Recent from talks
Nothing was collected or created yet.
Cloning
View on WikipediaIt has been suggested that this article be split out into a new article titled Cloning in fiction. (Discuss) (February 2025) |

Cloning is the process of producing individual organisms with identical genomes, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction; this reproduction of an organism by itself without a mate is known as parthenogenesis. In the field of biotechnology, cloning is the process of creating cloned organisms of cells and of DNA fragments.
The artificial cloning of organisms, sometimes known as reproductive cloning, is often accomplished via somatic-cell nuclear transfer (SCNT), a cloning method in which a viable embryo is created from a somatic cell and an egg cell. In 1996, Dolly the sheep achieved notoriety for being the first mammal cloned from a somatic cell. Another example of artificial cloning is molecular cloning, a technique in molecular biology in which a single living cell is used to clone a large population of cells that contain identical DNA molecules.
In bioethics, there are a variety of ethical positions regarding the practice and possibilities of cloning. The use of embryonic stem cells, which can be produced through SCNT, in some stem cell research has attracted controversy. Cloning has been proposed as a means of reviving extinct species. In popular culture, the concept of cloning—particularly human cloning—is often depicted in science fiction; depictions commonly involve themes related to identity, the recreation of historical figures or extinct species, or cloning for exploitation (e.g. cloning soldiers for warfare).
Etymology
[edit]Coined by Herbert J. Webber in 1903, the term clone derives from the Ancient Greek word κλών (klōn), twig, which is the process whereby a new plant is created from a twig. In botany, the term lusus was used.[1] In horticulture, the spelling clon was used until the early twentieth century; the final e came into use to indicate the vowel is a "long o" instead of a "short o".[2][3] Since the term entered the popular lexicon in a more general context, the spelling clone has been used exclusively.
Natural cloning
[edit]This section needs expansion. You can help by adding to it. (December 2022) |
Natural cloning is the production of clones without the involvement of genetic engineering techniques or human intervention (i.e. artificial cloning).[4] Natural cloning occurs through a variety of natural mechanisms, from single-celled organisms to complex multicellular organisms, and has allowed life forms to spread for hundreds of millions of years. Versions of this reproduction method are used by plants, fungi, and bacteria, and is also the way that clonal colonies reproduce themselves.[5][6] Some of the mechanisms are explored and used in plants and animals are binary fission, budding, fragmentation, and parthenogenesis.[7] It can also occur during some forms of asexual reproduction, when a single parent organism produces genetically identical offspring by itself.[8][9]
Many plants are well known for natural cloning ability, including blueberry plants, Hazel trees, the Pando trees,[10][11] the Kentucky coffeetree, Myrica, and the American sweetgum.
It also occurs accidentally in the case of identical twins, which are formed when a fertilized egg splits, creating two or more embryos that carry identical DNA.
Molecular cloning
[edit]Molecular cloning refers to the process of making multiple molecules. Cloning is commonly used to amplify DNA fragments containing whole genes, but it can also be used to amplify any DNA sequence such as promoters, non-coding sequences and randomly fragmented DNA. It is used in a wide array of biological experiments and practical applications ranging from genetic fingerprinting to large scale protein production. Occasionally, the term cloning is misleadingly used to refer to the identification of the chromosomal location of a gene associated with a particular phenotype of interest, such as in positional cloning. In practice, localization of the gene to a chromosome or genomic region does not necessarily enable one to isolate or amplify the relevant genomic sequence. To amplify any DNA sequence in a living organism, that sequence must be linked to an origin of replication, which is a sequence of DNA capable of directing the propagation of itself and any linked sequence. However, a number of other features are needed, and a variety of specialised cloning vectors (small piece of DNA into which a foreign DNA fragment can be inserted) exist that allow protein production, affinity tagging, single-stranded RNA or DNA production and a host of other molecular biology tools.
Cloning of any DNA fragment essentially involves four steps[12]
- fragmentation - breaking apart a strand of DNA
- ligation – gluing together pieces of DNA in a desired sequence
- transfection – inserting the newly formed pieces of DNA into cells
- screening/selection – selecting out the cells that were successfully transfected with the new DNA
Although these steps are invariable among cloning procedures a number of alternative routes can be selected; these are summarized as a cloning strategy.
Initially, the DNA of interest needs to be isolated to provide a DNA segment of suitable size. Subsequently, a ligation procedure is used where the amplified fragment is inserted into a vector (piece of DNA). The vector (which is frequently circular) is linearised using restriction enzymes, and incubated with the fragment of interest under appropriate conditions with an enzyme called DNA ligase. Following ligation, the vector with the insert of interest is transfected into cells. A number of alternative techniques are available, such as chemical sensitisation of cells, electroporation, optical injection and biolistics. Finally, the transfected cells are cultured. As the aforementioned procedures are of particularly low efficiency, there is a need to identify the cells that have been successfully transfected with the vector construct containing the desired insertion sequence in the required orientation. Modern cloning vectors include selectable antibiotic resistance markers, which allow only cells in which the vector has been transfected, to grow. Additionally, the cloning vectors may contain colour selection markers, which provide blue/white screening (alpha-factor complementation) on X-gal medium. Nevertheless, these selection steps do not absolutely guarantee that the DNA insert is present in the cells obtained. Further investigation of the resulting colonies must be required to confirm that cloning was successful. This may be accomplished by means of PCR, restriction fragment analysis and/or DNA sequencing.
Cell cloning
[edit]Cloning unicellular organisms
[edit]
Cloning a cell means to derive a population of cells from a single cell. In the case of unicellular organisms such as bacteria and yeast, this process is remarkably simple and essentially only requires the inoculation of the appropriate medium. However, in the case of cell cultures from multi-cellular organisms, cell cloning is an arduous task as these cells will not readily grow in standard media.
A useful tissue culture technique used to clone distinct lineages of cell lines involves the use of cloning rings (cylinders).[13] In this technique a single-cell suspension of cells that have been exposed to a mutagenic agent or drug used to drive selection is plated at high dilution to create isolated colonies, each arising from a single and potentially clonal distinct cell. At an early growth stage when colonies consist of only a few cells, sterile polystyrene rings (cloning rings), which have been dipped in grease, are placed over an individual colony and a small amount of trypsin is added. Cloned cells are collected from inside the ring and transferred to a new vessel for further growth.
Cloning stem cells
[edit]Somatic-cell nuclear transfer, popularly known as SCNT, can also be used to create embryos for research or therapeutic purposes. The most likely purpose for this is to produce embryos for use in stem cell research. This process is also called "research cloning" or "therapeutic cloning". The goal is not to create cloned human beings (called "reproductive cloning"), but rather to harvest stem cells that can be used to study human development and to potentially treat disease. While a clonal human blastocyst has been created, stem cell lines are yet to be isolated from a clonal source.[14]
Therapeutic cloning is achieved by creating embryonic stem cells in the hopes of treating diseases such as diabetes and Alzheimer's. The process begins by removing the nucleus (containing the DNA) from an egg cell and inserting a nucleus from the adult cell to be cloned.[15] In the case of someone with Alzheimer's disease, the nucleus from a skin cell of that patient is placed into an empty egg. The reprogrammed cell begins to develop into an embryo because the egg reacts with the transferred nucleus. The embryo will become genetically identical to the patient.[15] The embryo will then form a blastocyst which has the potential to form/become any cell in the body.[16]
The reason why SCNT is used for cloning is because somatic cells can be easily acquired and cultured in the lab. This process can either add or delete specific genomes of farm animals. A key point to remember is that cloning is achieved when the oocyte maintains its normal functions and instead of using sperm and egg genomes to replicate, the donor's somatic cell nucleus is inserted into the oocyte.[17] The oocyte will react to the somatic cell nucleus, the same way it would to a sperm cell's nucleus.[17]
The process of cloning a particular farm animal using SCNT is relatively the same for all animals. The first step is to collect the somatic cells from the animal that will be cloned. The somatic cells could be used immediately or stored in the laboratory for later use.[17] The hardest part of SCNT is removing maternal DNA from an oocyte at metaphase II. Once this has been done, the somatic nucleus can be inserted into an egg cytoplasm.[17] This creates a one-cell embryo. The grouped somatic cell and egg cytoplasm are then introduced to an electrical current.[17] This energy will hopefully allow the cloned embryo to begin development. The successfully developed embryos are then placed in surrogate recipients, such as a cow or sheep in the case of farm animals.[17]
SCNT is seen as a good method for producing agriculture animals for food consumption. It successfully cloned sheep, cattle, goats, and pigs. Another benefit is SCNT is seen as a solution to clone endangered species that are on the verge of going extinct.[17] However, stresses placed on both the egg cell and the introduced nucleus can be enormous, which led to a high loss in resulting cells in early research. For example, the cloned sheep Dolly was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.[18] As the procedure could not be automated, and had to be performed manually under a microscope, SCNT was very resource intensive. The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg was also far from being well understood. However, by 2014 researchers were reporting cloning success rates of seven to eight out of ten[19] and in 2016, a Korean Company Sooam Biotech was reported to be producing 500 cloned embryos per day.[20]
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus.
Organism cloning
[edit]Organism cloning (also called reproductive cloning) refers to the procedure of creating a new multicellular organism, genetically identical to another. In essence this form of cloning is an asexual method of reproduction, where fertilization or inter-gamete contact does not take place. Asexual reproduction is a naturally occurring phenomenon in many species, including most plants and some insects. Scientists have made some major achievements with cloning, including the asexual reproduction of sheep and cows. There is a lot of ethical debate over whether or not cloning should be used. However, cloning, or asexual propagation,[21] has been common practice in the horticultural world for hundreds of years.
Horticultural
[edit]

The term clone is used in horticulture to refer to descendants of a single plant which were produced by vegetative reproduction or apomixis. Many horticultural plant cultivars are clones, having been derived from a single individual, multiplied by some process other than sexual reproduction.[22] As an example, some European cultivars of grapes represent clones that have been propagated for over two millennia. Other examples are potatoes and bananas.[23]
Grafting can be regarded as cloning, since all the shoots and branches coming from the graft are genetically a clone of a single individual, but this particular kind of cloning has not come under ethical scrutiny and is generally treated as an entirely different kind of operation.
Many trees, shrubs, vines, ferns and other herbaceous perennials form clonal colonies naturally. Parts of an individual plant may become detached by fragmentation and grow on to become separate clonal individuals. A common example is in the vegetative reproduction of moss and liverwort gametophyte clones by means of gemmae. Some vascular plants e.g. dandelion and certain viviparous grasses also form seeds asexually, termed apomixis, resulting in clonal populations of genetically identical individuals.
Parthenogenesis
[edit]Clonal derivation exists in nature in some animal species and is referred to as parthenogenesis (reproduction of an organism by itself without a mate). This is an asexual form of reproduction that is only found in females of some insects, crustaceans, nematodes,[24] fish (for example the hammerhead shark[25]), Cape honeybees,[26] and lizards including the Komodo dragon[25] and several whiptails. The growth and development occurs without fertilization by a male. In plants, parthenogenesis means the development of an embryo from an unfertilized egg cell, and is a component process of apomixis. In species that use the XY sex-determination system, the offspring will always be female. An example of partenogenesis is the little fire ant (Wasmannia auropunctata), which is native to Central and South America but has spread throughout many tropical environments.
Artificial cloning of organisms
[edit]Artificial cloning of organisms may also be called reproductive cloning.
First steps
[edit]Hans Spemann, a German embryologist was awarded a Nobel Prize in Physiology or Medicine in 1935 for his discovery of the effect now known as embryonic induction, exercised by various parts of the embryo, that directs the development of groups of cells into particular tissues and organs. In 1924 he and his student, Hilde Mangold, were the first to perform somatic-cell nuclear transfer using amphibian embryos – one of the first steps towards cloning.[27]
Methods
[edit]Reproductive cloning generally uses "somatic cell nuclear transfer" (SCNT) to create animals that are genetically identical. This process entails the transfer of a nucleus from a donor adult cell (somatic cell) to an egg from which the nucleus has been removed, or to a cell from a blastocyst from which the nucleus has been removed.[28] If the egg begins to divide normally it is transferred into the uterus of the surrogate mother. Such clones are not strictly identical since the somatic cells may contain mutations in their nuclear DNA. Additionally, the mitochondria in the cytoplasm also contains DNA and during SCNT this mitochondrial DNA is wholly from the cytoplasmic donor's egg, thus the mitochondrial genome is not the same as that of the nucleus donor cell from which it was produced. This may have important implications for cross-species nuclear transfer in which nuclear-mitochondrial incompatibilities may lead to death.
Artificial embryo splitting or embryo twinning, a technique that creates monozygotic twins from a single embryo, is not considered in the same fashion as other methods of cloning. During that procedure, a donor embryo is split in two distinct embryos, that can then be transferred via embryo transfer. It is optimally performed at the 6- to 8-cell stage, where it can be used as an expansion of IVF to increase the number of available embryos.[29] If both embryos are successful, it gives rise to monozygotic (identical) twins.
Dolly the sheep
[edit]
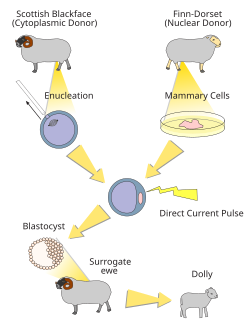
Dolly, a Finn-Dorset ewe, was the first mammal to have been successfully cloned from an adult somatic cell. Dolly was formed by taking a cell from the udder of her 6-year-old biological mother.[30] Dolly's embryo was created by taking the cell and inserting it into a sheep ovum. It took 435 attempts before an embryo was successful.[31] The embryo was then placed inside a female sheep that went through a normal pregnancy.[32] She was cloned at the Roslin Institute in Scotland by British scientists Sir Ian Wilmut and Keith Campbell and lived there from her birth in 1996 until her death in 2003 when she was six. She was born on 5 July 1996 but not announced to the world until 22 February 1997.[33] Her stuffed remains were placed at Edinburgh's Royal Museum, part of the National Museums of Scotland.[34]
Dolly was publicly significant because the effort showed that genetic material from a specific adult cell, designed to express only a distinct subset of its genes, can be redesigned to grow an entirely new organism. Before this demonstration, it had been shown by John Gurdon that nuclei from differentiated cells could give rise to an entire organism after transplantation into an enucleated egg.[35] However, this concept was not yet demonstrated in a mammalian system.
The first mammalian cloning (resulting in Dolly) had a success rate of 29 embryos per 277 fertilized eggs, which produced three lambs at birth, one of which lived. In a bovine experiment involving 70 cloned calves, one-third of the calves died quite young. The first successfully cloned horse, Prometea, took 814 attempts. Notably, although the first clones were frogs, no adult cloned frog has yet been produced from a somatic adult nucleus donor cell.[36]
There were early claims that Dolly had pathologies resembling accelerated aging. Scientists speculated that Dolly's death in 2003 was related to the shortening of telomeres, DNA-protein complexes that protect the end of linear chromosomes. However, other researchers, including Ian Wilmut who led the team that successfully cloned Dolly, argue that Dolly's early death due to respiratory infection was unrelated to problems with the cloning process. This idea that the nuclei have not irreversibly aged was shown in 2013 to be true for mice.[37]
Dolly was named after performer Dolly Parton because the cells cloned to make her were from a mammary gland cell, and Parton is known for her ample cleavage.[38]
Recent advances in biotechnology allowed the modification of wolf clones to make them appear similar to dire wolves by a company named Colossal Biosciences. "The company used a combination of gene-editing techniques and ancient DNA found in fossils to engineer the newborn pups."[39] It's currently contested whether this can be considered a true dire wolf[40], but this shows that gene modification and possibly cloning in the future could advance. They produced three of the white "dire wolves" and scientists are more interested in how this can be used for endangered animals.[citation needed]
Species cloned and applications
[edit]This section needs expansion. You can help by adding to it. (March 2023) |
The modern cloning techniques involving nuclear transfer have been successfully performed on several species. Notable experiments include:
- Tadpole: (1952) Robert Briggs and Thomas J. King successfully cloned northern leopard frogs: thirty-five complete embryos and twenty-seven tadpoles from one-hundred and four successful nuclear transfers.[41][42]
- Carp: (1963) In China, embryologist Tong Dizhou produced the world's first cloned fish by inserting the DNA from a cell of a male carp into an egg from a female carp.[43]
- Zebrafish: (1981) George Streisinger produced the first cloned vertebrate.[44]
- Sheep: (1984) Steen Willadsen produced the first cloned mammal from early embryonic cells.
- In June 1995, the Roslin Institute cloned Megan and Morag from differentiated embryonic cells.[45][46]
- In July 1996, PPL Therapeutics and the Roslin Institute cloned Dolly the sheep from a somatic cell.[47][43]
- Mouse: (1986) A mouse was successfully cloned from an early embryonic cell. In 1987, Soviet scientists Levon Chaylakhyan, Veprencev, Sviridova, and Nikitin cloned Masha, a mouse.[clarification needed][48][49][needs update]
- Rhesus monkey: (October 1999) The Oregon National Primate Research Center cloned Tetra from embryo splitting and not nuclear transfer: a process more akin to artificial formation of twins.[50][51]
- Pig: (March 2000) PPL Therapeutics cloned five piglets.[52] By 2014, BGI in China was producing 500 cloned pigs a year to test new medicines.[53]
- Gaur: (2001) was the first endangered species cloned.[54]
- Cattle:
- Alpha and Beta (males, 2001) and (2005), Brazil[55]
- In 2023, Chinese scientists reported the cloning of three supercows with a milk productivity "nearly 1.7 times the amount of milk an average cow in the United States produced in 2021" and a plan for 1,000 of such super cows in the near-term. According to a news report "[i]n many countries, including the United States, farmers breed clones with conventional animals to add desirable traits, such as high milk production or disease resistance, into the gene pool".[clarification needed][when?][56]
- Cat: CopyCat "CC" (female, late 2001), Little Nicky, 2004, was the first cat cloned for commercial reasons[57]
- Rat: Ralph, the first cloned rat (2003)[58]
- Mule: Idaho Gem, a john mule born 4 May 2003, was the first horse-family clone.[59]
- Horse: Prometea, a Haflinger female born 28 May 2003, was the first horse clone.[60]
- Przewalksi's Horse: An ongoing cloning program by the San Diego Zoo Wildlife Alliance and Revive & Restore attempts to reintroduce genetic diversity to this endangered species.[61]
- Dog:
- Snuppy, a male Afghan hound was the first cloned dog (2005).[64] In 2017, the world's first gene-editing clone dog, Apple, was created by Sinogene Biotechnology.[65] Sooam Biotech, South Korea, was reported in 2015 to have cloned 700 dogs to date for their owners, including two Yakutian Laika hunting dogs, which are seriously endangered due to crossbreeding.[66]
- Cloning of super sniffer dogs was reported in 2011, four years afterwards when the dogs started working.[67] Cloning of a successful rescue dog was also reported in 2009[68] and of a similar police dog in 2019.[69] Cancer-sniffing dogs have also been cloned. A review concluded that "qualified elite working dogs can be produced by cloning a working dog that exhibits both an appropriate temperament and good health."[70]
- Wolf: Snuwolf and Snuwolffy, the first two cloned female wolves (2005).[71]
- Water buffalo: Samrupa was the first cloned water buffalo. It was born on 6 February 2009, at India's Karnal National Dairy Research Institute but died five days later due to lung infection.[72]
- Pyrenean ibex: (2009) was the first extinct animal to be cloned back to life; the clone lived for seven minutes before dying of lung defects.[73] The extinct Pyrenean ibex is a sub-species of the extant Spanish ibex.[74]
- Camel: (2009) Injaz, was the first cloned camel.[75]
- Pashmina goat: (2012) Noori, is the first cloned pashmina goat. Scientists at the faculty of veterinary sciences and animal husbandry of Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir successfully cloned the first Pashmina goat (Noori) using the advanced reproductive techniques under the leadership of Riaz Ahmad Shah.[76]
- Goat: (2001) Scientists of Northwest A&F University successfully cloned the first goat which use the adult female cell.[77]
- Gastric brooding frog: (2013) The gastric brooding frog, Rheobatrachus silus, thought to have been extinct since 1983 was cloned in Australia, although the embryos died after a few days.[78]
- Macaque monkey: (2017) First successful cloning of a primate species using nuclear transfer, with the birth of two live clones named Zhong Zhong and Hua Hua. Conducted in China in 2017, and reported in January 2018.[79][80][81][82] In January 2019, scientists in China reported the creation of five identical cloned gene-edited monkeys, using the same cloning technique that was used with Zhong Zhong and Hua Hua and Dolly the sheep, and the gene-editing Crispr-Cas9 technique allegedly used by He Jiankui in creating the first ever gene-modified human babies Lulu and Nana. The monkey clones were made to study several medical diseases.[83][84]
- Black-footed ferret: (2020) A team of scientists cloned a female named Willa, who died in the mid-1980s and left no living descendants. Her clone, a female named Elizabeth Ann, was born on 10 December. Scientists hope that the contribution of this individual will alleviate the effects of inbreeding and help black-footed ferrets better cope with plague. Experts estimate that this female's genome contains three times as much genetic diversity as any of the modern black-footed ferrets.[85]
- First artificial parthenogenesis in mammals: (2022) Viable mice offspring was born from unfertilized eggs via targeted DNA methylation editing of seven imprinting control regions.[86]
Human cloning
[edit]Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissues. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass legislation regarding human cloning and its legality. As of right now, scientists have no intention of trying to clone people and they believe their results should spark a wider discussion about the laws and regulations the world needs to regulate cloning.[87]
Two commonly discussed types of theoretical human cloning are therapeutic cloning and reproductive cloning. Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants, and is an active area of research, but is not in medical practice anywhere in the world, as of 2024[update]. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and, more recently, pluripotent stem cell induction. Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.[88]
Ethical issues of cloning
[edit]There are a variety of ethical positions regarding the possibilities of cloning, especially human cloning. While many of these views are religious in origin, the questions raised by cloning are faced by secular perspectives as well. Perspectives on human cloning are theoretical, as human therapeutic and reproductive cloning are not commercially used; animals are currently cloned in laboratories and in livestock production.
