Recent from talks
Nothing was collected or created yet.
Gnetum
View on Wikipedia
| Gnetum | |
|---|---|

| |
| Gnetum luofuense in China | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Gymnospermae |
| Division: | Gnetophyta |
| Order: | Gnetales Mart |
| Family: | Gnetaceae Blume |
| Genus: | Gnetum L. |
| Type species | |
| Gnetum gnemon | |
| |
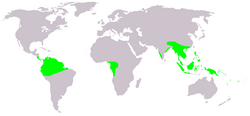
| Distribution | |
| Synonyms[1] | |
| |
Gnetum is a genus of gymnosperms, the sole genus in the family Gnetaceae within the Gnetophyta. They are tropical evergreen trees, shrubs and lianas. Unlike other gymnosperms, they possess vessel elements in the xylem. Some species have been proposed to have been the first plants to be insect-pollinated as their fossils occur in association with extinct pollinating scorpionflies.[2] Molecular phylogenies based on nuclear and plastid sequences from most of the species indicate hybridization among some of the Southeast Asian species. Fossil-calibrated molecular-clocks suggest that the Gnetum lineages now found in Africa, South America and Southeast Asia are the result of ancient long-distance dispersal across seawater.[3][4]
Their leaves are rich in phytochemicals such as flavonoids and stilbenes. Of the species studied so far, Gnetum have photosynthetic and transpiration capacities which are considerably lower than those of other seed plants, due to the absence of multiple chloroplast genes essential for photosynthesis, a trait they seem to share with the other living members of Gnetophyta, Ephedra and Welwitschia, as well as conifers.[5] There are over 50 different species of Gnetum.[citation needed]
Species
[edit]| Phylogeny of Gnetum[6] | |||||||||||||||||||||||||||||||||
|
| Phylogeny of Gnetum[7][8] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
There are around 50 different species of Gnetum. The Catalogue of Life lists 44 species.[9]
- Gnetum interruptum Biye
- Gnetum latispicum Biye
- Gnetum sect. Gnetum
- Gnetum subsect. Gnetum – 2 species of trees; Southeast Asia, Pacific Islands
- Gnetum gnemon – Tibet, Yunnan, Assam, Indochina, Nicobar Islands, Malaysia, Indonesia, Philippines, New Guinea, Melanesia, Micronesia
- Gnetum costatum – New Guinea, Solomon Islands
- Gnetum subsect. Micrognemones – 2 species of lianas; tropical west Africa
- Gnetum africanum – central Africa from Cameroon to Angola
- Gnetum buchholzianum – central Africa from Nigeria to Zaire
- Gnetum subsect. Araeognemones – 9 species of lianas; tropical South America and Central America - Ituá
- Gnetum camporum – Venezuela
- Gnetum leyboldii – Costa Rica, Panama, Venezuela, Colombia, Ecuador, Peru, Amazonian Brazil
- Gnetum nodiflorum – Guianas, Venezuela, Colombia, Ecuador, Peru, northwestern Brazil
- Gnetum paniculatum – Guianas, Venezuela, northwestern Brazil
- Gnetum schwackeanum – Amazonas State of southern Venezuela, northwestern Brazil
- Gnetum urens – Guianas, Venezuela, Peru, northwestern Brazil
- Gnetum venosum – Bolívar State of southern Venezuela, northwestern Brazil
- Gnetum subsect. Gnetum – 2 species of trees; Southeast Asia, Pacific Islands
- Gnetum sect. Scandentia [Gnetum sect. Cylindrostachys] - about 20 species of lianas; southern Asia
- Gnetum subsect. Stipitati
- Gnetum arboreum – Luzon in Philippines
- Gnetum contractum – southern India
- Gnetum edule – southern India
- Gnetum gracilipes – Yunnan + Guangxi in China
- Gnetum latifolium – Assam, much of Southeast Asia, New Guinea, Bismarck Archipelago
- Gnetum montanum – Himalayas, southern China, northern Indochina
- Gnetum oblongum – Bangladesh, Myanmar
- Gnetum pendulum – Tibet, southern China
- Gnetum tenuifolium – Peninsular Malaysia, Thailand, Sumatra
- Gnetum ula
- Gnetum subsect. Sessiles
- Gnetum acutum – Sarawak
- Gnetum bosavicum – Papua New Guinea
- Gnetum catasphaericum – southern China
