not absorbed into cells, so it is applied to tissues to enhance the visibility of mucosa. This leads to its use for examination and diagnosis of benign and malignant lesions and growths on mucosal surfaces of the body.[10]
Food, pharmaceutical, cosmetic, and scientific uses
[edit]Indigo carmine is one of the few blue food colorants. Others include the anthocyanidins and rare substances such as variegatic acid and popolohuanone.[11]
Safety and regulation
[edit]This article is missing information about legal status outside US/EU and most usages. (August 2024) |
Indigo carmine shows "genotoxicity, developmental toxicity or modifications of haematological parameters in chronic toxicity studies". Only at 17 mg/kg of body weight per day were effects on testes observed.[12]
References
[edit]- ^ a b "Indigo carmine". Sigma Aldrich. Retrieved 15 Feb 2022.
- ^ a b c d "Bludigo- indigotindisulfonate sodium injection". DailyMed. 7 November 2022. Retrieved 21 January 2023.
- ^ Summary of Color Additives for Use in United States in Foods, Drugs, Cosmetics, and Medical Devices, Food and Drug Administration
- ^ Current EU approved additives and their E Numbers, Food Standards Agency, 26 November 2010
- ^ "Regulatory Status of Color Additives: FD&C Blue No. 2". U.S. Department of Health and Human Services. Retrieved 29 April 2025.
- ^ a b Takeuchi K, Ibusuki T (March 1989). "Quantitative determination of aqueous-phase ozone by chemiluminescence using indigo-5,5'-disulfonate". Analytical Chemistry. 61 (6): 619–623. doi:10.1021/ac00181a025. PMID 2729594.
- ^ Kettle AJ, Clark BM, Winterbourn CC (April 2004). "Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone". The Journal of Biological Chemistry. 279 (18): 18521–18525. doi:10.1074/jbc.M400334200. PMID 14978029.
- ^ a b "NDA APPROVAL: Bludigo (indigotindisulfonate sodium) injection" (PDF). U.S. Food and Drug Administration. Archived from the original (PDF) on July 12, 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Craik JD, Khan D, Afifi R (January–February 2009). "The Safety of Intravenous Indigo Carmine to Assess Ureteric Patency During Transvaginal Uterosacral Suspension of the Vaginal Vault". Journal of Pelvic Medicine & Surgery. 15 (1): 11–15. doi:10.1097/SPV.0b013e3181986ace.
- ^ Jang JY (November 2015). "The Past, Present, and Future of Image-Enhanced Endoscopy". Clinical Endoscopy. 48 (6): 466–475. doi:10.5946/ce.2015.48.6.466. PMC 4676674. PMID 26668791.
- ^ Newsome AG, Culver CA, van Breemen RB (July 2014). "Nature's palette: the search for natural blue colorants". Journal of Agricultural and Food Chemistry. 62 (28): 6498–6511. Bibcode:2014JAFC...62.6498N. doi:10.1021/jf501419q. PMID 24930897.
- ^ Amchova P, Kotolova H, Ruda-Kucerova J (December 2015). "Health safety issues of synthetic food colorants". Regulatory Toxicology and Pharmacology. 73 (3): 914–922. doi:10.1016/j.yrtph.2015.09.026. PMID 26404013.
Indigo carmine
View on GrokipediaChemical properties
Molecular structure
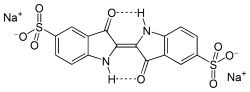
Indigo carmine is the disodium salt of indigo-5,5'-disulfonic acid, possessing the chemical formula CHNNaOS.[8] Its molecular weight is 466.35 g/mol.[9] The IUPAC name is disodium (2E)-3-oxo-2-(3-oxo-5-sulfonato-1H-indol-2-ylidene)-1H-indole-5-sulfonate.[8] The molecular structure consists of two indolinone (or indole-2,3-dione) rings linked by a central carbon-carbon double bond at their 2-positions, with keto groups at the 3-positions of each ring and sulfonate groups (-SONa) attached to the 5-positions of the benzene portions of the rings.[8] This arrangement forms a planar, conjugated system responsible for its characteristic blue color in solution, where the double bond conjugation between the rings and the electron-withdrawing sulfonate groups stabilize the chromophore.[5] Indigo carmine serves as a synthetic analog of natural indigo, a vat dye extracted from plants such as Indigofera tinctoria, but it is produced through sulfonation of indigo at the 5 and 5' positions, which introduces the sulfonic acid groups to improve water solubility while retaining the core bis-indole framework.[4]Physical characteristics
Indigo carmine is typically observed as a deep blue to dark purple powder or crystalline solid.[1][10] When dissolved in water, it produces a vibrant blue solution characteristic of its use in various applications.[11] The compound exhibits high solubility in water, approximately 10 g/L at 25°C, owing to the sulfonate groups enhancing its hydrophilic nature, while it is slightly soluble in ethanol, insoluble in acetone and most other organic solvents.[12] Its density is approximately 0.71 g/cm³ (bulk density at 29°C).[10] Indigo carmine decomposes at temperatures above 300°C without undergoing melting.[10] In terms of pH stability, indigo carmine remains stable and retains its blue color in acidic to neutral solutions (pH 4-7).[11] However, in strong alkaline conditions, its color fades or shifts. It functions as a pH indicator, transitioning from blue to yellow over the range of pH 11.4-14.[8] Optically, it absorbs light maximally at around 610 nm, accounting for its distinctive blue hue.[13]Chemical reactivity
Indigo carmine demonstrates significant stability under neutral conditions, where it resists oxidation effectively. However, it is susceptible to reduction by agents such as sodium dithionite, converting to its colorless leuco form through a two-step process involving direct interaction with the reductant.[14] This transformation highlights its redox properties, featuring a reversible cycle between the oxidized blue form and the reduced yellowish leuco form, which underpins its role as a redox indicator in analytical chemistry.[1] The compound exhibits poor lightfastness, undergoing photodegradation under UV exposure that leads to gradual color loss and structural breakdown.[11] Aqueous solutions of indigo carmine also fade upon prolonged standing in light, emphasizing its sensitivity to photolytic processes.[1] Due to its two sulfonic acid groups, indigo carmine behaves as a strong acid in its protonated form, though it is typically used as the disodium salt. It functions as a pH indicator with a color transition from blue at pH 11.5 to yellow at pH 14.0, corresponding to a pKa of approximately 12.8 for the relevant deprotonation event. Additionally, indigo carmine is incompatible with strong oxidizing agents like hydrogen peroxide or hypochlorite, which induce decomposition and discharge its color.[1]Production
Synthesis methods
Indigo carmine, also known as disodium 5,5'-indigotindisulfonate, is primarily synthesized through the sulfonation of indigo, a process that introduces two sulfonic acid groups at the 5 and 5' positions of the indigo molecule. The classical method employs fuming sulfuric acid (oleum) as the sulfonating agent, where indigo is heated in oleum at temperatures ranging from 80 to 100°C for several hours to form indigo disulfonic acid. This reaction leverages the electrophilic aromatic substitution facilitated by the excess sulfur trioxide in oleum, targeting the electron-rich positions on the indigo structure. Following sulfonation, the reaction mixture is diluted with water to precipitate unreacted indigo, and the disulfonic acid is isolated before neutralization with sodium hydroxide to yield the water-soluble disodium salt.[3][15] The key reaction steps can be summarized as follows:- Sulfonation: Indigo reacts with sulfuric acid to produce indigo disulfonic acid.
- Neutralization: The disulfonic acid is treated with sodium hydroxide to form the disodium salt.