Advocates support development of therapeutic cloning to generate tissues and whole organs to treat patients who otherwise cannot obtain transplants,[89] to avoid the need for immunosuppressive drugs,[88] and to stave off the effects of aging.[90] Advocates for reproductive cloning believe that parents who cannot otherwise procreate should have access to the technology.[91]
Opponents of cloning have concerns that technology is not yet developed enough to be safe[92] and that it could be prone to abuse (leading to the generation of humans from whom organs and tissues would be harvested),[93][94] as well as concerns about how cloned individuals could integrate with families and with society at large.[95][96] Cloning humans could lead to serious violations of human rights.[97]
Religious groups are divided, with some opposing the technology as usurping "God's place" and, to the extent embryos are used, destroying a human life; others support therapeutic cloning's potential life-saving benefits.[98][99] There is at least one religion, Raëlism, in which cloning plays a major role.[100][101][102]
Contemporary work on this topic is concerned with the ethics, adequate regulation and issues of any cloning carried out by humans, not potentially by extraterrestrials (including in the future), and largely also not replication – also described as mind cloning[103][104][105][106] – of potential whole brain emulations.
Cloning of animals is opposed by animal-groups due to the number of cloned animals that suffer from malformations before they die, and while food from cloned animals has been approved as safe by the US FDA,[107][108] its use is opposed by groups concerned about food safety.[109][110]
In practical terms, the inclusion of "licensing requirements for embryo research projects and fertility clinics, restrictions on the commodification of eggs and sperm, and measures to prevent proprietary interests from monopolizing access to stem cell lines" in international cloning regulations has been proposed, albeit e.g. effective oversight mechanisms or cloning requirements have not been described.[111]
Cloning extinct and endangered species
[edit]Cloning, or more precisely, the reconstruction of functional DNA from extinct species has, for decades, been a dream. Possible implications of this were dramatized in the 1984 novel Carnosaur and the 1990 novel Jurassic Park.[112][113] The best current cloning techniques have an average success rate of 9.4 percent[114] (and as high as 25 percent[37]) when working with familiar species such as mice,[note 1] while cloning wild animals is usually less than 1 percent successful.[117]
Conservation cloning
[edit]Several tissue banks have come into existence, including the "Frozen zoo" at the San Diego Zoo, to store frozen tissue from the world's rarest and most endangered species.[112][118][119][120] This is also referred to as "Conservation cloning".[121][122]
Engineers have proposed a "lunar ark" in 2021 – storing millions of seed, spore, sperm and egg samples from Earth's contemporary species in a network of lava tubes on the Moon as a genetic backup.[123][124][125] Similar proposals have been made since at least 2008.[126] These also include sending human customer DNA,[127] and a proposal for "a lunar backup record of humanity" that includes genetic information by Avi Loeb et al.[128]
In 2020, the San Diego Zoo began a number of projects in partnership with the conservation organization Revive & Restore and the ViaGen Pets and Equine Company to clone individuals of genetically-impoverished endangered species. A Przewalski's horse was cloned from preserved tissue of a stallion whose genes are absent in the surviving populations of the species, which descend from twenty individuals. The clone, named Kurt, had been born to a domestic surrogate mother, and was partnered with a natural-born Przewalski's mare in order to socialize him with the species' natural behavior before being introduced to the Zoo's breeding herd.[129] In 2023, a second clone of the original stallion, named Ollie, was born; this marked the first instance of multiple living clones of a single individual of an endangered species being alive at the same time.[130] Also in 2020, a clone named Elizabeth Ann was produced of a female black-footed ferret that had no living descendants.[131] While Elizabeth Ann became sterile due to secondary health complications, a pair of additional clones of the same individual, named Antonia and Noreen, were born to distinct surrogate mothers, and Antonia successfully reproduced later in the year.[132]
De-extinction
[edit]One of the most anticipated targets for cloning was once the woolly mammoth, but attempts to extract DNA from frozen mammoths have been unsuccessful, though a joint Russo-Japanese team is currently working toward this goal.[when?] In January 2011, it was reported by Yomiuri Shimbun that a team of scientists headed by Akira Iritani of Kyoto University had built upon research by Dr. Wakayama, saying that they will extract DNA from a mammoth carcass that had been preserved in a Russian laboratory and insert it into the egg cells of an Asian elephant in hopes of producing a mammoth embryo. The researchers said they hoped to produce a baby mammoth within six years.[133][134] The challenges are formidable. Extensively degraded DNA that may be suitable for sequencing may not be suitable for cloning; it would have to be synthetically reconstituted. In any case, with currently available technology, DNA alone is not suitable for mammalian cloning; intact viable cell nuclei are required. Patching pieces of reconstituted mammoth DNA into an Asian elephant cell nucleus would result in an elephant-mammoth hybrid rather than a true mammoth.[135] Moreover, true de-extinction of the wooly mammoth species would require a breeding population, which would require cloning of multiple genetically distinct but reproductively compatible individuals, multiplying both the amount of work and the uncertainties involved in the project. There are potentially other post-cloning problems associated with the survival of a reconstructed mammoth, such as the requirement of ruminants for specific symbiotic microbiota in their stomachs for digestion.[135]
Scientists at the University of Newcastle and University of New South Wales announced in March 2013 that the very recently extinct gastric-brooding frog would be the subject of a cloning attempt to resurrect the species.[136]
Many such "de-extinction" projects are being championed by the non-profit Revive & Restore.[137]
In 2022, scientists showed major limitations and the scale of challenge of genetic-editing-based de-extinction, suggesting resources spent on more comprehensive de-extinction projects such as of the woolly mammoth may currently not be well allocated and substantially limited. Their analyses "show that even when the extremely high-quality Norway brown rat (R. norvegicus) is used as a reference, nearly 5% of the genome sequence is unrecoverable, with 1,661 genes recovered at lower than 90% completeness, and 26 completely absent", complicated further by that "distribution of regions affected is not random, but for example, if 90% completeness is used as the cutoff, genes related to immune response and olfaction are excessively affected" due to which "a reconstructed Christmas Island rat would lack attributes likely critical to surviving in its natural or natural-like environment".[138]
In a 2021 online session of the Russian Geographical Society, Russia's defense minister Sergei Shoigu mentioned using the DNA of 3,000-year-old Scythian warriors to potentially bring them back to life. The idea was described as absurd at least at this point in news reports and it was noted that Scythians likely weren't skilled warriors by default.[139][140][141]
The idea of cloning Neanderthals or bringing them back to life in general is controversial but some scientists have stated that it may be possible in the future and have outlined several issues or problems with such as well as broad rationales for doing so.[142][143][144][145][146][147]
Unsuccessful attempts
[edit]In 2001, a cow named Bessie gave birth to a cloned Asian gaur, an endangered species, but the calf died after two days. In 2003, a banteng was successfully cloned, followed by three African wildcats from a thawed frozen embryo. These successes provided hope that similar techniques (using surrogate mothers of another species) might be used to clone extinct species. Anticipating this possibility, tissue samples from the last bucardo (Pyrenean ibex) were frozen in liquid nitrogen immediately after it died in 2000. Researchers are also considering cloning endangered species such as the Giant panda and Cheetah.[148][149][150][151]
In 2002, geneticists at the Australian Museum announced that they had replicated DNA of the thylacine (Tasmanian tiger), at the time extinct for about 65 years, using polymerase chain reaction.[152] However, on 15 February 2005 the museum announced that it was stopping the project after tests showed the specimens' DNA had been too badly degraded by the (ethanol) preservative. On 15 May 2005 it was announced that the thylacine project would be revived, with new participation from researchers in New South Wales and Victoria.[153]
In 2003, for the first time, an extinct animal, the Pyrenean ibex mentioned above was cloned, at the Centre of Food Technology and Research of Aragon, using the preserved frozen cell nucleus of the skin samples from 2001 and domestic goat egg-cells. The ibex died shortly after birth due to physical defects in its lungs.[154]
Lifespan
[edit]After an eight-year project involving the use of a pioneering cloning technique, Japanese researchers created 25 generations of healthy cloned mice with normal lifespans, demonstrating that clones are not intrinsically shorter-lived than naturally born animals.[37][155] Other sources have noted that the offspring of clones tend to be healthier than the original clones and indistinguishable from animals produced naturally.[156]
Some posited that Dolly the sheep may have aged more quickly than naturally born animals, as she died relatively early for a sheep at the age of six. Ultimately, her death was attributed to a respiratory illness, and the "advanced aging" theory is disputed.[157][dubious – discuss]
A 2016 study indicated that once cloned animals survive the first month or two of life they are generally healthy.[158] However, early pregnancy loss and neonatal losses are still greater with cloning than natural conception or assisted reproduction (IVF). Current research is attempting to overcome these problems.[38]
In popular culture
[edit]
Discussion of cloning in the popular media often presents the subject negatively. In an article in the 8 November 1993 article of Time, cloning was portrayed in a negative way, modifying Michelangelo's Creation of Adam to depict Adam with five identical hands.[159] Newsweek's 10 March 1997 issue also critiqued the ethics of human cloning, and included a graphic depicting identical babies in beakers.[160]
The concept of cloning, particularly human cloning, has featured a wide variety of science fiction works. An early fictional depiction of cloning is Bokanovsky's Process which features in Aldous Huxley's 1931 dystopian novel Brave New World. The process is applied to fertilized human eggs in vitro, causing them to split into identical genetic copies of the original.[161][162] Following renewed interest in cloning in the 1950s, the subject was explored further in works such as Poul Anderson's 1953 story UN-Man, which describes a technology called "exogenesis", and Gordon Rattray Taylor's book The Biological Time Bomb, which popularised the term "cloning" in 1963.[163]
Cloning is a recurring theme in a number of contemporary science fiction films, ranging from action films such as Anna to the Infinite Power, The Boys from Brazil, Jurassic Park (1993), Alien Resurrection (1997), The 6th Day (2000), Resident Evil (2002), Star Wars: Episode II – Attack of the Clones (2002), The Island (2005), Tales of the Abyss (2006), and Moon (2009) to comedies such as Woody Allen's 1973 film Sleeper.[164]
The process of cloning is represented variously in fiction. Many works depict the artificial creation of humans by a method of growing cells from a tissue or DNA sample; the replication may be instantaneous, or take place through slow growth of human embryos in artificial wombs. In the long-running British television series Doctor Who, the Fourth Doctor and his companion Leela were cloned in a matter of seconds from DNA samples ("The Invisible Enemy", 1977) and then – in an apparent homage to the 1966 film Fantastic Voyage – shrunk to microscopic size to enter the Doctor's body to combat an alien virus. The clones in this story are short-lived, and can only survive a matter of minutes before they expire.[165] Science fiction films such as The Matrix and Star Wars: Episode II – Attack of the Clones have featured scenes of human foetuses being cultured on an industrial scale in mechanical tanks.[166]
Cloning humans from body parts is also a common theme in science fiction. Cloning features strongly among the science fiction conventions parodied in Woody Allen's Sleeper, the plot of which centres around an attempt to clone an assassinated dictator from his disembodied nose.[167] In the 2008 Doctor Who story "Journey's End", a duplicate version of the Tenth Doctor spontaneously grows from his severed hand, which had been cut off in a sword fight during an earlier episode.[168]
After the death of her beloved 14-year-old Coton de Tulear named Samantha in late 2017, Barbra Streisand announced that she had cloned the dog, and was now "waiting for [the two cloned pups] to get older so [she] can see if they have [Samantha's] brown eyes and her seriousness".[169] The operation cost $50,000 through the pet cloning company ViaGen.[170]
In films such as Roger Spottiswoode's 2000 The 6th Day, which makes use of the trope of a "vast clandestine laboratory ... filled with row upon row of 'blank' human bodies kept floating in tanks of nutrient liquid or in suspended animation", clearly fear is to be incited. In Clark's view, the biotechnology is typically "given fantastic but visually arresting forms" while the science is either relegated to the background or fictionalised to suit a young audience.[171] Genetic engineering methods are weakly represented in film; Michael Clark, writing for The Wellcome Trust, calls the portrayal of genetic engineering and biotechnology "seriously distorted"[171]
Cloning and identity
[edit]Science fiction has used cloning, most commonly and specifically human cloning, to address questions of identity and eugenics.[172][173] A Number is a 2002 play by English playwright Caryl Churchill which addresses the subject of human cloning and identity, especially nature and nurture. The story, set in the near future, is structured around the conflict between a father (Salter) and his sons (Bernard 1, Bernard 2, and Michael Black) –the second two of whom are clones of the first. A Number was adapted by Caryl Churchill for television, in a co-production between the BBC and HBO Films.[174]
In 2012, a Japanese television series named "Bunshin" was created. The story's main character, Mariko, is a woman studying child welfare in Hokkaido. She grew up always doubtful about the love from her mother, who looked nothing like her and who died nine years before. One day, she finds some of her mother's belongings at a relative's house, and heads to Tokyo to seek out the truth behind her birth. She later discovered that she was a clone.[175]
In the 2013 television series Orphan Black, cloning is used as a scientific study on the behavioral adaptation of the clones.[176] In a similar vein, the book The Double by Nobel Prize winner José Saramago explores the emotional experience of a man who discovers that he is a clone.[177]
Cloning as resurrection
[edit]Cloning has been used in fiction as a way of recreating historical figures. In the 1976 Ira Levin novel The Boys from Brazil and its 1978 film adaptation, Josef Mengele uses cloning to create copies of Adolf Hitler.[178]
The Norman Spinrad's satirical The Iron Dream, published in 1972, concludes with 300 seven-foot-tall, blond, super-intelligent all-male SS clones in suspended animation launched into space to begin the Hitler analog's galactic empire in the aftermath of a genocidal race war. (They had won a Pyrrhic victory, the subhumans' leader having as his last act detonated a doomsday weapon, specifically a cobalt bomb, irredeemably contaminated the gene pool).[179]
In Michael Crichton's 1990 novel Jurassic Park, which spawned a series of Jurassic Park feature films, the bioengineering company InGen develops a technique to resurrect extinct species of dinosaurs by creating cloned creatures using DNA extracted from fossils. The cloned dinosaurs are used to populate the Jurassic Park wildlife park for the entertainment of visitors. The scheme goes disastrously wrong when the dinosaurs escape their enclosures.[180]
Cloning for warfare
[edit]The use of cloning for military purposes has also been explored in several fictional works. In Doctor Who, an alien race of armour-clad, warlike beings called Sontarans was introduced in the 1973 serial "The Time Warrior". Sontarans are depicted as squat, bald creatures who have been genetically engineered for combat. Their weak spot is a "probic vent", a small socket at the back of their neck which is associated with the cloning process.[181] The concept of cloned soldiers being bred for combat was revisited in "The Doctor's Daughter" (2008), when the Doctor's DNA is used to create a female warrior called Jenny.[182]
The 1977 film Star Wars was set against the backdrop of a historical conflict called the Clone Wars. The events of this war were not fully explored until the prequel films Attack of the Clones (2002) and Revenge of the Sith (2005), which depict a space war waged by a massive army of heavily armoured clone troopers that leads to the foundation of the Galactic Empire. Cloned soldiers are "manufactured" on an industrial scale, genetically conditioned for obedience and combat effectiveness. It is also revealed that the popular character Boba Fett originated as a clone of Jango Fett, a mercenary who served as the genetic template for the clone troopers.[183][184]
Cloning for exploitation
[edit]A recurring sub-theme of cloning fiction is the use of clones as a supply of organs for transplantation. The 2005 Kazuo Ishiguro novel Never Let Me Go and the 2010 film adaption[185] are set in an alternate history in which cloned humans are created for the sole purpose of providing organ donations to naturally born humans, despite the fact that they are fully sentient and self-aware. The 2005 film The Island[186] revolves around a similar plot, with the exception that the clones are unaware of the reason for their existence.
The exploitation of human clones for dangerous and undesirable work was examined in the 2009 British science fiction film Moon.[187] In the futuristic novel Cloud Atlas and subsequent film, one of the story lines focuses on a genetically engineered fabricant clone named Sonmi~451, one of millions raised in an artificial "wombtank", destined to serve from birth. She is one of thousands created for manual and emotional labor; Sonmi herself works as a server in a restaurant. She later discovers that the sole source of food for clones, called 'Soap', is manufactured from the clones themselves.[188]
In the film Us, at some point prior to the 1980s, the US Government creates clones of every citizen of the United States with the intention of using them to control their original counterparts, akin to voodoo dolls. This fails, as they were able to copy bodies, but unable to copy the souls of those they cloned. The project is abandoned and the clones are trapped exactly mirroring their above-ground counterparts' actions for generations. In the present day, the clones launch a surprise attack and manage to complete a mass-genocide of their unaware counterparts.[189][190]
See also
[edit]Notes
[edit]References
[edit]- ^ de Candolle, A. (1868). Laws of Botanical Nomenclature adopted by the International Botanical Congress held at Paris in August 1867; together with an Historical Introduction and Commentary by Alphonse de Candolle, Translated from the French. translated by H.A. Weddell. London: L. Reeve and Co.: 21, 43
- ^ "Torrey Botanical Club: Volumes 42–45". Torreya. 42–45: 133. 1942.
- ^ Herbert J. Webber (1903). Science. American Association for the Advancement of Science. pp. 502–503. Retrieved 8 October 2010.
- ^ "Natural Cloning: Definition & Examples | StudySmarter". StudySmarter US. Archived from the original on 5 May 2023. Retrieved 5 May 2023.
- ^ "Tasmanian bush could be oldest living organism". Discovery Channel. Archived from the original on 23 July 2006. Retrieved 7 May 2008.
- ^ "Ibiza's Monster Marine Plant". Ibiza Spotlight. Archived from the original on 26 December 2007. Retrieved 7 May 2008.
- ^ "Natural Cloning: Definition & Examples | StudySmarter".
- ^ "Cloning Fact Sheet". Genome.gov. Retrieved 5 May 2023.
- ^ "Cloning | Definition, Process, & Types | Britannica". www.britannica.com. Retrieved 5 May 2023.
- ^ DeWoody, J.; Rowe, C.A.; Hipkins, V.D.; Mock, K.E. (2008). ""Pando" Lives: Molecular Genetic Evidence of a Giant Aspen Clone in Central Utah". Western North American Naturalist. 68 (4): 493–497. Bibcode:2008WNAN...68..493D. doi:10.3398/1527-0904-68.4.493. ISSN 1944-8341. S2CID 59135424.