- Gnetum chinense Yang, Liu, & Chang – northern China
- Gnetum cleistostachyum – southern China
- Gnetum cuspidatum – Indochina, Indonesia, Malaysia, Philippines
- Gnetum diminutum – Borneo
- Gnetum formosum – Vietnam
- Gnetum giganteum – Guangxi in China
- Gnetum globosum – Pahang in Malaysia
- Gnetum gnemonoides – New Guinea, Bismarck Archipelago, Indonesia, Philippines
- Gnetum hainanense – southern China
- Gnetum klossii – Sabah
- Gnetum leptostachyum – Laos, Thailand, Vietnam, Borneo
- Gnetum loerzingii – Sumatra
- Gnetum luofuense – Fujian, Guangdong, Jiangxi
- Gnetum macrostachyum – Indochina, Indonesia, Malaysia, New Guinea
- Gnetum microcarpum – Myanmar, Thailand, Malaysia, Borneo, Sumatra
- Gnetum neglectum – Borneo
- Gnetum oxycarpum – Sumatra
- Gnetum parvifolium – Laos, Vietnam, southern China
- Gnetum raya – Borneo
- Gnetum ridleyi – Peninsular Malaysia
- Gnetum subsect. Stipitati
Uses
[edit]Many Gnetum species are edible, with the seeds being roasted, and the foliage used as a leaf vegetable.[10] The plant is harvested and yields a useful fiber.[clarification needed] There is no sense of danger in consuming the fruit or the seeds.[11]
There is also a study done on the plant to see if it has any medicinal properties, finding some anti-coagulation effects due to its stilbenoid content. The family Gnetaceae is well known as a rich source of plant-derived stilbenoids as well as Cyperaceae, Dipterocarpaceae, Fabaceae, and Vitaceae.[12]
Conservation
[edit]Some species of Gnetum are in danger of dying out. The habitats are being removed with the trees being cut down to create industry. The tropical rainforest are being destroyed so many of the species are going extinct such as Gnetum oxycarpum. The rainforests are being torn down and being turned into farmland. Gnetum live in only a small part of the rainforest.
Gallery
[edit]-
Gnetum gnemon carpellate/female cones
-
Gnetum latifolium staminate/male cones
-
Gathered leaves of Gnetum africanum
-
Gnetum gnemon seeds
References
[edit]- ^ Kew World Checklist of Selected Plant Families
- ^ Ren, Dong; Labandeira, Conrad C.; Santiago-Blay, Jorge A.; Rasnitsyn, Alexandr; Shih, Chungkun; Bashkuev, Alexei; Logan, M. Amelia V.; Hotton, Carol L.; Dilcher, David (2009). "A Probable Pollination Mode Before Angiosperms: Eurasian, Long-Proboscid Scorpionflies". Science. 326 (5954): 840–847. Bibcode:2009Sci...326..840R. doi:10.1126/science.1178338. PMC 2944650. PMID 19892981.
- ^ Won, Hyosig; Renner, Susanne S. (2005). "The internal transcribed spacer of nuclear ribosomal DNA in the gymnosperm Gnetum". Molecular Phylogenetics and Evolution. 36 (3): 581–597. Bibcode:2005MolPE..36..581W. doi:10.1016/j.ympev.2005.03.011. PMID 16099382.
- ^ Won, Hyosig; Renner, Susanne S. (2006). "Dating Dispersal and Radiation in the Gymnosperm Gnetum (Gnetales)—Clock Calibration when Outgroup Relationships Are Uncertain". Systematic Biology. 55 (4): 610–622. doi:10.1080/10635150600812619. PMID 16969937.
- ^ Deng, N.; Hou, C.; Liu, C.; Li, M.; Bartish, I.; Tian, Y.; Chen, W.; Du, C.; Jiang, Z.; Shi, S. (2019). "Significance of Photosynthetic Characters in the Evolution of Asian Gnetum (Gnetales)". Frontiers in Plant Science. 10: 39. doi:10.3389/fpls.2019.00039. PMC 6370715. PMID 30804953.
- ^ Hou, Chen; Humphreys, Aelys M.; Thureborn, Olle; Rydin, Catarina (April 2015). "New insights into the evolutionary history of Gnetum (Gnetales)". Taxon. 64 (2): 239–253. doi:10.12705/642.12.
- ^ Stull, Gregory W.; Qu, Xiao-Jian; Parins-Fukuchi, Caroline; Yang, Ying-Ying; Yang, Jun-Bo; Yang, Zhi-Yun; Hu, Yi; Ma, Hong; Soltis, Pamela S.; Soltis, Douglas E.; Li, De-Zhu; Smith, Stephen A.; Yi, Ting-Shuang; et al. (2021). "Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms". Nature Plants. 7 (8): 1015–1025. bioRxiv 10.1101/2021.03.13.435279. doi:10.1038/s41477-021-00964-4. PMID 34282286. S2CID 232282918.
