Recent from talks
Nothing was collected or created yet.
Guenon
View on Wikipedia
| Guenons[1] | |
|---|---|

| |
| Diana monkey (C. diana) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Suborder: | Haplorhini |
| Infraorder: | Simiiformes |
| Family: | Cercopithecidae |
| Subfamily: | Cercopithecinae |
| Tribe: | Cercopithecini |
| Genus: | Cercopithecus Linnaeus, 1758 |
| Type species | |
| Simia diana | |
| Species | |
|
See text | |
The guenons (UK: /ɡəˈnɒnz/, US: /ˈɡwɛn.ənz/) are Old World monkeys of the genus Cercopithecus (/ˌsɜːrkəˈpɪθəkəs/). Not all members of this genus have the word "guenon" in their common names; also, because of changes in scientific classification, some monkeys in other genera may have common names that include the word "guenon". Nonetheless, the use of the term guenon for monkeys of this genus is widely accepted.[citation needed]
All members of the genus are endemic to sub-Saharan Africa, and most are forest monkeys. Many of the species are quite local in their ranges, and some have even more local subspecies. Many are threatened or endangered because of habitat loss. The species currently placed in the genus Chlorocebus, such as vervet monkeys and green monkeys, were formerly considered as a single species in this genus, Cercopithecus aethiops.
In the English language, the word "guenon" is apparently of French origin.[2] In French, guenon was the common name for all species and individuals, both males and females, from the genus Cercopithecus. In all other monkey and apes species, the French word guenon designates only the females.[3] The three species such as the L'hoest's monkey, Preuss's monkey and the sun-tailed monkey were formerly included in the genus and now listed in a different genus Allochrocebus[1][4][5]
Classification
[edit]The genus name Cercopithecus comes from Ancient Greek κέρκος (kérkos), meaning "tail", and πίθηκος (píthēkos), meaning "ape". It was named by Carl Linnaeus in 1758.
Species list
[edit]| Common name | Scientific name and subspecies | Range | Size and ecology | IUCN status and estimated population |
|---|---|---|---|---|
| Blue monkey | C. mitis Wolf, 1822 Sixteen subspecies
|
Sub-Saharan Africa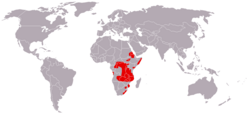
|
Size: 31–70 cm (12–28 in) long, plus 55–109 cm (22–43 in) tail[6] Habitat: Forest[7] Diet: Fruit and leaves, as well as invertebrates[8] |
LC
|
| Campbell's mona monkey | C. campbelli Waterhouse, 1838 |
Western Africa
|
Size: 36–55 cm (14–22 in) long, plus 49–85 cm (19–33 in) tail[9] Habitat: Forest, savanna, and shrubland[10] Diet: Fruit, leaves, seeds and grains, as well as birds, bird eggs, small reptiles, and insects[9] |
NT
|
| Crested mona monkey | C. pogonias Bennett, 1833 Three subspecies
|
Central Africa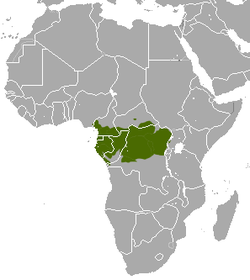
|
Size: 34–55 cm (13–22 in) long, plus 48–87 cm (19–34 in) tail[11] Habitat: Forest[12] Diet: Fruit and seeds, as well as leaves, flowers and insects[12] |
NT
|
| De Brazza's monkey | C. neglectus Schlegel, 1876 |
Central Africa
|
Size: 39–60 cm (15–24 in) long, plus 47–79 cm (19–31 in) tail[13] Habitat: Inland wetlands and forest[14] Diet: Fruit, as well as leaves, flowers, mushrooms, beetles, termites, and worms[15] |
LC
|
| Dent's mona monkey | C. denti Thomas, 1907 |
Central Africa
|
Size: 40–70 cm (16–28 in) long, plus 70–90 cm (28–35 in) tail[16] Habitat: Forest[17] Diet: Fruit and arthropods, as well as flowers, caterpillars, shoots, and leaves[17] |
LC
|
| Diana monkey | C. diana (Linnaeus, 1758) |
Western Africa
|
Size: 40–55 cm (16–22 in) long, plus 50–75 cm (20–30 in) tail[18] Habitat: Forest[19] Diet: Fruit, flowers, leaves, insects, and other invertebrates[18] |
EN
|
| Greater spot-nosed monkey | C. nictitans (Linnaeus, 1766) Five subspecies
|
Western Africa
|
Size: 40–57 cm (16–22 in) long, plus 56–100 cm (22–39 in) tail[20] Habitat: Forest[21] Diet: Fruits and seeds, as well as leaves and insects[22] |
NT
|
| Hamlyn's monkey | C. hamlyni Pocock, 1907 Two subspecies
|
Central Africa
|
Size: 43–63 cm (17–25 in) long, plus 49–63 cm (19–25 in) tail[23] Habitat: Forest[24] Diet: Shoots, leaves, plants, and herbs, as well as fruit and seeds[25] |
VU
|
| Lesser spot-nosed monkey | C. petaurista (Schreber, 1774) Two subspecies
|
Western Africa
|
Size: 29–53 cm (11–21 in) long, plus 57–78 cm (22–31 in) tail[26] Habitat: Forest[27] Diet: Fruit as well as insects[26] |
NT
|
| Lesula | C. lomamiensis Hart et al., 2012 |
Central Africa
|
Size: 40–65 cm (16–26 in) long, plus 40–65 cm (16–26 in) tail[28] Habitat: Forest[29] Diet: Leaves, fruits and flowers[30] |
VU
|
| Lowe's mona monkey | C. lowei Thomas, 1923 |
Western Africa (in green)
|
Size: 36–55 cm (14–22 in) long, plus 54–85 cm (21–33 in) tail[31] Habitat: Forest and savanna[32] Diet: Fruit and insects[31] |