- ^ Mock, K.E.; Rowe, C.A.; Hooten, M.B.; Dewoody, J.; Hipkins, V.D. (2008). "Blackwell Publishing Ltd Clonal dynamics in western North American aspen (Populus tremuloides)". U.S. Department of Agriculture, Oxford, UK : Blackwell Publishing Ltd. p. 17. Archived from the original on 11 August 2014. Retrieved 5 December 2013.
- ^ Peter J. Russel (2005). iGenetics: A Molecular Approach. San Francisco, California, United States of America: Pearson Education. ISBN 978-0-8053-4665-7.
- ^ McFarland, Douglas (2000). "Preparation of pure cell cultures by cloning". Methods in Cell Science. 22 (1): 63–66. doi:10.1023/A:1009838416621. PMID 10650336.
- ^ Gil, Gideon (17 January 2008). "California biotech says it cloned a human embryo, but no stem cells produced". Boston Globe.
- ^ a b Halim, N. (September 2002). "Extensive new study shows abnormalities in cloned animals". Massachusetts Institute of Technology. Retrieved 31 October 2011.
- ^ Plus, M. (2011). "Fetal development". Nlm.nih.gov. Retrieved 31 October 2011.
- ^ a b c d e f g Latham, K. E. (2005). "Early and delayed aspects of nuclear reprogramming during cloning" (PDF). Biology of the Cell. pp. 97, 119–132. Archived from the original (PDF) on 2 August 2014.
- ^ Campbell KH, McWhir J, Ritchie WA, Wilmut I (March 1996). "Sheep cloned by nuclear transfer from a cultured cell line". Nature. 380 (6569): 64–6. Bibcode:1996Natur.380...64C. doi:10.1038/380064a0. PMID 8598906. S2CID 3529638.
- ^ Shukman, David (14 January 2014) China cloning on an 'industrial scale' BBC News Science and Environment, Retrieved 10 April 2014
- ^ Zastrow, Mark (8 February 2016). "Inside the cloning factory that creates 500 new animals a day". New Scientist. Retrieved 23 February 2016.
- ^ "Asexual Propagation". Aggie-horticulture.tamu.edu. Retrieved 4 August 2010.
- ^ Sagers, Larry A. (2 March 2009) Proliferate favorite trees by grafting, cloning Archived 1 March 2014 at the Wayback Machine Utah State University, Deseret News (Salt Lake City) Retrieved 21 February 2014
- ^ Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J. -P.; Jenny, C.; Lebot, V.; Risterucci, A. -M.; Tomekpe, K.; Doutrelepont, H.; Ball, T.; Manwaring, J.; De Maret, P.; Denham, T. (2011). "Multidisciplinary perspectives on banana (Musa spp.) domestication". Proceedings of the National Academy of Sciences. 108 (28): 11311–11318. Bibcode:2011PNAS..10811311P. doi:10.1073/pnas.1102001108. PMC 3136277. PMID 21730145.
- ^ Castagnone-Sereno P, et al. Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic era Annu Rev Phytopathol. 2013;51:203-20
- ^ a b Shubin, Neil (24 February 2008). "Birds Do It. Bees Do It. Dragons Don't Need To". The New York Times. Retrieved 21 February 2014
- ^ Oldroyd, Benjamin P.; Yagound, Boris; Allsopp, Michael H.; Holmes, Michael J.; Buchmann, Gabrielle; Zayed, Amro; Beekman, Madeleine (9 June 2021). "Adaptive, caste-specific changes to recombination rates in a thelytokous honeybee population". Proceedings of the Royal Society B: Biological Sciences. 288 (1952) 20210729. doi:10.1098/rspb.2021.0729. ISSN 0962-8452. PMC 8187994. PMID 34102886. S2CID 235372422.
- News article about the study: "A single honeybee has cloned itself hundreds of millions of times". New Scientist. Retrieved 8 March 2023.
- ^ De Robertis, EM (April 2006). "Spemann's organizer and self-regulation in amphibian embryos". Nature Reviews. Molecular Cell Biology. 7 (4): 296–302. doi:10.1038/nrm1855. PMC 2464568. PMID 16482093.. See Box 1: Explanation of the Spemann-Mangold experiment
- ^ "Cloning Fact Sheet". Human Genome Project Information. Archived from the original on 2 May 2013. Retrieved 25 October 2011.
- ^ Illmensee K, Levanduski M, Vidali A, Husami N, Goudas VT (February 2009). "Human embryo twinning with applications in reproductive medicine". Fertil. Steril. 93 (2): 423–7. doi:10.1016/j.fertnstert.2008.12.098. PMID 19217091.
- ^ Rantala, M.; Milgram, Arthur (1999). Cloning: For and Against. Chicago, Illinois: Carus Publishing Company. p. 1. ISBN 978-0-8126-9375-1.
- ^ Swedin, Eric. "Cloning". CredoReference. Retrieved 23 September 2013.
- ^ Lassen, J.; Gjerris, M.; Sandøe, P. (2005). "After Dolly—Ethical limits to the use of biotechnology on farm animals" (PDF). Theriogenology. 65 (5): 992–1004. doi:10.1016/j.theriogenology.2005.09.012. PMID 16253321. S2CID 10466667. Archived from the original (PDF) on 28 July 2020. Retrieved 2 December 2019.
- ^ Swedin, Eric. "Cloning". CredoReference. Science in the Contemporary World. Retrieved 23 September 2013.
- ^ TV documentary Visions of the Future part 2 shows this process, explores the social implicatins of cloning and contains footage of monoculture in livestock
- ^ Gurdon J (April 1962). "Adult frogs derived from the nuclei of single somatic cells". Developmental Biology. 4 (2): 256–73. doi:10.1016/0012-1606(62)90043-x. PMID 13903027.
- ^ this is a source that was used in List of animals that have been cloned
- ^ a b c Wakayama S, Kohda T, Obokata H, Tokoro M, Li C, Terashita Y, Mizutani E, Nguyen VT, Kishigami S, Ishino F, Wakayama T (7 March 2013). "Successful serial recloning in the mouse over multiple generations". Cell Stem Cell. 12 (3): 293–7. doi:10.1016/j.stem.2013.01.005. PMID 23472871.
- ^ a b BBC. 22 February 2008. BBC On This Day: 1997: Dolly the sheep is cloned
- ^ Stening, Tanner (8 April 2025). "Did scientists genetically engineer the long-extinct dire wolf, or give gray wolf offspring its features?". Northeastern Global News. Retrieved 5 June 2025.
- ^ Mullin, Emily; Reynolds, Matt (7 April 2025). "Scientists Claim to Have Brought Back the Dire Wolf". Wired. Retrieved 12 August 2025.
- ^ "ThinkQuest". Archived from the original on 12 October 2009. Retrieved 3 May 2015.
- ^ "Robert W. Briggs". National Academies Press. Retrieved 1 December 2012.
- ^ a b "Bloodlines timeline". PBS.
- ^ Streisinger, George; Walker, C.; Dower, N.; Knauber, D.; Singer, F. (1981), "Production of clones of homozygous diploid zebra fish (Brachydanio rerio)", Nature, 291 (5813): 293–296, Bibcode:1981Natur.291..293S, doi:10.1038/291293a0, PMID 7248006, S2CID 4323945
- ^ Campbell, K. H. S.; McWhir, J.; Ritchie, W. A.; Wilmut, I. (7 March 1996). "Sheep cloned by nuclear transfer from a cultured cell line". Nature. 380 (6569): 64–66. Bibcode:1996Natur.380...64C. doi:10.1038/380064a0. PMID 8598906. S2CID 3529638.
- ^ "Gene Genie". BBC World Service. 1 May 2000. Retrieved 4 August 2010.
- ^ McLaren A (2000). "Cloning: pathways to a pluripotent future". Science. 288 (5472): 1775–80. doi:10.1126/science.288.5472.1775. PMID 10877698. S2CID 44320353.
- ^ Chaylakhyan, Levon (1987). "Электростимулируемое слияние клеток в клеточной инженерии". Биофизика (in Russian). 32 (5): 874–887. Archived from the original on 11 September 2016.
- ^ "Кто изобрел клонирование?". Archived from the original on 23 December 2004. (Russian)
- ^ "Researchers clone monkey by splitting embryo". CNN. 13 January 2000. Archived from the original on 13 August 2006. Retrieved 5 August 2008.
- ^ Dean Irvine (19 November 2007). "You, again: Are we getting closer to cloning humans?". CNN. Retrieved 4 August 2010.
- ^ Grisham, Julie (April 2000). "Pigs cloned for first time". Nature Biotechnology. 18 (4): 365. doi:10.1038/74335. PMID 10748477. S2CID 34996647.
- ^ Shukman, David (14 January 2014) China cloning on an 'industrial scale' BBC News Science and Environment, Retrieved 14 January 2014
- ^ Tobin, Kate (12 January 2001). "First cloned endangered species dies 2 days after birth". CNN. Archived from the original on 6 June 2009. Retrieved 30 April 2010.
- ^ Camacho, Keite (20 May 2005). "Embrapa clona raça de boi ameaçada de extinção". Agência Brasil (in Portuguese). Archived from the original on 21 April 2009. Retrieved 5 August 2008.
- ^ Gan, Nectar (2 February 2023). "China says it successfully cloned 3 highly productive 'super cows'". CNN Business. Archived from the original on 16 February 2023. Retrieved 17 February 2023.
- ^ "Americas | Pet kitten cloned for Christmas". BBC News. 23 December 2004. Retrieved 4 August 2010.
- ^ "Rat called Ralph is latest clone". BBC News. 25 September 2003. Retrieved 30 April 2010.
- ^ "Gordon Woods dies at 57; Veterinary scientist helped create first cloned mule". Los Angeles Times. Associated Press. 25 August 2009. Retrieved 4 August 2010.
- ^ "World's first cloned horse is born – 06 August 2003". New Scientist. Retrieved 4 August 2010.
- ^ "Przewalski's Horse Project - Revive & Restore". Retrieved 23 April 2023.
- ^ "Major Kurt Milestones - Revive & Restore". Retrieved 23 April 2023.
- ^ Tuff, Kika. "Przewalski's Foal Offers Further Hope for Conservation Cloning". Retrieved 23 April 2023.
- ^ "First Dog Clone". National Geographic Society. Archived from the original on 14 August 2005. Retrieved 4 August 2010.
- ^ Wang, Serenitie; Rivers, Matt; Wang, Shunhe (27 December 2017). "Chinese firm clones gene-edited dog in bid to treat cardiovascular disease". CNN. Retrieved 9 July 2020.
- ^ Bender, Kelli (27 June 2017). "You Look Familiar! Cloned Puppy Meets Her 'Mom' For the First Time". People. Archived from the original on 12 August 2022. Retrieved 12 August 2022.
- ^ Webster, George; Anderson, Becky (30 September 2011). "'Super clone' sniffer dogs: Coming to an airport near you?". CNN Business. Archived from the original on 30 September 2011. Retrieved 8 March 2023.
- ^ Pilkington, Ed (18 June 2009). "Dog hailed as hero cloned by California company". The Guardian. Retrieved 8 March 2023.
- ^ "China's first cloned police dog reports for duty". South China Morning Post. 19 March 2019. Retrieved 8 March 2023.
- ^ Kim, Min Jung; Oh, Hyun Ju; Hwang, Sun Young; Hur, Tai Young; Lee, Byeong Chun (1 September 2018). "Health and temperaments of cloned working dogs". Journal of Veterinary Science. 19 (5): 585–591. doi:10.4142/jvs.2018.19.5.585. ISSN 1229-845X. PMC 6167335. PMID 29929355. S2CID 49344658.
- ^ (1 September 2009) World's first cloned wolf dies Phys.Org, Retrieved 9 April 2015
- ^ Sinha, Kounteya (13 February 2009). "India clones world's first buffalo". The Times of India. Archived from the original on 11 August 2011. Retrieved 4 August 2010.
- ^ "Extinct ibex is resurrected by cloning". The Daily Telegraph. 31 January 2009. Archived from the original on 1 February 2009.
- ^ Maas, Peter H. J. (15 April 2012). "Pyrenean Ibex – Capra pyrenaica pyrenaica". The Sixth Extinction. Archived from the original on 27 June 2012.
- ^ Spencer, Richard (14 April 2009). "World's first cloned camel unveiled in Dubai". The Daily Telegraph. London. Retrieved 15 April 2009.
- ^ Ishfaq-ul-Hassan (15 March 2012). "India gets its second cloned animal Noorie, a pashmina goat". Daily News & Analysis. Kashmir, India.
- ^ "家畜体细胞克隆技术取得重大突破 ——成年体细胞克隆山羊诞生". Archived from the original on 10 November 2017. Retrieved 13 January 2018.
- ^ Hickman, L. (18 March 2013). "Scientists clone extinct frog – Jurassic Park here we come?". The Guardian. Retrieved 10 July 2016.
- ^ Liu, Zhen; et al. (24 January 2018). "Cloning of Macaque Monkeys by Somatic Cell Nuclear Transfer". Cell. 172 (4): 881–887.e7. doi:10.1016/j.cell.2018.01.020. PMID 29395327.
- ^ Normile, Dennis (24 January 2018). "These monkey twins are the first primate clones made by the method that developed Dolly". Science. doi:10.1126/science.aat1066. Retrieved 24 January 2018.
- ^ Briggs, Helen (24 January 2018). "First monkey clones created in Chinese laboratory". BBC News. Retrieved 24 January 2018.
- ^ "Scientists Successfully Clone Monkeys; Are Humans Up Next?". The New York Times. Associated Press. 24 January 2018. Retrieved 24 January 2018.
- ^ "Gene-edited disease monkeys cloned in China". EurekAlert!. Science China Press. 23 January 2019. Archived from the original on 24 January 2019. Retrieved 24 January 2019.
- ^ Mandelbaum, Ryan F. (23 January 2019). "China's Latest Cloned-Monkey Experiment Is an Ethical Mess". Gizmodo. Retrieved 24 January 2019.
- ^ "A black-footed ferret has been cloned, a first for a U.S. endangered species". Animals. 18 February 2021. Archived from the original on 18 February 2021. Retrieved 20 February 2021.
- ^ Wei, Yanchang; Yang, Cai-Rong; Zhao, Zhen-Ao (22 March 2022). "Viable offspring derived from single unfertilized mammalian oocytes". Proceedings of the National Academy of Sciences. 119 (12) e2115248119. Bibcode:2022PNAS..11915248W. doi:10.1073/pnas.2115248119. ISSN 0027-8424. PMC 8944925. PMID 35254875.
- News article about the study: Magazine, Smithsonian; Osborne, Margaret. "Mice Birthed From Unfertilized Eggs for the First Time". Smithsonian Magazine. Retrieved 18 April 2022.
- ^ Martinez, Bobby (26 January 2018). "Watch: Scientists clone monkeys using technique that created Dolly the sheep". Fox61. WTIC-TV. Retrieved 26 January 2018.
- ^ a b Kfoury, C (July 2007). "Therapeutic cloning: promises and issues". Mcgill J Med. 10 (2): 112–20. PMC 2323472. PMID 18523539.
- ^ "Cloning Fact Sheet". U.S. Department of Energy Genome Program. 11 May 2009. Archived from the original on 2 May 2013.
- ^ de Grey, Aubrey; Rae, Michael (September 2007). Ending Aging: The Rejuvenation Breakthroughs that Could Reverse Human Aging in Our Lifetime. New York, NY: St. Martin's Press, 416 pp. ISBN 0-312-36706-6.
- ^ Times Higher Education. 10 August 2001 In the news: Antinori and Zavos
- ^ "AAAS Statement on Human Cloning". Archived from the original on 11 September 2012. Retrieved 6 June 2007.
- ^ McGee, G. (October 2011). "Primer on Ethics and Human Cloning". American Institute of Biological Sciences. Archived from the original on 23 February 2013.
- ^ "Universal Declaration on the Human Genome and Human Rights". UNESCO. 11 November 1997. Retrieved 27 February 2008.
- ^ McGee, Glenn (2000). The Perfect Baby: Parenthood in the New World of Cloning and Genetics. Lanham: Rowman & Littlefield.
- ^ Havstad JC (2010). "Human reproductive cloning: a conflict of liberties". Bioethics. 24 (2): 71–7. doi:10.1111/j.1467-8519.2008.00692.x. PMID 19076121. S2CID 40051820.
- ^ Engineering, Berkeley Master of (11 May 2020). "Op-ed: The dangers of cloning". Fung Institute for Engineering Leadership. Retrieved 8 March 2023.
- ^ Bob Sullivan, Technology correspondent for NBC News. November 262003 Religions reveal little consensus on cloning – Health – Special Reports – Beyond Dolly: Human Cloning
- ^ Bainbridge, William Sims (October 2003). "Religious Opposition to Cloning" Archived 21 April 2018 at the Wayback Machine. Journal of Evolution and Technology. 13.
- ^ "Out of this world". The Guardian. 15 February 2002. Retrieved 8 March 2023.
- ^ "Raelian leader says cloning first step to immortality - Feb. 12, 2004". CNN. Archived from the original on 23 January 2023. Retrieved 8 March 2023.
- ^ Zitner, Aaron (29 March 2001). "Lawmakers Propose Human Cloning Ban, Alien Order Notwithstanding". Los Angeles Times. Retrieved 8 March 2023.
- ^ Huberman, Jenny (2 January 2018). "Immortality transformed: mind cloning, transhumanism and the quest for digital immortality". Mortality. 23 (1): 50–64. doi:10.1080/13576275.2017.1304366. ISSN 1357-6275. S2CID 152261367.
- ^ Mandelbaum, Eric (2022). "Everything and More: The Prospects of Whole Brain Emulation". Journal of Philosophy. 119 (8): 444–459. doi:10.5840/jphil2022119830. S2CID 252266788.
- ^ Godfrey, Alex (6 June 2019). "The science of Black Mirror's Ashley Too: will 'whole brain emulation' ever be possible?". The Telegraph. Retrieved 8 March 2023.
- ^ Hanson, Robin (2016). The age of em: work, love, and life when robots rule the Earth (First ed.). Oxford. ISBN 978-0-19-875462-6.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Watanabe, S (September 2013). "Effect of calf death loss on cloned cattle herd derived from somatic cell nuclear transfer: clones with congenital defects would be removed by the death loss". Animal Science Journal. 84 (9): 631–8. doi:10.1111/asj.12087. PMID 23829575.
- ^ "FDA says cloned animals are OK to eat". NBC News. Associated Press. 28 December 2006. Archived from the original on 13 June 2013.
- ^ "An HSUS Report: Welfare Issues with Genetic Engineering and Cloning of Farm Animals" (PDF). Humane Society of the United States. Archived (PDF) from the original on 24 December 2010.
- ^ Hansen, Michael (27 April 2007). "Comments of Consumers Union to US Food and Drug Administration on Docket No. 2003N-0573, Draft Animal Cloning Risk Assessment" (PDF). Consumers Union. Archived from the original (PDF) on 11 December 2009. Retrieved 16 October 2009.
- ^ Sandel, Michael J (2005). "The Ethical Implications of Human Cloning". Perspectives in Biology and Medicine. 48 (2): 241–247. doi:10.1353/pbm.2005.0063. ISSN 1529-8795. PMID 15834196. S2CID 32155922.
- ^ a b Holt, W. V., Pickard, A. R., & Prather, R. S. (2004) Wildlife conservation and reproductive cloning Archived 24 September 2015 at the Wayback Machine. Reproduction, 126.
- ^ Ehrenfeld, David (2006). "Transgenics and Vertebrate Cloning as Tools for Species Conservation". Conservation Biology. 20 (3): 723–732. Bibcode:2006ConBi..20..723E. doi:10.1111/j.1523-1739.2006.00399.x. PMID 16909565. S2CID 12798003.
- ^ Ono T, Li C, Mizutani E, Terashita Y, Yamagata K, Wakayama T (December 2010). "Inhibition of class IIb histone deacetylase significantly improves cloning efficiency in mice". Biol. Reprod. 83 (6): 929–37. doi:10.1095/biolreprod.110.085282. PMID 20686182.
- ^ Shukman, David (14 January 2014) China cloning on an 'industrial scale' BBC News Science and Environment, Retrieved 27 February 2016
- ^ Baer, Drake (8 September 2015). "This Korean lab has nearly perfected dog cloning, and that's just the start". Tech Insider. Retrieved 27 February 2016.