- ^ Stull, Gregory W.; et al. (2021). "main.dated.supermatrix.tree.T9.tre". Figshare. doi:10.6084/m9.figshare.14547354.v1.
- ^ "Gnetum L." Catalogue of Life. Retrieved October 24, 2024.
- ^ Hoe, V.B. and Siong, K.H., "The Nutritional Value of Indigenous Fruits and Vegetables in Sarawak,"Asia-Pacific Journal of Clinical Nutrition, Vol. 8, no. 1, 1998, pp 24-31
- ^ "Gnetum gnemon | plant | Britannica". Encyclopædia Britannica. Retrieved May 2, 2022.
- ^ Kloypan, Chiraphat; Jeenapongsa, Rattima; Sri-In, Piyawit; Chanta, Surin; Dokpuang, Dech; Tip-Pyang, Santi; Surapinit, Nattanan (2012). "Stilbenoids from Gnetum macrostachyum Attenuate Human Platelet Aggregation and Adhesion". Phytotherapy Research. 26 (10): 1564–1568. doi:10.1002/ptr.4605. PMID 22511550. S2CID 43249684.
External links
[edit]- Gymnosperm Database - Gnetum
- Sorting Gnetum names
- Uses of Gnetum in Africa (FAO) Archived January 11, 2019, at the Wayback Machine
- Kloypan, Chiraphat & Jeenapongsa, Rattima & Sri-In, Piyawit & Chanta, Surin & Dokpuang, Dech & Tip-Pyang, Santi & Surapinit, Serm. (2012). Stilbenoids from Gnetum macrostachyum Attenuate Human Platelet Aggregation and Adhesion. Phytotherapy research : PTR. 26. 1564–8. 10.1002/ptr.4605.
- https://bsapubs.onlinelibrary.wiley.com/doi/abs/10.1002/j.1537-2197.1916.tb05408.x Archived December 22, 2021, at the Wayback Machine
- http://www.theplantlist.org/tpl1.1/record/kew-334161...
Gnetum
View on GrokipediaTaxonomy and phylogeny
Classification
Gnetum constitutes the sole genus in the family Gnetaceae, which is the only family within the order Gnetales and part of the gymnosperm division Gnetophyta.[1] This placement reflects both morphological traits, such as compound ovules and opposite leaves, and molecular data supporting its distinction from other seed plant lineages.[1] The genus encompasses approximately 40 species, subject to ongoing taxonomic revisions; for instance, Gnetum chinense was formally described as a new lianoid species from southwestern China in 2020 based on morphological distinctions from congeners like G. montanum.[5][6] Early classifications faced challenges due to the presence of vessel elements in Gnetum's xylem, a conductive tissue feature long considered diagnostic of angiosperms and absent in most other gymnosperms, prompting suggestions of angiosperm affinity or convergence.[7] Molecular phylogenetic studies, including analyses of nuclear and organellar genes, have since affirmed Gnetum's gymnosperm status, demonstrating that vessel elements and other shared traits with angiosperms arose independently through convergence rather than shared ancestry.[8] This resolution underscores the role of genetic data in clarifying morphological ambiguities in gymnosperm taxonomy.[3]Phylogenetic position and debates
The phylogenetic position of Gnetum, as part of the order Gnetales, has been central to debates on seed plant evolution, with early hypotheses favoring close ties to angiosperms under the anthophyte model due to shared traits like vessel elements in xylem and compound reproductive structures.[3] This view posited Gnetales as sister to flowering plants, potentially illuminating angiosperm origins, but it relied heavily on morphological and limited molecular data prone to artifacts such as long-branch attraction in chloroplast phylogenies, which artificially pulled fast-evolving Gnetales toward distant angiosperms.[9] Empirical evidence from comprehensive nuclear and chloroplast genome analyses has refuted the anthophyte hypothesis, instead embedding Gnetales firmly within gymnosperms as a derived clade.[10] Phylogenomic studies since 2010, incorporating thousands of loci, consistently recover Gnetales as sister to Pinaceae (the pine family), supporting the "gnepine" topology and rendering conifers paraphyletic.[11] For instance, a 2018 analysis of 125 plastid and 82 nuclear genes across seed plants resolved Gnetales adjacent to Pinaceae with strong bootstrap support (>95%), attributing prior misplacements to chloroplast-specific biases like accelerated substitution rates in Gnetales lineages.[10] More recent consilience approaches, integrating phylogenomics with paleobotanical evidence from Mesozoic fossils, reinforce this placement by aligning molecular divergence estimates with gymnosperm fossil calibrations, while highlighting developmental homologies (e.g., in ovule enclosure) that distinguish Gnetales from angiosperms despite superficial convergences.[12] These findings underscore Gnetales, including Gnetum, as specialized gymnosperms rather than angiosperm precursors, with no credible genomic support persisting for anthophyte affinities after accounting for systematic errors.[9] Within Gnetum, a 2015 phylogeny based on nuclear ribosomal and chloroplast markers from 58 accessions across 27 species estimated major lineage divergences in the Late Cretaceous (circa 80–70 million years ago), contrasting earlier Miocene estimates and aligning with Gondwanan vicariance patterns.[13] South American clades emerge as basal in this framework, predating Old World radiations and reflecting tectonic fragmentation, with robust node support from Bayesian inference.[14] This timeline integrates with broader Gnetales evolution, where Gnetum–Welwitschia splits trace to the Early Cretaceous, emphasizing Gnetum's relictual status amid gymnosperm diversification.[12]Description
