Recent from talks
Nothing was collected or created yet.
Stem cell
View on Wikipedia| Stem cell | |
|---|---|
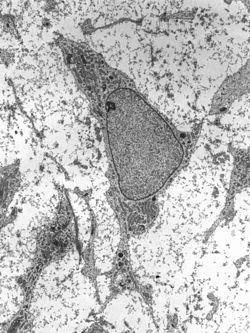
 Transmission electron micrograph of a mesenchymal stem cell displaying typical ultrastructural characteristics | |
| Details | |
| Identifiers | |
| Latin | cellula praecursoria |
| MeSH | D013234 |
| TH | H1.00.01.0.00028, H2.00.01.0.00001 |
| FMA | 63368 |
| Anatomical terminology | |
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage.[1] They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
In mammals, roughly 50 to 150 cells make up the inner cell mass during the blastocyst stage of embryonic development, around days 5–14. These have stem-cell capability. In vivo, they eventually differentiate into all of the body's cell types (making them pluripotent). This process starts with the differentiation into the three germ layers – the ectoderm, mesoderm and endoderm – at the gastrulation stage. However, when they are isolated and cultured in vitro, they can be kept in the stem-cell stage and are known as embryonic stem cells (ESCs).
Adult stem cells are found in a few select locations in the body, known as niches, such as those in the bone marrow or gonads. They exist to replenish rapidly lost cell types and are multipotent or unipotent, meaning they only differentiate into a few cell types or one type of cell. In mammals, they include, among others, hematopoietic stem cells, which replenish blood and immune cells, basal cells, which maintain the skin epithelium, and mesenchymal stem cells, which maintain bone, cartilage, muscle and fat cells. Adult stem cells are a small minority of cells; they are vastly outnumbered by the progenitor cells and terminally differentiated cells that they differentiate into.[1]
Research into stem cells grew out of findings by Canadian biologists Ernest McCulloch, James Till and Andrew J. Becker at the University of Toronto and the Ontario Cancer Institute in the 1960s.[2][3] As of 2016[update], the only established medical therapy using stem cells is hematopoietic stem cell transplantation,[4] first performed in 1958 by French oncologist Georges Mathé. Since 1998 however, it has been possible to culture and differentiate human embryonic stem cells (in stem-cell lines). The process of isolating these cells has been controversial, because it typically results in the destruction of the embryo. Sources for isolating ESCs have been restricted in some European countries and Canada, but others such as the UK and China have promoted the research.[5] Somatic cell nuclear transfer is a cloning method that can be used to create a cloned embryo for the use of its embryonic stem cells in stem cell therapy.[6] In 2006, a Japanese team led by Shinya Yamanaka discovered a method to convert mature body cells back into stem cells. These were termed induced pluripotent stem cells (iPSCs).[7]
History
[edit]The term stem cell was coined by Theodor Boveri and Valentin Haecker in late 19th century.[8] Pioneering works in theory of blood stem cell were conducted in the beginning of 20th century by Artur Pappenheim, Alexander A. Maximow, Franz Ernst Christian Neumann.[8]
The key properties of a stem cell were first defined by Ernest McCulloch and James Till at the University of Toronto and the Ontario Cancer Institute in the early 1960s. They discovered the blood-forming stem cell, the hematopoietic stem cell (HSC), through their pioneering work in mice. McCulloch and Till began a series of experiments in which bone marrow cells were injected into irradiated mice. They observed lumps in the spleens of the mice that were linearly proportional to the number of bone marrow cells injected. They hypothesized that each lump (colony) was a clone arising from a single marrow cell (stem cell). In subsequent work, McCulloch and Till, joined by graduate student Andrew John Becker and senior scientist Louis Siminovitch, confirmed that each lump did in fact arise from a single cell. Their results were published in Nature in 1963. In that same year, Siminovitch was a lead investigator for studies that found colony-forming cells were capable of self-renewal, which is a key defining property of stem cells that Till and McCulloch had theorized.[9]
The first therapy using stem cells was a bone marrow transplant performed by French oncologist Georges Mathé in 1956 on five workers at the Vinča Nuclear Institute in Yugoslavia who had been affected by a criticality accident. The workers all survived.[10]
In 1981, embryonic stem (ES) cells were first isolated and successfully cultured using mouse blastocysts by British biologists Martin Evans and Matthew Kaufman. This allowed the formation of murine genetic models, a system in which the genes of mice are deleted or altered in order to study their function in pathology. In 1991, a process that allowed the human stem cell to be isolated was patented by Ann Tsukamoto. By 1998, human embryonic stem cells were first isolated by American biologist James Thomson, which made it possible to have new transplantation methods or various cell types for testing new treatments. In 2006, Shinya Yamanaka's team in Kyoto, Japan converted fibroblasts into pluripotent stem cells by modifying the expression of only four genes. The feat represents the origin of induced pluripotent stem cells, known as iPS cells.[7]
In 2011, a female maned wolf, run over by a truck, underwent stem cell treatment at the Zoo Brasília, this being the first recorded case of the use of stem cells to heal injuries in a wild animal.[11][12]
Properties
[edit]The classical definition of a stem cell requires that it possesses two properties:
- Self-renewal: the ability to go through numerous cycles of cell growth and cell division, known as cell proliferation, while maintaining the undifferentiated state.
- Potency: the capacity to differentiate into specialized cell types. In the strictest sense, this requires stem cells to be either totipotent or pluripotent—to be able to give rise to any mature cell type, although multipotent or unipotent progenitor cells are sometimes referred to as stem cells. Apart from this, it is said that stem cell function is regulated in a feedback mechanism.
Self-renewal
[edit]Two mechanisms ensure that a stem cell population is maintained (does not shrink in size):
1. Asymmetric cell division: a stem cell divides into one mother cell, which is identical to the original stem cell, and another daughter cell, which is differentiated.
When a stem cell self-renews, it divides and disrupts the undifferentiated state. This self-renewal demands control of cell cycle as well as upkeep of multipotency or pluripotency, which all depends on the stem cell.[13]
H.
Stem cells use telomerase, a protein that restores telomeres, to protect their DNA and extend their cell division limit (the Hayflick limit).[14]
Potency meaning
[edit]
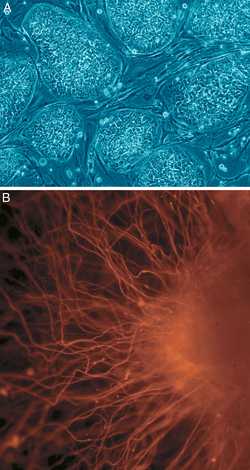
A: Stem cell colonies that are not yet differentiated.
B: Nerve cells, an example of a cell type after differentiation.
Potency specifies the differentiation potential (the potential to differentiate into different cell types) of the stem cell.[15]
- Totipotent (also known as omnipotent) stem cells can differentiate into embryonic and extraembryonic cell types. Such cells can construct a complete, viable organism.[15] These cells are produced from the fusion of an egg and sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent.[16]
- Pluripotent stem cells are the descendants of totipotent cells and can differentiate into nearly all cells,[15] i.e. cells derived from any of the three germ layers.[17]
- Multipotent stem cells can differentiate into a number of cell types, but only those of a closely related family of cells.[15]
- Oligopotent stem cells can differentiate into only a few cell types, such as lymphoid or myeloid stem cells.[15]
- Unipotent cells can produce only one cell type, their own,[15] but have the property of self-renewal, which distinguishes them from non-stem cells
Identification
[edit]In practice, stem cells are identified by whether they can regenerate tissue. For example, the defining test for bone marrow or hematopoietic stem cells (HSCs) is the ability to transplant the cells and save an individual without HSCs. This demonstrates that the cells can produce new blood cells over a long term. It should also be possible to isolate stem cells from the transplanted individual, which can themselves be transplanted into another individual without HSCs, demonstrating that the stem cell was able to self-renew.
Properties of stem cells can be illustrated in vitro, using methods such as clonogenic assays, in which single cells are assessed for their ability to differentiate and self-renew.[18][19] Stem cells can also be isolated by their possession of a distinctive set of cell surface markers. However, in vitro culture conditions can alter the behavior of cells, making it unclear whether the cells shall behave in a similar manner in vivo. There is considerable debate as to whether some proposed adult cell populations are truly stem cells.[20]
Embryonic
[edit]Embryonic stem cells (ESCs) are the cells of the inner cell mass of a blastocyst, formed prior to implantation in the uterus.[21] In human embryonic development the blastocyst stage is reached 4–5 days after fertilization, at which time it consists of 50–150 cells. ESCs are pluripotent and give rise during development to all derivatives of the three germ layers: ectoderm, endoderm and mesoderm. In other words, they can develop into each of the more than 200 cell types of the adult body when given sufficient and necessary stimulation for a specific cell type. They do not contribute to the extraembryonic membranes or to the placenta.
During embryonic development the cells of the inner cell mass continuously divide and become more specialized. For example, a portion of the ectoderm in the dorsal part of the embryo specializes as 'neurectoderm', which will become the future central nervous system (CNS).[22] Later in development, neurulation causes the neurectoderm to form the neural tube. At the neural tube stage, the anterior portion undergoes encephalization to generate or 'pattern' the basic form of the brain. At this stage of development, the principal cell type of the CNS is considered a neural stem cell.
The neural stem cells self-renew and at some point transition into radial glial progenitor cells (RGPs). Early-formed RGPs self-renew by symmetrical division to form a reservoir group of progenitor cells. These cells transition to a neurogenic state and start to divide asymmetrically to produce a large diversity of many different neuron types, each with unique gene expression, morphological, and functional characteristics. The process of generating neurons from radial glial cells is called neurogenesis. The radial glial cell, has a distinctive bipolar morphology with highly elongated processes spanning the thickness of the neural tube wall. It shares some glial characteristics, most notably the expression of glial fibrillary acidic protein (GFAP).[23][24] The radial glial cell is the primary neural stem cell of the developing vertebrate CNS, and its cell body resides in the ventricular zone, adjacent to the developing ventricular system. Neural stem cells are committed to the neuronal lineages (neurons, astrocytes, and oligodendrocytes), and thus their potency is restricted.[22]
Nearly all research to date has made use of mouse embryonic stem cells (mES) or human embryonic stem cells (hES) derived from the early inner cell mass. Both have the essential stem cell characteristics, yet they require very different environments in order to maintain an undifferentiated state. Mouse ES cells are grown on a layer of gelatin as an extracellular matrix (for support) and require the presence of leukemia inhibitory factor (LIF) in serum media. A drug cocktail containing inhibitors to GSK3B and the MAPK/ERK pathway, called 2i, has also been shown to maintain pluripotency in stem cell culture.[25] Human ESCs are grown on a feeder layer of mouse embryonic fibroblasts and require the presence of basic fibroblast growth factor (bFGF or FGF-2).[26] Without optimal culture conditions or genetic manipulation,[27] embryonic stem cells will rapidly differentiate.
A human embryonic stem cell is also defined by the expression of several transcription factors and cell surface proteins. The transcription factors Oct-4, Nanog, and Sox2 form the core regulatory network that ensures the suppression of genes that lead to differentiation and the maintenance of pluripotency.[28] The cell surface antigens most commonly used to identify hES cells are the glycolipids stage specific embryonic antigen 3 and 4, and the keratan sulfate antigens Tra-1-60 and Tra-1-81. The molecular definition of a stem cell includes many more proteins and continues to be a topic of research.[29]
By using human embryonic stem cells to produce specialized cells like nerve cells or heart cells in the lab, scientists can gain access to adult human cells without taking tissue from patients. They can then study these specialized adult cells in detail to try to discern complications of diseases, or to study cell reactions to proposed new drugs.
Because of their combined abilities of unlimited expansion and pluripotency, embryonic stem cells remain a theoretically potential source for regenerative medicine and tissue replacement after injury or disease.,[30] however, there are currently no approved treatments using ES cells. The first human trial was approved by the US Food and Drug Administration in January 2009.[31] However, the human trial was not initiated until October 13, 2010, in Atlanta for spinal cord injury research. On November 14, 2011, the company conducting the trial (Geron Corporation) announced that it will discontinue further development of its stem cell programs.[32] Differentiating ES cells into usable cells while avoiding transplant rejection are just a few of the hurdles that embryonic stem cell researchers still face.[33] Embryonic stem cells, being pluripotent, require specific signals for correct differentiation – if injected directly into another body, ES cells will differentiate into many different types of cells, causing a teratoma. Many nations currently have moratoria or limitations on either human ES cell research or the production of new human ES cell lines due to their ethical controversies.
-
Human embryonic stem cell colony on mouse embryonic fibroblast feeder layer
The use of embryonic stem cells has generated significant ethical and political controversy. Central to the debate is the moral status of the human embryo, as deriving ES typically involves the destruction of early-stage embryos. Critics argue that this practice violates the sanctity of human life, and therefore is unacceptable.
Mesenchymal stem cells
[edit]
Mesenchymal stem cells (MSC) or mesenchymal stromal cells, also known as medicinal signaling cells are known to be multipotent, which can be found in adult tissues, for example, in the muscle, liver, bone marrow and adipose tissue. Mesenchymal stem cells usually function as structural support in various organs as mentioned above, and control the movement of substances. MSC can differentiate into numerous cell categories as an illustration of adipocytes, osteocytes, and chondrocytes, derived by the mesodermal layer.[34] Where the mesoderm layer provides an increase to the body's skeletal elements, such as relating to the cartilage or bone. The term "meso" means middle, infusion originated from the Greek, signifying that mesenchymal cells are able to range and travel in early embryonic growth among the ectodermal and endodermal layers. This mechanism helps with space-filling thus, key for repairing wounds in adult organisms that have to do with mesenchymal cells in the dermis (skin), bone, or muscle.[35]
Mesenchymal stem cells are known to be essential for regenerative medicine. They are broadly studied in clinical trials. Since they are easily isolated and obtain high yield, high plasticity, which makes able to facilitate inflammation and encourage cell growth, cell differentiation, and restoring tissue derived from immunomodulation and immunosuppression. MSC comes from the bone marrow, which requires an aggressive procedure when it comes to isolating the quantity and quality of the isolated cell, and it varies by how old the donor. When comparing the rates of MSC in the bone marrow aspirates and bone marrow stroma, the aspirates tend to have lower rates of MSC than the stroma. MSC are known to be heterogeneous, and they express a high level of pluripotent markers when compared to other types of stem cells, such as embryonic stem cells.[34] MSCs injection leads to wound healing primarily through stimulation of angiogenesis.[36]
Cell cycle control
[edit]Embryonic stem cells (ESCs) have the ability to divide indefinitely while keeping their pluripotency, which is made possible through specialized mechanisms of cell cycle control.[37] Compared to proliferating somatic cells, ESCs have unique cell cycle characteristics—such as rapid cell division caused by shortened G1 phase, absent G0 phase, and modifications in cell cycle checkpoints—which leaves the cells mostly in S phase at any given time.[37][38] ESCs' rapid division is demonstrated by their short doubling time, which ranges from 8 to 10 hours, whereas somatic cells have doubling time of approximately 20 hours or longer.[39] As cells differentiate, these properties change: G1 and G2 phases lengthen, leading to longer cell division cycles. This suggests that a specific cell cycle structure may contribute to the establishment of pluripotency.[37]
Particularly because G1 phase is the phase in which cells have increased sensitivity to differentiation, shortened G1 is one of the key characteristics of ESCs and plays an important role in maintaining undifferentiated phenotype. Although the exact molecular mechanism remains only partially understood, several studies have shown insight on how ESCs progress through G1—and potentially other phases—so rapidly.[38]
The cell cycle is regulated by complex network of cyclins, cyclin-dependent kinases (Cdk), cyclin-dependent kinase inhibitors (Cdkn), pocket proteins of the retinoblastoma (Rb) family, and other accessory factors.[39] Foundational insight into the distinctive regulation of ESC cell cycle was gained by studies on mouse ESCs (mESCs).[38] mESCs showed a cell cycle with highly abbreviated G1 phase, which enabled cells to rapidly alternate between M phase and S phase. In a somatic cell cycle, oscillatory activity of Cyclin-Cdk complexes is observed in sequential action, which controls crucial regulators of the cell cycle to induce unidirectional transitions between phases: Cyclin D and Cdk4/6 are active in the G1 phase, while Cyclin E and Cdk2 are active during the late G1 phase and S phase; and Cyclin A and Cdk2 are active in the S phase and G2, while Cyclin B and Cdk1 are active in G2 and M phase.[39] However, in mESCs, this typically ordered and oscillatory activity of Cyclin-Cdk complexes is absent. Rather, the Cyclin E/Cdk2 complex is constitutively active throughout the cycle, keeping retinoblastoma protein (pRb) hyperphosphorylated and thus inactive. This allows for direct transition from M phase to the late G1 phase, leading to absence of D-type cyclins and therefore a shortened G1 phase.[38] Cdk2 activity is crucial for both cell cycle regulation and cell-fate decisions in mESCs; downregulation of Cdk2 activity prolongs G1 phase progression, establishes a somatic cell-like cell cycle, and induces expression of differentiation markers.[40]
In human ESCs (hESCs), the duration of G1 is dramatically shortened. This has been attributed to high mRNA levels of G1-related Cyclin D2 and Cdk4 genes and low levels of cell cycle regulatory proteins that inhibit cell cycle progression at G1, such as p21CipP1, p27Kip1, and p57Kip2.[37][41] Furthermore, regulators of Cdk4 and Cdk6 activity, such as members of the Ink family of inhibitors (p15, p16, p18, and p19), are expressed at low levels or not at all. Thus, similar to mESCs, hESCs show high Cdk activity, with Cdk2 exhibiting the highest kinase activity. Also similar to mESCs, hESCs demonstrate the importance of Cdk2 in G1 phase regulation by showing that G1 to S transition is delayed when Cdk2 activity is inhibited and G1 is arrest when Cdk2 is knocked down.[37] However unlike mESCs, hESCs have a functional G1 phase. hESCs show that the activities of Cyclin E/Cdk2 and Cyclin A/Cdk2 complexes are cell cycle-dependent and the Rb checkpoint in G1 is functional.[39]
ESCs are also characterized by G1 checkpoint non-functionality, even though the G1 checkpoint is crucial for maintaining genomic stability. In response to DNA damage, ESCs do not stop in G1 to repair DNA damages but instead, depend on S and G2/M checkpoints or undergo apoptosis. The absence of G1 checkpoint in ESCs allows for the removal of cells with damaged DNA, hence avoiding potential mutations from inaccurate DNA repair.[37] Consistent with this idea, ESCs are hypersensitive to DNA damage to minimize mutations passed onto the next generation.[39]
Fetal
[edit]The primitive stem cells located in the organs of fetuses are referred to as fetal stem cells.[42]
There are two types of fetal stem cells:
- Fetal proper stem cells come from the tissue of the fetus proper and are generally obtained after an abortion. These stem cells are not immortal but have a high level of division and are multipotent.