- ^ Ferris Jabr for Scientific American. 11 March 2013. Will cloning ever saved endangered species?
- ^ Heidi B. Perlman (8 October 2000). "Scientists Close on Extinct Cloning". The Washington Post. Associated Press. Archived from the original on 8 July 2016. Retrieved 25 August 2017.
- ^ Pence, Gregory E. (2005). Cloning After Dolly: Who's Still Afraid?. Rowman & Littlefield. ISBN 978-0-7425-3408-7.
- ^ Strickland, Ashley (2 April 2022). "'Frozen zoos' may be a Noah's Ark for endangered animals". CNN. Retrieved 8 March 2023.
- ^ Marshall, Andrew (2000). "Cloning for conservation". Nature Biotechnology. 18 (11): 1129. doi:10.1038/81057. PMID 11062403. S2CID 11078427.
- ^ "Conservation cloning". 2 December 2015.
- ^ Baker, Harry (14 March 2021). "Scientists want to store DNA of 6.7 million species on the moon, just in case – The 'lunar ark' would be hidden in lava tubes". Live Science. Retrieved 14 March 2021.
- ^ Woodyatt, Amy (16 March 2021). "Scientists want to build a doomsday vault on the moon". CNN. Retrieved 8 March 2023.
- ^ "Engineers Propose Solar-Powered Lunar Ark as 'Modern Global Insurance Policy'". University of Arizona. 8 March 2021. Retrieved 14 March 2021.
- ^ "Plans for 'doomsday ark' on the moon". The Telegraph. Retrieved 8 March 2023.
- ^ Werner, Debra (24 October 2022). "Sending DNA-infused Space Crystals to the moon". SpaceNews. Retrieved 8 March 2023.
- ^ Ezell, Carson; Lazarian, Alexandre; Loeb, Abraham (December 2022). "A Lunar Backup Record of Humanity". Signals. 3 (4): 823–829. arXiv:2209.11155. doi:10.3390/signals3040049. ISSN 2624-6120.
- Article about the study: Tognetti, Laurence. "The moon is the perfect spot for humanity's offsite backup". Universe Today via phys.org. Retrieved 8 March 2023.
- ^ "A Clone and His Mentor". sandiegozoo.org. San Diego Zoo. 20 October 2022. Archived from the original on 29 February 2024. Retrieved 29 February 2024.
- ^ Novak, Ben J.; Ryder, Oliver A.; Houck, Marlys L.; Putnam, Andrea S.; Walker, Kelcey; Russell, Lexie; Russell, Blake; Walker, Shawn; Arenivas, Sanaz Sadeghieh; Aston, Lauren; Veneklasen, Gregg; Ivy, Jamie A.; Koepfli, Klaus-Peter; Rusnak, Anna; Simek, Jaroslav; Zhuk, Anna; Phelan, Ryan (9 January 2024). "Endangered Przewalski's horse, Equus przewalskii, cloned from historically cryopreserved cells". bioRxiv 10.1101/2023.12.20.572538.
- ^ Szuszwalak, Joe (18 February 2021). "Innovative Genetic Research Boosts Black-footed Ferret Conservation Efforts by USFWS and Partners". U.S. Fish and Wildlife Service. Retrieved 22 February 2025.
- ^ Welk, Martin (1 November 2024). "Cloned ferret gives birth in Va., making history, U.S. officials say". The Washington Post. Yahoo. Retrieved 16 November 2024.
- ^ "Scientists 'to clone mammoth'". BBC News. 18 August 2003.
- ^ "BBC News". BBC. 7 December 2011. Retrieved 19 August 2012.
- ^ a b "Когда вернутся мамонты" Archived 30 June 2015 at the Wayback Machine ("When the Mammoths Return"), 5 February 2015 (retrieved 6 September 2015)
- ^ Yong, Ed (15 March 2013). "Resurrecting the Extinct Frog with a Stomach for a Womb". National Geographic. Archived from the original on 18 March 2013. Retrieved 15 March 2013.
- ^ "Long Now Foundation, Revive and Restore Project". 25 May 2017.
- ^ Lin, Jianqing; Duchêne, David; Carøe, Christian; Smith, Oliver; Ciucani, Marta Maria; Niemann, Jonas; Richmond, Douglas; Greenwood, Alex D.; MacPhee, Ross; Zhang, Guojie; Gopalakrishnan, Shyam; Gilbert, M. Thomas P. (11 April 2022). "Probing the genomic limits of de-extinction in the Christmas Island rat". Current Biology. 32 (7): 1650–1656.e3. Bibcode:2022CBio...32E1650L. doi:10.1016/j.cub.2022.02.027. ISSN 0960-9822. PMC 9044923. PMID 35271794. S2CID 247323087.
- News report on the study: Ahmed, Issam. "Forget mammoths, study shows how to resurrect Christmas Island rats". phys.org. Retrieved 19 April 2022.
- ^ "Russia Is Going to Try to Clone an Army of 3,000-Year-Old Scythian Warriors". Popular Mechanics. 11 May 2021. Retrieved 8 March 2023.
- ^ Wehner, Mike (24 May 2021). "Does Russia really think it can clone ancient warriors?". BGR. Retrieved 8 March 2023.
- ^ Lang, Fabienne (14 May 2021). "Russia's Defense Minister Wants to Clone 3000-Year-Old Scythian Army". interestingengineering.com. Retrieved 8 March 2023.
- ^ "Yes, We Should Clone Neanderthals". Discover Magazine. Retrieved 8 March 2023.
- ^ "Could we clone a Neanderthal?". BBC Science Focus Magazine. Retrieved 8 March 2023.
- ^ "Return of the Neanderthals". Science. 6 March 2013. Archived from the original on 17 February 2021. Retrieved 8 March 2023.
- ^ Lupkin, Sydney. "Harvard Prof Says Neanderthal Clones Possible but Experts Doubt It". ABC News. Retrieved 8 March 2023.
- ^ Brown, Andrew (23 June 2011). "Should we clone Neanderthals?". The Guardian. Retrieved 8 March 2023.
- ^ Lallanilla, Marc (21 January 2013). "Could a Surrogate Mother Deliver a Neanderthal Baby?". livescience.com. Retrieved 8 March 2023.
- ^ "CNN – Nature – First cloned endangered species dies 2 days after birth – January 12, 2001". CNN. Archived from the original on 15 April 2021. Retrieved 25 October 2020.
- ^ "Fresh effort to clone extinct animal". BBC News. 22 November 2013. Retrieved 25 October 2020.
- ^ "Endangered Species Cloning". www.elements.nb.ca. Archived from the original on 21 September 2015. Retrieved 25 October 2020.
- ^ Khan, Firdos Alam (3 September 2018). Biotechnology Fundamentals. CRC Press. ISBN 978-1-315-36239-7.
- ^ Holloway, Grant (28 May 2002). "Cloning to revive extinct species". CNN.
- ^ "Researchers revive plan to clone the Tassie tiger". The Sydney Morning Herald. 15 May 2005. Retrieved 1 February 2019.
- ^ Gray, Richard; Dobson, Roger (31 January 2009). "Extinct ibex is resurrected by cloning". The Telegraph. London. Archived from the original on 1 February 2009. Retrieved 1 February 2009.
- ^ "Generations of Cloned Mice With Normal Lifespans Created: 25th Generation and Counting". Science Daily. 7 March 2013. Retrieved 8 March 2013.
- ^ Carey, Nessa (2012). The Epigenetics Revolution. London, UK: Icon Books Ltd. pp. 149–150. ISBN 978-184831-347-7.
- ^ Burgstaller, Jörg Patrick; Brem, Gottfried (2017). "Aging of Cloned Animals: A Mini-Review". Gerontology. 63 (5): 419. doi:10.1159/000452444. ISSN 0304-324X. PMID 27820924.
- ^ Sinclair, K. D.; Corr, S. A.; Gutierrez, C. G.; Fisher, P. A.; Lee, J.-H.; Rathbone, A. J.; Choi, I.; Campbell, K. H. S.; Gardner, D. S. (26 July 2016). "Healthy ageing of cloned sheep". Nature Communications. 7 12359. Bibcode:2016NatCo...712359S. doi:10.1038/ncomms12359. ISSN 2041-1723. PMC 4963533. PMID 27459299.
- ^ "Cloning Humans". Time. Vol. 142, no. 19. 8 November 1993. Archived from the original on 5 November 2017. Retrieved 5 November 2017.
- ^ "Today The Sheep ..." Newsweek. 9 March 1997. Retrieved 5 November 2017.
- ^ Huxley, Aldous; "Brave New World and Brave New World Revisited"; p. 19; HarperPerennial, 2005.
- ^ Bhelkar, Ratnakar D. (2009). Science Fiction: Fantasy and Reality. Atlantic Publishers & Dist. p. 58. ISBN 978-81-269-1036-6. Retrieved 4 November 2017.
- ^ Stableford, Brian M. (2006). "Clone". Science Fact and Science Fiction: An Encyclopedia. Taylor & Francis. pp. 91–92. ISBN 978-0-415-97460-8.
- ^ "Sleeper (1973)". IMDb. 17 December 1973. Retrieved 3 May 2015.
- ^ Muir, John Kenneth (2007). A Critical History of Doctor Who on Television. McFarland. pp. 258–9. ISBN 978-1-4766-0454-1. Retrieved 4 November 2017.
- ^ Mumford, James (2013). Ethics at the Beginning of Life: A Phenomenological Critique. OUP Oxford. p. 108. ISBN 978-0-19-967396-4. Retrieved 6 November 2017.
- ^ Humber, James M.; Almeder, Robert (1998). Human Cloning. Springer Science & Business Media. p. 10. ISBN 978-1-59259-205-0. Retrieved 6 November 2017.
- ^ Lewis, Courtland; Smithka, Paula (2010). "What's Continuity without Persistence?". Doctor Who and Philosophy: Bigger on the Inside. Open Court. pp. 32–33. ISBN 978-0-8126-9725-4. Retrieved 4 November 2017.
- ^ "Twice as nice: Barbra Streisand cloned her beloved dog and has 2 new pups". CBC News. Retrieved 1 March 2018.
- ^ Streisand, Barbra (2 March 2018). "Barbra Streisand Explains: Why I Cloned My Dog". The New York Times. ISSN 0362-4331. Archived from the original on 1 January 2022. Retrieved 23 June 2020.
- ^ a b Clark, Michael. "Genetic themes in fiction films: Genetics meets Hollywood". The Wellcome Trust. Archived from the original on 18 May 2012. Retrieved 19 July 2018.
- ^ Hopkins, Patrick (1998). "How Popular media represent cloning as an ethical problem" (PDF). The Hastings Center Report. 28 (2): 6–13. doi:10.2307/3527566. JSTOR 3527566. PMID 9589288.[permanent dead link]
- ^ "Yvonne A. De La Cruz Science Fiction Storytelling and Identity: Seeing the Human Through Android Eyes" (PDF). Archived (PDF) from the original on 28 September 2011. Retrieved 19 August 2012.
- ^ "Uma Thurman, Rhys Ifans and Tom Wilkinson star in two plays for BBC Two" (Press release). BBC. 19 June 2008. Retrieved 9 September 2008.
- ^ "Review of Bunshin". 9 June 2012.
- ^ "Orphan Black (TV Series 2013– )". IMDb. Retrieved 3 May 2015.
- ^ Banville, John (10 October 2004). "'The Double': The Tears of a Clone". The New York Times. Retrieved 14 January 2015.
- ^ Christian Lee Pyle (12 October 1978). "The Boys from Brazil (1978)". IMDb. Retrieved 3 May 2015.
- ^ Le Guin, Ursula K. (Spring 1973). "On Norman Spinrad's The Iron Dream". Science Fiction Studies. Retrieved 9 February 2007.
- ^ Cohen, Daniel (2002). Cloning. Millbrook Press. ISBN 978-0-7613-2802-5. Retrieved 4 November 2017.
- ^ Thompson, Dave (2013). Doctor Who FAQ: All That's Left to Know About the Most Famous Time Lord in the Universe. Hal Leonard Corporation. ISBN 978-1-4803-4295-8. Retrieved 4 November 2017.
- ^ Barr, Jason; Mustachio, Camille D. G. (2014). The Language of Doctor Who: From Shakespeare to Alien Tongues. Rowman & Littlefield. p. 219. ISBN 978-1-4422-3481-9. Retrieved 5 November 2017.
- ^ McDonald, Paul F. (2013). "The Clones". The Star Wars Heresies: Interpreting the Themes, Symbols and Philosophies of Episodes I, II and III. McFarland. pp. 167–171. ISBN 978-0-7864-7181-2. Retrieved 4 November 2017.
- ^ "Star Wars: The Clone Wars (2008)". IMDb. 15 August 2008. Retrieved 3 May 2015.
- ^ "Never Let Me Go (2010)". IMDb. 11 February 2011. Retrieved 3 May 2015.
- ^ "The Island (2005)". IMDb. 22 July 2005. Retrieved 3 May 2015.
- ^ "Moon (2009)". IMDb. 17 July 2009. Retrieved 3 May 2015.
- ^ "Technology News – 2017 Innovations and Future Tech". Archived from the original on 5 January 2014. Retrieved 15 December 2013.
- ^ Daniel Kurland (23 May 2019). "Us: Who Are the Tethered?". Retrieved 11 July 2019.
- ^ Jason Spiegel (23 March 2019). "'Us' Ending Explained: Could There Be A Sequel?". The Hollywood Reporter. Retrieved 11 July 2019.
Further reading
[edit]- Guo, Owen. "World's Biggest Animal Cloning Center Set for '16 in a Skeptical China". The New York Times, 26 November 2015
- Lerner, K. Lee. "Animal cloning". The Gale Encyclopedia of Science, edited by K. Lee Lerner and Brenda Wilmoth Lerner, 5th ed., Gale, 2014. Science in Context, link[permanent dead link]
- Dutchen, Stephanie (11 July 2018). "Rise of the Clones". Harvard Medical School.
External links
[edit]- Fieser, James; Dowden, Bradley (eds.). "Cloning". Internet Encyclopedia of Philosophy. ISSN 2161-0002. OCLC 37741658.
- Cloning Fact Sheet Archived 2 May 2013 at the Wayback Machine from Human Genome Project Information website.
- 'Cloning' Freeview video by the Vega Science Trust and the BBC/OU
- Cloning in Focus, an accessible and comprehensive look at cloning research from the University of Utah's Genetic Science Learning Center
- Click and Clone. Try it yourself in the virtual mouse cloning laboratory, from the University of Utah's Genetic Science Learning Center
- "Cloning Addendum: A statement on the cloning report issues by the President's Council on Bioethics" Archived 8 January 2009 at the Wayback Machine. National Review, 15 July 2002 8:45 am
Cloning
View on GrokipediaDefinitions and Fundamentals
Terminology and Etymology
The term "clone" originates from the Ancient Greek word klōn (κλών), meaning "twig" or "branch," alluding to the horticultural practice of propagating plants from cuttings or slips.[11] This etymological root reflects early associations with asexual reproduction in botany, where a new plant develops from a vegetative part of the parent.[12] In 1903, American botanist Herbert J. Webber formally introduced "clone" in a letter published in the journal Science, defining it as a group of plants descended asexually from a single progenitor via vegetative propagation, such as cuttings, bulbs, or buds.[13] Webber's usage emphasized populations of genetically uniform individuals, distinguishing them from sexually reproduced variants, and the term initially remained confined to plant agriculture and botany.[14] By the mid-20th century, "clone" expanded in biological contexts to describe any set of genetically identical cells or organisms derived asexually from a common ancestor, including applications in microbiology and animal reproduction.[13] "Cloning" denotes the process—natural or artificial—by which such identical copies are produced, encompassing mechanisms like binary fission in bacteria, budding in yeasts, or laboratory techniques yielding replicas of DNA, cells, or whole organisms.[15] A "clone" specifically refers to the resulting entity with matching genetic material to the source, though environmental factors can introduce non-genetic variations.[1] Key distinctions include reproductive cloning, which generates a viable organism genetically identical to the donor (e.g., via somatic cell nuclear transfer), versus therapeutic cloning or molecular cloning, the latter focused on amplifying specific DNA segments for research without producing organisms.[2] These terms underscore cloning's dual scope: organismal replication mirroring natural asexual propagation, and recombinant DNA methods for gene isolation and expression in vectors like plasmids.[15] In Arabic, the term "istinsākh" (استنساخ) is used for cloning, derived from the root "نسخ" (naskh), meaning "to copy" or "transcribe," and can also denote copying or duplication in broader contexts.[16]Core Principles and Mechanisms
Cloning fundamentally relies on the principle of genomic equivalence, wherein somatic cells contain the complete genetic blueprint necessary to generate an entire organism, provided epigenetic restrictions are overcome. This principle underpins both natural and artificial cloning processes, enabling the production of genetically identical copies without genetic recombination from sexual reproduction. The mechanism exploits DNA's capacity for precise semi-conservative replication, ensuring fidelity in copying genetic information during cell division or propagation in host systems.[1][2] At the cellular level, cloning mechanisms hinge on totipotency or induced pluripotency, where a single cell's nucleus directs full embryonic development. In somatic cell nuclear transfer (SCNT), the core technique for reproductive cloning, an oocyte is enucleated to remove its haploid nucleus, followed by insertion of a diploid somatic nucleus via micromanipulation or electrofusion. The recipient cytoplasm then reprograms the donor nucleus by altering epigenetic marks, such as DNA methylation and histone modifications, to restore developmental potential akin to a zygote. Activation via chemical agents or electrical pulses initiates embryonic cleavage, though efficiency remains low—typically under 5% in mammals—due to incomplete reprogramming and aberrant gene expression.[17][18] For molecular cloning, the principle involves recombinant DNA assembly, where a target gene is excised using restriction endonucleases and ligated into a vector plasmid possessing an origin of replication, selectable markers, and promoter elements. Transformation into competent bacterial hosts, such as Escherichia coli, leverages the bacterium's replication machinery to amplify the insert exponentially during logarithmic growth phases. Selection via antibiotic resistance ensures propagation of recombinant clones, with verification through sequencing or restriction digest confirming insert integrity. This process, established since the 1970s, enables isolation of pure DNA segments for downstream applications while minimizing mutations through high-fidelity polymerases.[19][20] Epigenetic reprogramming represents a unifying mechanism across cloning types, addressing the causal barrier of cellular differentiation: somatic cells' silenced pluripotency genes must be reactivated, often imperfectly, leading to phenomena like large offspring syndrome in cloned animals from disrupted imprinting. Empirical data from mammalian clones, such as the 1996 sheep Dolly derived from an adult mammary cell via SCNT, validate these principles but highlight persistent challenges in achieving full-term viability without anomalies.[17][18]Natural Cloning Phenomena
In Plants and Fungi
Many plant species reproduce clonally through vegetative propagation, producing genetically identical offspring known as ramets from a single parental genet via structures such as rhizomes, stolons, tubers, bulbs, or root suckers.[21] This asexual mechanism supplements sexual reproduction by seeds, offering reproductive assurance in stable or stressful environments where pollinators or mates may be scarce.[22] Apomixis, another form, enables clonal seed production without fertilization, preserving maternal genotypes in species like certain dandelions and grasses.[23] A prominent example is the Pando clonal colony of quaking aspen (Populus tremuloides) in Utah's Fishlake National Forest, consisting of approximately 47,000 interconnected stems arising from a single root system, spanning over 43 hectares and representing one organism by dry weight exceeding 6,000 tonnes.[24] Genetic analysis confirms its clonal nature through uniform mitochondrial DNA across stems, with estimates suggesting origins up to 14,000 years ago, though recent studies explore mechanisms protecting it from mutational accumulation.[25] Fungi frequently engage in clonal reproduction via asexual spore formation, such as conidia produced by mitosis, or through mycelial growth and fragmentation, allowing rapid expansion of genetically identical networks.[26] This enables formation of vast subterranean colonies, as seen in Armillaria species, which spread via rhizomorphs and degrade wood. The largest documented clonal fungus, Armillaria ostoyae in Oregon's Malheur National Forest, covers approximately 965 hectares, weighs an estimated 35,000 tonnes in mycelial mass, and may exceed 8,000 years in age based on growth modeling.[27][28] Such clones persist through resource acquisition and evasion of competition, though genetic uniformity risks vulnerability to pathogens.[29]In Animals and Invertebrates
Asexual reproduction via fission, budding, and fragmentation represents primary mechanisms of natural cloning in many invertebrates and basal animals, yielding offspring genetically identical to the parent. These processes bypass gamete fusion, relying instead on mitotic division and regeneration to propagate clones, which confers advantages in stable environments but limits genetic diversity.[1][30] Fission entails the parent organism splitting into two or more viable parts, each maturing into an independent clone. While common in prokaryotes, it occurs in certain invertebrate animals such as some sea anemones through longitudinal or transverse division. Planarians, free-living flatworms, also employ fission-like processes where the body constricts and separates, with each segment regenerating fully.[30][31] Budding involves the outgrowth of a bud from the parent's body, which develops organs and detaches as a clone. This is widespread in cnidarians like hydra, where environmental cues trigger bud formation on the parent's column, leading to genetically identical polyps. Sponges (poriferans) similarly produce buds that either detach or form gemmules, resistant structures for cloning under adverse conditions. Colonial invertebrates such as corals and bryozoans extend budding to form clonal polyps or zooids, expanding via interconnected clones.[30][31][32] Fragmentation occurs when the parent fractures into pieces, each regenerating into a complete organism through dedifferentiation and proliferation. Starfish (echinoderms) exemplify this, as an arm fragment with a portion of the central disk can regrow the entire body, producing clones. Annelid worms and planarians also fragment intentionally or via injury, with high regenerative capacity enabling clone formation from non-reproductive tissues. These methods underscore regeneration's role in cloning, distinct from embryonic development.[33][30] In vertebrates, natural cloning manifests less through direct asexual means and more via monozygotic twinning, where a single fertilized zygote divides early in development, yielding identical genetic copies. This occurs across mammals, including humans, as a stochastic event during cleavage stages.[1][34]Parthenogenesis and Virgin Birth
Parthenogenesis is the process by which an embryo develops from an unfertilized ovum, resulting in asexual reproduction without genetic contribution from a male. In apomictic parthenogenesis, meiosis is suppressed, yielding offspring that are exact genetic clones of the mother through endoreduplication of the egg's chromosomes. Automictic parthenogenesis involves meiosis followed by restoration of diploidy, which may introduce some genetic variation via recombination but still produces predominantly female offspring closely related to the parent. This natural mechanism contrasts with artificial cloning techniques like somatic cell nuclear transfer, as it utilizes the egg's intrinsic developmental machinery rather than transferring a somatic nucleus, though both can achieve genetic identity to the progenitor.[35][36] In vertebrates, parthenogenesis is rare and typically facultative, serving as a reproductive fallback in isolated or male-scarce environments, with true obligate parthenogenesis—where populations consist solely of females—documented in about 39 species of squamate reptiles, such as lizards and snakes. The New Mexico whiptail lizard (Aspidoscelis neomexicana), first described in parthenogenetic form in the mid-20th century, exemplifies obligate thelytoky, where females produce diploid eggs via premeiotic genome duplication, generating clonal daughters that perpetuate all-female lineages. In fish, the Amazon molly (Poecilia formosa) was the first vertebrate identified with natural gynogenesis—a parthenogenesis variant requiring sperm for egg activation but not genetic fusion—reported in 1932. Facultative cases include birds like domestic turkeys, where unfertilized eggs occasionally develop parthenogenones, though survival to hatching is low at under 1% without intervention.[37][38][39] Recent documentation highlights its adaptability in threatened species, such as the first confirmed recurrent facultative parthenogenesis in the endangered common smooth-hound shark (Mustelus mustelus) in 2024, where captive females produced multiple litters without males, verified via genetic analysis showing maternal inheritance. No natural parthenogenesis occurs in mammals due to genomic imprinting conflicts, where certain paternal genes are essential for embryonic viability; experimental induction in mice has yielded live births since the 2000s, but these require genetic modifications to bypass imprinting barriers. In cloning contexts, parthenogenetic clones demonstrate nature's capacity for self-replication but underscore limitations like reduced genetic diversity and potential accumulation of deleterious mutations, as observed in long-term lizard lineages.[40][41][42]History of Artificial Cloning