Morphology
Gnetum species are tropical evergreen plants that grow as woody lianas, shrubs, or trees, with some reaching heights of 10 to 20 meters or more in the case of arborescent forms like G. gnemon.[15][16] Lianas can extend up to 30 meters in length, featuring cylindrical branches with smooth surfaces and enlarged nodes, while exhibiting simple, elongated shoots and decussate branching patterns atypical among most gymnosperms.[17][18] Leaves are arranged opposite or decussate, broad and leathery, with pinnate-reticulate venation comprising multiple vein orders, a feature convergent with many angiosperms and distinguishing Gnetum from needle-like or scale-like foliage in other gymnosperms.[2][15] Each leaf typically bears two primary veins connected to axial stem vascular bundles, contributing to their angiosperm-like appearance.[19] The vascular system includes xylem composed of tracheids with bordered pits, vessels up to 300 micrometers in diameter (a rare gymnosperm trait via convergent evolution), and xylem parenchyma, alongside phloem with sieve cells and parenchyma but lacking sieve tubes.[20][21][18] Plants are dioecious, with separate male and female individuals bearing these vegetative structures.[22]Reproduction
Gnetum species are dioecious, with plants producing either male or female compound strobili organized into inflorescences. Male strobili consist of collars of bracts bearing microsporophylls with two microsporangia each, while female strobili feature paired ovules enveloped by integuments and surrounded by bract-like collars.[20][23] These strobili lack the perianth and fused carpels characteristic of angiosperm flowers, confirming their gymnosperm nature despite superficial resemblances such as vessel elements in vascular tissue.[24] Pollination in Gnetum occurs primarily through anemophily (wind) supplemented by entomophily (insects), with both male and female strobili secreting pollination droplets from ovules—fertile in females and sterile in males—to facilitate pollen capture and attract vectors. Insects are drawn by odors and nectar rewards, as observed in species like Gnetum cuspidatum, where nocturnal activity peaks in the evening.[25][26] Pollen grains germinate on the droplet, forming pollen tubes that deliver sperm cells to the ovule micropyle.[27] Fertilization involves a form of double fertilization unique among gymnosperms, where two sperm cells from a single pollen tube participate: one fuses with the egg to form a zygote, and the other with another cell to produce a second zygote rather than endosperm. This process, documented in Gnetum gnemon, yields two potential embryos per ovule but no nutritive endosperm tissue, distinguishing it from angiosperm double fertilization.[28] Developmental genetic studies reveal conserved gymnosperm pathways in ovule formation, with genes like MADS-box factors expressed in patterns underscoring the absence of angiosperm-specific innovations.[29][30] Mature seeds are naked, lacking a fruit enclosure, but feature an outer fleshy aril derived from the integument or surrounding tissue, which aids in animal-mediated dispersal, particularly by birds attracted to the nutrient-rich covering.[31] This aril contrasts with the exposed seeds of other gymnosperms, enhancing dispersal efficiency in tropical habitats without evolving true fruits.[32]Distribution and ecology
Geographic range
The genus Gnetum encompasses approximately 40 species exhibiting a pantropical distribution, primarily confined to humid tropical forests in Africa, Southeast Asia, and northern South America, with no native presence in Australia or oceanic interiors.[23] In Africa, only two species occur—G. africanum and G. buchholzianum—predominantly in West and Central regions from Nigeria eastward to the Central African Republic and southward to Angola. The Asian clade, representing the majority of diversity with 28–32 species, centers in Malesia (Southeast Asia), including undescribed taxa, extending from southern China and India through Indonesia and Papuasia.[33] In the Neotropics, around seven species inhabit northern South America and adjacent Central America, exemplified by G. leyboldii in Andean foothills.[17] Phylogenetic analyses calibrated with fossils indicate that these disjunct distributions trace to ancient vicariance events dating to the Cretaceous, with no evidence of recent range expansions or contractions altering the core pantropical pattern.[13] Species richness gradients align with regional paleoclimate stability in equatorial lowlands, underscoring long-term persistence without significant latitudinal shifts.[34]Habitat preferences and ecological role