VU
|
| Mona monkey | C. mona (Schreber, 1774) |
Western Africa
|
Size: 32–53 cm (13–21 in) long, plus 67–90 cm (26–35 in) tail[33] Habitat: Forest[34] Diet: Fruit, sprouts, leaves, and invertebrates[33] |
NT
|
| Moustached guenon | C. cephus (Linnaeus, 1758) Three subspecies
|
Western Africa
|
Size: 44–60 cm (17–24 in) long, plus 66–99 cm (26–39 in) tail[35] Habitat: Forest[36] Diet: Fruit, as well as seeds, leaves, insects, and eggs[37] |
LC
|
| Red-eared guenon | C. erythrotis Waterhouse, 1838 Two subspecies
|
Western Africa
|
Size: 36–55 cm (14–22 in) long, plus 46–77 cm (18–30 in) tail[38] Habitat: Forest[39] Diet: Fruit, as well as leaves, shoots and arthropods[39] |
VU
|
| Red-tailed monkey | C. ascanius (Audebert, 1799) Five subspecies
|
Central Africa
|
Size: 34–55 cm (13–22 in) long, plus 67–92 cm (26–36 in) tail[40] Habitat: Forest[41] Diet: Fruit, as well as leaves, insects, flowers, buds, and tree gum[42] |
LC
|
| Roloway monkey | C. roloway (Schreber, 1774) |
Western Africa
|
Size: 44–62 cm (17–24 in) long, plus 70–91 cm (28–36 in) tail[43] Habitat: Forest[44] Diet: Insects, as well as seeds, fruit, and leaves[43] |
CR
|
| Sclater's guenon | C. sclateri Pocock, 1904 |
Western Africa
|
Size: 32–38 cm (13–15 in) long, plus 61–85 cm (24–33 in) tail[45] Habitat: Forest[46] Diet: Fruit, as well as insects, flowers and leaves[47] |
EN
|
| White-throated guenon | C. erythrogaster Gray, 1866 Two subspecies
|
Western Africa
|
Size: 38–46 cm (15–18 in) long, plus 58–70 cm (23–28 in) tail[26] Habitat: Forest and inland wetlands[48] Diet: Fruit[48] |
EN
|
| Wolf's mona monkey | C. wolfi Meyer, 1891 Three subspecies
|
Central Africa | Size: 44–52 cm (17–20 in) long, plus 69–83 cm (27–33 in) tail[49] Habitat: Forest[50] Diet: Fruit, leaves, seeds, and flowers[49] |
NT
|
Hybrids
[edit]The red-tailed monkey (Cercopithecus ascanius) is known to hybridize with the blue monkey (C. mitis) in several locations in the wild in Africa.[51]
References
[edit]- ^ a b Groves, C. P. (2005). "GENUS Cercopithecus". In Wilson, D. E.; Reeder, D. M (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. pp. 154–158. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ^ guenon /gəˈnoʊn/ n. M19. [Fr., of uncertain origin.] (The New Shorter Oxford English Dictionary, Clarendon Press, Oxford, Vol. 1 A-M, 1993 edition, see page 1,157)
- ^ guenon [gənɔ̃] n. f. - 1505; o. i.; p.-ê même rad. que guenille 1. vx Cercopithèque, mâle ou femelle. 2. MOD. Singe femelle. [...] (Le Petit Robert, grand format, Dictionnaires Le Robert, Paris, first edition: 1967, Nouveau Petit Robert edition: 1993, grand format edition: 1996, ISBN 2-85036-469-X, see page 1,056)
- ^ "Allochrocebus". ITIS. Retrieved 2019-07-18.
- ^ "Allochrocebus". Mammal Diversity Database. Retrieved 2019-07-18.
- ^ Kingdon 2015, p. 175
- ^ a b Butynski, T. M.; de Jong, Y. A. (2021) [errata version of 2019 assessment]. "Cercopithecus mitis". IUCN Red List of Threatened Species. 2019 e.T4221A196007901. doi:10.2305/IUCN.UK.2019-3.RLTS.T4221A196007901.en.
- ^ Strawder, Nicole (2001). "Cercopithecus mitis". Animal Diversity Web. University of Michigan. Archived from the original on August 12, 2023. Retrieved July 24, 2023.
- ^ a b Leinberger, Kaitlynn (2022). "Cercopithecus campbelli". Animal Diversity Web. University of Michigan. Archived from the original on August 12, 2023. Retrieved July 24, 2023.
- ^ a b Matsuda Goodwin, R.; Gonedelé Bi, S.; Koné, I. (2020). "Cercopithecus campbelli". IUCN Red List of Threatened Species. 2020 e.T136930A92374066. doi:10.2305/IUCN.UK.2020-2.RLTS.T136930A92374066.en.
- ^ Kingdon 2015, p. 168
- ^ a b c Maisels, F.; Cronin, D. T.; Hart, J.; Etiendem, D.; Oates, J. F.; Butynski, T. M.; Linder, J. (2021) [errata version of 2020 assessment]. "Cercopithecus pogonias". IUCN Red List of Threatened Species. 2020 e.T92411527A197301301. doi:10.2305/IUCN.UK.2020-1.RLTS.T92411527A197301301.en.
- ^ Kingdon 2015, p. 161
- ^ a b Mwenja, I.; Maisels, F.; Hart, J. A. (2019). "Cercopithecus neglectus". IUCN Red List of Threatened Species. 2019 e.T4223A17947167. doi:10.2305/IUCN.UK.2019-3.RLTS.T4223A17947167.en.
- ^ Stein, Joshua (2002). "Cercopithecus neglectus". Animal Diversity Web. University of Michigan. Archived from the original on June 26, 2023. Retrieved August 16, 2023.
- ^ Kingdon 2015, p. 166
- ^ a b c Detwiler, K. M.; Hart, J. A.; Hicks, T. C. (2020). "Cercopithecus denti". IUCN Red List of Threatened Species. 2020 e.T136885A92413658. doi:10.2305/IUCN.UK.2020-2.RLTS.T136885A92413658.en.
- ^ a b Kennedy, Karen (2023). "Cercopithecus diana". Animal Diversity Web. University of Michigan. Archived from the original on March 6, 2016. Retrieved July 24, 2023.