Early Experiments and Milestones
In 1938, embryologist Hans Spemann proposed a theoretical experiment involving the transfer of a differentiated cell nucleus into an enucleated egg cell to test nuclear totipotency, an idea that foreshadowed modern cloning techniques but remained unfeasible with contemporary methods.[43] The first practical nuclear transfer experiments occurred in 1952, when Robert Briggs and Thomas J. King successfully transplanted nuclei from blastula-stage cells of Rana pipiens frog embryos into enucleated eggs, yielding viable tadpoles that developed to swimming stages.[43] Their technique involved ultraviolet irradiation to remove the host egg's nucleus and microsurgical injection of donor nuclei, demonstrating that early embryonic nuclei retained full developmental potential despite cytoplasmic removal.[44] However, attempts with nuclei from more differentiated gastrula or later stages resulted in abnormal development or arrest, suggesting progressive restrictions on nuclear potency during differentiation.[43] Building on this foundation, John B. Gurdon advanced the field in the late 1950s using Xenopus laevis eggs, which tolerated enucleation better due to their larger size and opaque pigmentation for verifying nuclear removal.[45] In 1962, Gurdon reported the first cloning of fertile adult frogs from transplanted nuclei of differentiated intestinal epithelial cells extracted from feeding tadpoles, achieving success rates of approximately 1-2% after serial nuclear transfers to select for reprogrammed clones.[45] This milestone established that somatic cell nuclei could be reprogrammed by egg cytoplasm to support full organismal development, challenging earlier doubts about irreversible differentiation and providing empirical evidence for genomic equivalence across cell types.[46] Gurdon's serial transplantation method, involving up to five sequential transfers, amplified rare successful reprogrammings, with cloned frogs proving genetically identical to donors and capable of producing viable offspring.[46]Development of Key Techniques
The concept of nuclear transplantation as a method to assess cellular totipotency was first proposed by Hans Spemann in 1938, suggesting the transfer of a nucleus from a differentiated cell into an enucleated egg cell to study developmental potential.[47] This laid the theoretical groundwork for artificial cloning techniques beyond simple embryo splitting, which had been demonstrated as early as 1885 through manual separation of blastomeres in sea urchins and amphibians.[48] Practical implementation began in amphibians with Robert Briggs and Thomas J. King in 1952, who developed the nuclear transfer technique using Rana pipiens frog embryos; they successfully produced viable tadpoles by injecting blastula-stage nuclei into enucleated eggs but observed declining success with nuclei from more differentiated gastrula stages, indicating progressive restrictions on nuclear reprogramming.[49] John B. Gurdon advanced this in the late 1950s using Xenopus laevis, refining enucleation via ultraviolet irradiation and serial nuclear transfers to bypass early developmental blocks; by 1962, he achieved fertile adult frogs from transplanted intestinal epithelial cell nuclei of feeding tadpoles, confirming that differentiated somatic nuclei could be reprogrammed by oocyte cytoplasm, though efficiency remained low at about 1-2%.[49] These amphibian experiments established core procedural elements, including donor nucleus isolation, microinjection, and host egg activation, while highlighting epigenetic barriers that accumulate during differentiation.[47] Transitioning to mammals proved challenging due to smaller oocyte size, opaque cytoplasm complicating enucleation, and stricter cell cycle synchronization requirements. Early attempts, such as John McKinnell's 1960s rabbit transfers using frog-derived methods, yielded no viable offspring.[50] In 1981, Karl Illmensee reported mouse cloning from eight-cell embryo nuclei, producing live offspring, but the claim faced scrutiny over methodological reproducibility and potential parthenogenetic contamination.[50] Steen Willadsen's 1986 breakthrough with sheep involved electrofusion of eight-cell blastomere nuclei into enucleated oocytes embedded in agar to stabilize manipulation, yielding live lambs from embryonic cells and demonstrating mammalian embryo cloning viability, though limited to undifferentiated blastomeres.[49] Subsequent refinements focused on somatic cells, with key techniques including quiescence induction of donor cells (e.g., serum starvation to arrest in G0 phase), use of metaphase II-arrested oocytes for reprogramming factors, and post-transfer activation via calcium ionophores or electrical pulses to mimic fertilization.[49] By the early 1990s, Ian Wilmut's team at the Roslin Institute cultured fetal and embryonic cells prior to transfer, improving nuclear compatibility and paving the way for adult somatic cell nuclear transfer (SCNT), though early mammalian efficiencies hovered below 1% due to incomplete epigenetic erasure.[47] These developments underscored the oocyte's role in chromatin remodeling and gene expression reset, informed by amphibian precedents but adapted for mammalian placentation and imprinting demands.[49]Molecular and Cellular Cloning
Molecular Cloning Processes
Molecular cloning refers to the laboratory process of isolating a specific DNA sequence, inserting it into a vector, and propagating it within a host organism to produce multiple identical copies, enabling the study, manipulation, or expression of the DNA fragment.[51] This technique, foundational to recombinant DNA technology, typically involves bacterial hosts like Escherichia coli for amplification due to their rapid growth and ease of genetic manipulation.[52] The traditional process begins with isolation of the target DNA fragment, often via restriction enzyme digestion, which cleaves DNA at specific recognition sites to generate compatible ends.[53] Vectors, such as plasmids or bacteriophages, are similarly digested to create matching ends, followed by ligation using DNA ligase to join the insert to the vector, forming recombinant DNA.[54] Common plasmids include pUC19, featuring multiple cloning sites and selectable markers like ampicillin resistance for subsequent screening.[19] Transformation introduces the ligated recombinant DNA into competent host cells, typically via heat shock or electroporation, allowing uptake and replication.[55] Selection then identifies successful clones through markers: cells with recombinant plasmids survive on media containing antibiotics, while blue-white screening distinguishes inserts from empty vectors using lacZ gene disruption, where white colonies indicate insertion.[52] Verification confirms the clone via PCR, restriction mapping, or sequencing to ensure the insert's integrity and orientation.[19] Modern variants enhance efficiency and reduce restrictions. PCR-based cloning amplifies inserts with primers adding restriction sites or overhangs for direct ligation, bypassing full genomic digests.[56] Seamless methods like Gibson assembly use exonuclease, polymerase, and ligase activities to join overlapping DNA fragments without restriction enzymes, ideal for multi-fragment constructs.[57] These approaches, developed since the 2000s, minimize scar sequences and improve fidelity, with success rates often exceeding 90% for simple assemblies.[58]Cloning Unicellular Organisms
Unicellular organisms, such as bacteria and yeast, naturally produce clones through asexual reproduction mechanisms like binary fission in prokaryotes and budding in eukaryotes.[59] This process generates genetically identical daughter cells from a single progenitor, enabling rapid population expansion under favorable conditions.[60] In laboratory settings, artificial cloning of bacteria involves isolating a single cell via techniques such as streak plating or spread plating on solid agar media, where individual colonies arise from the proliferation of that cell, each representing a clonal population.[61] Serial dilutions ensure single-cell isolation by reducing cell density to achieve well-separated colonies, typically visible after 24-48 hours of incubation at optimal temperatures like 37°C for Escherichia coli.[61] These methods exploit the organisms' high growth rates, with bacteria dividing every 20-30 minutes under ideal nutrient availability.[62] For recombinant cloning, bacterial transformation introduces foreign DNA, often via plasmids, into competent cells using chemical methods like calcium chloride treatment followed by heat shock or electroporation to permeabilize membranes.[63] Transformed cells are then plated on selective media containing antibiotics, where only clones harboring the recombinant plasmid survive and form colonies; this process, foundational to molecular biology, was first demonstrated in 1973 when Stanley Cohen and Herbert Boyer successfully inserted and propagated antibiotic resistance genes in E. coli.[64][63] Yeast, particularly Saccharomyces cerevisiae, are cloned similarly by isolating single cells through plating on selective agar, leveraging their budding reproduction to form visible colonies in 2-3 days.[65] Transformation in yeast employs lithium acetate for chemical uptake or electroporation, often followed by selection with markers like auxotrophic complements or antibiotics.[66] Advanced techniques exploit yeast's homologous recombination efficiency for seamless cloning, enabling assembly of large DNA fragments or even entire bacterial genomes, as achieved in 2010 with Mycoplasma genitalium and Mycoplasma pneumoniae genomes maintained as single molecules in S. cerevisiae.[67][68] These cloning approaches underpin biotechnology applications, including gene library construction, protein expression, and synthetic biology, where unicellular hosts amplify specific DNA sequences or produce recombinant proteins at scales unattainable in multicellular systems.[60] Transformation efficiencies vary, with optimized E. coli protocols yielding 10^6 to 10^9 transformants per microgram of DNA, while yeast methods achieve 10^3 to 10^5, influenced by cell competency and vector design.[62][66]Stem Cell Cloning and iPSCs
Stem cell cloning, often termed therapeutic cloning, employs somatic cell nuclear transfer (SCNT) to generate patient-specific embryonic stem cell lines for potential regenerative therapies. In SCNT, the nucleus of a somatic cell is inserted into an enucleated oocyte, which is then stimulated to divide and form a blastocyst from which embryonic stem cells can be harvested.[17] This approach aims to produce histocompatible cells avoiding immune rejection, but it faces low efficiency rates, typically below 5% in mammals, and ethical concerns over embryo destruction.[9] Early SCNT experiments succeeded in amphibians in the 1950s, with Briggs and King demonstrating nuclear reprogramming in frogs, but mammalian applications lagged until refinements enabled derivation of embryonic stem cells.[69] Induced pluripotent stem cells (iPSCs) offer an alternative to SCNT by reprogramming differentiated somatic cells, such as fibroblasts, into a pluripotent state without creating embryos. Japanese researchers Kazutoshi Takahashi and Shinya Yamanaka first generated mouse iPSCs in 2006 by introducing four transcription factors—Oct4, Sox2, Klf4, and c-Myc—via retroviral vectors into fibroblasts, restoring pluripotency akin to embryonic stem cells.[70] Human iPSCs followed in 2007 using the same factors on adult dermal fibroblasts, enabling self-renewal and differentiation into all three germ layers. Unlike SCNT, iPSC generation avoids oocyte donation and ethical embryo issues, allowing autologous cell production from a patient's own tissues, though initial methods risked insertional mutagenesis from viral integration, potentially leading to tumors.[71] iPSCs surpass therapeutic cloning in scalability and accessibility, with reprogramming efficiencies improved to over 1% through non-integrating methods like mRNA or small molecules by the 2010s, reducing oncogenic risks.[72] Applications include disease modeling, such as generating neurons from patient fibroblasts to study Parkinson's, and drug screening for toxicity.[71] However, iPSCs retain epigenetic memory from donor cells, potentially biasing differentiation, and exhibit incomplete reprogramming in some lines, limiting full equivalence to embryonic stem cells.[73] Therapeutic cloning via SCNT provides more faithful nuclear reprogramming but remains technically challenging, with human embryonic stem cell derivation from SCNT achieved only sporadically, as in primate models yielding viable lines in 2013.[17] Both techniques advance toward clinical use, yet iPSCs dominate research due to fewer regulatory hurdles and broader applicability in personalized medicine.[74]Organismal Cloning Techniques
Plant and Horticultural Methods
Plant cloning through horticultural methods relies on vegetative propagation, which exploits the totipotency of plant cells to generate genetically identical copies from parent tissues, preserving traits like fruit quality or disease resistance without genetic recombination from seeds.[75] These techniques, practiced since antiquity but refined in modern agriculture, include cuttings, layering, grafting, division, and micropropagation, enabling mass production for commercial horticulture.[76] Unlike sexual propagation, they minimize variability, though success depends on factors such as plant species, season, and hormonal treatments like auxins to stimulate rooting.[77] Cuttings involve excising stems, leaves, or roots from a healthy parent and inducing adventitious roots under controlled conditions, such as mist propagation or hormone dips with indole-3-butyric acid (IBA). Stem cuttings, severed below a node, root readily in species like roses (Rosa spp.) and figs (Ficus carica), with success rates exceeding 80% in optimal setups using perlite-vermiculite media.[77] Leaf cuttings, as in African violets (Saintpaulia spp.), generate new plantlets from leaf veins or petioles placed on sterile soil, while root cuttings from plants like oriental poppy (Papaver orientale) are buried horizontally to sprout shoots.[76] These methods, scalable for nurseries, produce clones within weeks to months but risk transmitting pathogens if not sanitized.[75] Layering promotes rooting of a stem or branch while still attached to the parent, ensuring nutrient supply until independence. In simple layering, a low shoot is wounded, treated with rooting hormone, and buried, as practiced with blackberries (Rubus spp.); separation occurs after root formation in 4-6 weeks.[76] Air layering, suitable for tropical trees like citrus (Citrus spp.), involves girdling bark, applying hormone, and wrapping in moist sphagnum moss sealed with plastic, yielding roots in 2-3 months for propagation of hard-to-root species.[78] Compound layering suits vining plants like grapes (Vitis vinifera), where multiple tips are layered sequentially.[78] Grafting unites a scion (upper shoot) with a compatible rootstock to clone superior varieties onto vigorous or disease-resistant bases, common in fruit horticulture since the Roman era but standardized in the 19th century. Techniques include whip-and-tongue grafting for similar-diameter parts, achieving 90% take in apples (Malus domestica), and T-budding, where a single bud is inserted under rootstock bark during dormancy.[75] This method clones cultivars like the 'Honeycrisp' apple, propagated on dwarfing rootstocks such as M9 to control tree size and enhance yield.[79] Division separates clustered crowns or rhizomes of perennials like daylilies (Hemerocallis spp.), instantly yielding multiple clones with established roots.[76] Micropropagation via tissue culture, pioneered in the 1930s with callus induction but commercialized post-1960 with Murashige-Skoog medium, multiplies explants (meristems or buds) in vitro under sterile conditions.[80] Stages include establishment on cytokinin-rich media for shoot proliferation, subculturing every 3-4 weeks to yield thousands of clones, followed by rooting on auxin media and acclimatization in greenhouses.[81] Applied to bananas (Musa spp.) and orchids, it produces virus-free stock at rates up to 10^6 plants per explant annually, though costs limit it to high-value crops.[82] These methods collectively underpin horticultural industries, with global propagation of woody ornamentals and fruits relying on them for uniformity and efficiency.[79]Animal Cloning via Somatic Cell Nuclear Transfer
Somatic cell nuclear transfer (SCNT) is a reproductive cloning technique used to create genetically identical copies of animals by transferring the nucleus from a differentiated somatic cell into an enucleated oocyte, followed by reprogramming to develop into an embryo.[17] The process begins with the isolation of a donor somatic cell, typically from skin or other tissues, whose nucleus contains the genetic material to be cloned.[83] An oocyte is then enucleated by removing its own nucleus, usually via micromanipulation under a microscope, to create a cytoplast.[17] The key step involves inserting the somatic nucleus into the enucleated oocyte, either by direct injection or electrofusion, which merges the donor cell with the oocyte.[17] Following nuclear transfer, the reconstructed embryo is activated using chemical agents or electrical pulses to initiate embryonic development, mimicking natural fertilization.[17] The embryo is cultured in vitro to the blastocyst stage before transfer into a surrogate mother's uterus for gestation.[84] This method has been applied primarily to mammals, including sheep, cattle, pigs, and dogs, though success varies by species.[85] Efficiency of SCNT remains low due to incomplete epigenetic reprogramming of the somatic nucleus, leading to aberrant gene expression and high rates of embryonic loss or developmental abnormalities in clones.[86] In mammals, live birth rates typically range from 1-5% of transferred embryos, with even lower efficiencies in some species like mice (around 1-2%) and higher in others like cattle (up to 5-20% in optimized protocols).[87] Recent advancements, such as histone deacetylase inhibitors or improved activation protocols, have increased blastocyst formation to 30-50% in some studies, but full-term development often stays below 10%.[18] Cloned animals frequently exhibit health issues, including large offspring syndrome, immune deficiencies, and shortened telomeres, attributed to faulty reprogramming rather than the cloning process per se.[88] Variations in SCNT include handmade cloning (HMC), which simplifies micromanipulation by bisecting oocytes and fusing multiple fragments, improving throughput and pregnancy rates in bovines to over 40% blastocysts and higher live births compared to traditional methods.[89] Interspecies SCNT (iSCNT) uses oocytes from a different but related species to clone endangered animals, though it faces additional barriers like mitochondrial-nuclear incompatibilities.[90] Despite challenges, SCNT enables precise genetic replication for research and agriculture, with ongoing refinements targeting reprogramming fidelity to boost viability.[91]Other Reproductive Cloning Approaches
Embryo splitting, also known as artificial twinning or blastomere separation, represents the primary alternative to somatic cell nuclear transfer (SCNT) for reproductive cloning in animals. This technique involves mechanically dividing an early-stage embryo—typically at the 2-, 4-, or 8-cell stage—into individual blastomeres or groups of cells, each of which is then cultured to form a complete embryo capable of implantation and development into a genetically identical offspring.[92][93] Unlike SCNT, which reprograms differentiated somatic cells, embryo splitting relies on totipotent cells from the zygote, preserving natural developmental potency but limiting the number of clones per starting embryo to the stage at which division occurs.[93] The method has been applied successfully across various mammals, including cattle, sheep, pigs, and primates. In livestock, embryo splitting enabled the production of identical twins or multiples as early as the 1980s, facilitating genetic uniformity in breeding programs for traits like milk production or disease resistance.[93] A notable achievement occurred in 2000 when a rhesus monkey was cloned via embryo splitting, yielding a viable offspring named Tet, demonstrating feasibility in non-human primates despite challenges like reduced embryo viability post-division.[1] Success rates vary by species and embryo stage; for instance, splitting at the 2-cell stage in mice yields higher implantation rates (up to 50-60%) compared to later stages, where incomplete cell compensation can lead to developmental abnormalities.[94][92] Efforts to induce artificial parthenogenesis—activating unfertilized oocytes to develop without sperm—have been explored as another potential avenue but have not yielded live mammalian births due to genomic imprinting issues, where paternal gene expression is essential for placental and fetal development.[95] In mice and rabbits, parthenogenetic embryos reach blastocyst stages but fail to progress beyond mid-gestation, highlighting inherent barriers in mammals absent in some invertebrates or reptiles.[96] Recent advances, such as chemical activation combined with genetic modifications to bypass imprinting, have produced short-term embryonic development in mice but no full-term clones, underscoring parthenogenesis's limitations for reproductive cloning.[97] Emerging techniques involving induced pluripotent stem cells (iPSCs) derived from somatic cells show promise for generating gamete-like cells or tetraploid complements to facilitate cloning, but as of 2023, they have not resulted in live reproductive clones in mammals, remaining experimental and focused on chimeras or organoids rather than whole organisms.[98] Overall, embryo splitting remains the most established non-SCNT method, valued for its simplicity and lower technical demands, though it offers less flexibility than SCNT for cloning from adult donors.[93]Key Achievements and Case Studies
Dolly the Sheep and Mammalian Cloning