- Extraembryonic fetal stem cells come from extraembryonic membranes, and are generally not distinguished from adult stem cells. These stem cells are acquired after birth, they are not immortal but have a high level of cell division, and are pluripotent.[43]
Adult
[edit]
Adult stem cells, also called somatic (from Greek σωματικóς, "of the body") stem cells, are stem cells which maintain and repair the tissue in which they are found.[44]
There are four known accessible sources of autologous adult stem cells in humans:
- Bone marrow, which requires extraction by harvesting, usually from pelvic bones via surgery.[45]
- Adipose tissue (fat cells), which requires extraction by liposuction.[46]
- Blood, which requires extraction through apheresis, wherein blood is drawn from the donor (similar to a blood donation), and passed through a machine that extracts the stem cells and returns other portions of the blood to the donor.[47]
- Menstrual fluid[48]
Stem cells can also be taken from umbilical cord blood just after birth. Of all stem cell types, autologous harvesting involves the least risk. By definition, autologous cells are obtained from one's own body, just as one may bank their own blood for elective surgical procedures.[citation needed]
Pluripotent adult stem cells are rare and generally small in number, but they can be found in umbilical cord blood and other tissues.[49] Bone marrow is a rich source of adult stem cells,[50] which have been used in treating several conditions including liver cirrhosis,[51] chronic limb ischemia[52] and endstage heart failure.[53] The quantity of bone marrow stem cells declines with age and is greater in males than females during reproductive years.[54] Much adult stem cell research to date has aimed to characterize their potency and self-renewal capabilities.[55] DNA damage accumulates with age in both stem cells and the cells that comprise the stem cell environment. This accumulation is considered to be responsible, at least in part, for increasing stem cell dysfunction with aging (see DNA damage theory of aging).[56]
Most adult stem cells are lineage-restricted (multipotent) and are generally referred to by their tissue origin (mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell, dental pulp stem cell, etc.).[57][58] Muse cells (multi-lineage differentiating stress enduring cells) are a recently discovered pluripotent stem cell type found in multiple adult tissues, including adipose, dermal fibroblasts, and bone marrow. While rare, muse cells are identifiable by their expression of SSEA-3, a marker for undifferentiated stem cells, and general mesenchymal stem cells markers such as CD90, CD105. When subjected to single cell suspension culture, the cells will generate clusters that are similar to embryoid bodies in morphology as well as gene expression, including canonical pluripotency markers Oct4, Sox2, and Nanog.[59]
Adult stem cell treatments have been successfully used for many years to treat leukemia and related bone/blood cancers through bone marrow transplants.[60] Adult stem cells are also used in veterinary medicine to treat tendon and ligament injuries in horses.[61]
The use of adult stem cells in research and therapy is not as controversial as the use of embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo. Additionally, in instances where adult stem cells are obtained from the intended recipient (an autograft), the risk of rejection is essentially non-existent. Consequently, more US government funding is being provided for adult stem cell research.[62]
With the increasing demand of human adult stem cells for both research and clinical purposes (typically 1–5 million cells per kg of body weight are required per treatment) it becomes of utmost importance to bridge the gap between the need to expand the cells in vitro and the capability of harnessing the factors underlying replicative senescence. Adult stem cells are known to have a limited lifespan in vitro and to enter replicative senescence almost undetectably upon starting in vitro culturing.[63]
Hematopoietic stem cells
[edit]Hematopoietic stem cells (HSCs) are vulnerable to DNA damage and mutations that increase with age.[64] This vulnerability may explain the increased risk of slow growing blood cancers (myeloid malignancies) in the elderly.[64] Several factors appear to influence HSC aging including responses to the production of reactive oxygen species that may cause DNA damage and genetic mutations as well as altered epigenetic profiling.[65]
Amniotic
[edit]Also called perinatal stem cells, these multipotent stem cells are found in amniotic fluid and umbilical cord blood. These stem cells are very active, expand extensively without feeders and are not tumorigenic. Amniotic stem cells are multipotent and can differentiate in cells of adipogenic, osteogenic, myogenic, endothelial, hepatic and also neuronal lines.[66] Amniotic stem cells are a topic of active research.
Use of stem cells from amniotic fluid overcomes the ethical objections to using human embryos as a source of cells. Roman Catholic teaching forbids the use of embryonic stem cells in experimentation; accordingly, the Vatican newspaper "Osservatore Romano" called amniotic stem cells "the future of medicine".[67]
It is possible to collect amniotic stem cells for donors or for autologous use: the first US amniotic stem cells bank[68][69] was opened in 2009 in Medford, MA, by Biocell Center Corporation[70][71][72] and collaborates with various hospitals and universities all over the world.[73]
Induced pluripotent
[edit]Adult stem cells have limitations with their potency; unlike embryonic stem cells (ESCs), they are not able to differentiate into cells from all three germ layers. As such, they are deemed multipotent.
However, reprogramming allows for the creation of pluripotent cells, induced pluripotent stem cells (iPSCs), from adult cells. These are not adult stem cells, but somatic cells (e.g. epithelial cells) reprogrammed to give rise to cells with pluripotent capabilities. Using genetic reprogramming with protein transcription factors, pluripotent stem cells with ESC-like capabilities have been derived.[74][75][76] The first demonstration of induced pluripotent stem cells was conducted by Shinya Yamanaka and his colleagues at Kyoto University.[77] They used the transcription factors Oct3/4, Sox2, c-Myc, and Klf4 to reprogram mouse fibroblast cells into pluripotent cells.[74][78] Subsequent work used these factors to induce pluripotency in human fibroblast cells.[79] Junying Yu, James Thomson, and their colleagues at the University of Wisconsin–Madison used a different set of factors, Oct4, Sox2, Nanog and Lin28, and carried out their experiments using cells from human foreskin.[74][80] However, they were able to replicate Yamanaka's finding that inducing pluripotency in human cells was possible.
Induced pluripotent stem cells differ from embryonic stem cells. They share many similar properties, such as pluripotency and differentiation potential, the expression of pluripotency genes, epigenetic patterns, embryoid body and teratoma formation, and viable chimera formation,[77][78] but there are many differences within these properties. The chromatin of iPSCs appears to be more "closed" or methylated than that of ESCs.[77][78] Similarly, the gene expression pattern between ESCs and iPSCs, or even iPSCs sourced from different origins.[77] There are thus questions about the "completeness" of reprogramming and the somatic memory of induced pluripotent stem cells. Despite this, inducing somatic cells to be pluripotent appears to be viable.
As a result of the success of these experiments, Ian Wilmut, who helped create the first cloned animal Dolly the Sheep, has announced that he will abandon somatic cell nuclear transfer as an avenue of research.[81]
The ability to induce pluripotency benefits developments in tissue engineering. By providing a suitable scaffold and microenvironment, iPSC can be differentiated into cells of therapeutic application, and for in vitro models to study toxins and pathogenesis.[82]
Induced pluripotent stem cells provide several therapeutic advantages. Like ESCs, they are pluripotent. They thus have great differentiation potential; theoretically, they could produce any cell within the human body (if reprogramming to pluripotency was "complete").[77] Moreover, unlike ESCs, they potentially could allow doctors to create a pluripotent stem cell line for each individual patient.[83] Frozen blood samples can be used as a valuable source of induced pluripotent stem cells.[84] Patient specific stem cells allow for the screening for side effects before drug treatment, as well as the reduced risk of transplantation rejection.[83] Despite their current limited use therapeutically, iPSCs hold great potential for future use in medical treatment and research.
Cell cycle control
[edit]The key factors controlling the cell cycle also regulate pluripotency. Thus, manipulation of relevant genes can maintain pluripotency and reprogram somatic cells to an induced pluripotent state.[39] However, reprogramming of somatic cells is often low in efficiency and considered stochastic.[85]
With the idea that a more rapid cell cycle is a key component of pluripotency, reprogramming efficiency can be improved. Methods for improving pluripotency through manipulation of cell cycle regulators include: overexpression of Cyclin D/Cdk4, phosphorylation of Sox2 at S39 and S253, overexpression of Cyclin A and Cyclin E, knockdown of Rb, and knockdown of members of the Cip/Kip family or the Ink family.[39] Furthermore, reprogramming efficiency is correlated with the number of cell divisions happened during the stochastic phase, which is suggested by the growing inefficiency of reprogramming of older or slow diving cells.[86]
Lineage
[edit]Lineage is an important procedure to analyze developing embryos. Since cell lineages shows the relationship between cells at each division. This helps in analyzing stem cell lineages along the way which helps recognize stem cell effectiveness, lifespan, and other factors. With the technique of cell lineage mutant genes can be analyzed in stem cell clones that can help in genetic pathways. These pathways can regulate how the stem cell perform.[87]
To ensure self-renewal, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives rise to two identical daughter cells both endowed with stem cell properties. Asymmetric division, on the other hand, produces only one stem cell and a progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before terminally differentiating into a mature cell. It is possible that the molecular distinction between symmetric and asymmetric divisions lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells.[88]
An alternative theory is that stem cells remain undifferentiated due to environmental cues in their particular niche. Stem cells differentiate when they leave that niche or no longer receive those signals. Studies in Drosophila germarium have identified the signals decapentaplegic and adherens junctions that prevent germarium stem cells from differentiating.[89][90]
In the United States, Executive Order 13505 established that federal money can be used for research in which approved human embryonic stem-cell (hESC) lines are used, but it cannot be used to derive new lines.[91] The National Institutes of Health (NIH) Guidelines on Human Stem Cell Research, effective July 7, 2009, implemented the Executive Order 13505 by establishing criteria which hESC lines must meet to be approved for funding.[92] The NIH Human Embryonic Stem Cell Registry can be accessed online and has updated information on cell lines eligible for NIH funding.[93] There are 486 approved lines as of January 2022.[94]
Therapies
[edit]Stem cell therapy is the use of stem cells to treat or prevent a disease or condition. Bone marrow transplant is a form of stem cell therapy that has been used for many years because it has proven to be effective in clinical trials.[95][96] Stem cell implantation may help in strengthening the left-ventricle of the heart, as well as retaining the heart tissue to patients who have suffered from heart attacks in the past.[97]
For over 50 years, hematopoietic stem cell transplantation (HSCT) has been used to treat people with conditions such as leukaemia and lymphoma; this is the only widely practiced form of stem-cell therapy.[95][96][98] As of 2016[update], the only established therapy using stem cells is hematopoietic stem cell transplantation.[4] This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases[99][30]>[100] and conditions such as diabetes and heart disease.
Advantages
[edit]Stem cell treatments may lower symptoms of the disease or condition that is being treated. The lowering of symptoms may allow patients to reduce the drug intake of the disease or condition. Stem cell treatment may also provide knowledge for society to further stem cell understanding and future treatments.[101] The physicians' creed would be to do no injury, and stem cells make that simpler than ever before. Surgical processes by their character are harmful. Tissue has to be dropped as a way to reach a successful outcome. One may prevent the dangers of surgical interventions using stem cells. Additionally, there's a possibility of disease, and whether the procedure fails, further surgery may be required. Risks associated with anesthesia can also be eliminated with stem cells.[102] On top of that, stem cells have been harvested from the patient's body and redeployed in which they're wanted. Since they come from the patient's own body, this is referred to as an autologous treatment. Autologous remedies are thought to be the safest because there's likely zero probability of donor substance rejection.
Disadvantages
[edit]Stem cell treatments may require immunosuppression because of a requirement for radiation before the transplant to remove the person's previous cells, or because the patient's immune system may target the stem cells. One approach to avoid the second possibility is to use stem cells from the same patient who is being treated.
Pluripotency in certain stem cells could also make it difficult to obtain a specific cell type. It is also difficult to obtain the exact cell type needed, because not all cells in a population differentiate uniformly. Undifferentiated cells can create tissues other than desired types.[103]
Some stem cells form tumors after transplantation;[104] pluripotency is linked to tumor formation especially in embryonic stem cells, fetal proper stem cells, induced pluripotent stem cells. Fetal proper stem cells form tumors despite multipotency.[105]
Ethical concerns are also raised about the practice of using or researching embryonic stem cells. Harvesting cells from the blastocyst results in the death of the blastocyst. The concern is whether or not the blastocyst should be considered as a human life.[106] The debate on this issue is mainly a philosophical one, not a scientific one.