- ^ a b Koné, I.; McGraw, S.; Gonedelé Bi, S.; Oates, J. F. (2019). "Cercopithecus diana". IUCN Red List of Threatened Species. 2019 e.T4245A92384250. doi:10.2305/IUCN.UK.2019-3.RLTS.T4245A92384250.en.
- ^ Kingdon 2015, p. 174
- ^ a b Cronin, D. T.; Maisels, F.; Gadsby, E. L.; Gonedelé Bi, S.; Ikemeh, R.; Imong, I. (2022) [errata version of 2020 assessment]. "Cercopithecus nictitans". IUCN Red List of Threatened Species. 2020 e.T4224A222904443. doi:10.2305/IUCN.UK.2020-1.RLTS.T4224A222904443.en.
- ^ Neinast, Alexandra (2012). "Cercopithecus nictitans". Animal Diversity Web. University of Michigan. Archived from the original on July 26, 2023. Retrieved August 16, 2023.
- ^ Kingdon 2015, p. 170
- ^ a b Hart, J.; Maisels, F. (2020) [amended version of 2019 assessment]. "Cercopithecus hamlyni". IUCN Red List of Threatened Species. 2020 e.T4219A166615690. doi:10.2305/IUCN.UK.2020-1.RLTS.T4219A166615690.en.
- ^ Bharti, Nita (2000). "Cercopithecus hamlyni". Animal Diversity Web. University of Michigan. Archived from the original on July 24, 2023. Retrieved July 24, 2023.
- ^ a b c Kingdon 2015, p. 179
- ^ a b Matsuda Goodwin, R.; Segniagbeto, G.; Wiafe, E.; Osei, D.; Koné, I.; Gonedelé Bi, S.; Oates, J. F. (2020). "Cercopithecus petaurista". IUCN Red List of Threatened Species. 2020 e.T4225A17945536. doi:10.2305/IUCN.UK.2020-2.RLTS.T4225A17945536.en.
- ^ Kingdon 2015, p. 171
- ^ a b Detwiler, K. M.; Hart, J. A. (2020). "Cercopithecus lomamiensis". IUCN Red List of Threatened Species. 2020 e.T92401376A92401776. doi:10.2305/IUCN.UK.2020-2.RLTS.T92401376A92401776.en.
- ^ Antosh, Bonnie (2013). "Cercopithecus lomamiensis". Animal Diversity Web. University of Michigan. Archived from the original on August 16, 2023. Retrieved August 16, 2023.
- ^ a b Kingdon 2015, p. 165
- ^ a b Wiafe, E.; Oates, J. F.; Gonedelé Bi, S.; Koné, I.; Matsuda Goodwin, R.; Osei, D. (2019). "Cercopithecus lowei". IUCN Red List of Threatened Species. 2019 e.T136931A92373680. doi:10.2305/IUCN.UK.2019-1.RLTS.T136931A92373680.en.
- ^ a b Liu, Sonia (2000). "Cercopithecus mona". Animal Diversity Web. University of Michigan. Archived from the original on February 20, 2023. Retrieved July 24, 2023.
- ^ a b Matsuda Goodwin, R.; Segniagbeto, G.; Nobimè, G.; Imong, I. (2020). "Cercopithecus mona". IUCN Red List of Threatened Species. 2020 e.T4222A17946672. doi:10.2305/IUCN.UK.2020-2.RLTS.T4222A17946672.en.
- ^ Kingdon 2015, p. 181
- ^ a b Abernethy, K.; Maisels, F. (2020) [amended version of 2019 assessment]. "Cercopithecus cephus". IUCN Red List of Threatened Species. 2020 e.T4214A166614362. doi:10.2305/IUCN.UK.2020-1.RLTS.T4214A166614362.en.
- ^ Miretti, Juan (2006). "Cercopithecus cephus". Animal Diversity Web. University of Michigan. Archived from the original on July 24, 2023. Retrieved July 24, 2023.
- ^ Kingdon 2015, p. 182
- ^ a b c Hofner, A.; Cronin, D. T.; Imong, I.; Gadsby, E. L.; Ndeloh, D. (2020). "Cercopithecus erythrotis". IUCN Red List of Threatened Species. 2020 e.T4218A17946043. doi:10.2305/IUCN.UK.2020-2.RLTS.T4218A17946043.en.
- ^ Kingdon 2015, p. 183
- ^ a b de Jong, Y. A.; Butynski, T. M. (2019). "Cercopithecus ascanius". IUCN Red List of Threatened Species. 2019 e.T4212A17947340. doi:10.2305/IUCN.UK.2019-3.RLTS.T4212A17947340.en.
- ^ Davis, Sarah (2002). "Cercopithecus ascanius". Animal Diversity Web. University of Michigan. Archived from the original on April 1, 2023. Retrieved July 24, 2023.
- ^ a b Johnson, Kelsey (2015). "Cercopithecus roloway". Animal Diversity Web. University of Michigan. Archived from the original on June 18, 2021. Retrieved July 24, 2023.
- ^ a b Koné, I.; Oates, J. F.; Dempsey, A.; Gonedelé Bi, S.; McGraw, S.; Wiafe, E. (2019). "Cercopithecus roloway". IUCN Red List of Threatened Species. 2019 e.T4232A92384429. doi:10.2305/IUCN.UK.2019-1.RLTS.T4232A92384429.en.
- ^ Kingdon 2015, p. 180
- ^ a b Baker, L.; Oates, J. F.; Ikemeh, R.; Gadsby, E. (2019). "Cercopithecus sclateri". IUCN Red List of Threatened Species. 2019 e.T4229A17945814. doi:10.2305/IUCN.UK.2019-1.RLTS.T4229A17945814.en.
- ^ Law, Jason (2004). "Cercopithecus sclateri". Animal Diversity Web. University of Michigan. Archived from the original on July 26, 2023. Retrieved August 16, 2023.
- ^ a b c Matsuda Goodwin, R.; Oates, J. F.; Nobimè, G.; Segniagbeto, G. H.; Ikemeh, R.; Mittermeier, R. A. (2020). "Cercopithecus erythrogaster". IUCN Red List of Threatened Species. 2020 e.T4217A17946182. doi:10.2305/IUCN.UK.2020-2.RLTS.T4217A17946182.en.