Dolly, a Finn-Dorset ewe, became the first mammal cloned from an adult somatic cell through somatic cell nuclear transfer (SCNT) at the Roslin Institute near Edinburgh, Scotland.[4] The procedure involved extracting a nucleus from a mammary gland cell of a six-year-old Finn-Dorset sheep and inserting it into an enucleated oocyte from a Scottish Blackface ewe, followed by electrical fusion and chemical activation to initiate development.[99] The resulting embryo was implanted into a surrogate Scottish Blackface mother, leading to Dolly's birth on July 5, 1996.[100] This achievement demonstrated that specialized adult cells could be reprogrammed to a totipotent state, challenging prior assumptions about irreversible cellular differentiation.[4] The cloning effort required extensive trials, with only one live birth from 277 fused couplets, highlighting the technique's initial low efficiency of approximately 0.4% for producing a viable offspring.[101] Dolly's creation was announced publicly on February 22, 1997, via a paper in Nature by Ian Wilmut and colleagues, sparking global interest and ethical debates on reproductive cloning.[100] Dolly matured normally, producing a lamb via natural mating in 1998 and another in 1999, confirming her fertility, though her telomeres were shorter than age-matched controls, raising questions about potential accelerated aging.[4] She was euthanized on February 14, 2003, at age 6.5 years due to progressive lung disease and arthritis, conditions also observed in her donor flock but occurring earlier than typical for sheep averaging 10-12 years.[99] Post-mortem analysis attributed her health issues partly to environmental factors at the research facility rather than solely cloning artifacts.[4] Following Dolly, SCNT enabled cloning of diverse mammals, including mice in 1998, cattle and goats shortly thereafter, and later cats (2001), water buffalo (2003), horses (2003), dogs (2005), and wolves (2007).[102] These successes expanded applications in agriculture and biomedical research, such as producing genetically identical animals for consistent drug testing or transgenic models.[103] However, efficiencies remained low, typically 1-5% viable births per transferred embryo, due to incomplete epigenetic reprogramming, leading to high rates of developmental abnormalities like large offspring syndrome.[101] Refinements, including improved donor cell preparation and oocyte quality, have incrementally raised success rates in species like sheep to 5-15% in some protocols, though variability persists across taxa.[104] Mammalian cloning via SCNT has informed stem cell research by revealing mechanisms of nuclear reprogramming, yet persistent health challenges in clones underscore limitations in mimicking natural embryonic development.[102] While Dolly's case proved the feasibility of mammalian reproductive cloning, subsequent efforts prioritized therapeutic cloning for tissue generation over widespread reproduction, constrained by technical inefficiencies and ethical concerns.[103]Cloned Species Across Taxa
Somatic cell nuclear transfer (SCNT) and related nuclear transfer techniques have enabled reproductive cloning in species across multiple vertebrate classes, though successes diminish outside mammals due to reprogramming inefficiencies and developmental barriers. Amphibians were among the first taxa cloned via nuclear transfer, with fertile clones produced from embryonic or larval donor nuclei in species such as the northern leopard frog (Lithobates pipiens) in 1957 and African clawed frog (Xenopus laevis) in 1960.[105] Additional amphibian species cloned include the axolotl (Ambystoma mexicanum) in 1965, Iberian ribbed newt (Pleurodeles waltl) in 1970, and various Rana and Pelophylax frogs through the 1970s, often yielding fertile adults when using less differentiated donors.[105] However, transfers from fully differentiated adult somatic cells frequently result in developmental arrest at early stages like gastrulation, with no healthy adults reported from such donors in amphibians since the 1980s amid declining research efforts.[105] Fish cloning via nuclear transfer has also produced fertile offspring, starting with early successes in the common carp (Cyprinus carpio), goldfish (Carassius auratus), and bitterling (Rhodeus sinensis) in 1963 using embryonic nuclei.[105] Later achievements include the grass carp (Ctenopharyngodon idellus) in 1984, loach (Paramisgurnus dabryanus) in 1990, medaka (Oryzias latipes) in 1999, and zebrafish (Danio rerio) in 2002, demonstrating viability across teleost orders despite cross-species oocyte challenges in conservation contexts.[105] Mammalian cloning dominates modern SCNT applications, with verified successes in at least 20 species across orders including Artiodactyla (e.g., sheep Ovis aries in 1996, cattle Bos taurus in 1998, goats Capra hircus in 1998, pigs Sus scrofa in 2000), Rodentia (mice Mus musculus in 1998), Carnivora (cats Felis catus in 2002, dogs Canis familiaris in 2005, black-footed ferrets Mustela nigripes in 2021), and Perissodactyla (horses Equus caballus in 2003).[106][105] Clones in these species often reach reproductive maturity, though early mortality and health issues persist; for instance, over 1,500 dogs from diverse breeds have been cloned commercially since 2005, with many exhibiting normal longevity.[107] Conservation cloning has extended to endangered mammals like the mouflon (2001), gaur (2001), and Przewalski's horse (2020), where clones have integrated into wild populations or breeding programs.[105] No reproductive clones have been viably produced in birds via SCNT, owing to the unique avian reproductive system involving large-yolked eggs and meroblastic cleavage, which complicates enucleation and reprogramming.[105] Reptilian cloning remains unverified at the organismal level, with technical hurdles similar to those in birds, including eggshell barriers and limited oocyte availability. Invertebrate successes are scarce, limited primarily to the fruit fly (Drosophila melanogaster) in 2004 using pole cell nuclear transfer, yielding fertile adults.[105]| Taxonomic Group | Representative Cloned Species | First Success Year | Notes on Fertility/Reproduction |
|---|---|---|---|
| Amphibia | Xenopus laevis | 1960 | Fertile clones from tadpole donors; adult somatic challenges[105] |
| Actinopterygii | Cyprinus carpio | 1963 | Fertile; viable in multiple cyprinid species[105] |
| Mammalia | Ovis aries | 1996 | Fertile; foundational for SCNT in mammals[5] |
| Mammalia | Canis familiaris | 2005 | Fertile; commercial scale with normal lifespans[107] |
| Insecta | Drosophila melanogaster | 2004 | Fertile via specialized transfer[105] |
Agricultural and Pet Cloning Successes
In agricultural cloning, successes have centered on livestock reproduction to propagate elite genetic traits for enhanced milk, meat, and breeding efficiency. The first cloned calf, Gene, was born on December 23, 1997, at facilities operated by the U.S.-based cattle-breeding company ABS Global in Deforest, Wisconsin, using cells from a cow fetus. Subsequent milestones included the cloning of the first bull from an adult donor cell line by researchers at Texas A&M University, with the calf born on September 13, 1999, demonstrating viability for adult somatic cell nuclear transfer in bovines. Commercial applications have proliferated through companies like ViaGen, which has produced cloned cattle, pigs, and goats since the early 2000s, enabling farmers to rapidly multiply high-value animals; for instance, clones of superior dairy bulls have been used to improve herd productivity without traditional breeding timelines. The U.S. Food and Drug Administration assessed in 2008 that meat and milk from clones and their progeny pose no unique risks compared to conventional livestock, facilitating integration into food production chains.[108][109][110] Pet cloning has achieved commercial viability primarily for dogs and cats, driven by demand to replicate deceased companions. ViaGen Pets, a division of ViaGen launched in 2015, pioneered routine cloning services in North America, completing hundreds of procedures by 2022 and over 1,000 dogs and cats by 2024, with annual growth reflecting improved success rates and client satisfaction. The process involves somatic cell nuclear transfer into donor eggs, followed by surrogate gestation, yielding clones that typically exhibit genetically identical traits, including appearance and temperament, though epigenetic factors can introduce variations. South Korean firm Sooam Biotech has similarly cloned hundreds of dogs since 2006, including high-profile cases for celebrities, with clones reported to live full lifespans comparable to non-clones. These efforts underscore cloning's reliability for pet replication, despite costs exceeding $50,000 per animal and occasional health challenges like large offspring syndrome in surrogates.[111][112][113][114]Applications in Biotechnology and Conservation
Livestock and Food Production
Cloning technologies, particularly somatic cell nuclear transfer (SCNT), have been applied to livestock species such as cattle, pigs, sheep, and goats to replicate animals exhibiting superior genetic traits for breeding purposes, thereby accelerating improvements in traits like milk yield, growth rate, and disease resistance.[110][115] This approach enables the rapid dissemination of elite genetics without the limitations of traditional breeding cycles, which can span years, allowing producers to enhance herd quality and productivity more efficiently.[110][84] Cloning also supports the production of transgenic clones, where genetically modified somatic cells are used in SCNT to create animals expressing novel traits, such as livestock engineered to produce human proteins in milk for pharmaceutical purposes, serving as bioreactors for therapeutics like antithrombin or lactoferrin.[116][117] These transgenic approaches provide research models and preserve elite traits for biotechnology applications. The first successful cloning of cattle via SCNT occurred in 1998, with Trans Ova Genetics producing the initial Holstein heifer, marking a milestone for dairy applications where clones of high-producing females or sires can generate semen or embryos for widespread use.[118] Subsequent advancements extended to pigs in 2000 and other livestock, with commercial entities like Trans Ova Genetics (which acquired ViaGen in 2012) offering cloning services to preserve genetics from top performers, such as record-setting gilts or bulls with exceptional marbling.[119][120] In practice, clones serve primarily as breeding stock rather than direct food sources due to high costs—often exceeding $10,000 per clone—and low success rates of 5-10% from reconstructed embryos to live births.[110][121] In the United States, where cloning is integrated into agriculture, an estimated 600 cloned livestock animals existed by the mid-2000s, predominantly cattle used for elite breeding, comprising a tiny fraction of the national herd (e.g., fewer than 150 cloned dairy cows amid nine million total).[122][123] Companies like Trans Ova produce around 100 cloned calves annually, focusing on dairy and beef sectors to propagate traits such as polled horns or feed efficiency.[121] The offspring of clones, rather than the clones themselves, enter conventional production chains, contributing indirectly to food output without distinct labeling requirements.[110] Regulatory assessments, including the U.S. Food and Drug Administration's 2008 guidance, determined that meat and milk from healthy clones and their progeny of cattle, swine, and goats pose no unique food safety risks compared to conventionally bred animals, based on compositional analyses showing equivalent nutritional profiles and absence of anomalies in examined samples.[124][125] This stance supports cloning's role in bolstering food production resilience, though adoption remains limited by expense and efficiency constraints, with primary value in conserving rare breeds or amplifying genetic progress in commercial herds.[115][126] Chimeric cloning techniques, involving the integration of cells from different species, hold potential for advancing biotechnology by enabling the growth of human organs in animal hosts to alleviate transplant shortages.[127]Endangered Species Preservation
Cloning has been investigated as a tool for preserving endangered species by reproducing individuals from cryopreserved genetic material, particularly to restore lost genetic diversity in bottlenecked populations. This approach aims to supplement wild or captive breeding programs rather than replace habitat restoration or anti-poaching efforts, while preserving valuable genetics and propagating elite traits to enhance population resilience.[128] Success rates remain low, typically under 5%, due to technical difficulties in somatic cell nuclear transfer (SCNT), including incomplete nuclear reprogramming and surrogate compatibility issues.[129][10] A notable success occurred with the black-footed ferret (Mustela nigripes), declared endangered in the United States. In December 2020, Elizabeth Ann became the first cloned U.S. endangered species, derived from frozen skin cells of Willa, a female ferret who died in the 1980s and whose lineage was absent from the modern population descended from just seven founders captured in 1981. This cloning addressed genetic bottlenecks exacerbating vulnerability to sylvatic plague. Elizabeth Ann's clone, Antonia, produced healthy offspring in 2023, with three litters born by September 2025, demonstrating reproductive viability and advancing conservation genetics. Ongoing black-footed ferret cloning efforts, as of 2026, continue to boost genetic diversity in endangered populations using advanced techniques.[130][131][132] The U.S. Fish and Wildlife Service highlighted cloning's role in countering disease threats like canine distemper, though it emphasized integration with habitat reintroduction.[133] Earlier attempts yielded mixed results. In January 2001, Noah, the first cloned endangered mammal, was born via SCNT using cells from a gaur (Bos gaurus), a vulnerable Southeast Asian bovid; gestated in a domestic cow surrogate, Noah died two days later from dysentery, underscoring health risks like infections in interspecies cloning. The Pyrenean ibex (Capra pyrenaica pyrenaica), extinct since January 2000, was cloned in July 2003 from skin cells of the last individual, Celia; the kid survived only minutes due to respiratory failure, marking the first de-extinction effort but highlighting persistent cloning defects such as lung malformations.[134][135] Limitations persist, including epigenetic instability leading to developmental abnormalities and the scarcity of suitable surrogates, often requiring related domestic species that introduce immunological mismatches. As of 2026, advancements such as non-invasive blood-based cloning developed by Colossal Biosciences, utilizing endothelial progenitor cells from simple blood draws, have made the process faster, more efficient, and less harmful to animals, supporting conservation efforts.[136] Critics argue resources for cloning—costly and labor-intensive—divert from proven strategies like protected areas, with cloned animals comprising a tiny fraction of populations and failing to resolve underlying threats like habitat loss. Nonetheless, cryopreserved cell banks, such as those at the San Diego Frozen Zoo, enable future applications, provided advancements in SCNT efficiency mitigate current 1% viability rates for wild species.[137][138] In plant conservation and forestry, clonal hybrids offer uniform propagation of elite traits, enhancing productivity while aiding preservation efforts in agriculture-related biodiversity.De-Extinction Initiatives
De-extinction initiatives seek to revive extinct species through biotechnological methods, including somatic cell nuclear transfer (SCNT) cloning and CRISPR-based genome editing, often applied to closely related living surrogates due to the degradation of ancient DNA samples.[139] These efforts prioritize species with recoverable genetic material and viable host species, but face limitations in achieving exact genetic replicas, resulting in hybrid organisms rather than pure clones.[140] Proponents argue that such proxies can restore ecological functions, while skeptics emphasize that true resurrection remains unfeasible given epigenetic mismatches and incomplete genomes.[140][141] Colossal Biosciences, founded in 2021, leads commercial de-extinction efforts by combining SCNT cloning with gene editing to insert extinct traits into extant species' cells.[142] For the woolly mammoth (Mammuthus primigenius), extinct around 4,000 years ago, the company edits Asian elephant (Elephas maximus) genomes using preserved mammoth DNA from permafrost specimens, aiming for hybrid calves by 2028 via elephant surrogates.[143] In the dire wolf (Aenocyon dirus), extinct for about 10,000 years, Colossal reported the birth of three pups in April 2025 through genetic engineering of gray wolf cells, marking the first claimed de-extinct mammal via these techniques, though the animals exhibit hybrid traits. Gene resurrection techniques, recognized as a 2026 breakthrough, enable the incorporation of ancient DNA into living species via cloning and gene editing, as exemplified by Colossal's engineering of gray wolves with dire wolf traits.[144][145][146] Similar projects target the thylacine (Tasmanian tiger) using fat-tailed dunnart marsupials and the dodo bird via Nicobar pigeon primordial germ cells, with a September 2025 breakthrough in culturing pigeon germ cells to facilitate editing.[147] Colossal secured $200 million in funding in January 2025 to advance these initiatives, focusing on Arctic rewilding for mammoths.[148] Non-invasive blood-based cloning methods further support these de-extinction efforts by improving efficiency and reducing animal harm.[136] Revive & Restore, established in 2012, integrates cloning with genetic rescue for near-extinct and extinct taxa, emphasizing biodiversity enhancement over pure revival.[149] The organization cloned a black-footed ferret in 2021 from cryopreserved cells of an individual dead since 1988, using domestic ferrets as intermediaries, to boost genetic diversity in the endangered population.[141] For the passenger pigeon (Ectopistes migratorius), extinct since 1914, efforts involve editing band-tailed pigeon genomes with pigeon-like traits, though full cloning remains exploratory due to tissue scarcity.[150] Revive & Restore also cloned a Przewalski's horse in 2020 from domestic horse cells to aid conservation, demonstrating cloning's utility for amplifying founder populations.[151] These projects highlight cloning's role in preventing further losses but underscore technical hurdles, such as low SCNT success rates (under 5% in mammals) and surrogate incompatibilities.[139] Early precedents include the 2003 cloning of a Pyrenean ibex (Capra pyrenaica pyrenaica), extinct since 2000, via goat-ibex hybrid embryos, yielding one live kid that survived seven minutes due to respiratory failure.[139] Such outcomes illustrate persistent health risks in clones, including genomic instability, prompting initiatives to refine protocols through iterative editing and surrogate optimization.[152] Despite progress, no initiative has produced self-sustaining populations, and ecological integration remains untested, with critics noting potential diversion of resources from habitat preservation.[140]Human Cloning Efforts
Therapeutic and Research Cloning
Therapeutic cloning, also known as somatic cell nuclear transfer (SCNT) for stem cell production, involves transferring the nucleus from a patient's somatic cell into an enucleated human oocyte to create a genetically identical embryo, which is then cultured to the blastocyst stage for deriving embryonic stem cells (ESCs).[1] These patient-matched ESCs can differentiate into various cell types for regenerative therapies, potentially treating conditions like Parkinson's disease, spinal cord injuries, or diabetes by replacing damaged tissues without triggering immune rejection.[9] Unlike reproductive cloning, which intends to implant the embryo for gestation and birth of a cloned organism, therapeutic cloning halts development at the early embryonic stage to harvest stem cells, destroying the embryo in the process.[9] [1] Research cloning encompasses broader applications of SCNT-derived embryos or cells for scientific investigation, including modeling human development, studying genetic diseases, and testing drug responses in genetically precise systems.[2] In mammals, SCNT has enabled the production of cloned embryos yielding viable ESCs since the late 1990s, following Dolly the sheep's creation in 1996, with successes in mice, cows, and monkeys providing insights into nuclear reprogramming and epigenetic mechanisms.[48] Human applications faced early setbacks, such as the 2004 claim by South Korean researcher Hwang Woo-suk of deriving patient-specific ESCs, which was retracted in 2006 due to fabricated data and ethical violations, underscoring challenges in verification and oversight.[153] The first verified derivation of human ESCs via SCNT occurred in 2013, when Shoukhrat Mitalipov's team at Oregon Health & Science University used fetal somatic cells and caffeine-supplemented media to achieve reprogramming, producing ESC lines with normal karyotypes and pluripotency markers.[154] In 2014, researchers extended this to adult somatic cells, generating ESCs from a 35-year-old male's fibroblasts, confirming SCNT's feasibility for personalized medicine despite efficiencies below 5%—far lower than induced pluripotent stem cell (iPSC) reprogramming, which avoids embryo creation.[155] These advances support research into mitochondrial disorders, as SCNT allows nuclear transfer to healthy oocytes, preserving patient genetics while mitigating maternal inheritance defects.[156] Technical limitations persist, including incomplete genomic reprogramming leading to abnormalities, high oocyte requirements (often 10-20 per ESC line), and ethical restrictions in many jurisdictions prohibiting federal funding for embryo-destructive research.[9] While iPSCs have largely supplanted SCNT for routine applications due to ethical neutrality and scalability, therapeutic cloning retains value for specific cases like histocompatibility testing and studying early embryogenesis, with ongoing refinements in reprogramming factors improving yields as of 2014.[155] No clinical trials using SCNT-derived human cells have reached approval by 2025, reflecting persistent efficiency and safety hurdles over therapeutic promise.[157]Reproductive Cloning Claims and Attempts