Stem cell tourism
[edit]Stem cell tourism is the part of the medical tourism industry in which patients travel to obtain stem cell procedures.[107]
The United States has had an explosion of "stem cell clinics".[108] Stem cell procedures are highly profitable for clinics. The advertising sounds authoritative but the efficacy and safety of the procedures is unproven. Patients sometimes experience complications, such as spinal tumors[109] and death. The high expense can also lead to financial problems.[109] According to researchers, there is a need to educate the public, patients, and doctors about this issue.[110]
According to the International Society for Stem Cell Research, the largest academic organization that advocates for stem cell research, stem cell therapies are under development and cannot yet be said to be proven.[111][112] Doctors should inform patients that clinical trials continue to investigate whether these therapies are safe and effective but that unethical clinics present them as proven.[113]
Research
[edit]Some of the fundamental patents covering human embryonic stem cells are owned by the Wisconsin Alumni Research Foundation (WARF) – they are patents 5,843,780, 6,200,806, and 7,029,913 invented by James A. Thomson. WARF does not enforce these patents against academic scientists, but does enforce them against companies.[114]
In 2006, a request for the US Patent and Trademark Office (USPTO) to re-examine the three patents was filed by the Public Patent Foundation[115] on behalf of its client, the non-profit patent-watchdog group Consumer Watchdog (formerly the Foundation for Taxpayer and Consumer Rights).[114] In the re-examination process, which involves several rounds of discussion between the USPTO and the parties, the USPTO initially agreed with Consumer Watchdog and rejected all the claims in all three patents,[116] however in response, WARF amended the claims of all three patents to make them more narrow, and in 2008 the USPTO found the amended claims in all three patents to be patentable. The decision on one of the patents (7,029,913) was appealable, while the decisions on the other two were not.[117][118] Consumer Watchdog appealed the granting of the '913 patent to the USPTO's Board of Patent Appeals and Interferences (BPAI) which granted the appeal, and in 2010 the BPAI decided that the amended claims of the '913 patent were not patentable.[119] However, WARF was able to re-open prosecution of the case and did so, amending the claims of the '913 patent again to make them more narrow, and in January 2013 the amended claims were allowed.[120]
In July 2013, Consumer Watchdog announced that it would appeal the decision to allow the claims of the '913 patent to the US Court of Appeals for the Federal Circuit (CAFC), the federal appeals court that hears patent cases.[121] At a hearing in December 2013, the CAFC raised the question of whether Consumer Watchdog had legal standing to appeal; the case could not proceed until that issue was resolved.[122]
Conditions
[edit]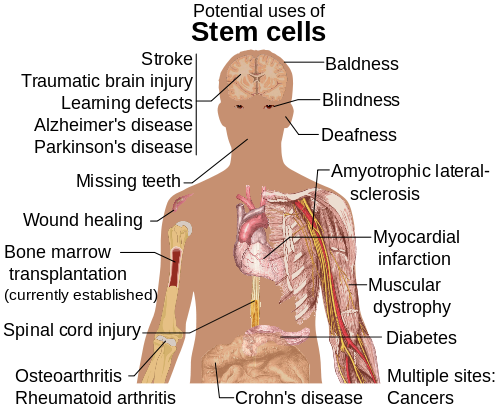
Diseases and conditions where stem cell treatment is being investigated include:
- Diabetes[123]
- Androgenic Alopecia and hair loss[124][125]
- Rheumatoid arthritis[123]
- Parkinson's disease[123]
- Alzheimer's disease[123]
- Respiratory disease[126]
- Osteoarthritis[123]
- Stroke and traumatic brain injury repair[127]
- Learning disability due to congenital disorder[128]
- Spinal cord injury repair[129]
- Heart infarction[130]
- Anti-cancer treatments[127]
- Baldness reversal[131]
- Replace missing teeth[132]
- Repair hearing[133]
- Restore vision[134] and repair damage to the cornea[135]
- Amyotrophic lateral sclerosis[136]
- Crohn's disease[137]
- Wound healing[138]
- Male infertility due to absence of spermatogonial stem cells.[139] In recent studies, scientists have found a way to solve this problem by reprogramming a cell and turning it into a spermatozoon. Other studies have proven the restoration of spermatogenesis by introducing human iPSC cells in mice testicles. This could mean the end of azoospermia.[140]
- Female infertility: oocytes made from embryonic stem cells. Scientists have found the ovarian stem cells, a rare type of cells (0.014%) found in the ovary. They could be used as a treatment not only for infertility, but also for premature ovarian insufficiency (POI).[141] New research posted in Science Direct suggests that ovarian follicles could be triggered to grow in the ovarian environment by using stem cells present in bone marrow. This study was conducted by infusing human bone marrow stem cells into immune-deficient mice to improve fertilization.[142] Another study conducted using mice with damaged ovarian function from chemothearpy found that in vivo therapy with bone marrow stem cells can heal the damaged ovaries.[143] Both of these studies are proof-of-concept and need to be furthered tested, but they have the possibility improve fertility for individuals who have POI from chemotherapy treatment.
- Critical Limb Ischemia[144]
Production
[edit]Research is underway to develop various sources for stem cells.[145]
Organoids
[edit]Research is attempting to generating organoids using stem cells, which would allow for further understanding of human development, organogenesis, and modeling of human diseases.[146] Engineered 'synthetic organizer' (SO) cells can instruct stem cells to grow into specific tissues and organs. The program used native and synthetic cell adhesion protein molecules (CAMs) that help make cells sticky. The organizer cells self-assembled around mouse ESCs. These cells were engineered to produce morphogens (signaling molecules) that direct cellular development based on their concentration. Delivered morphogens disperse, leaving higher concentrations closer to the source and lower concentrations further away. These gradients signal cells' ultimate roles, such as nerve, skin cell, or connective tissue. The engineered organizer cells were also fitted with a chemical switch that enabled the researchers to turn the delivery of cellular instructions on and off, as well as a 'suicide switch' for eliminating the cells when needed. SOs carry spatial and biochemical information, allowing considerable discretion in organoid formation.[147]
Risks
[edit]Hepatotoxicity and drug-induced liver injury account for a substantial number of failures of new drugs in development and market withdrawal, highlighting the need for screening assays such as stem cell-derived hepatocyte-like cells, that are capable of detecting toxicity early in the drug development process.[148]
Dormancy
[edit]In August 2021, researchers in the Princess Margaret Cancer Centre at the University Health Network published their discovery of a dormancy mechanism in key stem cells which could help develop cancer treatments in the future.[149]
See also
[edit]References
[edit]- ^ a b Atala A, Lanza R (2012). Handbook of Stem Cells. Academic Press. ISBN 978-0-12-385943-3.
- ^ Becker AJ, McCulloch EA, Till JE (February 1963). "Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells". Nature. 197 (4866): 452–4. Bibcode:1963Natur.197..452B. doi:10.1038/197452a0. hdl:1807/2779. ISSN 0028-0836. PMID 13970094. S2CID 11106827.
- ^ Siminovitch L, McCulloch EA, Till JE (December 1963). "The distribution of colony-forming cells among spleen colonies". Journal of Cellular and Comparative Physiology. 62 (3): 327–336. doi:10.1002/jcp.1030620313. hdl:1807/2778. PMID 14086156. S2CID 43875977.
- ^ a b Müller AM, Huppertz S, Henschler R (July 2016). "Hematopoietic Stem Cells in Regenerative Medicine: Astray or on the Path?". Transfusion Medicine and Hemotherapy. 43 (4): 247–254. doi:10.1159/000447748. PMC 5040947. PMID 27721700.
- ^ Ralston, Michelle (17 July 2008). "Stem Cell Research Around the World". Pew Research Center's Religion & Public Life Project.
- ^ Tuch, B. E. (September 2006). "Stem cells: a clinical update". Australian Family Physician. 35 (9): 719–721. PMID 16969445. ProQuest 216301343.
- ^ a b Ferreira L (2014-01-03). "Stem Cells: A Brief History and Outlook". Stem Cells: A Brief History and Outlook – Science in the News. WordPress. Retrieved 3 December 2019.
- ^ a b Ramalho-Santos, Miguel; Willenbring, Holger (June 2007). "On the Origin of the Term 'Stem Cell'". Cell Stem Cell. 1 (1): 35–38. doi:10.1016/j.stem.2007.05.013. PMID 18371332.
- ^ MacPherson C. "The Accidental Discovery of Stem Cells". USask News. University of Saskatchewan. Retrieved 3 December 2019.
- ^ Johnston, Wm. Robert. "Vinca reactor accident, 1958". www.johnstonsarchive.net. Archived from the original on 27 January 2011. Retrieved 2024-12-28.
- ^ Boyle, Rebecca (2011-01-15). "Injured Brazilian Wolf Is First Wild Animal Treated With Stem Cells". Popular Science. Retrieved 2024-12-28.
- ^ suporte (2011-01-11). "Tratamento". CFMV (in Brazilian Portuguese). Conselho Federal de Medicina Veterinária. Retrieved 2024-12-28.
- ^ He S, Nakada D, Morrison SJ (2009). "Mechanisms of stem cell self-renewal". Annu Rev Cell Dev Biol. 25: 377–406. doi:10.1146/annurev.cellbio.042308.113248. PMID 19575646.
- ^ Cong YS, Wright WE, Shay JW (September 2002). "Human telomerase and its regulation". Microbiology and Molecular Biology Reviews. 66 (3): 407–425, table of contents. doi:10.1128/MMBR.66.3.407-425.2002. PMC 120798. PMID 12208997.
- ^ a b c d e f Schöler HR (2007). "The Potential of Stem Cells: An Inventory". In Nikolaus Knoepffler, Dagmar Schipanski, Stefan Lorenz Sorgner (eds.). Humanbiotechnology as Social Challenge. Ashgate Publishing. p. 28. ISBN 978-0-7546-5755-2.
- ^ Mitalipov S, Wolf D (2009). "Totipotency, pluripotency and nuclear reprogramming". Engineering of Stem Cells. Advances in Biochemical Engineering/Biotechnology. Vol. 114. Springer. pp. 185–199. Bibcode:2009esc..book..185M. doi:10.1007/10_2008_45. ISBN 978-3-540-88805-5. PMC 2752493. PMID 19343304.
- ^ Ulloa-Montoya F, Verfaillie CM, Hu WS (July 2005). "Culture systems for pluripotent stem cells". Journal of Bioscience and Bioengineering. 100 (1): 12–27. doi:10.1263/jbb.100.12. PMID 16233846.
- ^ Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA (1974). "Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method". Experimental Hematology. 2 (2): 83–92. PMID 4455512. INIST PASCAL7536501060 NAID 10025700209.
- ^ Friedenstein AJ, Gorskaja JF, Kulagina NN (September 1976). "Fibroblast precursors in normal and irradiated mouse hematopoietic organs". Experimental Hematology. 4 (5): 267–274. PMID 976387.
- ^ Sekhar L, Bisht N (2006-09-01). "Stem Cell Therapy". Apollo Medicine. 3 (3): 271–6. doi:10.1016/S0976-0016(11)60209-3.
- ^ Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (November 1998). "Embryonic stem cell lines derived from human blastocysts". Science. 282 (5391): 1145–47. Bibcode:1998Sci...282.1145T. doi:10.1126/science.282.5391.1145. PMID 9804556.
- ^ a b Gilbert, Scott F. (2014). Developmental Biology. Sinauer Associates. ISBN 978-0-87893-978-7.[page needed]
- ^ Rakic P (October 2009). "Evolution of the neocortex: a perspective from developmental biology". Nature Reviews. Neuroscience. 10 (10): 724–735. doi:10.1038/nrn2719. PMC 2913577. PMID 19763105.
- ^ Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (February 2001). "Neurons derived from radial glial cells establish radial units in neocortex". Nature. 409 (6821): 714–720. Bibcode:2001Natur.409..714N. doi:10.1038/35055553. PMID 11217860. S2CID 3041502.
- ^ Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A (May 2008). "The ground state of embryonic stem cell self-renewal". Nature. 453 (7194): 519–523. Bibcode:2008Natur.453..519Y. doi:10.1038/nature06968. PMC 5328678. PMID 18497825.
- ^ "Culture of Human Embryonic Stem Cells (hESC)". National Institutes of Health. Archived from the original on 2010-01-06. Retrieved 2010-03-07.
- ^ Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (May 2003). "Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells". Cell. 113 (5): 643–655. doi:10.1016/S0092-8674(03)00392-1. hdl:1842/843. PMID 12787505. S2CID 2236779.
- ^ Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA (September 2005). "Core transcriptional regulatory circuitry in human embryonic stem cells". Cell. 122 (6): 947–956. doi:10.1016/j.cell.2005.08.020. PMC 3006442. PMID 16153702.
- ^ Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, et al. (The International Stem Cell Initiative) (July 2007). "Characterization of human embryonic stem cell lines by the International Stem Cell Initiative". Nature Biotechnology. 25 (7): 803–816. doi:10.1038/nbt1318. PMID 17572666. S2CID 13780999.
- ^ a b Mahla RS (2016). "Stem Cells Applications in Regenerative Medicine and Disease Therapeutics". International Journal of Cell Biology. 2016 (7): 1–24. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.
- ^ Winslow R, Mundy A (23 January 2009). "First Embryonic Stem-Cell Trial Gets Approval from the FDA". The Wall Street Journal.
- ^ "Embryonic Stem Cell Therapy At Risk? Geron Ends Clinical Trial". ScienceDebate.com. Archived from the original on 2014-08-22. Retrieved 2011-12-11.
- ^ Wu DC, Boyd AS, Wood KJ (May 2007). "Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine". Frontiers in Bioscience. 12 (8–12): 4525–35. doi:10.2741/2407. PMID 17485394. S2CID 6355307.
- ^ a b Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE (2015-09-28). "Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives". Stem Cells and Cloning: Advances and Applications. 8: 125–134. doi:10.2147/SCCAA.S88036. PMC 4592031. PMID 26451119.
- ^ Caplan AI (September 1991). "Mesenchymal stem cells". Journal of Orthopaedic Research. 9 (5): 641–650. doi:10.1002/jor.1100090504. PMID 1870029. S2CID 22606668.
- ^ Krasilnikova, O. A.; Baranovskii, D. S.; Lyundup, A. V.; Shegay, P. V.; Kaprin, A. D.; Klabukov, I. D. (2022-04-27). "Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action". Stem Cell Reviews and Reports. 18 (6): 1974–85. doi:10.1007/s12015-022-10379-z. ISSN 2629-3277. PMID 35476187. S2CID 248402820.
- ^ a b c d e f Koledova Z, Krämer A, Kafkova LR, Divoky V (November 2010). "Cell-cycle regulation in embryonic stem cells: centrosomal decisions on self-renewal". Stem Cells and Development. 19 (11): 1663–78. doi:10.1089/scd.2010.0136. PMID 20594031.
- ^ a b c d Barta T, Dolezalova D, Holubcova Z, Hampl A (March 2013). "Cell cycle regulation in human embryonic stem cells: links to adaptation to cell culture". Experimental Biology and Medicine. 238 (3): 271–5. doi:10.1177/1535370213480711. PMID 23598972. S2CID 2028793.
- ^ a b c d e f g Zaveri L, Dhawan J (2018). "Cycling to Meet Fate: Connecting Pluripotency to the Cell Cycle". Frontiers in Cell and Developmental Biology. 6 57. doi:10.3389/fcell.2018.00057. PMC 6020794. PMID 29974052.
- ^ Koledova Z, Kafkova LR, Calabkova L, Krystof V, Dolezel P, Divoky V (February 2010). "Cdk2 inhibition prolongs G1 phase progression in mouse embryonic stem cells". Stem Cells and Development. 19 (2): 181–194. doi:10.1089/scd.2009.0065. PMID 19737069.
- ^ Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS (December 2006). "Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase". Journal of Cellular Physiology. 209 (3): 883–893. doi:10.1002/jcp.20776. PMID 16972248. S2CID 24908771.
- ^ Ariff Bongso; Eng Hin Lee, eds. (2005). "Stem cells: their definition, classification and sources". Stem Cells: From Benchtop to Bedside. World Scientific. p. 5. ISBN 978-981-256-126-8. OCLC 443407924.
- ^ Moore, Keith L.; Persaud, T. V. N.; Torchia, Mark G. (2013). Before We are Born: Essentials of Embryology and Birth Defects. Saunders/Elsevier. ISBN 978-1-4377-2001-3.[page needed]
- ^ "What is a stem cell". anthonynolan.org. Anthony Nolan. Retrieved 17 February 2022.
- ^ "Bone marrow (stem cell) donation: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 2021-10-17.
- ^ Coughlin RP, Oldweiler A, Mickelson DT, Moorman CT (October 2017). "Adipose-Derived Stem Cell Transplant Technique for Degenerative Joint Disease". Arthroscopy Techniques. 6 (5): e1761 – e1766. doi:10.1016/j.eats.2017.06.048. PMC 5795060. PMID 29399463.
- ^ "autologous stem cell transplant". www.cancer.gov. 2011-02-02. Retrieved 2022-06-26.
- ^ Lv, Haining; Hu, Yali; Cui, Zhanfeng; Jia, Huidong (2018). "Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine". Stem Cell Research & Therapy. 9 (1) 325. doi:10.1186/s13287-018-1067-y. PMC 6249727. PMID 30463587.
- ^ Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M (May 2007). "A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues". Leukemia. 21 (5): 860–7. doi:10.1038/sj.leu.2404630. PMID 17344915. S2CID 21433689.
- ^ Narasipura SD, Wojciechowski JC, Charles N, Liesveld JL, King MR (January 2008). "P-Selectin coated microtube for enrichment of CD34+ hematopoietic stem and progenitor cells from human bone marrow". Clinical Chemistry. 54 (1): 77–85. doi:10.1373/clinchem.2007.089896. PMID 18024531.
- ^ Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, Okita K, Sakaida I (October 2006). "Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy". Stem Cells. 24 (10): 2292–98. doi:10.1634/stemcells.2005-0542. PMID 16778155. S2CID 5649484.
- ^ Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S, Manjunath SR, Senthilkumar R, Murugan P, Srinivasan VR, Abraham S (September 2011). "Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience". Cytotherapy. 13 (8): 993–9. doi:10.3109/14653249.2011.579961. PMID 21671823. S2CID 27251276.
- ^ Madhusankar N (2007). "Use of Bone Marrow derived Stem Cells in Patients with Cardiovascular Disorders". Journal of Stem Cells and Regenerative Medicine. 3 (1): 28–29. PMC 3908115. PMID 24693021.
- ^ Dedeepiya VD, Rao YY, Jayakrishnan GA, Parthiban JK, Baskar S, Manjunath SR, Senthilkumar R, Abraham SJ (2012). "Index of CD34+ Cells and Mononuclear Cells in the Bone Marrow of Spinal Cord Injury Patients of Different Age Groups: A Comparative Analysis". Bone Marrow Research. 2012: 1–8. doi:10.1155/2012/787414. PMC 3398573. PMID 22830032.