- ^ a b Platter, Branden (2008). "Cercopithecus wolfi". Animal Diversity Web. University of Michigan. Archived from the original on July 24, 2023. Retrieved July 24, 2023.
- ^ a b Hart, J. A.; Detwiler, K. M.; Maisels, F. (2020) [amended version of 2019 assessment]. "Cercopithecus wolfi". IUCN Red List of Threatened Species. 2020 e.T92466239A166601223. doi:10.2305/IUCN.UK.2020-1.RLTS.T92466239A166601223.en.
- ^ Rowe, N. (1996). The Pictorial Guide to the Living Primates. Pogonias Press. pp. 139, 143, 154, 185, 223. ISBN 0-9648825-0-7.
Sources
[edit]- Kingdon, Jonathan (2015). The Kingdon Field Guide to African Mammals (Second ed.). Bloomsbury Publishing. ISBN 978-1-4729-2531-2.
External links
[edit]Guenon
View on GrokipediaTaxonomy
Classification
Guenons are classified within the kingdom Animalia, phylum Chordata, class Mammalia, order Primates, family Cercopithecidae, subfamily Cercopithecinae, tribe Cercopithecini, and genus Cercopithecus (Linnaeus, 1758).[2] The type species for the genus is Simia diana (now Cercopithecus diana), designated by Stiles and Orleman in 1926.[2] Phylogenetically, guenons belong to the Old World monkeys and are closely related to other cercopithecines, with the tribe Cercopithecini diverging approximately 12.3 million years ago.[3] Molecular studies, including mitochondrial and nuclear DNA analyses, generally support the monophyly of the core Cercopithecus genus for arboreal species, though mitochondrial data indicate paraphyly due to nesting of terrestrial genera like Erythrocebus within it.[3] However, nuclear markers confirm monophyly, suggesting incongruences may arise from hybridization or incomplete lineage sorting, while species like those in Chlorocebus (vervets) have been separated into a distinct genus based on genetic evidence.[3][2] Historical taxonomic revisions have refined the genus boundaries, excluding certain species previously included in Cercopithecus. For instance, Allen's swamp monkey (Allenopithecus nigroviridis) was reclassified into its own genus Allenopithecus due to distinct morphological and genetic traits, such as periodic perineal swelling.[2][4] Similarly, the sun-tailed guenon (Allochrocebus solatus), along with L'Hoest's monkey (A. lhoesti) and Preuss's monkey (A. preussi), has been moved to the genus Allochrocebus following phylogenetic analyses that highlight their terrestrial adaptations and closer relations to other non-Cercopithecus cercopithecines.[5][6] These changes, informed by works like Groves (1989) and Grubb et al. (2003), emphasize the rapid radiation and adaptive diversity within the tribe.[2][3]Etymology and nomenclature
The term "guenon" derives from the French word guenon, which specifically denotes a female monkey or ape.[7] Borrowed into English in the 19th century, it came to refer collectively to the diverse species within the primate genus Cercopithecus, emphasizing their arboreal and often colorful nature in sub-Saharan African forests.[8] The precise origin of the French guenon remains obscure, though some linguistic analyses link it to earlier terms like guenipe (a dirty scrap of cloth), potentially alluding to the monkey's trailing tail or unkempt appearance.[9] The scientific genus name Cercopithecus was coined by Carl Linnaeus in his 1758 Systema Naturae, encompassing slender, long-tailed Old World monkeys.[10] Its etymology traces to Ancient Greek kerkopíthēkos, combining kérkos ("tail") and píthēkos ("ape" or "monkey"), thus literally meaning "long-tailed ape" to highlight the prominent tails characteristic of these primates.[11] Historically, Linnaeus's initial classification applied Cercopithecus broadly to various African cercopithecine monkeys, but subsequent taxonomic refinements in the 19th and 20th centuries narrowed it to exclude groups like the talapoins (Miopithecus) and savanna monkeys (Chlorocebus), based on morphological and later genetic distinctions.[12] In French nomenclature, guenon originally served as a general common name for all Cercopithecus individuals irrespective of sex during early European descriptions of African fauna, though modern French usage typically limits it to females of non-Cercopithecus monkey species.[13] Common names for Cercopithecus species often incorporate descriptive, regional, or eponymous elements that evoke their appearance or discovery context. For instance, the mona monkey (C. mona) derives its name from a term rooted in Moorish or Portuguese influences, possibly referencing the species' long tail rather than vocalizations, as popularly misconstrued.[14] Similarly, Campbell's guenon (C. campbelli) honors the 19th-century British naturalist Henry Dundas Campbell, who contributed to early documentation of West African primates, while de Brazza's guenon (C. neglectus) commemorates the Italian-French explorer Pierre Savorgnan de Brazza for his role in central African expeditions.[15] These names reflect a blend of local linguistic traditions and colonial-era explorations that shaped European understanding of guenon diversity.Physical characteristics
Morphology and size
Guenons, members of the genus Cercopithecus, exhibit a range of body sizes typical of medium-sized Old World monkeys, with adult males generally measuring 40–70 cm in head-body length and weighing 3–9 kg, while females are notably smaller, often about half the weight of males at 1.5–5 kg. Tail length typically spans 50–75 cm, exceeding the head-body length in most species and serving primarily for balance during locomotion. These dimensions vary across the 24-26 recognized species, with larger forms like De Brazza's guenon (C. neglectus) reaching up to 7 kg in males, highlighting the genus's diversity in scale.[1] Structurally, guenons possess adaptations suited to their predominantly arboreal lifestyle, including elongated limbs that facilitate quadrupedal movement and leaping through forest canopies. Their tails are nonprehensile, lacking the gripping capability seen in some New World monkeys but aiding in postural stability. The skull is rounded with a short face, and the dental formula is the standard for cercopithecids: 2.1.2.3 (upper and lower), consisting of 32 teeth adapted for a frugivorous and folivorous diet. Ischial callosities—hardened, hairless pads on the buttocks—provide cushioning for prolonged sitting on branches.[16] Across the genus, guenons typically display a slender, graceful build optimized for agility in trees, though variations exist; for instance, De Brazza's guenon has a stockier form compared to the more lithe species like the moustached guenon (C. cephus). Sexual dimorphism is pronounced in size, with males consistently larger than females, a trait that influences social dynamics but is evident in morphological measurements from the outset of adulthood. These features underscore the genus's evolutionary success in diverse African habitats.[17]Coloration and sexual dimorphism