Claims of successful human reproductive cloning emerged shortly after the 1996 birth of Dolly the sheep, but all have lacked independent verification and scientific substantiation.[1] Proponents, often operating outside mainstream regulatory frameworks, cited somatic cell nuclear transfer techniques adapted from animal models, yet high failure rates, embryo abnormalities, and ethical barriers have precluded confirmed live births.[6] As of 2025, no peer-reviewed evidence supports the production of a viable human clone via this method, with claims typically dismissed due to methodological opacity and ties to non-credible entities.[158] In December 2002, Clonaid, a company affiliated with the Raëlian Movement—a group espousing extraterrestrial origins of humanity—announced the birth of "Eve," purportedly the first cloned human infant, delivered via an American client using nuclear transfer from the mother's skin cells.[159] Clonaid's CEO, Brigitte Boisselier, claimed the procedure succeeded after prior animal cloning trials but refused independent DNA testing, citing client privacy, which fueled widespread scientific skepticism.[160] Subsequent offers for verification, including by the FDA, went unheeded, and no further evidence materialized; experts attributed the announcement to publicity-seeking by a fringe organization rather than empirical achievement.[161] Clonaid later alleged additional clones but provided no documentation.[162] Italian reproductive specialist Severino Antinori, known for prior work in infertility treatments, declared intentions in 2001 to initiate human cloning for infertile couples, predicting the first cloned baby by early 2003.[163] Collaborating with clients unable to produce gametes, Antinori claimed embryo transfers had occurred by 2002, but no births were confirmed, and Italian authorities investigated his activities amid international backlash.[164] Peers criticized the venture as premature and risky, given animal cloning inefficiencies like developmental failures exceeding 99% in mammals.[165] Cypriot-American andrologist Panayiotis Zavos pursued similar efforts, announcing in 2002–2004 the implantation of cloned human embryos derived from somatic cells of deceased donors or infertile individuals.[166] By 2009, Zavos reported creating 14 cloned embryos and transferring 11 into four women's uteri, asserting viable pregnancies ensued, though none resulted in verified live births.[167] A planned publication on these claims was withdrawn by Fertility and Sterility due to insufficient evidence and ethical concerns.[168] Critics highlighted Zavos's reliance on unproven techniques and evasion of oversight, with no genomic or phenotypic data released to affirm cloning success.[169] Other sporadic assertions, such as unverified embryo cloning interruptions in South Korea (1998) and isolated reports from private clinics, similarly failed scrutiny, often conflating therapeutic cloning—aimed at stem cell derivation—with reproductive intent.[1] Post-2010, claims diminished amid reinforced global bans, including UN declarations and national laws prohibiting reproductive cloning, reflecting consensus on its unfeasibility and hazards like genomic instability observed in animal proxies.[170] No advancements reported between 2020 and 2025 indicate successful human reproductive cloning, underscoring persistent biological and technical impediments.[171]Regulatory Frameworks and Bans
The United Nations General Assembly adopted the Declaration on Human Cloning on March 8, 2005, a non-binding resolution urging member states to prohibit all forms of human cloning incompatible with human dignity and the protection of human life.[172] The declaration passed with 84 votes in favor, 34 against, and 37 abstentions, reflecting divisions over whether to target only reproductive cloning or include therapeutic applications.[172] It stopped short of a binding convention due to disagreements, with some nations favoring a comprehensive ban and others preferring to allow cloning for biomedical research.[173] At the national level, over 50 countries have enacted laws explicitly banning reproductive human cloning, which involves implanting a cloned embryo to initiate pregnancy and birth.[174] These include Australia, Austria, Belgium, Brazil, Canada, France, Germany, Japan, South Korea, and the United Kingdom, where penalties range from fines to imprisonment.[175] In France, reproductive cloning is classified as a crime against the human species, punishable by up to 20 years in prison under bioethics laws revised as of July 2025.[176] The Council of Europe Additional Protocol to the Convention on Human Rights and Biomedicine, signed by 19 European nations in 1998 and effective from 2001, prohibits any intervention to create genetically identical human beings, influencing EU-wide opposition to reproductive cloning.[177] Therapeutic cloning, involving the creation of cloned embryos for stem cell research without implantation, faces fewer universal restrictions but remains prohibited in several jurisdictions. Germany and Austria ban it outright, while the United Kingdom permits it under strict licensing by the Human Fertilisation and Embryology Authority since the Human Reproductive Cloning Act 2001.[175] In the United States, no comprehensive federal ban exists as of 2025, though federal funding for human cloning research is prohibited under appropriations riders since 1997, and at least six states—California, Iowa, Louisiana, Michigan, Rhode Island, and Virginia—have enacted reproductive cloning bans.[178] Legislative attempts, such as the Human Cloning Prohibition Act of 2003, failed to pass, leaving private sector activities unregulated federally but subject to ethical oversight by bodies like the National Institutes of Health.[179] Enforcement challenges persist due to the technology's accessibility and cross-border research, with some nations like China regulating but not fully banning embryo cloning for therapeutic purposes since 2004.[180] These frameworks prioritize preventing births of cloned humans over research bans, driven by empirical evidence of health risks in animal clones and concerns over embryo destruction, though proponents argue regulatory gaps hinder potential medical advances.Biological Limitations and Risks
Health Defects in Clones
Cloned mammals produced via somatic cell nuclear transfer (SCNT) exhibit elevated rates of developmental abnormalities compared to naturally reproduced offspring, primarily due to incomplete epigenetic reprogramming of the donor nucleus. These defects often manifest as high embryonic and fetal loss, with success rates for live births typically below 5% in many species. Perinatal complications include large offspring syndrome (LOS), characterized by macrosomia, organomegaly, and placental overgrowth, which contribute to respiratory distress, cardiovascular failures, and immune deficiencies in survivors.[86][181][84] Postnatal health issues in surviving clones frequently involve respiratory, hepatic, and renal dysfunctions, alongside higher susceptibility to infections and tumors. For instance, early bovine clones displayed thymic hypoplasia and altered immune responses, leading to increased mortality within the first months of life. In sheep, placental defects such as hydroallantois and abnormal vascularization have been recurrent, often resulting in abortion or weak neonates. These anomalies stem from faulty gene expression patterns, including aberrant imprinting and X-chromosome inactivation, which persist despite attempts at nuclear reprogramming.[182][183][184] The iconic case of Dolly the sheep, born in 1996, highlighted potential premature aging concerns, as she developed arthritis by age 5 and euthanized at 6.5 years due to progressive lung disease from jaagsiekte sheep retrovirus (JSRV). However, subsequent analyses of clones from the same cell line, aged to equivalent of 70 human years, showed no accelerated osteoarthritis or metabolic disorders, suggesting Dolly's ailments may reflect environmental factors or viral predisposition rather than inherent cloning defects. Telomere lengths in these clones were comparable to age-matched controls, countering early fears of replicative senescence. Nonetheless, across species, clones display a 20-30% higher incidence of neoplasms and organ failures, underscoring ongoing risks despite procedural refinements.[185][186][187] Improvements in SCNT techniques, such as histone deacetylase inhibitors and oocyte selection, have mitigated some defects, enabling healthier clones in cattle and pigs for agricultural use. Yet, epigenetic instability remains a core limitation, with studies indicating persistent DNA methylation errors that impair long-term viability. In mice, cloned embryos often suffer chromosomal aberrations from cell cycle mismatches, amplifying aneuploidy rates. Overall, while a subset of clones achieves normal lifespan and fertility, the preponderance of data affirms elevated health risks, necessitating rigorous screening and welfare considerations in cloning protocols.[188][189][183]Telomere Shortening and Lifespan Issues
In somatic cell nuclear transfer (SCNT), the primary cloning technique for mammals, telomeres—the protective caps at chromosome ends derived from an aged donor somatic cell—often enter the process already shortened due to prior cell divisions in the donor.[190] This raised early concerns that clones might inherit accelerated cellular aging, as telomere attrition is causally linked to replicative senescence and organismal lifespan limits in mammals.[187] However, the oocyte's reprogramming machinery can reactivate telomerase, an enzyme that elongates telomeres, potentially restoring or even extending their length during embryogenesis.[191] The cloned sheep Dolly exemplified initial telomere concerns: her telomeres were approximately 20% shorter than those in age-matched controls at 3–6 years old, correlating with her death at age 6.5 from progressive ovine pulmonary adenocarcinoma and arthritis, conditions atypical before age 10–12 in sheep.[190] This fueled speculation of premature aging, as somatic donor cells from a 6-year-old ewe carried division-induced telomere erosion not fully reset by SCNT.[192] Yet, telomere length alone does not dictate lifespan; Dolly's issues may reflect cumulative SCNT inefficiencies, including incomplete reprogramming, rather than telomeres per se.[187] Subsequent studies mitigated these fears. Four cloned sheep derived from the same cell line as Dolly (aged 7–9 years in 2016) exhibited shorter telomeres than controls but displayed no signs of premature aging beyond mild osteoarthritis, with blood values, bone density, and glucose tolerance comparable to non-clones.[185] In cattle, embryonic cell-derived clones often had telomeres 15–20% longer than age-matched controls, while adult-derived ones varied, with some restoration via telomerase.[193] Across species like pigs, goats, and mice, most post-2000 SCNT studies report telomere lengths equivalent to controls, attributed to refined protocols enhancing reprogramming.[184] Empirical data indicate telomere shortening does not consistently shorten clone lifespans. A 2016 review found reduced telomeres in one-third to half of clone studies, yet many animals achieved normal longevity, suggesting compensatory mechanisms or telomere-independent aging drivers dominate.[190] In livestock, cloned cattle and pigs reaching reproductive age without accelerated aging further support this; isolated premature deaths likely stem from multi-factorial defects like imprinting errors, not telomeres alone.[194] Recent analyses (up to 2023) affirm that while donor age influences initial telomere status, SCNT viability improves with telomere-positive selections, underscoring technique optimization over inherent doom.[195]Epigenetic and Genomic Instability
Cloned organisms produced via somatic cell nuclear transfer (SCNT) frequently exhibit epigenetic instability due to incomplete reprogramming of the donor somatic nucleus, where established patterns of DNA methylation, histone modifications, and chromatin structure fail to reset fully to an embryonic state. This results in aberrant gene expression, particularly affecting imprinted genes and developmental regulators, contributing to high rates of embryonic lethality and postnatal defects; for instance, cloning efficiency remains below 5% in most mammals, with the majority of embryos arresting early or developing placental abnormalities.[196][197] Specific epigenetic errors include persistent histone H3 lysine 9 trimethylation (H3K9me3) and barriers from H3K27me3 imprinting in somatic cells, which impede post-implantation development unless artificially mitigated.[183][198] Genomic instability in clones manifests as elevated rates of chromosomal aberrations, aneuploidy, and somatic mutations arising from the stress of nuclear reprogramming and enucleated oocyte environment, which disrupts normal mitotic fidelity. Studies in cloned mice and pigs reveal variations in gene expression profiles that diverge from the donor animal, with clones showing altered methylation patterns across the genome despite sequence identity, leading to phenotypes like large offspring syndrome or organ dysfunction.[199][189] In cloned dogs, genomic analyses of over 1,000 individuals identified congenital defects such as cleft palate occurring at a 2.9% rate, linked to subtle genetic and epigenetic variances not present in donors.[107] These instabilities persist even in apparently healthy clones, as evidenced by unstable epigenetic states in embryonic stem cells derived from them, underscoring the intrinsic challenges of SCNT beyond mere technical optimization.[197][200] Clonal hybrids, chimeric clones, and transgenic clones introduce additional biological risks. In animals, these approaches often involve high failure rates, pregnancy losses, neonatal morbidity and mortality, and health defects such as organ abnormalities and shortened lifespan. Chimeric methods, in particular, risk developmental malformations due to genomic incompatibilities. In plants, clonal propagation reduces genetic diversity, increasing vulnerability to diseases and the potential for plantation failures in forestry and agriculture.[201]Ethical and Philosophical Debates
Arguments in Favor: Scientific Progress and Utility
Therapeutic cloning, utilizing somatic cell nuclear transfer (SCNT), enables the generation of patient-matched embryonic stem cells, minimizing risks of immune rejection in regenerative therapies.[1] This approach holds promise for treating degenerative diseases such as Parkinson's, where cloned stem cells could differentiate into dopamine-producing neurons to replace damaged tissue.[202] Similarly, it offers potential for spinal cord injury repair by producing specialized neural cells tailored to the individual.[202] The technique advances scientific understanding of cellular reprogramming and developmental biology, as demonstrated by the 1996 cloning of Dolly the sheep, which confirmed that differentiated adult cells could be reverted to a totipotent state.[99] Ian Wilmut, lead researcher in Dolly's creation, advocated for therapeutic cloning to develop treatments for conditions like motor neuron disease, arguing that restricting such research would hinder medical progress.[203] SCNT-derived stem cells also facilitate precise drug testing and disease modeling, accelerating therapeutic development without relying on scarce donor tissues.[204] In agriculture and animal models, cloning produces genetically uniform organisms for consistent research outcomes, reducing variability in studies of transgenics or disease pathology.[205] For human applications, it supports organoid and tissue engineering, potentially alleviating organ shortages by cultivating compatible grafts.[206] Proponents emphasize that these utilities stem from empirical successes in non-human cloning, projecting scalable benefits for human health absent viable alternatives.[207]Objections: Moral Status of Clones and Embryos
Opponents of therapeutic cloning contend that cloned embryos warrant significant moral protection due to their biological equivalence to fertilized embryos, possessing the inherent potential to develop into full human beings. This perspective, advanced by bioethicists who ascribe moral status to human organisms from the earliest developmental stages, views the creation of embryos via somatic cell nuclear transfer (SCNT) followed by their dissociation for stem cell harvesting as a grave ethical violation, akin to the destruction of nascent human life.[208][209] Such arguments emphasize that the totipotent nature of cloned blastocysts—capable of forming all tissues, including extra-embryonic structures—confers upon them a status demanding respect beyond mere cellular utility, with proponents citing the continuity of human development as grounds for prohibiting research that necessitates embryo sacrifice.[210] Religious and philosophical traditions reinforcing this objection include those asserting personhood at fertilization or implantation, irrespective of cloning method; for example, the conviction that embryos embody inviolable human dignity underpins calls for bans on embryo-destructive cloning, as articulated in analyses of international bioethics debates where embryo status equates to that of born persons.[211] Critics of permissive policies highlight that even cloned embryos exhibit genetic individuality through mitochondrial variations and epigenetic factors, challenging utilitarian dismissals of their moral worth and arguing that according them lesser status reflects a commodification of human origins driven by therapeutic ambitions.[212] For reproductive cloning, objections rarely dispute the full moral status of a viable clone post-birth, recognizing it as a distinct human individual with equivalent rights and dignity to others; however, the process itself is decried for presuming to manufacture human life asexually, thereby undermining the relational and procreative essence of humanity. Leon Kass, in his ethical critique, invoked the "wisdom of repugnance"—a visceral public aversion to cloning—as evidence of its incompatibility with human flourishing, positing that clones, though morally equal, would embody a manufactured identity that erodes personal autonomy and uniqueness.[213][214] The President's Council on Bioethics, in its 2002 report, explored typologies of embryo and clone status but ultimately recommended prohibiting reproductive cloning to safeguard dignity, cautioning that treating clones as genetic replicas risks psychological harms and societal devaluation of individuality, even if legal personhood is granted at birth.[215][216] Ethical concerns in chimeric cloning, particularly human-animal chimeras for organ production, include risks of human-like consciousness or features emerging in host animals due to substantial human cell contributions, alongside developmental malformations and welfare issues from physiological uncertainties.[217][218] These moral status objections persist amid empirical realities: cloned embryos in animal models demonstrate viability comparable to natural ones, yet high failure rates in implantation underscore risks that ethicists frame not merely as technical but as symptomatic of hubristic interference with natural generation.[8] While consequentialist defenses prioritize potential medical benefits, deontological critiques, attributing intrinsic value to embryonic and cloned human forms, maintain that no instrumental gain justifies the antecedent ethical breach, a stance echoed in policy recommendations for moratoriums or outright bans.[219]Identity, Dignity, and Psychological Impacts
Clones produced through reproductive cloning would possess the same nuclear DNA as the donor individual but would constitute genetically identical yet ontologically distinct persons, akin to monozygotic twins, whose shared genetics do not preclude the formation of unique personal identities shaped by differential environmental experiences and epigenetic factors.[220] Empirical studies of identical twins demonstrate that, despite genetic equivalence, they exhibit divergent psychological development, including distinct self-concepts, ambitions, and anxiety profiles by adolescence, underscoring that identity emerges from the interplay of heredity and postnatal influences rather than genetics alone.[220] This evidence suggests that human clones would similarly forge independent identities, avoiding the misconception of being mere extensions or duplicates of the original.[221] Psychological impacts on clones remain largely speculative absent verified human cases, but analogies from identical twins and cloned animals provide causal insights: twins often navigate challenges in achieving autonomy and differentiation, such as heightened self-consciousness or relational enmeshment, yet longitudinal data reveal no inherent psychopathology attributable to genetic duplication itself, with identity formation proceeding robustly through individuation processes.[222] In cloned mammals, behavioral phenotypes vary significantly among clones of the same donor and diverge from the original, as observed in dogs displaying similar but not identical exploratory and cognitive patterns influenced by rearing environments, indicating that personality and psychological traits are not faithfully replicated by somatic cell nuclear transfer.[223] Pigs cloned from the same cell line likewise show inter-clone variability in food preferences and social behaviors, further evidencing that non-genetic factors—such as mitochondrial DNA differences, uterine conditions, and post-natal experiences—drive psychological divergence.[224] Concerns over dignity in cloning often center on the potential commodification of human life, where clones might be perceived or treated as manufactured replicas lacking intrinsic uniqueness, thereby eroding their equal moral worth—a view articulated in ethical analyses positing that reproductive cloning confounds natural procreation with technological replication, risking the instrumentalization of persons as means to parental or societal ends.[225] Proponents of this objection, including reports from bioethics bodies, argue that such practices could foster societal attitudes viewing clones as second-class individuals, predicated on a repugnance toward asexual origins that philosophers like Leon Kass have framed as intuitive safeguards against dehumanization, though these claims rest on normative rather than empirical grounds.[214] Counterarguments grounded in twin analogies maintain that dignity inheres in individuality irrespective of origins, with no causal evidence from animal cloning indicating diminished psychological welfare or self-regard in clones compared to naturally propagated counterparts.[226] Until human reproductive cloning occurs, these dignity impacts hinge on cultural and rearing contexts, potentially amplified by stigma but mitigated by recognition of clones as full persons with autonomous agency.[225]Societal Controversies and Criticisms
Eugenics Fears and Slippery Slope Claims