- ^ Gardner RL (March 2002). "Stem cells: potency, plasticity and public perception". Journal of Anatomy. 200 (Pt 3): 277–282. doi:10.1046/j.1469-7580.2002.00029.x. PMC 1570679. PMID 12033732.
- ^ Behrens A, van Deursen JM, Rudolph KL, Schumacher B (March 2014). "Impact of genomic damage and ageing on stem cell function". Nature Cell Biology. 16 (3): 201–7. doi:10.1038/ncb2928. PMC 4214082. PMID 24576896.
- ^ Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC (November 2006). "Review: ex vivo engineering of living tissues with adult stem cells". Tissue Engineering. 12 (11): 3007–19. CiteSeerX 10.1.1.328.2873. doi:10.1089/ten.2006.12.3007. PMID 17518617.
- ^ Gimble JM, Katz AJ, Bunnell BA (May 2007). "Adipose-derived stem cells for regenerative medicine". Circulation Research. 100 (9): 1249–60. doi:10.1161/01.RES.0000265074.83288.09. PMC 5679280. PMID 17495232.
- ^ Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M (May 2010). "Unique multipotent cells in adult human mesenchymal cell populations". Proceedings of the National Academy of Sciences of the United States of America. 107 (19): 8639–43. Bibcode:2010PNAS..107.8639K. doi:10.1073/pnas.0911647107. PMC 2889306. PMID 20421459.
- ^ "Bone Marrow Transplant". ucsfchildrenshospital.org.
- ^ Kane, Ed (2008-05-01). "Stem-cell therapy shows promise for horse soft-tissue injury, disease". DVM Newsmagazine. Archived from the original on 2008-12-11. Retrieved 2008-06-12.
- ^ "Stem Cell FAQ". US Department of Health and Human Services. 2004-07-14. Archived from the original on 2009-01-09.
- ^ Oliveira PH, da Silva CL, Cabral JM (2014). "Genomic Instability in Human Stem Cells: Current Status and Future Challenges". Stem Cells. 32 (11): 2824–32. doi:10.1002/stem.1796. PMID 25078438. S2CID 41335566.
- ^ a b Zhang L, Mack R, Breslin P, Zhang J (November 2020). "Molecular and cellular mechanisms of aging in hematopoietic stem cells and their niches". J Hematol Oncol. 13 (1) 157. doi:10.1186/s13045-020-00994-z. PMC 7686726. PMID 33228751.
- ^ Montazersaheb S, Ehsani A, Fathi E, Farahzadi R (2022). "Cellular and Molecular Mechanisms Involved in Hematopoietic Stem Cell Aging as a Clinical Prospect". Oxid Med Cell Longev. 2022 2713483. doi:10.1155/2022/2713483. PMC 8993567. PMID 35401928.
- ^ De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (January 2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biotechnology. 25 (1): 100–6. doi:10.1038/nbt1274. PMID 17206138. S2CID 6676167.
- ^ "Vatican newspaper calls new stem cell source 'future of medicine'". Catholic News Agency. 3 February 2010.
- ^ "European Biotech Company Biocell Center Opens First U.S. Facility for Preservation of Amniotic Stem Cells in Medford, Massachusetts". Reuters. 2009-10-22. Archived from the original on 2009-10-30. Retrieved 2010-03-14.
- ^ "Europe's Biocell Center opens Medford office – Daily Business Update". The Boston Globe. 2009-10-22. Retrieved 2010-03-14.
- ^ "The Ticker". Boston Herald. BostonHerald.com. 2009-10-22. Retrieved 2010-03-14.
- ^ "Biocell Center opens amniotic stem cell bank in Medford". Mass High Tech Business News. 2009-10-23. Archived from the original on 2012-10-14. Retrieved 2012-08-26.
- ^ "World's First Amniotic Stem Cell Bank Opens In Medford". wbur.org. 22 October 2009. Retrieved 2010-03-14.
- ^ "Biocell Center Corporation Partners with New England's Largest Community-Based Hospital Network to Offer a Unique..." (Press release). Medford, Mass.: Prnewswire.com. Retrieved 2010-03-14.
- ^ a b c "Making human embryonic stem cells". The Economist. 2007-11-22.
- ^ Brand M, Palca J, Cohen A (2007-11-20). "Skin Cells Can Become Embryonic Stem Cells". National Public Radio.
- ^ "Breakthrough Set to Radically Change Stem Cell Debate". News Hour with Jim Lehrer. 2007-11-20. Archived from the original on 2014-01-22. Retrieved 2017-09-15.
- ^ a b c d e Kimbrel EA, Lanza R (December 2016). "Pluripotent stem cells: the last 10 years". Regenerative Medicine. 11 (8): 831–847. doi:10.2217/rme-2016-0117. PMID 27908220.
- ^ a b c Takahashi K, Yamanaka S (August 2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell. 126 (4): 663–676. doi:10.1016/j.cell.2006.07.024. hdl:2433/159777. PMID 16904174. S2CID 1565219.
- ^ Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (November 2007). "Induction of pluripotent stem cells from adult human fibroblasts by defined factors". Cell. 131 (5): 861–872. doi:10.1016/j.cell.2007.11.019. hdl:2433/49782. PMID 18035408. S2CID 8531539.
- ^ Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (December 2007). "Induced pluripotent stem cell lines derived from human somatic cells". Science. 318 (5858): 1917–20. Bibcode:2007Sci...318.1917Y. doi:10.1126/science.1151526. PMID 18029452. S2CID 86129154.
- ^ "His inspiration comes from the research by Prof Shinya Yamanaka at Kyoto University, which suggests a way to create human embryo stem cells without the need for human eggs, which are in extremely short supply, and without the need to create and destroy human cloned embryos, which is bitterly opposed by the pro life movement." Highfield R (2007-11-16). "Dolly creator Prof Ian Wilmut shuns cloning". The Telegraph. London. Archived from the original on 2009-07-30.
- ^ Maldonado M, Luu RJ, Ico G, Ospina A, Myung D, Shih HP, Nam J (September 2017). "Lineage- and developmental stage-specific mechanomodulation of induced pluripotent stem cell differentiation". Stem Cell Research & Therapy. 8 (1) 216. doi:10.1186/s13287-017-0667-2. PMC 5622562. PMID 28962663.
- ^ a b Robinton, DA; Daley, GQ (January 2012). "The promise of induced pluripotent stem cells in research and therapy". Nature. 481 (7381): 295–305. Bibcode:2012Natur.481..295R. doi:10.1038/nature10761. PMC 3652331. PMID 22258608.
- ^ Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R (July 2010). "Reprogramming of human peripheral blood cells to induced pluripotent stem cells". Cell Stem Cell. 7 (1): 20–24. doi:10.1016/j.stem.2010.06.002. PMC 2917234. PMID 20621045.
- Lay summary in: "Frozen blood a source of stem cells, study finds". NewsDaily. Reuters. 2010-07-01. Archived from the original on 2010-07-03.
- ^ Chen X, Hartman A, Guo S (2015-09-01). "Choosing Cell Fate Through a Dynamic Cell Cycle". Current Stem Cell Reports. 1 (3): 129–138. doi:10.1007/s40778-015-0018-0. PMC 5487535. PMID 28725536.
- ^ Hindley C, Philpott A (April 2013). "The cell cycle and pluripotency". The Biochemical Journal. 451 (2): 135–143. doi:10.1042/BJ20121627. PMC 3631102. PMID 23535166.
- ^ Fox, D.T.; Morris, L.X.; Nystul, T.; Spradling, A.C. (2008). "Lineage analysis of stem cells". StemBook. doi:10.3824/stembook.1.33.1. PMID 20614627.
- ^ Beckmann, Julia; Scheitza, Sebastian; Wernet, Peter; Fischer, Johannes C.; Giebel, Bernd (15 June 2007). "Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins". Blood. 109 (12): 5494–5501. doi:10.1182/blood-2006-11-055921. PMID 17332245.
- ^ Xie, Ting; Spradling, Allan C (July 1998). "decapentaplegic Is Essential for the Maintenance and Division of Germline Stem Cells in the Drosophila Ovary". Cell. 94 (2): 251–260. doi:10.1016/s0092-8674(00)81424-5. PMID 9695953. S2CID 11347213.
- ^ Song, X.; Zhu, CH; Doan, C; Xie, T (7 June 2002). "Germline Stem Cells Anchored by Adherens Junctions in the Drosophila Ovary Niches". Science. 296 (5574): 1855–7. Bibcode:2002Sci...296.1855S. doi:10.1126/science.1069871. PMID 12052957. S2CID 25830121.
- ^ "Executive Order: Removing barriers to responsible scientific research involving human stem cells". whitehouse.gov. 9 March 2009 – via National Archives.
- ^ "National Institutes of Health Guidelines on Human Stem Cell Research". Retrieved 24 April 2014.
- ^ "NIH Human Embryonic Stem Cell Registry". Retrieved 1 March 2017.
- ^ "NIH Human Embryonic Stem Cell Registry". Retrieved 10 February 2022.
- ^ a b Murnaghan, Ian. "Why Perform a Stem Cell Transplant? – Explore Stem Cells". www.explorestemcells.co.uk. Retrieved 2024-12-28.
- ^ a b "Stem Cell and Bone Marrow Transplants for Cancer - NCI". www.cancer.gov. 2015-04-29. Retrieved 2024-12-28.
- ^ Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. (January 2003). "Autologous bone-marrow stem-cell transplantation for myocardial regeneration". Lancet. 361 (9351): 45–46. doi:10.1016/S0140-6736(03)12110-1. PMID 12517467. S2CID 23858666.
- ^ Karanes C, Nelson GO, Chitphakdithai P, Agura E, Ballen KK, Bolan CD, Porter DL, Uberti JP, King RJ, Confer DL (2008). "Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program". Biology of Blood and Marrow Transplantation. 14 (9 Suppl): 8–15. doi:10.1016/j.bbmt.2008.06.006. PMID 18721775.
- ^ Lyon, Louisa (2018-10-01). "Stem cell therapies in neurology: the good, the bad and the unknown". Brain. 141 (10): e77. doi:10.1093/brain/awy221. ISSN 0006-8950. PMID 30202947.
- ^ "Tế bào gốc là gì". Mirai Care (in Vietnamese). Retrieved 2023-11-27.
- ^ Master Z, McLeod M, Mendez I (March 2007). "Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson's disease". Journal of Medical Ethics. 33 (3): 169–173. doi:10.1136/jme.2005.013169. JSTOR 27719821. PMC 2598267. PMID 17329391.
- ^ "Comprehensive Stem Cell Training Course". R3 Medical Training. Retrieved 2021-05-15.
- ^ Moore KL, Persaud TV, Torchia MG (2000-03-04). "Before We Are Born: Essentials of Embryology and Birth Defects, 5th edition". Journal of Midwifery & Women's Health. 45 (2). Philadelphia, PA: Saunders, Elsevier: 192. doi:10.1016/s1526-9523(99)00026-4. ISSN 1526-9523.
- ^ Healy, Bernadine. "Why Embryonic Stem Cells are obsolete". US News and world report. Retrieved Aug 17, 2015.
- ^ Ballantyne, Coco. "Fetal stem cells cause tumor in a teenage boy". Scientific American.
- ^ Lo, Bernard; Parham, Lindsay (May 2009). "Ethical Issues in Stem Cell Research". Endocrine Reviews. 30 (3). NIH: 204–213. doi:10.1210/er.2008-0031. PMC 2726839. PMID 19366754.
- ^ Bauer, G; Elsallab, M; Abou-El-Enein, M (September 2018). "Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions". Stem Cells Translational Medicine. 7 (9): 676–685. doi:10.1002/sctm.17-0282. PMC 6127222. PMID 30063299.
- ^ Blackwell, Tom (2016-07-13). "Study reveals explosion of unproven stem-cell treatment in U.S. — and many Canadians are seeking them out". National Post. Retrieved 2022-03-22.
- ^ a b Berkowitz, Aaron L.; Miller, Michael B.; Mir, Saad A.; Cagney, Daniel; Chavakula, Vamsidhar; Guleria, Indira; Aizer, Ayal; Ligon, Keith L.; Chi, John H. (14 July 2016). "Glioproliferative Lesion of the Spinal Cord as a Complication of 'Stem-Cell Tourism'". New England Journal of Medicine. 375 (2): 196–8. doi:10.1056/NEJMc1600188. PMID 27331440.
- ^ Bowman, Michelle; Racke, Michael; Kissel, John; Imitola, Jaime (1 November 2015). "Responsibilities of Health Care Professionals in Counseling and Educating Patients With Incurable Neurological Diseases Regarding 'Stem Cell Tourism': Caveat Emptor". JAMA Neurology. 72 (11): 1342–5. doi:10.1001/jamaneurol.2015.1891. PMID 26322563.
- ^ "Communicating About Unproven Stem Cell Treatments to the Public".
- ^ Tsou, Amy (February 2015). "Ethical Considerations When Counseling Patients About Stem Cell Tourism". Continuum: Lifelong Learning in Neurology. 21 (1 Spinal Cord Disorders): 201–5. doi:10.1212/01.CON.0000461094.76563.be. PMID 25651226.
- ^ Du, Li; Rachul, Christen; Guo, Zhaochen; Caulfield, Timothy (9 March 2016). "Gordie Howe's 'Miraculous Treatment': Case Study of Twitter Users' Reactions to a Sport Celebrity's Stem Cell Treatment". JMIR Public Health and Surveillance. 2 (1): e8. doi:10.2196/publichealth.5264. PMC 4869214. PMID 27227162.
- ^ a b "How a University's Patents May Limit Stem-Cell Research". The Wall Street Journal. Retrieved 2024-12-28.
- ^ "Public Patent Foundation - GuideStar Profile". www.guidestar.org. Retrieved 2024-12-28.
- ^ Jenei, Stephen (April 3, 2007). "WARF Stem Cell Patents Knocked Down in Round One". Patent Baristas. Retrieved 2024-12-28.
- ^ Jenei, Stephen (March 3, 2008). "Ding! WARF Wins Round 2 As Stem Cell Patent Upheld". Patent Baristas. Retrieved 2024-12-28.
- ^ Holden, Constance (March 12, 2008). "WARF Goes 3 for 3 on Patents". Science Now. Retrieved 2024-12-28.
- ^ McKeown, Scott (2010-05-10). "BPAI Rejects WARF Stem Cell Patent Claims in Inter Partes Reexamination Appeal". Patents Post-Grant. Retrieved 2024-12-28.
- ^ "The Foundation For Taxpayer & Consumer Rights, Requester And Appellant V. Patent Of Wisconsin Alumni Research Foundation, Appeal 2012-011693, Reexamination Control 95/000,154 Patent 7,029,913". e-foia.uspto.gov. Archived from the original on 2013-02-20. Retrieved 2024-12-28.
- ^ "Consumer Watchdog, PPF Seek Invalidation of WARF's Stem Cell Patent". GenomeWeb. 2013-07-03. Retrieved 2024-12-28.
- ^ Antoinette Konski for Personalized Medicine Bulletin. February 3, 2014 U.S. Government and USPTO Urges Federal Circuit to Dismiss Stem Cell Appeal
- ^ a b c d e "Stemcells Redirection". stemcells.nih.gov. 2009. Archived from the original on 2018-06-19. Retrieved 2024-12-28.
- ^ "Treating Hair Loss with Stem Cell & PRP Therapy". Stem Cells LA. 2019-02-20. Retrieved 2020-05-30.
- ^ Gentile, Pietro; Garcovich, Simone; Bielli, Alessandra; Scioli, Maria Giovanna; Orlandi, Augusto; Cervelli, Valerio (November 2015). "The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial". Stem Cells Translational Medicine. 4 (11): 1317–23. doi:10.5966/sctm.2015-0107. ISSN 2157-6564. PMC 4622412. PMID 26400925.
- ^ Hynds, R (2022). "Exploiting the potential of lung stem cells to develop pro-regenerative therapies". Biology Open. 11 (10) bio059423. doi:10.1242/bio.059423. PMC 9581519. PMID 36239242.
- ^ a b Steinberg, Douglas (26 November 2000). "Stem Cells Tapped to Replenish Organs". The Scientist Magazine.
- ^ "Israeli scientists reverse brain birth defects using stem cells". ISRAEL21c. 2008-12-25. Retrieved 2024-12-28.
- ^ Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H (2005). "A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study". Cytotherapy. 7 (4): 368–373. doi:10.1080/14653240500238160. PMID 16162459. S2CID 33471639.
- ^ Strauer BE, Schannwell CM, Brehm M (April 2009). "Therapeutic potentials of stem cells in cardiac diseases". Minerva Cardioangiologica. 57 (2): 249–267. PMID 19274033.
- ^ DeNoon, Daniel J. (4 November 2004). "Hair Cloning Nears Reality as Baldness Cure". WebMD.