Guenons exhibit highly diverse pelage coloration across species, typically ranging from subdued olive-gray or dark brown tones on the body to more vibrant accents such as reddish-brown saddles on the lower back or bright blue hues on the limbs and rumps in certain taxa like the blue monkey (Cercopithecus mitis).[18] This variation in fur patterns provides effective camouflage against the dappled light and foliage of their arboreal forest environments. Facial features are particularly species-specific and conspicuous, often including colorful skin patches (e.g., blue, red, or orange), elongated beards, crests, and brow bands—such as the white, crescent-shaped bands in the Diana monkey (Cercopithecus diana)—which enhance visual distinctiveness among sympatric species.[19][20] Sexual dimorphism in coloration and markings is generally subtle within guenon species, with males and females sharing similar overall pelage and facial patterns in most cases, unlike the pronounced size differences where males are typically 30–50% larger.[21] However, males may display slightly brighter or more extensive markings in select species to facilitate display during mating, while females tend toward duller tones; for instance, the white chest patch in the Diana monkey is present in both sexes but appears more prominent in adult males.[22] Analyses of facial patterns across 22 guenon species confirm minimal sexual dichromatism, with no reliable differences in hue or structure between sexes that would impede pooling data for species-level comparisons.[19] The adaptive significance of guenon coloration balances crypsis and communication: earthy body tones reduce detection by predators in dense understory habitats, whereas bold facial elements serve as social signals for intraspecific recognition and coordination, particularly in multispecies assemblages where character displacement amplifies differences to prevent hybridization.[19] Additionally, some populations display pelage color polymorphism, as observed in samango monkeys (C. mitis) with distinct morphs (e.g., black, gray, or reddish variants) that may confer advantages in varying microhabitats or enhance individual fitness through disruptive coloration.[23]Distribution and habitat
Geographic range
Guenons of the genus Cercopithecus are endemic to sub-Saharan Africa, with their collective range spanning from western regions including Senegal and Guinea to eastern areas reaching Ethiopia and Tanzania, and extending southward into Angola and Zambia.[24] This distribution covers much of the continent's tropical and subtropical zones but excludes arid desert areas like the Sahara to the north and the extreme southern Cape region.[24] The greatest species diversity occurs in key hotspots such as the Upper and Lower Guinean forests of West Africa and the Congo Basin in Central Africa, where ancestral lineages originated and radiated.[24] However, many species exhibit fragmented distributions today, resulting from extensive habitat destruction and conversion for agriculture and logging, which have isolated populations across their former continuous ranges.[24] Historically, guenon ranges were more expansive and interconnected, particularly in forested refugia during Pleistocene climatic fluctuations, prior to accelerated human impacts from pre-colonial expansion through modern deforestation.[24] Current estimates indicate that while the overall extent of occurrence for the genus remains broad across sub-Saharan Africa, viable habitat patches for many species have contracted significantly due to these pressures.Habitat preferences and adaptations
Guenons (genus Cercopithecus) predominantly occupy forested habitats across sub-Saharan Africa, favoring environments that support their arboreal lifestyle. Primary and secondary rainforests form the core of their preferred habitats, providing dense canopies for movement and foraging, while gallery forests along watercourses and mangroves in coastal or riverine areas offer additional suitable niches for certain species, such as the roloway guenon (C. diana roloway) in the Tanoé forest of Ivory Coast. Some guenons, including De Brazza's guenon (C. neglectus), extend into swampy wetlands and seasonally flooded savanna woodlands, where proximity to water enhances their access to resources.[25][26][27] These monkeys exhibit remarkable physiological and behavioral adaptations to their forested environments, emphasizing their arboreal nature. With strong limbs and prehensile tails, guenons demonstrate superior climbing prowess, allowing them to traverse vertical trunks and leap between branches in the forest canopy, often at heights of 10–30 meters. They display flexibility in tolerating disturbed or secondary forests, where human-modified landscapes still provide sufficient cover, but severe habitat fragmentation poses challenges by limiting dispersal and increasing isolation risks. Altitudinally, guenons range from sea level to elevations up to 3,800 meters, as seen in the blue monkey (C. mitis) in the Rwenzori Mountains of Uganda, adapting to cooler montane conditions through denser fur and adjusted activity patterns.[25][25][25] Ecologically, guenons contribute significantly to forest dynamics as seed dispersers and insect population regulators. Their frugivorous habits, consuming a variety of fruits, facilitate seed transport over distances of up to several hundred meters, promoting plant regeneration in rainforests, as documented in studies of guenons including the samango monkey (C. mitis) in Afromontane forests.[28][29] Additionally, their omnivorous diet includes substantial insectivory, helping control arthropod populations and maintaining balance in forest understories.[25]Behavior and ecology
Social structure and groups