Gnetum species primarily occupy the understory and canopy layers of humid tropical rainforests, extending from lowland elevations to montane forests up to around 1200–2000 meters above sea level, depending on the taxon.[35][15] They thrive in environments with high rainfall (750–5000 mm annually) and consistent moisture, often favoring well-drained, slightly acidic to neutral soils near rivers, riparian zones, or swampy areas in ever-wet tropical climates.[36][37] As woody lianas or small trees, they exhibit shade tolerance, enabling persistence in dimly lit primary forest peripheries, though their dependence on stable humidity and forest cover renders them sensitive to seasonal dry spells or canopy gaps that reduce moisture retention.[38][39] This niche restriction stems from physiological adaptations to saturated conditions, where competition from faster-growing angiosperms limits expansion into drier or more variable habitats.[15] Ecologically, Gnetum contributes to forest dynamics through vertebrate-mediated seed dispersal, with birds primarily consuming and distributing the fleshy, drupe-like seeds that mimic angiosperm fruits for attraction.[32][40] Some species appear in secondary forests and farm fallows, suggesting a capacity for early colonization in disturbed sites, though empirical data indicate stronger association with primary shade than true pioneer traits like rapid gap-filling.[41][38] Mycorrhizal associations, such as with ectomycorrhizal fungi like Scleroderma sinnamariense, may facilitate nutrient uptake in nutrient-poor tropical soils, but symbiotic nitrogen fixation—often speculated due to endophytic bacteria—lacks confirmation in peer-reviewed studies and contrasts with well-documented bacterial symbioses in other gymnosperms or angiosperms.[42][43] Overall, their roles enhance biodiversity in intact humid forests by supporting frugivore food webs, but low photosynthetic and hydraulic efficiencies relative to co-occurring angiosperms constrain dominance in successional sequences.[21][2]Species diversity
Number and distribution of species
The genus Gnetum includes 44 accepted species, as recognized by the Plants of the World Online database maintained by the Royal Botanic Gardens, Kew.[5] This count reflects ongoing taxonomic refinements, with earlier phylogenetic studies from 2015 incorporating data from approximately 27 species to resolve evolutionary relationships. Species richness is concentrated in tropical regions, exhibiting a pantropical distribution pattern without evidence of recent extinctions. The majority of species, over 20, occur in Southeast Asia and Malesia, where diverse lianescent forms predominate in humid forests.[1] Tropical Africa hosts 6 to 8 species, primarily in West and Central regions such as Cameroon, Gabon, and the Democratic Republic of Congo.[44] In the Americas, around 6 species are found in northern South America and Central America, often as climbers in lowland rainforests.[4] Taxonomic challenges arise from the dioecious nature of most species, complicating identification and delimitation, particularly among morphologically similar climbers. Recent discoveries, such as Gnetum chinense described in 2020 from southwestern China and adjacent Vietnam, highlight continued exploration in understudied areas.[45]Notable species
Gnetum gnemon stands out as one of the few arborescent species in the genus, forming a slender evergreen tree typically 5-10 meters tall but reaching up to 18 meters, with a conical crown and fern-like leaves.[46] It is extensively cultivated in Southeast Asian home gardens and agroforestry systems due to its rapid growth, shade tolerance, and cyclone resistance.[47] Gnetum africanum, a dioecious liana endemic to Central African rainforests, is empirically distinguished by its nutrient-dense leaves, containing 13-18% protein, 28-37% fiber, 38-44% carbohydrates, and high levels of essential minerals such as calcium, iron, magnesium, and zinc across varieties.[48][49] These leaves provide a critical source of essential amino acids and vitamins, supporting local diets in regions where protein scarcity prevails.[50] Indian endemics like Gnetum ula and G. montanum, both woody climbers restricted to the Western Ghats and northeastern hills, remain underutilized despite unique traits such as bark fibers from G. montanum suitable for ropes, nets, and gunny bags, and seeds yielding edible oil.[51][52] In contrast to more widespread tropical congeners, these species exhibit localized adaptations, including phytochemical profiles with potential anti-inflammatory compounds, though empirical data on their distinctiveness is limited compared to African and Southeast Asian counterparts.[53]