- ^ Yen AH, Sharpe PT (January 2008). "Stem cells and tooth tissue engineering". Cell and Tissue Research. 331 (1): 359–372. doi:10.1007/s00441-007-0467-6. PMID 17938970. S2CID 23765276.
- ^ "Gene therapy is first deafness 'cure'". New Scientist. February 14, 2005.
- ^ "Stem cells used to restore vision". BBC News. 2005-04-28.
- ^ Hanson C, Hardarson T, Ellerström C, Nordberg M, Caisander G, Rao M, Hyllner J, Stenevi U (March 2013). "Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro". Acta Ophthalmologica. 91 (2): 127–130. doi:10.1111/j.1755-3768.2011.02358.x. PMC 3660785. PMID 22280565.
- ^ Vastag B (April 2001). "Stem cells step closer to the clinic: paralysis partially reversed in rats with ALS-like disease". JAMA. 285 (13): 1691–93. doi:10.1001/jama.285.13.1691. PMID 11277806.
- ^ Anderson, Querida (15 June 2008). "Osiris Trumpets Its Adult Stem Cell Product". Genetic Engineering and Biotechnology News. 28 (12).
- ^ Gurtner, Geoffrey C.; Callaghan, Matthew J.; Longaker, Michael T. (February 2007). "Progress and Potential for Regenerative Medicine". Annual Review of Medicine. 58 (1): 299–312. doi:10.1146/annurev.med.58.082405.095329. PMID 17076602.
- ^ Hanna V, Gassei K, Orwig KE (2015). "Stem Cell Therapies for Male Infertility: Where Are We Now and Where Are We Going?". In Carrell D, Schlegel P, Racowsky C, Gianaroli L (eds.). Biennial Review of Infertility. Springer. pp. 17–39. doi:10.1007/978-3-319-17849-3_3. ISBN 978-3-319-17849-3. Bone marrow transplantation is, as of 2009, the only established use of stem cells.
- ^ Valli H, Phillips BT, Shetty G, Byrne JA, Clark AT, Meistrich ML, Orwig KE (January 2014). "Germline stem cells: toward the regeneration of spermatogenesis". Fertility and Sterility. 101 (1): 3–13. doi:10.1016/j.fertnstert.2013.10.052. PMC 3880407. PMID 24314923.
- ^ White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL (February 2012). "Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women". Nature Medicine. 18 (3): 413–421. doi:10.1038/nm.2669. PMC 3296965. PMID 22366948.
- ^ Herraiz, Sonia; Buigues, Anna; Díaz-García, César; Romeu, Mónica; Martínez, Susana; Gómez-Seguí, Inés; Simón, Carlos; Hsueh, Aaron J.; Pellicer, Antonio (May 2018). "Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion". Fertility and Sterility. 109 (5): 908–918.e2. doi:10.1016/j.fertnstert.2018.01.004. ISSN 0015-0282. PMID 29576341.
- ^ Badawy, Ahmed; Sobh, Mohamed A.; Ahdy, Mohamed; Abdelhafez, Mohamed Sayed (2017-06-15). "Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model". International Journal of Women's Health. 9: 441–7. doi:10.2147/IJWH.S134074. PMC 5479293. PMID 28670143.
- ^ Liew, Aaron; O'Brien, Timothy (2012-07-30). "Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia". Stem Cell Research & Therapy. 3 (4): 28. doi:10.1186/scrt119. ISSN 1757-6512. PMC 3580466. PMID 22846185.
- ^ Bubela T, Li MD, Hafez M, Bieber M, Atkins H (November 2012). "Is belief larger than fact: expectations, optimism and reality for translational stem cell research". BMC Medicine. 10 133. doi:10.1186/1741-7015-10-133. PMC 3520764. PMID 23131007.
- ^ Ader M, Tanaka EM (December 2014). "Modeling human development in 3D culture". Current Opinion in Cell Biology. 31: 23–28. doi:10.1016/j.ceb.2014.06.013. PMID 25033469.
- ^ McClure, Paul (2024-12-27). "Stem cells 'instructed' to form specific tissues and organs". New Atlas. Retrieved 2024-12-28.
- ^ Greenhough, Sebastian; Hay, David C. (April 2012). "Stem Cell-Based Toxicity Screening: Recent Advances in Hepatocyte Generation". Pharmaceutical Medicine. 26 (2): 85–89. doi:10.1007/BF03256896. S2CID 15893493.
- ^ García-Prat, Laura; Kaufmann, Kerstin B.; Schneiter, Florin; Voisin, Veronique; Murison, Alex; Chen, Jocelyn; Chan-Seng-Yue, Michelle; Gan, Olga I.; McLeod, Jessica L.; Smith, Sabrina A.; Shoong, Michelle C.; Parris, Darrien; Pan, Kristele; Zeng, Andy G.X.; Krivdova, Gabriela; Gupta, Kinam; Takayanagi, Shin-Ichiro; Wagenblast, Elvin; Wang, Weijia; Lupien, Mathieu; Schroeder, Timm; Xie, Stephanie Z.; Dick, John E. (August 2021). "TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate". Cell Stem Cell. 28 (10): 1838–50.e10. doi:10.1016/j.stem.2021.07.003. hdl:20.500.11850/510219. PMID 34343492. S2CID 236915618.
Further reading
[edit]- Manzo, Carlo; Torreno-Pina, Juan A.; Massignan, Pietro; Lapeyre, Gerald J.; Lewenstein, Maciej; Garcia Parajo, Maria F. (25 February 2015). "Weak Ergodicity Breaking of Receptor Motion in Living Cells Stemming from Random Diffusivity". Physical Review X. 5 (1) 011021. arXiv:1407.2552. Bibcode:2015PhRvX...5a1021M. doi:10.1103/PhysRevX.5.011021. S2CID 73582473.
External links
[edit]- "National Institutes of Health: Stem Cell Information". stemcells.nih.gov. Retrieved 2024-12-28.
- "Stem cells — Latest research and news". Nature. Retrieved 2024-12-28.
Stem cell
View on GrokipediaFundamental Properties
Self-Renewal and Proliferation
Self-renewal is defined as the process by which stem cells undergo division to produce at least one daughter cell that retains the undifferentiated, multipotent, or pluripotent state of the parent cell, thereby sustaining the stem cell population over multiple generations.[10] This capability distinguishes stem cells from differentiated cells and is essential for tissue homeostasis, repair, and development.[11] Proliferation, in this context, refers to the mitotic division that enables the expansion of the stem cell pool, often coupled with self-renewal to prevent exhaustion or uncontrolled growth.[12] Stem cell division can occur symmetrically, yielding two identical stem cells to amplify the population, or asymmetrically, producing one stem cell and one committed progenitor to balance maintenance and differentiation.[11] Symmetric division predominates during embryonic development or injury response to rapidly expand stem cell numbers, while asymmetric division maintains steady-state tissue renewal in adult organisms, such as in the hematopoietic system where hematopoietic stem cells (HSCs) generate ~10^11 blood cells daily in humans without depleting the stem pool.[13] These modes are regulated by intrinsic factors like unequal partitioning of determinants (e.g., Numb protein in neural stem cells) and extrinsic niche signals that dictate division outcomes.[14] Key signaling pathways orchestrate self-renewal and proliferation. In mouse embryonic stem cells, leukemia inhibitory factor (LIF) activates the STAT3 pathway to promote symmetric self-renewal and inhibit differentiation.[15] Wnt/β-catenin signaling enhances proliferation and maintains undifferentiated states in various stem cell types, including intestinal and HSCs, by stabilizing β-catenin to transcriptionally repress differentiation genes.[15][13] Notch signaling supports self-renewal in HSCs and neural progenitors by lateral inhibition, preventing premature differentiation, while FGF/ERK pathways fine-tune proliferation rates in human pluripotent stem cells.[15][16] Dysregulation of these pathways, such as hyperactive Wnt or Notch, can shift proliferation toward uncontrolled expansion, as observed in cancer stem cells, underscoring their precise calibration in normal physiology.[17] Metabolic and redox states further modulate these processes; for instance, low reactive oxygen species (ROS) levels favor quiescent self-renewal in HSCs, whereas moderate ROS promote proliferation via redox-sensitive transcription factors.[18] Epigenetic modifiers, including Polycomb group proteins like BMI1, sustain proliferative capacity by repressing differentiation loci, as demonstrated in BMI1-deficient mice exhibiting impaired HSC self-renewal.[16] Overall, self-renewal and proliferation integrate environmental cues from the niche—such as stromal cell-secreted factors—with intracellular networks to ensure long-term tissue integrity without oncogenic risk.[19]Potency, Plasticity, and Differentiation Potential
Stem cell potency denotes the range of cell types a stem cell can differentiate into, forming a hierarchy from totipotent to unipotent. Totipotent cells, exemplified by the zygote and early blastomeres up to the 4- to 8-cell stage in mammals, can generate all embryonic cell lineages as well as extraembryonic tissues like the placenta.[20] Pluripotent stem cells, such as embryonic stem cells derived from the inner cell mass of the blastocyst, possess the potential to form derivatives of all three germ layers—ectoderm, mesoderm, and endoderm—but not extraembryonic structures.[1] Multipotent stem cells, typically found in adult tissues, can differentiate into multiple specialized cell types within a particular lineage; for instance, hematopoietic stem cells produce various blood cells including erythrocytes, leukocytes, and platelets.[20] Unipotent stem cells, like those in the epidermis or spermatogonia, are restricted to generating a single mature cell type while retaining self-renewal capacity.[21] Differentiation potential realizes this potency through orchestrated processes involving asymmetric division, where one daughter cell retains stemness and the other commits to specialization, influenced by genetic and epigenetic mechanisms. Key regulators include transcription factors such as Oct4, Sox2, and Nanog in pluripotent cells, which maintain undifferentiated states until external signals trigger lineage commitment via pathways like Wnt, Notch, and BMP.[22] Extrinsic factors, including soluble growth factors, extracellular matrix composition, and mechanical cues from the microenvironment, further direct differentiation; for example, stiffness of the substrate can bias mesenchymal stem cells toward osteogenic versus adipogenic fates.[23] This potential diminishes progressively as cells specialize, reflecting epigenetic silencing of unused genes and activation of lineage-specific programs.[4] Stem cell plasticity describes the observed ability of certain adult stem cells to adopt phenotypes outside their tissue of origin, potentially via transdifferentiation—direct conversion between differentiated states—or cell fusion events. Early evidence from bone marrow transplants showed donor-derived cells in non-hematopoietic tissues like liver and neurons, suggesting broad plasticity.[24] However, subsequent critiques have challenged these findings, attributing many instances to fusion with host cells or experimental artifacts rather than genuine reprogramming, with limited reproducible transdifferentiation under physiological conditions.[25] While induced pluripotency via Yamanaka factors demonstrates engineered plasticity in somatic cells, innate adult stem cell plasticity remains controversial and insufficiently robust for reliable therapeutic exploitation as of 2025.[26] This debate underscores the need for rigorous single-cell tracking and clonal assays to distinguish true potency extension from alternative mechanisms.[27]Identification, Isolation, and Molecular Markers
Stem cells are identified primarily through functional assays that confirm their capacity for self-renewal and differentiation into multiple cell lineages, supplemented by detection of specific molecular markers via techniques such as immunofluorescence, flow cytometry, or gene expression analysis.[28] These markers vary by stem cell type but generally include transcription factors and cell surface proteins that correlate with undifferentiated states and proliferative potential.[29] Isolation methods depend on the tissue source and cell type, often beginning with enzymatic or mechanical dissociation to release cells from embryos, bone marrow, or other tissues, followed by enrichment using density gradient centrifugation or immunoselection.[30] Flow cytometry-based fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS) exploit surface markers for high-purity isolation, enabling prospective identification without prior culture.[31] For instance, hematopoietic stem cells (HSCs) from bone marrow are routinely isolated using CD34 as a key positive selector, achieving populations with long-term repopulating activity upon transplantation.[32]| Stem Cell Type | Key Molecular Markers | Notes |
|---|---|---|
| Pluripotent (ESCs/iPSCs) | OCT4, SOX2, NANOG (transcription factors); SSEA-4, TRA-1-60, CD133 (surface) | Essential for maintaining undifferentiated state; OCT4 and SOX2 form a regulatory network with NANOG to prevent differentiation.[33] |
| Hematopoietic (HSCs) | CD34+, CD38-, CD90+, CD45RA-, Lin- (surface) | CD34 expression identifies primitive progenitors; combination with negativity for lineage markers enhances HSC purity for clinical use.[36][37] |
| Mesenchymal (MSCs) | CD73+, CD90+, CD105+; CD34-, CD45- (surface); plastic adherence, CFU-F formation | Isolated from adipose or bone marrow via adherence; markers confirm multipotency without hematopoietic contamination.[38] |
Classification of Stem Cells
Totipotent and Early Embryonic Stem Cells
Totipotent cells represent the most versatile stage of cellular potency, defined as the capacity of a single cell to generate a complete viable organism, encompassing both embryonic lineages and extra-embryonic structures such as the placenta and yolk sac.[41] This property is inherently tied to the zygote, the fertilized ovum formed immediately following sperm-egg fusion, which undergoes asymmetric division to produce daughter cells retaining this full developmental repertoire.[42] In mammalian embryos, totipotency persists transiently through early cleavage stages; for instance, in mice, blastomeres at the 2-cell stage exhibit pronounced totipotent features, including the activation of a unique transcriptome dominated by Zscan4 and MERVL endogenous retroviruses, enabling contributions to all tissue types when reintroduced into host embryos.[43] Beyond this window, typically by the 4- to 8-cell stage, cells lose the ability to form extra-embryonic trophoblast lineages independently, transitioning toward pluripotency.[44] Early embryonic stem cells, often synonymous with totipotent blastomeres from pre-morula stages, are characterized by minimal epigenetic restrictions, featuring open chromatin configurations and low levels of DNA methylation that facilitate broad gene accessibility.[45] Unlike pluripotent embryonic stem cells derived from the inner cell mass of the blastocyst, which exclude extra-embryonic fates, totipotent cells demonstrate causal potency through experimental tetraploid complementation assays, where a single diploid blastomere can rescue a tetraploid host embryo to produce live offspring.[46] Key molecular hallmarks include the expression of factors like Oct4 and Nanog at low basal levels, coupled with transient bursts of lineage-specifying genes, reflecting a poised state for lineage segregation driven by cell-cell interactions and positional cues during compaction.[47] Isolation of these cells remains challenging due to their scarcity and ethical constraints on human embryos, but studies in mice have quantified their proliferative capacity, with 2-cell blastomeres dividing symmetrically to yield up to 4-8 equivalent daughters before potency restriction.[48] Efforts to recapitulate totipotency in vitro have identified culture conditions mimicking the 2-cell embryo environment, such as dual inhibition of MAPK and GSK3 pathways supplemented with retinoic acid signaling modulators, yielding totipotency-like states in extended pluripotent stem cells.[46] However, authentic totipotent stem cell lines capable of independent embryo formation remain elusive in humans, with mouse models showing that sustained totipotency requires precise regulation of histone modifications like H3K9me3 demethylation to prevent premature lineage commitment.[41] These cells' defining trait—global developmental coordination rather than mere multi-lineage potential—underscores their distinction from pluripotent counterparts, as evidenced by failure of inner cell mass cells to form trophoblast in chimera assays.[42]Pluripotent Stem Cells: Embryonic and Induced
Pluripotent stem cells possess the capacity for indefinite self-renewal and differentiation into derivatives of all three primary germ layers, enabling formation of any cell type in the body except extra-embryonic tissues.[49] This potency distinguishes them from multipotent adult stem cells, which are lineage-restricted.[50] Embryonic stem cells (ESCs) are derived from the inner cell mass of pre-implantation blastocysts, typically 4-5 days post-fertilization in humans.[50] Mouse ESCs were first isolated in 1981 by Gail Martin, and independently by Martin Evans and Matthew Kaufman, through explantation and culture of inner cell mass cells on feeder layers.[51] Human ESCs (hESCs) were successfully derived in 1998 by James Thomson's team from surplus in vitro fertilization embryos, marking a milestone in human pluripotent cell research despite ensuing ethical debates over embryo destruction.[52] hESCs maintain pluripotency in culture via signaling pathways like LIF/STAT3 in mice or Activin/Nodal in humans, expressing markers such as Oct4, Nanog, and Sox2, and forming teratomas in vivo to confirm potency.[49] Induced pluripotent stem cells (iPSCs) are generated by reprogramming somatic cells, such as fibroblasts, to an embryonic-like state through ectopic expression of defined transcription factors.[53] Shinya Yamanaka's group achieved this in mouse fibroblasts in 2006 using four factors—Oct4, Sox2, Klf4, and c-Myc (collectively OSKM)—introducing them via retroviral vectors, resulting in cells capable of germline transmission and chimera formation.[53] Human iPSCs followed in 2007, independently reported by Yamanaka and Thomson teams, exhibiting gene expression profiles, epigenetic marks, and differentiation potential akin to hESCs.[54] Reprogramming efficiency remains low, around 0.01-0.1%, involving epigenetic remodeling and mesenchymal-to-epithelial transition, with risks of incomplete reprogramming leading to aberrant differentiation or oncogenesis from c-Myc integration.[26] Both ESCs and iPSCs demonstrate equivalent pluripotency, validated by teratoma assays, directed differentiation into lineages like neurons or cardiomyocytes, and contribution to all germ layers in animal models.[54] However, hESC derivation necessitates embryo sacrifice, prompting ethical opposition from pro-life perspectives and regulatory restrictions, such as U.S. federal funding limits until 2009.[3] iPSCs circumvent these issues by using accessible adult cells, enabling patient-matched lines to mitigate immune rejection, though they carry epigenetic memory from donor cells and higher variability due to reprogramming artifacts.[3][55] ESCs offer a more stable, "naive" pluripotent state in some protocols, while iPSCs facilitate disease modeling from patient biopsies, as in Parkinson's or genetic disorders.[56] Ongoing refinements, including non-integrating vectors and chemical cocktails, aim to enhance iPSC safety and fidelity to ESC standards.[57]Multipotent Adult Stem Cells