Guenons typically form multi-female, one-male social units consisting of 5 to 30 individuals, including a single adult male, several adult females, and their dependent offspring, which serve as the core social structure for most species in the genus Cercopithecus.[30] These groups are often supplemented by all-male bachelor troops comprising juvenile or subadult males that have dispersed from natal units and await opportunities to challenge resident males for group leadership.[31] Some species, such as the blue monkey (C. mitis), exhibit fission-fusion dynamics, where subgroups temporarily split and reform based on resource availability or social affiliations, allowing flexibility in group cohesion while maintaining overall stability.[32] Social dynamics within guenon groups are characterized by female philopatry, where females remain in their natal groups for life, forming the stable core of social bonds, while males disperse at maturity to reduce inbreeding and competition.[30] Dominance hierarchies are prevalent, particularly among females, which are often linear and influenced by age, body size, and matrilineal kinship, though these ranks do not always correlate strongly with fitness outcomes like survival or reproduction in species like the blue monkey.[33] Intergroup interactions frequently involve conflicts over territorial resources, with resident males employing loud vocalizations and displays to deter intruders, escalating to physical aggression when necessary.[34] Variations in social structure exist across guenon species; for instance, De Brazza's guenon (C. neglectus) forms smaller groups of 5 to 16 individuals with a single adult male and fewer females, and solitary adult males are commonly observed outside these units, reflecting a more dispersed and less cohesive organization compared to other congeners.[35] Cooperative behaviors, such as allogrooming, play a key role in maintaining intragroup harmony and reinforcing affiliative ties, particularly among females in species like the red-tailed guenon (C. ascanius) and spot-nosed guenon (C. petaurista), where grooming follows patterns of reciprocity and hierarchy.[36] These interactions, often involving tactile contact and vocal communication, underscore the emphasis on female-bonded relationships in guenon societies.[37]Diet, foraging, and predation
Guenons are primarily frugivorous, with fruit constituting 40-60% of their caloric intake across species.[38] They exhibit folivorous tendencies, consuming leaves as fallback foods when fruit availability declines seasonally.[39] Insectivory supplements their diet, with insects comprising up to 25% in some populations such as the Diana guenon (C. diana), providing essential proteins and fats, while opportunistic omnivory includes seeds, flowers, exudates, and occasional small vertebrates.[40] Seasonal shifts occur, with increased reliance on seeds and flowers during fruit scarcity, enabling dietary flexibility in variable forest environments.[39] Guenons employ diurnal foraging strategies, active primarily during daylight hours in arboreal settings, where they navigate the forest canopy using quadrupedal locomotion, climbing, and leaping to access food resources.[41] Foraging occurs in small parties, often 10-30 individuals depending on species and habitat, allowing coordinated scanning for ripe fruits and insects while minimizing competition.[22] They selectively target preferred items like figs when available, using cheek pouches in some species to store food temporarily for safe consumption away from immediate threats.[42] Natural predators of guenons include leopards (Panthera pardus), crowned hawk-eagles (Stephanoaetus coronatus), and African rock pythons (Python sebae), which pose risks during both terrestrial and arboreal activities.[43] Anti-predator defenses encompass alarm calls, such as short "hack" or "chirp" vocalizations to alert the group, mobbing by adult males who aggressively approach aerial threats like eagles, and cryptic behaviors including increased vigilance and hiding in dense foliage.[42] These tactics, including aggregation for dilution of risk, enhance survival by balancing foraging needs with predator avoidance.[44]Reproduction and development
Mating systems
Guenons primarily exhibit polygynous mating systems, in which a single resident adult male maintains exclusive or primary access to multiple females within a social group, often through aggressive defense against intruders.[45] This male dominance is frequently disrupted by takeovers, where extragroup males challenge and displace the resident, leading to shifts in reproductive control.[46] During periods of female estrus, mating becomes more promiscuous, with females soliciting copulations from the resident male and occasionally from subordinate or extragroup males, increasing opportunities for multiple paternity within litters.[47] An exception occurs in De Brazza's guenon (Cercopithecus neglectus), where small family groups sometimes form stable monogamous pairs consisting of one adult male and one female, particularly in regions with limited resources that favor pair-bonding over larger harems; however, larger groups in this species revert to polygyny.[27] In most guenon species, female reproductive cycles last approximately 30-35 days, marked by subtle behavioral cues such as increased genital swelling and proceptivity during the fertile phase.[48] [49] Reproductive seasonality varies by geographic location and environmental cues. In equatorial forests with stable climates, mating occurs year-round, allowing continuous breeding opportunities.[50] In contrast, populations in more seasonal habitats time conceptions to align with resource peaks, such as the onset of rainy seasons, resulting in birth peaks 5-7 months later to coincide with food abundance.[51] Mate selection in guenons involves both intersexual and intrasexual competition. Females typically prefer mating with dominant resident males, who sire the majority of offspring due to their control over group access and demonstrated genetic quality through prior tenure.[52] Males compete for mating rights primarily through ritualized displays, such as vocalizations, branch-shaking, and charging, escalating to physical fights during takeovers or estrus periods to establish or maintain dominance.[46]Life cycle and parental care
Guenons typically have a gestation period of 5 to 7 months, varying slightly among species such as the mona monkey (Cercopithecus mona) at 5–6 months and the red-tailed monkey (C. ascanius) at approximately 6 months.[14][53] Births usually occur at night in elevated positions within trees, with females delivering a single offspring; twins are rare across the genus, though documented in species like the mona monkey.[14][45] Newborn guenon infants are precocial, with fur and open eyes, and immediately cling ventrally to their mother's abdomen for protection and nursing during the initial weeks.[54] This ventral clinging persists for 3 to 6 months, after which infants transition to dorsal riding on the mother's back while beginning to explore independently and consume solid foods around 2 to 3 months of age.[55] Weaning occurs between 6 and 12 months, with full nutritional independence by about 1 year, as seen in the de Brazza's monkey (C. neglectus) and mona monkey.[54][14] Sexual maturity is reached at 3 to 7 years, with females maturing earlier (around 3–6 years) than males (4.5–7 years).[56] In the wild, guenons have a lifespan of 20 to 30 years, influenced by predation and habitat factors.[14][57] Parental care is primarily provided by the mother, who nurses, grooms, and transports the infant for the first year, fostering bonding and survival skills.[58] Allomothering is common among female group members, who carry, groom, and protect non-offspring infants, particularly in guenon species like the blue monkey (C. mitis), reducing maternal energetic costs and enhancing infant safety.[59] In some multi-male groups, resident males contribute to protection by defending infants against predators or potential infanticide, as observed in de Brazza's guenons.[54]Conservation