Multipotent adult stem cells, also termed somatic or tissue-specific stem cells, are undifferentiated cells present in postnatal tissues capable of self-renewal and differentiation into a restricted set of mature cell types belonging to the same tissue or organ lineage. These cells maintain tissue homeostasis and contribute to repair following injury, but their potency is limited compared to pluripotent stem cells, as they typically derive from and generate progeny within one embryonic germ layer, such as mesoderm for blood or connective tissues.[20] [21] Identification relies on functional assays demonstrating multilineage differentiation and surface markers specific to each type, alongside in vivo reconstitution capacity where applicable.[58] Hematopoietic stem cells (HSCs), primarily residing in bone marrow, exemplify multipotent adult stem cells by generating all mature blood cell types through myeloid and lymphoid lineages via hierarchical differentiation. Discovered in 1961 by James Till and Ernest McCulloch through mouse bone marrow transplantation experiments revealing clonal spleen colony-forming units, HSCs are enriched by markers including CD34 positivity, alongside CD38 negativity and CD90 expression for long-term repopulating subsets.[59] [32] Clinical utility is established in bone marrow transplants, where donor HSCs reconstitute recipient hematopoiesis, with success rates exceeding 80% in matched sibling donors for conditions like leukemia.[58] Mesenchymal stem cells (MSCs), or multipotent stromal cells, sourced from bone marrow, adipose tissue, and other mesenchymal depots, differentiate into osteoblasts, chondrocytes, adipocytes, and sometimes myocytes or fibroblasts. First isolated in the 1960s by Alexander Friedenstein as plastic-adherent, colony-forming units with osteogenic potential, MSCs are defined by International Society for Cellular Therapy criteria: adherence to plastic, expression of CD73, CD90, and CD105 (>95% positive), absence of CD34, CD45, and HLA-DR (<2% positive), and trilineage differentiation in vitro.[60] [61] Adipose-derived MSCs, demonstrated multipotent in 2002, yield higher cell numbers per harvest than bone marrow equivalents, facilitating autologous applications.[62] Other multipotent adult stem cells include neural stem cells in the subventricular zone and hippocampus, which produce neurons, astrocytes, and oligodendrocytes; epidermal stem cells in hair follicle bulges generating skin appendages; and intestinal crypt base columnar cells marked by Lgr5, sustaining epithelial renewal. These cells underscore tissue-specific multipotency, with limited evidence of translineage plasticity under standard conditions, prioritizing endogenous repair over broad reprogramming.[20] [63]Other Stem Cell Sources: Fetal, Amniotic, and Perinatal
Fetal stem cells are derived from tissues of developing fetuses during gestation, including blood, liver, bone marrow, and extra-embryonic structures such as the placenta and membranes.[64] These cells exhibit multipotency, with greater proliferative capacity, differentiation potential, and in vitro expansion ease compared to adult stem cells, while possessing lower immunogenicity and enhanced homing and engraftment abilities.[65] [66] Isolation typically occurs via enzymatic digestion or mechanical dissociation from discarded fetal tissues post-termination or miscarriage, though ethical concerns limit widespread sourcing.[67] Unlike embryonic stem cells, fetal stem cells lack totipotency and do not form teratomas, but they demonstrate broader lineage potential than adult counterparts, differentiating into mesodermal, endodermal, and limited ectodermal derivatives.[66] Amniotic fluid stem cells (AFSCs) originate from the amniotic fluid surrounding the fetus, collected noninvasively via amniocentesis between weeks 12-35 of gestation or from term fluid post-delivery.[68] These cells express markers such as c-kit (CD117) and stage-specific embryonic antigen-4 (SSEA-4), enabling selection and expansion without karyotypic abnormalities or tumorigenic risk.[69] AFSCs display broad multipotency, differentiating into lineages from all three germ layers—including osteocytes, adipocytes, chondrocytes, hepatocytes, and neurons—but fall short of full pluripotency seen in embryonic stem cells.[70] [71] Relative to adult mesenchymal stem cells, AFSCs proliferate faster and retain higher neurogenic and cardiogenic potential, with gene expression profiles shifting toward maturity over passages.[72] Their derivation from discarded fluid positions them as an ethically favorable alternative, though yield varies with gestational age.[66] Perinatal stem cells encompass those harvested at birth from umbilical cord blood, Wharton's jelly in cord tissue, and placental components like the amnion, chorion, and villi.[73] Cord blood primarily yields hematopoietic stem cells capable of reconstituting blood lineages, as demonstrated in transplants since 1988 for conditions like Fanconi anemia.[74] Placental and cord-derived mesenchymal stem cells exhibit immune-privileged properties, higher proliferation rates than adult MSCs, and trilineage differentiation (adipogenic, osteogenic, chondrogenic), with some studies showing superior expansion from cord tissue over placental sources.[75] [76] These cells surpass adult stem cells in potency and telomere length but lack the pluripotency of embryonic cells, avoiding ethical issues tied to embryo destruction while enabling cryopreservation from routinely discarded tissues.[77] Clinical banking of perinatal cells has expanded since the early 2000s, supported by evidence of multilineage engraftment in preclinical models.[74]Historical Development
Pre-20th Century Observations and Early Concepts
In the 18th century, experimental observations of regeneration in simple organisms provided initial insights into cellular capacities resembling later stem cell properties. Abraham Trembley, a Genevan naturalist, reported in 1740 that freshwater polyps (Hydra vulgaris) could regenerate entire bodies from fragments as small as one-eighth of the original, with severed parts developing heads or feet as needed, demonstrating robust proliferative and differentiative potential in animal tissues.[78] These findings, published in 1744, challenged preformationist doctrines favoring fixed developmental blueprints and suggested intrinsic organizational powers in biological matter.[79] Building on such work, Lazzaro Spallanzani conducted systematic experiments in 1768 on regeneration across species, including salamanders, frogs, snails, and worms. He documented limb and tail regrowth in salamanders via blastema formation—a mass of undifferentiated cells at the amputation site that proliferated and specialized into structured tissues—while noting limited regenerative ability in higher vertebrates like frogs, where only tadpole tails reformed. Spallanzani's histological examinations of the stump-regrowth interface emphasized empirical causation over vitalistic forces, attributing regeneration to cellular proliferation rather than mystical influences, though he acknowledged variability tied to species and injury site.[80] By the mid-19th century, advances in microscopy enabled observations of blood cell origins, foreshadowing stem-like progenitors. In 1870, German pathologist Albert von Neumann proposed the bone marrow as the primary site of leukocyte production, based on stains revealing maturing white blood cells within marrow cavities.[81] Giulio Bizzozero further detailed in the 1870s that marrow hosted both erythropoiesis (red cell formation from nucleated precursors) and leukopoiesis, positing it as a dynamic site of blood genesis and turnover, though he erroneously linked large marrow cells to destruction rather than solely production.[82] These hematological insights culminated in early conceptual framing of precursor cells. In 1896, German hematologist Arthur Pappenheim introduced the term "stem cell" (Stammzelle) to describe a primitive marrow element capable of yielding both erythrocytes and leukocytes, drawing analogies to plant meristems for their generative hierarchy.[83] This notion, rooted in observable lineage commitment without full appreciation of self-renewal or quiescence, represented a proto-stem cell idea amid broader cell theory debates, yet lacked experimental isolation or potency assays available only later.[84] Such pre-20th century work, while descriptive, empirically grounded later understandings of tissue homeostasis through renewable cellular pools.Mid-20th Century Discoveries in Hematopoiesis and Tissue Regeneration
In the 1950s, experiments with bone marrow transplantation provided early empirical evidence for the regenerative capacity of hematopoietic tissues. Following World War II atomic bomb survivals and radiation accident cases, researchers irradiated animals to mimic lethal bone marrow ablation and tested intravenous bone marrow infusions from donors. In 1956, E. Donnall Thomas performed the first successful human bone marrow transplant in a child with acute leukemia, achieving temporary hematopoietic recovery, though graft rejection limited long-term success.[85] By 1957, clinical trials expanded, confirming that donor cells could repopulate blood lineages in myeloablated hosts, establishing bone marrow as a source of regenerative progenitors.[86] These findings, grounded in observable colony formation and blood count restoration, shifted focus from mere transfusion to cellular engraftment mechanisms.[87] The seminal 1961 experiments by James E. Till and Ernest A. McCulloch formalized the hematopoietic stem cell (HSC) concept through quantitative assays. Irradiating mice to near-lethality and injecting limiting dilutions of bone marrow cells, they observed macroscopic nodules (colony-forming units, CFU-S) in recipient spleens, each deriving from a single progenitor capable of proliferating into multilineage blood cells.[88] Serial transplantation assays further demonstrated self-renewal, as CFU-S from primary colonies regenerated secondary nodules, proving clonal expansion without exhaustion.[89] This direct measurement of radiation sensitivity—yielding a survival curve with a D0 of approximately 100 rads for CFU-S—provided causal evidence that rare, quiescent cells (about 1 in 10^4-10^5 marrow nucleated cells) underpin hematopoiesis, challenging prior views of fixed lineage commitments.[90] These discoveries extended to tissue regeneration by revealing HSCs' role in systemic repair beyond blood formation. Radiation chimerism studies showed donor-derived cells contributing to non-hematopoietic tissues under stress, hinting at plasticity, though later verified as limited to fusion or contamination artifacts in most cases.[91] In 1958, Georges Mathé's allograft of autologous bone marrow to radiation-exposed workers restored hematopoiesis, validating transplantation's therapeutic potential for acute radiation syndrome and foreshadowing leukemia treatments.[92] By the mid-1960s, HSC models informed scalable protocols, with over 100 murine transplants confirming dose-response relationships for engraftment (e.g., 10^5-10^6 cells minimum for survival).[93] This era's empirical focus—prioritizing reproducible colony assays over speculative hierarchies—laid causal foundations for distinguishing stem from progenitor cells, influencing regenerative paradigms despite institutional delays in human application due to histocompatibility barriers.[94]Late 20th to Early 21st Century: Isolation of Embryonic and Adult Stem Cells
In 1981, Matthew Evans and Martin Kaufman established the first lines of pluripotential cells derived directly from mouse blastocysts cultured in vitro, demonstrating their ability to contribute to chimeric embryos upon injection into host blastocysts.[95] Independently in the same year, Gail Martin isolated a pluripotent cell line from early mouse embryos using medium conditioned by teratocarcinoma stem cells, and introduced the term "embryonic stem (ES) cells" to describe these self-renewing, undifferentiated populations capable of differentiation into multiple cell types.[96] These mouse ES cells enabled genetic manipulation techniques, such as homologous recombination for gene targeting, which later contributed to the creation of knockout mice for studying gene function.[97] Progress toward human embryonic stem cells accelerated in the late 1990s. On November 6, 1998, James Thomson and colleagues reported the derivation of five human ES cell lines from discarded IVF blastocysts, maintained on mouse embryonic fibroblast feeders, with evidence of pluripotency shown by in vitro differentiation into trophoblasts, derivatives of all three embryonic germ layers, and teratoma formation in immunodeficient mice.[98] These cells expressed markers like Oct-4 and SSEA-4, and could be propagated indefinitely without differentiation, marking a pivotal advance for potential regenerative therapies, though raising ethical concerns due to embryo destruction.[99] Parallel efforts isolated and purified adult stem cells during this period. In 1988, Gerald Spangrude, Scott Heimfeld, and Irving Weissman achieved the first purification of mouse hematopoietic stem cells (HSCs) from bone marrow using fluorescence-activated cell sorting based on low Hoechst dye staining (side population) and absence of lineage markers, combined with stem cell antigen-1 expression, confirming their multilineage repopulation potential via serial transplantation assays. Human HSCs were similarly isolated in the early 1990s using CD34 surface marker enrichment, enabling clinical applications like bone marrow transplants refined through these techniques.[100] In 1992, Brent Reynolds and Samuel Weiss isolated multipotent neural stem cells from the adult mouse forebrain subventricular zone, demonstrating their capacity for self-renewal as neurospheres and differentiation into neurons, astrocytes, and oligodendrocytes in culture. For mesenchymal stem cells (MSCs), Alexander Friedenstein's earlier stromal cell isolations from guinea pig bone marrow in the 1970s were advanced in the 1990s; by 1999, Mark Pittenger et al. cloned human bone marrow MSCs and rigorously demonstrated their trilineage differentiation into osteoblasts, chondrocytes, and adipocytes under specific inductive conditions, supported by gene expression and functional assays. These adult stem cell isolations highlighted tissue-specific plasticity, contrasting with the broader potency of embryonic cells, and laid groundwork for autologous therapies avoiding ethical issues.[101]2006 Onward: Induced Pluripotency and Clinical Translation
In 2006, Kazutoshi Takahashi and Shinya Yamanaka demonstrated that mouse embryonic and adult fibroblasts could be reprogrammed into pluripotent stem cells, termed induced pluripotent stem cells (iPSCs), through retroviral delivery of four transcription factors: Oct4 (also known as Oct3/4), Sox2, Klf4, and c-Myc.00976-7) This breakthrough established a method to generate pluripotent cells from somatic tissues without relying on embryos or oocytes, enabling patient-specific cell lines for research and potential therapy.00976-7) By 2007, Yamanaka's group extended the technique to human dermal fibroblasts using the same four factors, producing iPSCs capable of forming teratomas and differentiating into multiple lineages, confirming their pluripotency.[102] Concurrently and independently, James Thomson's team generated human iPSCs from fibroblasts via Oct4, Sox2, Nanog, and Lin28, validating the approach with an alternative factor combination and highlighting the robustness of reprogramming across species and cell types.[103] These human iPSCs exhibited gene expression and epigenetic profiles akin to embryonic stem cells, though initial efficiencies remained low at under 0.1% and integration of viral vectors posed risks of mutagenesis.[102][103] The significance of iPSC reprogramming was recognized in 2012 when Yamanaka and John Gurdon received the Nobel Prize in Physiology or Medicine for proving that mature somatic cells could revert to a pluripotent state, building on Gurdon's earlier nuclear transfer experiments in frogs.[104] Post-2007 advancements focused on enhancing safety and scalability: c-Myc was omitted in some protocols to reduce oncogenicity, reprogramming efficiency improved to 1-10% via optimized culture conditions and small-molecule enhancers like valproic acid, and non-integrating methods emerged, including Sendai virus vectors (2008-2009), episomal plasmids, and mRNA transfection by the early 2010s.[105] These refinements minimized genomic insertion risks, with CRISPR-Cas9 integration from 2013 onward enabling precise editing for disease modeling and correction of mutations in iPSC lines.[26] Clinical translation accelerated in the 2010s, with Japan's regulatory framework enabling early approvals. In 2013, the first iPSC-derived cell therapy trial was authorized for age-related macular degeneration, involving autologous retinal pigment epithelial cells transplanted into a patient in 2014; however, it was suspended after one case due to detected genetic abnormalities raising tumorigenicity concerns.[106] Subsequent shifts to allogeneic iPSCs from universal donor lines or banks mitigated variability and costs, leading to Japan's 2019 approval of iPSC-derived corneal epithelial sheets for limbal stem cell deficiency, marking the first commercial iPSC product.[106] By 2023, over 50 iPSC-based trials were registered globally, targeting conditions like Parkinson's disease (dopaminergic neurons), spinal cord injury (neural progenitors), and heart failure (cardiomyocytes), with phase I/II data showing feasibility in engraftment but variable long-term efficacy pending larger studies.[107] Preclinical successes, such as iPSC-derived beta islets reversing diabetes in nonhuman primates, underscore potential, though challenges like immune compatibility and scalability persist.[106]Therapeutic Applications and Clinical Evidence
Established Treatments Using Hematopoietic and Mesenchymal Stem Cells
Hematopoietic stem cell transplantation (HSCT) is the cornerstone established therapy utilizing hematopoietic stem cells, primarily sourced from bone marrow, peripheral blood, or umbilical cord blood, to treat hematologic malignancies and severe non-malignant disorders. This procedure involves high-dose chemotherapy or radiation to eradicate diseased cells, followed by infusion of stem cells to reconstitute the blood and immune systems. Allogeneic HSCT, employing donor cells, offers curative potential for acute leukemias, with 5-year overall survival rates of 40-60% in high-risk acute myeloid leukemia (AML) patients transplanted in first complete remission, outperforming chemotherapy alone in eligible cases.[108] Autologous HSCT, using the patient's own cells, is standard for multiple myeloma and non-Hodgkin lymphoma, enabling tolerance of intensified conditioning regimens and yielding relapse-free survival improvements of 10-20% over conventional therapy in randomized trials.[109] Over 50,000 HSCT procedures occur globally each year, with engraftment typically within 2-4 weeks and long-term efficacy verified through decades of prospective data from registries like the Center for International Blood and Marrow Transplant Research.[110] Indications extend to inherited disorders such as severe combined immunodeficiency and sickle cell disease, where HSCT corrects genetic defects via donor-derived hematopoiesis, achieving event-free survival rates exceeding 80% with matched sibling donors.[111] Risks include infection, graft failure (1-5% incidence), and graft-versus-host disease (GVHD) in allogeneic settings (20-50% acute form), mitigated by immunosuppressive protocols and HLA matching, yet causal benefits stem from the graft-versus-leukemia effect, where donor T cells target residual malignancy.[109] Empirical outcomes underscore HSCT's role as first-line curative intent for fit patients under 70, per guidelines from the European Society for Blood and Marrow Transplantation. Mesenchymal stem cells (MSCs), multipotent cells typically isolated from bone marrow stroma or adipose tissue, have one primary established therapeutic application as of 2025: adjunctive treatment for steroid-refractory acute GVHD following HSCT. In December 2024, the U.S. Food and Drug Administration approved Ryoncil (remestemcel-L), an off-the-shelf allogeneic MSC product derived from umbilical cord tissue, for pediatric patients aged 2 months and older with severe, treatment-resistant GVHD involving the lower gastrointestinal tract or liver.[112] Approval followed phase III trials demonstrating overall response rates of 70% at day 28, with MSCs' paracrine secretion of anti-inflammatory factors like PGE2 and IDO enabling tissue-specific immunomodulation without systemic immunosuppression.[113] Infused intravenously at doses of 2 million cells/kg twice weekly for four weeks, Ryoncil reduces mortality in this high-risk cohort, where untreated GVHD exceeds 80% fatality.[114] Beyond GVHD, MSCs lack broad regulatory approvals for routine clinical use, though they support hematopoietic engraftment in co-transplantation settings by fostering niche vascularization and cytokine modulation, reducing time to neutrophil recovery by 2-5 days in pilot studies.[115] Orthopedic applications, such as intra-articular injection for knee osteoarthritis, show symptomatic relief in meta-analyses (pain reduction of 20-30 mm on VAS scales), but remain unapproved by major regulators like the FDA due to inconsistent cartilage regeneration evidence and heterogeneity in cell potency.[116] Causal efficacy derives from MSCs' trophic support rather than transdifferentiation, with durable benefits limited to inflammatory modulation in verified trials.[114]Experimental Therapies and Ongoing Clinical Trials
Experimental stem cell therapies encompass applications beyond established hematopoietic stem cell transplants, targeting conditions such as neurodegenerative diseases, spinal cord injuries, diabetes, and heart failure using mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and embryonic stem cell-derived products. These approaches aim to replace damaged tissues, modulate immune responses, or promote regeneration, but most remain in early to mid-stage clinical trials with preliminary safety data outweighing definitive efficacy evidence. As of January 2025, over 100 interventional human pluripotent stem cell (hPSC) trials worldwide have reported safety profiles without severe adverse events in many cases, though long-term outcomes and scalability challenges persist.[117] In Parkinson's disease, iPSC-derived dopamine neuron transplants have advanced significantly. A phase 1 trial at Memorial Sloan Kettering Cancer Center, initiated from patient-derived iPSCs reprogrammed into midbrain dopaminergic neurons, demonstrated safety and modest symptom improvement in initial patients as of April 2025, with no tumorigenicity observed after surgical implantation.[118] In Japan, a clinical study involving seven patients aged 50-69 transplanted with 5-10 million iPSC-derived cells reported no serious side effects over two years, prompting regulatory approval applications in August 2025 for commercial use.[119] [120] A phase 3 trial for similar iPSC-based therapy is slated to begin later in 2025 at institutions like UCI Health, focusing on efficacy endpoints such as motor function scores.[121] For spinal cord injury, ongoing phase II/III trials evaluate neural stem cell implants. The Neuro-Cells trial (NCT03935724) assesses safety and efficacy of intrathecal neural stem cell administration in subacute injuries, with phase II data indicating feasibility but requiring phase III for confirmatory outcomes on ambulation and sensory recovery.[122] In diabetes, MSCs from bone marrow or umbilical cord target beta-cell regeneration or immunomodulation; a review of trials up to 2025 shows phase II studies in type 1 diabetes achieving temporary insulin independence in subsets of patients, though sustained glycemic control remains inconsistent across cohorts.[114] Cardiovascular applications include MSC infusions for heart failure, with 27 trials across phases I-III analyzed as of 2025 demonstrating improved ejection fractions in some phase II arms (e.g., 5-10% gains post-infarction), but phase III results highlight variable long-term benefits and risks of arrhythmias.[123] Autoimmune disease trials, numbering over 50 registered by January 2025, primarily use MSCs for conditions like rheumatoid arthritis and multiple sclerosis, reporting reduced inflammation markers in phase II but no universal remission rates.[124] Overall, while safety is bolstered by extensive preclinical data, efficacy hinges on trial maturation, with meta-analyses indicating 20-50% response rates in select neurological and metabolic indications, tempered by placebo effects and patient heterogeneity.[125]Empirical Outcomes: Success Rates and Verifiable Efficacy Data
Hematopoietic stem cell transplantation (HSCT) demonstrates the highest verifiable success rates among stem cell therapies, primarily for hematological malignancies where it replaces defective blood-forming cells. In acute myeloid leukemia (AML), 5-year overall survival (OS) post-allogeneic HSCT reaches 74% in recent cohorts, though high-risk cases yield 43.7% (95% CI, 37.9-49.3).[126][108] For patients in first complete remission, 4-year OS with allogeneic HSCT is 79%, versus 42% without transplantation.[127] Haploidentical HSCT for acute leukemia achieves 60-month OS of 64.1% (95% CI, 53.8-74.4), with 96.4% neutrophil engraftment.[128] These outcomes reflect causal efficacy in reconstituting hematopoiesis, though influenced by donor match, patient age, and minimal residual disease status, with MRD-negative cases showing 3-year OS up to 88.7%.[129] Mesenchymal stem cell (MSC) therapies exhibit more modest, condition-specific efficacy in clinical trials, often limited to immunomodulatory effects rather than tissue regeneration. In pediatric steroid-refractory acute graft-versus-host disease (SR-aGVHD), remestemcel-L (Ryoncil), the first FDA-approved MSC product in 2024, yields overall response rates of approximately 70% in phase III trials, supporting its conditional approval for this indication.[130] Meta-analyses of MSC infusions for knee osteoarthritis report significant pain reduction (visual analogue scale improvements) and functional gains (WOMAC scores) at 6-12 months, with standardized mean differences favoring treatment over controls, though long-term cartilage repair remains unverified.[131][132] For multiple sclerosis, meta-analyses indicate trends toward reduced disability progression, but randomized trials show no consistent superiority over placebo in large cohorts.[133] Beyond HSCT, verifiable efficacy data for experimental stem cell applications reveal low to moderate success rates, with many trials failing to demonstrate durable benefits. In orthopedic conditions like osteoarthritis, phase II/III studies report 50-80% patient-reported improvements in pain and mobility at 1-2 years, but placebo-controlled data often attribute gains to paracrine signaling rather than engraftment, with regression to baseline in 20-30% of cases.[131] Heart failure trials, including large phase III efforts with MSCs or cardiac progenitors, show reduced scar tissue and modest ejection fraction gains (3-5%) in subsets, yet overall survival benefits are inconsistent, with some meta-analyses finding no significant hazard ratio reduction versus standard care.[123] Unapproved therapies, comprising most clinic-offered treatments, lack rigorous data and carry failure rates exceeding 50% for claimed regenerative outcomes, compounded by risks like multiorgan failure in stem cell tourism cases.[134]| Condition | Therapy Type | Key Metric | Reported Rate | Source |
|---|---|---|---|---|
| AML (high-risk) | Allogeneic HSCT | 5-year OS | 43.7% | JCO 2025 |
| AML (first CR) | Allogeneic HSCT | 4-year OS | 79% | PMC 2024 |
| SR-aGVHD (pediatric) | MSC (remestemcel-L) | Response rate | ~70% | REPROCELL 2025 |
| Knee OA | MSC injection | Pain reduction (VAS) | Significant SMD favor | Stem Cell Res Ther 2025 |
Scientific Limitations and Risks
Tumorigenicity, Immune Rejection, and Genetic Instability
Tumorigenicity remains a primary safety concern in pluripotent stem cell therapies, particularly with embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), as undifferentiated cells can proliferate uncontrollably and form teratomas—benign tumors containing derivatives of all three germ layers—when transplanted into animal models. This risk arises from the core self-renewal capacity of pluripotent cells, which, if not fully differentiated or purified prior to administration, leads to ectopic tissue formation; studies in immunocompromised mice demonstrate teratoma incidence rates exceeding 80% for human ESCs and iPSCs injected subcutaneously. Adult stem cells, such as mesenchymal stem cells (MSCs), exhibit lower tumorigenic potential due to their multipotent rather than pluripotent nature, with rare spontaneous transformation reported only after prolonged in vitro expansion beyond 50-100 population doublings. Clinical evidence underscores this hazard: in early trials, such as a 2014 Japanese study using autologous iPSCs for macular degeneration, no tumors formed after one year of monitoring, but broader preclinical data indicate incomplete purification protocols fail to eliminate risks entirely, with detection limits around 1 in 10^6 cells.[135][136] Mitigation strategies, including cell sorting via surface markers (e.g., TRA-1-60 for pluripotency) and genetic reporters, reduce but do not abolish tumorigenicity, as residual undifferentiated cells or reprogramming-induced epigenetic anomalies can still drive oncogenesis; for instance, vector integration in early iPSC methods increased leukemia-like events in mouse models by activating proto-oncogenes. Recent advancements, such as non-integrating reprogramming via mRNA or small molecules, lower these risks, yet empirical data from 2022-2024 reviews highlight persistent challenges, with genomic alterations correlating to higher tumor formation rates in differentiated derivatives.[137][138] Immune rejection complicates allogeneic stem cell transplantation, where donor cells mismatched at human leukocyte antigen (HLA) loci provoke host T-cell and antibody responses, leading to graft clearance or inflammation; this affects over 90% of unmatched pairs, necessitating lifelong immunosuppression that elevates infection and malignancy risks. Autologous iPSCs circumvent personal rejection but face scalability issues, while allogeneic approaches, dominant in hematopoietic stem cell transplants, succeed mainly with HLA-identical donors (e.g., 10-20% match rate in unrelated registries), yet even then, minor antigen mismatches trigger chronic rejection in 20-30% of cases. In regenerative therapies, MSCs show immunomodulatory properties that dampen rejection—secreting factors like IDO and PGE2 to suppress T-cells—but preclinical models reveal variable efficacy, with human trials reporting 10-15% acute rejection rates without adjunct therapies.[139][140][141] Emerging solutions include CRISPR-Cas9 editing to knock out HLA class I/II genes or overexpress immune checkpoints like PD-L1, enabling "universal" donor cells evading recognition; a 2025 review notes these hypoimmunogenic iPSCs persist in xenogeneic models without immunosuppression, though off-target edits and neoantigen formation pose secondary risks. Graft-versus-host disease (GVHD), prevalent in 30-50% of mismatched transplants, reverses the dynamic as donor immune components attack host tissues, underscoring causal trade-offs in immune modulation.[142][139] Genetic instability manifests in pluripotent stem cells through accumulated mutations during reprogramming and extended culture, with iPSCs acquiring 2-10 single-nucleotide variants (SNVs) and copy number variations (CNVs) per reprogramming event, often at hotspots like chromosome 17q21 (affecting tumor suppressors) and 12p (amplifying MYC). Reprogramming induces replication stress and DNA damage, elevating instability 5-10 fold over somatic cells, as evidenced by whole-genome sequencing of serially passaged lines showing progressive aneuploidy after 20-50 passages. ESCs display similar vulnerabilities, with culture media lacking nucleosides exacerbating double-strand breaks and translocations linked to leukemia in long-term derivatives.[138][143][137] These alterations compromise therapeutic safety, as mutated clones expand clonally—up to 20% prevalence in high-passage cultures—and correlate with impaired differentiation or oncogenic potential; a 2024 analysis found non-random CNVs in 15-25% of clinical-grade iPSC lines, delaying regulatory approval. Mitigation via episomal vectors or short-term reprogramming reduces initial mutations by 50-70%, but ongoing monitoring via karyotyping and sequencing remains essential, revealing that genetic fidelity deteriorates causally with proliferation demands exceeding physiological rates.[135][144][145]Challenges in Scalability, Delivery, and Long-Term Integration
Scalability in stem cell production for therapeutic applications is constrained by the inherent variability in cell expansion protocols, high costs associated with good manufacturing practice (GMP) compliance, and difficulties in achieving consistent quality across batches. Autologous approaches, which use patient-derived cells, exacerbate these issues due to individual differences in donor cell yield and proliferative capacity, often resulting in insufficient quantities for widespread clinical dosing—typically requiring billions of cells per treatment. Allogeneic strategies, while potentially more scalable through off-the-shelf banking, demand rigorous genetic and functional standardization to mitigate donor-recipient mismatches, yet legacy bioreactor and cryopreservation methods drive up expenses, with manufacturing costs frequently exceeding $100,000 per dose.[146][147][148] Delivery of stem cells to precise injury sites remains inefficient, with systemic intravenous routes leading to widespread entrapment in non-target organs such as the lungs and liver, where up to 90% of administered mesenchymal stem cells (MSCs) may be lost within hours due to mechanical filtration and immune clearance. Local injection methods improve targeting but are invasive and limited to accessible tissues, while engineered carriers like hydrogels or nanoparticles show promise for enhanced retention yet face biocompatibility and scalability hurdles in clinical translation. Homing signals, reliant on chemokine gradients and adhesion molecules, often prove unreliable in diseased states, resulting in engraftment rates below 5% in many preclinical models of cardiac or neurological repair.[149][150][151] Long-term integration of transplanted stem cells into host tissues is impeded by poor survival post-engraftment, immune-mediated rejection, and incomplete functional maturation, with studies reporting median persistence of only weeks to months rather than years in human trials for conditions like spinal cord injury. Even when initial differentiation occurs, cells frequently fail to establish vascular connections or synaptic integrations necessary for sustained tissue contribution, compounded by epigenetic drift and microenvironmental cues that promote senescence or aberrant signaling. In hematopoietic stem cell transplants, long-term complications including graft-versus-host disease affect up to 50% of allogeneic recipients, underscoring the need for advanced immunomodulation strategies without compromising therapeutic efficacy.[152][153][154]Comparative Efficacy: Embryonic vs. Non-Embryonic Approaches
Embryonic stem cells (ESCs), derived from early-stage embryos, possess true pluripotency, enabling differentiation into all three germ layers and theoretically offering broad therapeutic potential for tissue regeneration. However, their clinical efficacy remains largely unproven due to persistent challenges including high tumorigenicity, with rates of teratoma formation exceeding 10-20% in preclinical models, and the need for immunosuppression to mitigate rejection.[155] In contrast, non-embryonic approaches—encompassing adult stem cells such as hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), as well as induced pluripotent stem cells (iPSCs)—demonstrate superior translational success, with HSCs achieving cure rates of 50-80% in hematological malignancies via bone marrow transplantation, supported by over 50 years of clinical data.[156][157] HSCs and MSCs, sourced from bone marrow or umbilical cord blood, have established efficacy in specific indications; for instance, allogeneic HSC transplants yield 3-year survival rates of approximately 79% in leukemia patients, outperforming experimental pluripotent cell therapies where long-term engraftment and functional integration remain inconsistent.[156] MSCs exhibit immunomodulatory effects, achieving complete remission in 55% of graft-versus-host disease cases in phase II trials, with overall 2-year survival rates around 55%, though efficacy varies by dosing and patient condition.[158] iPSCs, reprogrammed from somatic cells since 2006, circumvent ethical barriers of ESCs while mimicking pluripotency, yet clinical trials as of 2025 show limited efficacy data, with only preliminary safety in applications like macular degeneration, where visual improvements occurred in small cohorts but lacked controls for placebo effects.[159][26]| Stem Cell Type | Key Clinical Applications | Reported Success Metrics | Limitations |