Threats and population status
Guenons face significant anthropogenic threats across their range in sub-Saharan African forests. The primary risks include habitat destruction driven by deforestation for agriculture, logging, and infrastructure development, which fragments and degrades their forest habitats. Hunting for bushmeat and the illegal pet trade further exacerbate population declines, as guenons are targeted for consumption and live capture in regions with high human population density. Climate change poses an additional emerging threat by altering forest ecosystems through shifts in temperature and precipitation patterns, potentially restricting suitable habitats and increasing vulnerability to disease and resource scarcity.[60][61] Population trends for guenons are predominantly declining, with overall numbers estimated to be low and fragmented. The genus Cercopithecus comprises approximately 20 recognized species, all assessed by the IUCN. As of the IUCN Red List version 2025-2, five species are classified as Critically Endangered, three as Endangered, five as Vulnerable, and seven as Least Concern.[62] Several guenon species, including the Roloway, Dryas, and Sclater's guenons, are featured in the IUCN SSC's 'Primates in Peril: The World's 25 Most Endangered Primates 2023–2025' report.[63] For example, the Roloway guenon (C. roloway) has an estimated wild population of fewer than 2,000 individuals.[64] Total population estimates for many threatened guenon species fall below 100,000, reflecting ongoing losses without comprehensive range-wide surveys. While many IUCN Red List assessments for guenon species date from before 2021, updates through 2025 have refined statuses, with increasing threats noted. Specific species statuses, such as those for the Roloway guenon, underscore the genus-wide vulnerability but are detailed further in discussions of species diversity.[60]Conservation measures
Conservation measures for guenons primarily involve the establishment and management of protected areas, international trade regulations, and targeted conservation initiatives coordinated by global organizations. Key protected areas include Taï National Park in Ivory Coast, a UNESCO World Heritage Site that safeguards multiple guenon species through habitat preservation and research on their ecological associations.[65] Similarly, Lopé National Park in Gabon, another UNESCO site, protects endemic guenons such as the sun-tailed guenon amid diverse primate populations, with ongoing monitoring to mitigate logging pressures. Many guenon species, including the Preuss's monkey, red-eared guenon, and white-nosed guenon, are listed under Appendix II of the Convention on International Trade in Endangered Species (CITES), which regulates commercial trade to prevent overexploitation.[66][67][68] Active conservation efforts encompass reintroduction programs guided by IUCN standards for nonhuman primates, anti-poaching patrols in reserves, and community education campaigns to reduce human-wildlife conflict. The IUCN Species Survival Commission's Primate Specialist Group (PSG) plays a central role, developing action plans, prioritizing endangered primates, and facilitating collaborative research and policy advocacy across Africa.[69][70] Anti-poaching initiatives in areas like Taï National Park have strengthened enforcement, while community programs emphasize sustainable livelihoods to curb habitat encroachment.[71] Proposed habitat corridors aim to connect fragmented forests, enhancing guenon dispersal and genetic diversity, as seen in Nigerian primate conservation projects involving native tree planting. Some guenon populations remain stable within well-managed reserves, demonstrating the efficacy of these protections, though persistent funding gaps hinder broader implementation and long-term monitoring.[72][73]Species diversity
List of recognized species
The genus Cercopithecus includes approximately 26 recognized species of guenons, arboreal Old World monkeys distributed across sub-Saharan Africa, primarily in forested habitats.[1] These species exhibit diverse pelage patterns for species recognition and are generally omnivorous, feeding on fruits, leaves, insects, and small vertebrates. The list below details selected species (not exhaustive due to ongoing taxonomic revisions), including geographic range, primary habitat, approximate adult body size and weight (males larger than females), IUCN Red List status as of 2025, and notable notes such as subspecies or recent discoveries, based on authoritative taxonomic and conservation assessments. For a complete list, refer to IUCN Red List.| Common Name | Scientific Name | Geographic Range | Primary Habitat | Size and Weight | IUCN Status | Notes |
|---|---|---|---|---|---|---|
| Blue monkey | Cercopithecus mitis | Widespread across central, eastern, and southern Africa, from Cameroon to South Africa | Montane and lowland rainforests, woodlands | Body 42–60 cm, tail 50–80 cm, 5–9 kg | Least Concern | Recognizes 10 subspecies (e.g., C. m. stuhlmanni); adaptable to forest edges; diet includes fruits and foliage. |
| Campbell's mona monkey | Cercopithecus campbelli | West Africa, from Guinea to Ghana | Lowland rainforests, gallery forests | Body 40–50 cm, tail 50–70 cm, 3.5–5.5 kg | Near Threatened | Subspecies include C. c. lowei (sometimes separate); frugivorous with insect supplement; social groups of 10–40; threatened by habitat loss.[74] |
| Mona monkey | Cercopithecus mona | West and central Africa, including islands like Bioko | Rainforests, mangroves, secondary forests | Body 40–50 cm, tail 50–75 cm, 3.5–6 kg | Least Concern | Up to 4 subspecies; known for black-and-white facial markings; diet heavy on fruits and seeds. |
| Wolf's guenon | Cercopithecus wolfi | Central Africa, Democratic Republic of Congo | Lowland rainforests, swamp forests | Body 45–55 cm, tail 60–80 cm, 5–8 kg | Near Threatened | Limited data; sympatric with other guenons; frugivore-insectivore; population decline inferred.[75] |
| Lowe's monkey | Cercopithecus lowei | West Africa, Ghana and Togo | Rainforests, forest-savanna mosaics | Body 40–48 cm, tail 50–65 cm, 3–5 kg | Vulnerable | Sometimes considered subspecies of C. campbelli; threatened by habitat loss; diet includes insects and fruits. |
| Sclater's guenon | Cercopithecus sclateri | Southern Nigeria | Lowland rainforests | Body 40–50 cm, tail 50–70 cm, 4–6 kg | Endangered | Rare, with small fragmented populations; frugivorous; faces severe deforestation threats. |
| De Brazza's monkey | Cercopithecus neglectus | Central Africa, from Cameroon to Kenya | Swamp forests, riverine forests, montane forests | Body 40–60 cm, tail 50–75 cm, 3–7 kg | Least Concern | Distinctive orange forehead; secretive, often near water; diet of fruits, leaves, and aquatic prey. |
| Diana monkey | Cercopithecus diana | West Africa, from [Sierra Leone](/page/Sierra Leone) to Ghana | Primary rainforests, up to 500 m elevation | Body 40–55 cm, tail 55–80 cm, 4–5.5 kg | Endangered | Vocal species; hunted for bushmeat; formerly included Roloway as subspecies.[76] |
| Roloway monkey | Cercopithecus roloway | West Africa, Ivory Coast and Ghana | Primary rainforests | Body 40–50 cm, tail 50–70 cm, 4–5 kg | Critically Endangered | Formerly subspecies of C. diana; highly threatened by hunting and habitat loss; very small populations.[77] |
| Crowned monkey | Cercopithecus pogonias | West and central Africa, from Guinea to DRC | Lowland and montane rainforests | Body 40–50 cm, tail 60–80 cm, 4–7 kg | Least Concern | 5 subspecies (e.g., C. p. pogonias); crowned facial pattern; omnivorous diet. |
| Moustached monkey | Cercopithecus cephus | Central Africa, from Nigeria to DRC | Lowland rainforests, secondary forests | Body 35–45 cm, tail 50–70 cm, 3–5 kg | Least Concern | Subspecies include black-nosed variant; highly social; feeds mainly on fruits and insects. |
| Red-tailed monkey | Cercopithecus ascanius | Central and eastern Africa, from DRC to Tanzania | Rainforests, woodland forests | Body 45–60 cm, tail 60–90 cm, 4–6.5 kg | Least Concern | 5 subspecies; red tail diagnostic; often in polyspecific associations; frugivore. |
| Putty-nosed monkey | Cercopithecus nictitans | West and central Africa, from Senegal to Uganda | Rainforests, montane forests up to 2,000 m | Body 45–60 cm, tail 70–80 cm, 4–7 kg | Least Concern | Includes subspecies C. n. ludio; white nose patch; diet of fruits, leaves, insects. |
| Lesser spot-nosed monkey | Cercopithecus petaurista | West and central Africa, from Guinea to DRC | Lowland rainforests, swamp forests | Body 35–45 cm, tail 55–75 cm, 2.5–4 kg | Least Concern | 3 subspecies; spotted forehead; smallest guenon; insectivorous tendencies higher. |
| Red-eared guenon | Cercopithecus erythrotis | West Africa, Cameroon, Equatorial Guinea, Nigeria | Lowland rainforests, gallery forests | Body 40–50 cm, tail 50–70 cm, 3.5–5 kg | Vulnerable | Subspecies C. e. erythrotis; red ear tufts; threatened by logging and hunting. |
| Nigerian white-throated monkey | Cercopithecus erythrogaster | Southwest Nigeria, Benin | Lowland forests, mangroves | Body 40–50 cm, tail 50–70 cm, 3–5 kg | Endangered | 2 subspecies; white throat and belly; critically low populations due to habitat fragmentation. |
| Lesula | Cercopithecus lomamiensis | Central DRC, Lomami Basin | Primary rainforests | Body 40–50 cm, tail 50–60 cm, 4–6 kg | Vulnerable | Discovered in 2007, formally described in 2012; distinctive human-like face; diet includes fruits and invertebrates; threatened by bushmeat trade. |
| Hamlyn's monkey | Cercopithecus hamlyni | Eastern DRC | Montane rainforests, bamboo forests | Body 45–55 cm, tail 70–80 cm, 5–7 kg | Endangered | Blue face; rare, with declining populations; folivorous diet. |
| Dryas monkey | Cercopithecus dryas | Central DRC, near Lake Tumba | Swamp forests, primary rainforests | Body 40–50 cm, tail 60–70 cm, 4–6 kg | Critically Endangered | Extremely rare, known from few specimens; possibly extinct in wild; frugivorous. |
| Preuss's monkey | Cercopithecus preussi | West and central Africa, Cameroon to Nigeria | Montane forests, lowland rainforests | Body 40–50 cm, tail 50–70 cm, 3–5 kg | Vulnerable | Two subspecies; threatened by logging; diet mainly fruits and insects.[78] |
| L'Hoest's monkey | Cercopithecus lhoesti | Central Africa, from Cameroon to Uganda | Montane forests | Body 45–60 cm, tail 70–90 cm, 4–7 kg | Near Threatened | Includes subspecies like C. l. lhoesti; distinctive beard; folivorous.[79] |
Hybrids and subspecies
Hybrids between guenon species have been documented primarily in the wild, with the most studied case involving the red-tailed monkey (Cercopithecus ascanius) and the blue monkey (C. mitis) in overlapping forest habitats of East Africa. These interspecific crosses occur in limited zones, such as Gombe National Park in Tanzania, where hybrids exhibit intermediate morphological traits like pelage coloration and are viable and fertile, indicating ongoing gene flow between the parental species.[80] Observations of such hybrids remain rare across most surveyed forests, though a fertile hybrid between two Cercopithecus species was also reported in Uganda's Budongo Forest, suggesting sporadic natural hybridization events beyond captivity.[81] The genus Cercopithecus encompasses over 50 recognized subspecies distributed across its approximately 26 species, reflecting high levels of geographic and morphological variation adapted to diverse African forest environments.[1] For instance, Stuhlmann's blue monkey (C. m. stuhlmanni) is a subspecies of the blue monkey complex, characterized by its occurrence in montane forests of East Africa and distinct grayish pelage with a reddish tail base.[82] Genetic analyses have provided evidence that certain subspecies warrant elevation to full species status due to substantial divergence in mitochondrial DNA and nuclear markers, highlighting cryptic diversity within traditionally delimited taxa.[3] Hybrid zones among guenons are increasingly noted in fragmented habitats, where habitat loss from deforestation and human activity brings previously allopatric populations into secondary contact, potentially increasing unnatural interbreeding rates. This phenomenon raises conservation concerns regarding genetic purity and the integrity of distinct lineages, as persistent hybridization could dilute adaptive traits in small, isolated populations, complicating efforts to preserve biodiversity in rapidly degrading tropical forests.[83]References
- https://en.wiktionary.org/wiki/Cercopithecus
