|---|---|---|---|
| Embryonic (ESCs) | Experimental: Spinal cord injury, macular degeneration | Teratoma risk >10%; no FDA-approved therapies; halted trials (e.g., Geron 2011) due to cysts | High immunogenicity; ethical sourcing constraints; poor scalability |
| Hematopoietic (HSCs) | Leukemia, lymphoma, anemias | 50-80% cure rates; 79% 3-year survival in transplants | Graft-vs-host disease (20-50% incidence); donor matching required |
| Mesenchymal (MSCs) | Graft-vs-host disease, osteoarthritis | 55% remission in GVHD; pain reduction in 60-70% of OA trials | Variable potency across donors; transient effects in many cases |
| Induced Pluripotent (iPSCs) | Retinal disorders, Parkinson's (phase I/II) | Safety in ~90% of early trials; modest efficacy (e.g., 10-20% vision gain in AMD) | Epigenetic memory; reprogramming mutations; <1% of trials report robust outcomes |
Ethical Controversies and Societal Debates
Moral Status of Embryos and Destruction in Research
The derivation of human embryonic stem cells (hESCs) requires the destruction of early-stage embryos, typically blastocysts produced via in vitro fertilization, which has fueled intense ethical debate over the moral status of these entities.[163][8] Biologically, fertilization initiates a new human organism, as affirmed by 95% of surveyed biologists who recognize this event as the beginning of a human's life due to the formation of a unique genome and the onset of self-directed development.[164] This scientific consensus underscores the embryo's status as a distinct human entity from conception, rather than mere cellular material, challenging views that equate it to tissue or property.[165] Philosophically, advocates for full moral status, such as those employing the argument from potentiality, contend that the embryo's intrinsic capacity to develop into a mature human—absent interference—confers equivalent rights to those of born persons, as potentiality reflects the same essence realized over time.[166] Critics, often gradualists, argue that moral status accrues progressively with traits like sentience or viability, typically post-implantation or later, dismissing early embryos as lacking personhood despite biological humanity.[167] This divide persists without empirical resolution, as moral claims hinge on metaphysical premises rather than observable data; however, the potentiality view aligns more directly with the embryo's causal trajectory toward personhood, avoiding arbitrary thresholds that ignore continuous development.[168] Religious perspectives, particularly from the Catholic Church, assert the embryo's inviolable dignity from fertilization, equating its destruction to homicide and prohibiting any research entailing such acts, while endorsing non-destructive stem cell sources like adult or induced pluripotent cells.[169][170] Similar stances appear in some Protestant and Islamic traditions emphasizing life's sanctity at conception, though interpretations vary.[171] In policy terms, the U.S. Dickey-Wicker Amendment, enacted annually since 1996, bars federal funding for research creating or destroying human embryos, a prohibition upheld in 2010 court rulings that halted hESC funding under prior administrations.[172][173] Despite executive orders expanding access to existing lines—such as George W. Bush's 2001 restriction to pre-existing derivations and Barack Obama's 2009 reversal—these maneuvers faced legal challenges for circumventing statutory intent against incentivizing destruction.[174] State-level bans, like Arkansas's 2019 prohibition on public funding for destructive embryo research, reflect ongoing contention.[174] The advent of induced pluripotent stem cells (iPSCs) in 2006 has mitigated reliance on embryos by enabling reprogramming without destruction, shifting focus yet not resolving foundational moral questions for residual hESC pursuits.[3] Institutions favoring permissive policies often exhibit ideological biases prioritizing therapeutic promise over embryo protections, as evidenced by selective emphasis on potential benefits amid limited clinical successes.[175]Stem Cell Tourism: Exploitation, Risks, and Regulatory Gaps
Stem cell tourism refers to the practice where patients travel internationally to receive unproven or experimental stem cell interventions not approved or available in their home countries, often targeting conditions such as multiple sclerosis, spinal cord injuries, autism, and Parkinson's disease.[176] This phenomenon exploits regulatory disparities, with clinics in nations like Ukraine, Mexico, and Thailand offering treatments lacking rigorous clinical evidence or safety data.[177] [176] Exploitation arises as clinics market these therapies through aggressive online advertising, promising cures with anecdotal testimonials while charging fees ranging from $10,000 to $50,000 per treatment, despite minimal scientific backing.[178] Vulnerable patients, driven by desperation and limited options in regulated systems, are targeted, leading to financial burdens without therapeutic benefits.[179] The International Society for Stem Cell Research (ISSCR) has condemned such practices, noting that providers often lack credentials and prioritize profit over patient safety.[180] For instance, in Poland, clinics have leveraged legal loopholes to administer unproven interventions, exploiting both patient naivety and inadequate medical oversight.[181] Risks include severe adverse events such as infections, immune rejection, tumor formation, and multiorgan failure, with documented cases resulting in permanent disability or death.[176] A 2021 case involved a 48-year-old patient who, after receiving mesenchymal stem cell infusions in Ukraine, developed disseminated skin ulcers, acute hepatitis, and cardiomyopathy, culminating in fatal multiorgan failure.[177] Broader analyses report 360 adverse events from 2004 to 2020, including 21 deaths linked to unapproved stem cell procedures, alongside complications like blindness and chronic pain.[182] Earlier reviews identified at least 35 instances of harm or fatality, underscoring the causal link between untested cellular products and tissue damage or systemic inflammation.[183] Regulatory gaps persist due to inconsistent international standards, where some countries permit direct-to-consumer stem cell offerings without phase I-III trials or ethical review, contrasting with stringent requirements from bodies like the FDA or EMA.[178] In the U.S., while domestic clinics face enforcement, patients bypass restrictions by traveling abroad, complicating post-treatment liability and follow-up care.[176] Crackdowns in Australia and Canada since 2019 have reduced unproven offerings by enhancing oversight and penalties, demonstrating that targeted regulation can mitigate exploitation, though global coordination remains elusive.[184] The ISSCR advocates for patient education and international harmonization to close these voids, emphasizing pre-travel counseling to avert harm.[185]Overhype, Funding Biases, and Media Distortions in Efficacy Claims
Despite initial enthusiasm following the isolation of human embryonic stem cells in 1998, proponents forecasted rapid clinical breakthroughs for conditions like Parkinson's disease and spinal cord injuries, yet as of 2023, no therapies derived from embryonic stem cells have achieved widespread approval or demonstrated curative efficacy after over 25 years of research.[186] This persistent gap between anticipated and realized outcomes has been attributed to technical hurdles such as tumorigenicity and immune rejection, compounded by exaggerated projections that prioritized potential over empirical validation.[187] Media coverage has often amplified unverified efficacy claims, as seen in the 2004-2005 Hwang Woo-suk scandal, where South Korean researcher Hwang claimed patient-specific embryonic stem cell lines from cloned embryos, earning international acclaim and national hero status before revelations of fabricated data in 2005 exposed the fraud, eroding public trust and highlighting peer-review vulnerabilities under hype-driven pressure.[188][189] Similar distortions persist in reporting on unproven interventions, where outlets promote stem cell treatments for autism, multiple sclerosis, and COVID-19 without rigorous evidence, fostering misconceptions of imminent cures while downplaying risks like infections and tumors reported in clinical cases.[190][191] Funding allocations reveal biases favoring embryonic and pluripotent stem cell research, despite adult stem cells—particularly hematopoietic ones—yielding established treatments for blood disorders since the 1960s and mesenchymal applications in limited orthopedic contexts.[192] California's Proposition 71, approved in 2004, authorized $3 billion in bonds for the California Institute for Regenerative Medicine (CIRM), promising accelerated cures but yielding only four therapies in clinical use by 2018 amid administrative controversies and modest returns on investment.[193] Subsequent Proposition 14 in 2020 extended $5.5 billion more, perpetuating emphasis on high-risk embryonic approaches over scalable adult stem cell expansions, potentially influenced by institutional prestige tied to novelty rather than proven outcomes.[194] These patterns underscore systemic incentives in academia and funding bodies, where grant competition and publication pressures incentivize optimistic preliminary data over cautious reporting, often sidelining critiques of adult stem cell viability despite their lower ethical barriers and higher translation rates to approved therapies.[186] Unregulated clinics exploit this narrative, marketing mesenchymal stem cell injections for unvalidated uses like cerebral palsy, with media echoes amplifying anecdotal successes while underreporting adverse events such as blindness and paraplegia documented in patient registries.[190][182]Current Research Frontiers
Recent Advancements in iPSCs, Organoids, and Gene Editing (2023-2025)
In 2023–2025, induced pluripotent stem cells (iPSCs) advanced toward clinical application, with a comprehensive 2025 review documenting over 50 interventional human pluripotent stem cell (hPSC) trials worldwide, emphasizing safety profiles such as low tumorigenicity rates below 1% in early-phase studies and preliminary efficacy in conditions like macular degeneration and Parkinson's disease.[117] Innovations included scalable production of iPSC-derived natural killer (NK) cells for cancer immunotherapy, achieving uniform effector function and reduced alloreactivity through gene knockout of endogenous receptors, as reported in mid-2025 preclinical models.[195] For ischemic stroke, iPSC-derived neural stem cells (NSCs) demonstrated neuroprotective effects in rodent models, with transplantation yielding up to 30% lesion volume reduction and improved motor function scores by 2025 studies.[196] Organoid technology progressed with enhanced vascularization and maturation, exemplified by a June 2025 Stanford breakthrough using human pluripotent stem cells to generate scalable heart and liver organoids incorporating endothelial and smooth muscle cells via novel growth factor cocktails and a triple-reporter fluorescent line for real-time imaging.[197] This enabled modeling of early human organ development with functional blood vessel networks, validated by single-cell transcriptomics showing gene expression profiles matching fetal tissues. Concurrently, human blood-brain barrier (BBB) organoids derived from pluripotent stem cells recapitulated neurovascular unit architecture, facilitating 2025 studies on drug permeability and neuroinflammation with over 90% tight junction integrity.[198] Gene editing via CRISPR/Cas9 integrated deeply with iPSCs and organoids, enabling precise mutation correction; for instance, 2025 work edited APP/PS1 mutations in iPSCs, reducing amyloid-beta (Aβ) accumulation by 40–60% and tau hyperphosphorylation in derived neurons, alongside base and prime editing for scarless modifications.[199] In organoids, CRISPR facilitated genome-wide screenings, as adapted in 2025 protocols for brain and kidney models, identifying therapeutic targets with hit rates improved by isogenic controls and achieving allele-specific editing efficiencies above 80%.[200] These synergies supported personalized regenerative approaches, such as CRISPR-modified mesenchymal stem cells evading immune detection (>85% B2M knockout efficiency) in Alzheimer's models, correlating with cognitive score improvements in phase 2 trials (e.g., -3.98 points on ADAS-cog at 36 weeks).[199] Challenges persist in editing off-target effects (typically <0.1% but requiring validation) and organoid reproducibility.[202]Innovations in Disease Modeling and Precision Regenerative Medicine
Induced pluripotent stem cells (iPSCs) have enabled the generation of patient-specific cellular models that recapitulate disease phenotypes in vitro, facilitating high-throughput drug screening and mechanistic studies. Derived from somatic cells via reprogramming factors discovered in 2006 and refined through subsequent optimizations, iPSCs allow for the creation of disease-relevant cell types without ethical concerns associated with embryonic sources. For instance, iPSC-derived cardiomyocytes have modeled genetic cardiomyopathies, revealing arrhythmia mechanisms through single-cell RNA sequencing that correlates with clinical outcomes.[203][26] Organoids, three-dimensional structures grown from stem cells that mimic organ architecture and function, represent a breakthrough in disease modeling by simulating tissue-level interactions absent in 2D cultures. Advances in 2023-2024 include iPSC-derived lung organoids that replicate cystic fibrosis pathophysiology, enabling precision testing of CFTR modulators with improved predictive accuracy over animal models. Brain organoids from iPSCs have modeled neurodevelopmental disorders like autism spectrum conditions, identifying disrupted neuronal connectivity via integrated CRISPR editing to validate causal variants. These models reduce reliance on preclinical animal testing, where species differences often limit translatability, as evidenced by organoid platforms predicting human-specific toxicities in 80-90% of cases for hepatic diseases.[204][205][202] In precision regenerative medicine, stem cell innovations integrate autologous iPSCs with gene editing for tailored therapies, minimizing immune rejection and targeting root genetic causes. CRISPR-Cas9 editing of patient iPSCs has corrected mutations in conditions like sickle cell disease, with edited hematopoietic progenitors showing restored function in preclinical xenografts. By December 2024, over 115 clinical trials had tested human pluripotent stem cell-derived products, primarily for ocular, neurological, and metabolic disorders, with allogeneic approaches incorporating immune evasion strategies like HLA matching. Mesenchymal stem cell (MSC) therapies, approved by the FDA for conditions like graft-versus-host disease (e.g., Ryoncil in 2023), demonstrate 70-80% response rates in refractory cases, attributed to paracrine effects rather than long-term engraftment.[159][130][157] These advancements converge in hybrid platforms, such as iPSC-organoid biobanks for personalized drug response prediction, where patient-derived models forecast efficacy with 75% concordance to clinical outcomes in oncology trials. Challenges persist, including variability in differentiation efficiency and off-target editing risks, but iterative refinements—such as single-cell profiling to select homogeneous populations—enhance reliability. Overall, these innovations shift regenerative medicine toward causal interventions, prioritizing empirical validation over empirical extrapolation from heterogeneous donor cells.[206][156]Future Prospects: Overcoming Dormancy, Immune Modulation, and Ethical Alternatives
Research into overcoming stem cell dormancy focuses on strategies to reactivate quiescent cells for enhanced therapeutic efficacy, particularly in regenerative contexts like spermatogenesis recovery post-chemotherapy. A 2024 study demonstrated that dormant spermatogonial stem cells (SSCs), which survive high-dose chemoradiotherapy due to their quiescent state, can be activated via chromatin remodeling to restore fertility in infertile mice, suggesting potential for human applications in preserving reproductive potential during cancer treatment.[207] Similarly, rejuvenation techniques for autologous adult stem cells, such as hematopoietic or mesenchymal types, have shown promise in extending lifespan and improving organ function in aged animal models by countering dormancy-induced functional decline, with prospects for clinical translation in age-related diseases.[208] Advancements in immune modulation leverage mesenchymal stem cells (MSCs) for their paracrine effects that suppress excessive immune responses while promoting tolerance, positioning them as key for treating autoimmune disorders and graft-versus-host disease. As of 2025, MSCs exhibit low immunogenicity and broad immunomodulatory potential through cytokine secretion and interaction with immune cells, with ongoing trials exploring their use in conditions like multiple sclerosis and inflammatory bowel disease to achieve long-term remission.[209] Future prospects include engineering MSCs for targeted delivery, enhancing their efficacy in modulating T-cell and macrophage activity, as evidenced by preclinical models where preconditioned MSCs reduced inflammation more effectively than unmodified cells.[210] Ethical alternatives to embryonic stem cells, notably induced pluripotent stem cells (iPSCs), circumvent moral concerns over embryo destruction by reprogramming adult somatic cells into pluripotent states without ethical compromise. Since the 2006 discovery of iPSCs, refinements like non-integrating vectors (e.g., Sendai virus) have minimized genomic integration risks, enabling safer clinical-grade production for disease modeling and transplantation.[26] By 2025, iPSC-derived therapies have advanced toward precision medicine, with autologous iPSCs showing reduced rejection risks in trials for macular degeneration and Parkinson's, offering scalable, patient-specific alternatives that align with ethical standards while matching embryonic stem cell pluripotency in differentiation potential.[211] Other sources, such as umbilical cord blood stem cells, further expand ethical options for hematopoietic reconstitution without embryo involvement.[212]References
- https://www.[sigmaaldrich](/page/Sigma-Aldrich).com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/stem-cell-markers-antibodies
- https://clinicaltrials.gov/ct2/show/NCT04388982