Critics of human reproductive cloning have raised concerns that it could revive eugenic practices by enabling the systematic selection and replication of genetically "superior" individuals, potentially leading to discrimination against those with less desirable traits.[227] Following the 1996 announcement of Dolly the sheep's cloning, ethicists warned that cloning humans from elite donors—such as accomplished scientists or athletes—might prioritize certain heritable qualities like intelligence or physical prowess, echoing early 20th-century eugenics movements that sterilized or restricted reproduction among the "unfit."[228] The U.S. National Bioethics Advisory Commission (NBAC) in its 1997 report highlighted how cloning could erode social norms against eugenics by tempting parents or states to manipulate lineage for enhancement, though it emphasized safety risks as the primary barrier to pursuit.[229] Slippery slope arguments posit that permitting even limited cloning for research or therapy would inexorably progress to reproductive applications and genetic engineering, normalizing the commodification of human genomes.[213] Leon Kass, in his 1997 essay "The Wisdom of Repugnance," contended that public aversion to cloning signals deeper ethical violations, such as manufacturing children as means to ends, which could slide into widespread acceptance of designer offspring akin to consumer products.[230] This view gained traction in policy debates; the President's Council on Bioethics, chaired by Kass from 2001 to 2005, argued in 2002 that embryonic cloning for stem cells creates tools for broader germline alterations, fostering a "Brave New World" where human variation is engineered out.[214] Empirical precedents, such as the expansion from in vitro fertilization (introduced in 1978) to preimplantation genetic diagnosis by the 1990s, illustrate how initial restrictions erode under therapeutic rationales.[212] Proponents of bans, including the Council of Europe's 1997 recommendation against nuclear transfer cloning, invoked eugenics as a rationale alongside dignity concerns, fearing it would instrumentalize humans for genetic optimization.[231] In U.S. congressional hearings, such as the 2001 Senate discussion on human cloning, witnesses testified that without comprehensive prohibitions, cloning could enable "positive eugenics" through multiple copies of favored genomes, exacerbating inequalities as affluent groups access enhancements unavailable to others.[232] These claims persist despite low technical feasibility, as evidenced by high failure rates in animal cloning (e.g., over 90% embryonic loss in sheep trials post-Dolly), yet ethicists maintain that moral precedents set today could enable future abuses once efficiencies improve.[228] Counterarguments from some bioethicists dismiss eugenics fears as speculative, citing voluntary parental choices rather than coercive programs, but historical eugenics in the U.S. (e.g., 60,000 forced sterilizations by 1930s) underscores the plausibility of slippery escalations under state or market pressures.[227]Resource Allocation and Overhype Debunking
Cloning research, particularly somatic cell nuclear transfer (SCNT), has demanded substantial financial and infrastructural resources since the 1996 birth of Dolly the sheep, yet yields persistently low practical outcomes relative to investment. Animal cloning experiments typically achieve success rates of 5-15% for live births among transferred embryos, with overall viability often below 1% when accounting for early failures and abnormalities.[233] For instance, commercial pet cloning costs approximately $50,000 per dog or cat, involving hundreds of oocyte donations and surrogate pregnancies, with success probabilities remaining around 15-30% even after optimizations.[234][235] These inefficiencies extend to livestock, where cloning a cow averages $15,000 and a pig $4,000, primarily for elite breeding stock, but widespread adoption is hindered by high failure rates and health complications in clones.[236] Therapeutic cloning, hyped in the early 2000s as a pathway to patient-matched stem cells for treating degenerative diseases, has failed to deliver transformative medical advances despite targeted funding. Proponents anticipated circumventing immune rejection via personalized embryonic stem cells, but persistent scientific barriers—including incomplete epigenetic reprogramming, mitochondrial heteroplasmy, and tumorigenicity—have stymied progress.[9] By 2006, fraud scandals like the Hwang Woo-suk case exposed fabricated claims of human therapeutic cloning successes, eroding credibility and redirecting scrutiny to ethical and technical flaws.[237] The advent of induced pluripotent stem cells (iPSCs) in 2006 offered a non-embryonic alternative for generating patient-specific cells, rendering therapeutic cloning largely obsolete for regenerative applications without the need for embryo destruction or cloning inefficiencies.[216] Critics argue that allocating resources to cloning diverts funds from more efficacious biomedical pursuits, such as adult stem cell therapies or gene editing, where empirical returns are higher. Human cloning attempts could require hundreds of failed embryos per viable outcome, imposing exorbitant costs and ethical burdens on surrogate systems, while yielding speculative benefits overshadowed by alternatives.[216] Legislative restrictions in many nations, including U.S. federal bans on funding certain cloning variants, have curtailed public investment, yet private and international efforts persist with marginal gains, underscoring opportunity costs: for example, the emphasis on cloning primate models in the early 2000s advanced basic science but not scalable human therapies.[238] Over two decades post-Dolly, cloning's core promise—ubiquitous organ regeneration or infertility solutions—remains unfulfilled, attributable to intrinsic biological limits rather than mere regulatory hurdles, as evidenced by stagnant efficiencies in peer-reviewed animal studies.[239][240] This pattern invites skepticism toward narratives framing cloning as an imminent panacea, prioritizing instead evidence-based resource stewardship in medical research.Political and Religious Opposition
In the United States, political efforts to ban human reproductive cloning gained momentum following the 1997 announcement of Dolly the sheep, with lawmakers citing risks to human dignity and embryo destruction. Senator Sam Brownback (R-KS) introduced the Human Cloning Prohibition Act (S. 790) in 2001, which sought to criminalize the creation of human embryos via cloning for any purpose, including research, arguing that cloning commodifies human life.[241] Similar bills, such as the 2002 Brownback-Weldon Human Cloning Prohibition Act (H.R. 2505), passed the House but stalled in the Senate amid debates over distinguishing reproductive from therapeutic cloning, with opponents like the National Academy of Sciences warning of high risks of injury or death to clones.[242] No federal ban on reproductive cloning exists as of 2025, though 14 states prohibit it outright, often framed as protecting the unique genetic identity and rights of potential clones.[243] Internationally, the United Nations General Assembly adopted the non-binding United Nations Declaration on Human Cloning on March 8, 2005, by a vote of 84 in favor, 34 against, and 37 abstentions, urging member states to prohibit "all forms of human cloning inasmuch as they are incompatible with human dignity and the protection of human life."[172] Proponents, including the Holy See and many developing nations, emphasized ethical boundaries against creating human life solely for experimentation, while opponents like Belgium and the United Kingdom argued it unduly restricted therapeutic applications.[244] This declaration reflected broader consensus in Europe, where the European Parliament called for a moratorium on cloning in 1997, and most nations, including Germany and France, enacted strict bans on reproductive cloning by the early 2000s, prioritizing public safety and moral concerns over potential biomedical benefits.[245] Religious opposition has been near-universal among major faiths, rooted in doctrines affirming the sanctity of procreation as a divine or natural process not to be replicated artificially. The Catholic Church, through the Congregation for the Doctrine of the Faith's 1987 instruction Donum Veritatis (more precisely, Donum Vitae), explicitly condemned human cloning as an illicit attempt to manipulate human generation, violating the right to an "openness to life" and treating embryos as mere objects.[246] Pope John Paul II reiterated this in 1997 reflections on cloning, stating it undermines the "unrepeatable identity" of each person derived from natural origins, a position echoed by the Pontifical Academy for Life.[247] Evangelical Protestant groups, such as those affiliated with the Southern Baptist Convention, have similarly opposed cloning, viewing it as an arrogant usurpation of God's creative role and a pathway to devaluing individual souls, as articulated in U.S. congressional testimonies.[232] In Islam, leading scholars from bodies like the Islamic Fiqh Council have ruled reproductive cloning impermissible (haram), as it interferes with Allah's decree on human creation and lineage, potentially leading to social confusion over kinship; fatwas from Saudi Arabia's Permanent Committee for Scholarly Research and Ifta in the early 2000s equated it to forbidden tampering with divine will.[248] Judaism's Orthodox branches, per rabbinical opinions from the 1997 cloning era, reject cloning for disrupting natural reproduction and risking defective offspring, though some Reform perspectives tolerate therapeutic uses under strict oversight. These stances persist without significant evolution through 2025, influencing policy in religiously conservative nations and underscoring cloning's conflict with beliefs in inherent human uniqueness.[249]Recent Advances (2020–2026)
Integration with CRISPR and Gene Editing
The integration of CRISPR-Cas9 gene editing with somatic cell nuclear transfer (SCNT) cloning has facilitated the production of genetically modified animals by enabling targeted edits in donor somatic cells prior to nuclear transfer, thereby generating uniform cloned lineages with specific traits or disease models. This approach leverages CRISPR's precision to knock out or insert genes in fibroblasts or other accessible cells, which are then screened and cloned, addressing limitations of direct embryo injection such as mosaicism and low editing uniformity. Between 2020 and 2025, applications have focused on agricultural enhancements, biomedical research, and xenotransplantation, with success rates constrained by SCNT's inherent inefficiencies, typically 1-5% live birth rates, compounded by potential off-target edits and incomplete epigenetic reprogramming.[250][251] In equine cloning, a 2020 study demonstrated CRISPR/Cas9 editing of the myostatin (MSTN) gene in horse fibroblasts, achieving 87-90% indels across guide RNAs, with clonal lines showing monoallelic or biallelic knockouts and no detectable off-target mutations in sequenced blastocysts. SCNT of these edited cells yielded embryos developing to blastocyst stage at rates lower than unedited controls (p < 0.05), highlighting potential for breeding horses with enhanced muscle mass or corrected genetic defects while maintaining breed integrity.[250] Similar efforts in buffalo targeted MSTN for improved meat yield; in 2023, electroporation-delivered CRISPR/Cas9 produced edited fibroblast clones with 8-nucleotide deletions or frameshifts, followed by handmade SCNT, resulting in 83-86% cleavage and 22% blastocyst formation—viable for tropical livestock optimization but evidencing reduced developmental competence versus wild-type (33% blastocysts).[252] Biomedical applications advanced with canine models; a 2022 report detailed the first CRISPR-edited dogs via SCNT, targeting the DJ-1 gene (linked to Parkinson's disease) in beagle fibroblasts, yielding two healthy knockouts from 68 transferred embryos (3% efficiency). The clones exhibited confirmed biallelic indels, repressed DJ-1 expression, and no off-target effects at 14 months post-birth, enabling faithful disease modeling in purebred animals without altering broader genetics. In xenotransplantation, porcine SCNT integrated multiplex CRISPR edits (e.g., knocking out alpha-gal and other immunogenic genes) to mitigate hyperacute rejection; 2025 reviews note homozygous multi-gene modifications in donor cells prior to cloning, accelerating production of transplant-compatible pigs, though long-term viability requires further validation against complement activation and thrombosis risks.[251][253] Therapeutically, this synergy holds promise for human applications by editing patient-derived somatic cells with CRISPR before SCNT to derive isogenic embryonic stem cells, potentially bypassing immunogenicity in regenerative therapies; however, ethical prohibitions on human reproductive cloning and the ascendancy of induced pluripotent stem cells (iPSCs) for editing have limited progress to animal proxies, with no verified human trials by 2025. Persistent challenges include CRISPR off-target risks (mitigated by high-fidelity variants) and SCNT's epigenetic barriers, which cause developmental abnormalities in 90-95% of attempts, underscoring the need for improved reprogramming factors. Gene resurrection, recognized as a 2026 breakthrough by MIT Technology Review, enables the incorporation of ancient DNA into living species via cloning and gene editing.[254] These developments, grounded in empirical animal data, prioritize causal mechanisms like gene dosage effects over speculative narratives, with source credibility favoring peer-reviewed veterinary and transplantation journals over broader media claims.[253]Stem Cell and Organoid Developments
Therapeutic cloning via somatic cell nuclear transfer (SCNT) has seen technical refinements aimed at improving the derivation of nuclear transfer embryonic stem cells (ntESCs), which offer potential for patient-matched therapies without immune rejection. In 2023, researchers successfully derived ntESCs from wild-derived mouse strains, demonstrating feasibility for genetic diversity in cloning applications and enhancing models for studying complex traits.[255] By July 2025, advances addressed both pre- and post-implantation epigenetic barriers in SCNT, boosting embryo development rates and reprogramming efficiency through targeted histone modifications and small-molecule inhibitors.[256] These improvements, primarily validated in animal models, underscore ongoing efforts to overcome low success rates historically plaguing SCNT, which remain below 5% for viable blastocyst formation in mammals.[257] In the context of organoid development, SCNT-derived stem cells enable the generation of personalized 3D tissue models by differentiating ntESCs into organ-specific progenitors. While direct applications from cloned human stem cells are limited by ethical constraints on human embryo creation, animal studies illustrate the potential: SCNT has been used to produce stem cell lines capable of forming organoids for drug screening and disease modeling, as noted in reviews of cloning techniques.[257] For instance, ntESCs bypass the genetic aberrations sometimes seen in induced pluripotent stem cells (iPSCs), potentially yielding more faithful organoid representations of patient tissues.[258] However, empirical progress from 2020–2025 prioritizes iPSC-based organoids due to accessibility, with SCNT reserved for scenarios requiring exact nuclear genome matching, such as mitochondrial disease research. No large-scale human trials using SCNT-organoids were reported by 2025, reflecting persistent technical hurdles like incomplete reprogramming.[259] These developments highlight SCNT's niche role in stem cell research, where empirical data favor it for high-fidelity reprogramming over iPSCs in select cases, though broader adoption awaits efficiency gains beyond current animal benchmarks.[256]De-Extinction Breakthroughs
De-extinction efforts have advanced through genetic engineering and cloning techniques, focusing on proxy species edited with traits from extinct relatives rather than direct cloning of ancient DNA, which remains infeasible due to degradation. Companies like Colossal Biosciences have pioneered these methods, targeting species such as the woolly mammoth, thylacine, and dodo by inserting extinct genes into living surrogates using CRISPR-Cas9. In 2026, Colossal Biosciences developed a non-invasive blood-based cloning method using endothelial progenitor cells from simple blood draws, making cloning faster, more efficient, and less harmful to animals; this technique was recognized as a top invention of 2025 by TIME and supports conservation and de-extinction initiatives.[260] These approaches aim to create viable hybrids capable of ecological roles similar to their extinct counterparts, though full genomic resurrection is projected beyond 2028.[261] In the woolly mammoth project, Colossal Biosciences achieved a milestone in March 2025 by engineering laboratory mice with mammoth-derived traits, including thick, curly coats for insulation and enhanced cold tolerance, via targeted gene edits in Asian elephant stem cells adapted to rodents.[262] This demonstrated functional integration of up to 50 mammoth genes, advancing toward elephant-mammoth hybrids for gestation in artificial wombs or surrogate elephants, with initial calves potentially viable by 2028 if developmental hurdles are cleared.[263] Parallel progress includes 2024 advancements in induced pluripotent stem cells from elephant fibroblasts edited with mammoth DNA, enabling organoid formation for testing viability.[264] For the dodo and related birds, Colossal reported a breakthrough on September 18, 2025, with the world's first successful editing and culturing of primordial germ cells (PGCs) in pigeons, allowing germline transmission of dodo-specific genes for beak morphology and flight adaptations into Nicobar pigeon surrogates.[265] This built on 2025 gene edits in band-tailed pigeons to mimic passenger pigeon flocking behavior and genome resilience, yielding embryos with hybrid traits, though hatching and rearing challenges persist due to incomplete epigenetic reprogramming.[266] Revive & Restore's complementary work has sequenced over 99% of the passenger pigeon genome by 2024, enabling proxy breeding programs to restore flock dynamics in eastern U.S. forests.[267] Thylacine de-extinction has progressed via near-complete genome assembly (99.9% by 2024), with Colossal inserting thylacine genes for pouch development and predatory traits into fat-tailed dunnart cells, producing chimeric embryos in 2025 that survived to blastocyst stage.[268] These efforts highlight cloning's limitations—low success rates from somatic cell nuclear transfer (SCNT), historically under 5% viable births even in mammals like sheep—and underscore the need for artificial wombs to bypass surrogate incompatibilities.[269] Despite hype, critics note that proxies may not fully replicate extinct behaviors or ecosystems, prioritizing conservation of endangered species like red wolves, where Colossal cloned litters in April 2025 to bolster genetic diversity. Colossal also engineered gray wolves with dire wolf traits, such as larger skulls and white fur, through gene editing and cloning. Ongoing black-footed ferret cloning efforts by Colossal aim to enhance genetic diversity in this endangered population.[270][271]Future Prospects and Challenges
Technological Hurdles and Improvements
Somatic cell nuclear transfer (SCNT), the primary technique for reproductive cloning, faces persistent low efficiency rates, typically below 5% in mammals, stemming from incomplete epigenetic reprogramming of the donor nucleus.[272] This reprogramming failure results in aberrant DNA methylation and histone modifications, leading to dysregulated gene expression and high rates of embryonic lethality or congenital defects in surviving clones.[273] For instance, the original cloning of Dolly the sheep in 1996 required 277 attempts, yielding a success rate of approximately 0.3%, with many embryos exhibiting developmental abnormalities due to faulty erasure of somatic epigenetic marks.[274] Additional hurdles include mitochondrial incompatibilities between donor cells and recipient oocytes, as well as mechanical stresses from micromanipulation during enucleation and nuclear injection, which contribute to pre- and post-implantation losses exceeding 90% in many protocols.[86] Cell cycle mismatches between the quiescent somatic donor nucleus and the M-phase arrested oocyte further exacerbate reprogramming inefficiencies, often causing premature chromosome condensation and spindle assembly errors.[182] Cloned offspring frequently suffer from large offspring syndrome, respiratory distress, and immune deficiencies, attributed to these unresolved epigenetic and cytoplasmic factors.[275] Improvements have focused on enhancing epigenetic remodeling through chemical inhibitors and optimized protocols. Treatment with histone deacetylase inhibitors like trichostatin A (TSA) or scriptaid has increased blastocyst formation rates in bovine SCNT by up to 2-3 fold by facilitating chromatin relaxation and gene activation.[272] Donor cell preparation advancements, such as using fetal or neonatal fibroblasts instead of adult cells, have boosted efficiencies due to their more totipotent epigenetic states, with some studies reporting pregnancy rates doubling compared to adult donors.[276] Recent progress includes the application of G9a inhibitors like BIX-01294, which improved pig cloning success by reducing H3K9 methylation anomalies and enhancing embryo quality.[277] In 2022, analysis of over 1,000 cloned dogs revealed that refined oocyte synchronization and culture media reduced abnormality rates, achieving viable litters with efficiencies approaching 10-20% in optimized canine protocols.[107] A 2025 study demonstrated overcoming preimplantation barriers via targeted epigenetic modulation, yielding higher developmental rates in mammalian SCNT without gene editing.[256] Despite these gains, full-term success remains species-dependent and below natural reproduction levels, underscoring the need for further causal dissection of reprogramming kinetics.[86]Potential Societal Transformations
Therapeutic cloning holds the potential to transform organ transplantation by generating patient-specific tissues and organs, thereby eliminating the risk of immune rejection that affects approximately 10-20% of current transplants and contributes to waiting lists exceeding 100,000 patients annually in the United States alone.[9] [207] This approach could address chronic shortages, as demonstrated by early successes in cloning embryonic stem cells for regenerative therapies, such as repairing spinal cord injuries affecting hundreds of thousands worldwide or treating genetic disorders like sickle cell anemia through customized cell lines.[6] By enabling scalable production of compatible biological materials, societal healthcare systems might see reduced long-term costs from complications and prolonged patient lifespans, shifting reliance from cadaveric donors to engineered solutions.[205] In reproduction, successful human reproductive cloning could fundamentally alter family dynamics and demographic patterns by offering alternatives to traditional conception for infertile individuals or those seeking genetic continuity, potentially increasing birth rates in aging populations where fertility declines after age 35 for women.[205] Proponents argue it might preserve exceptional genetic traits, such as those of intellectually eminent figures, to accelerate societal progress in fields like science or arts, though empirical evidence from animal cloning reveals high failure rates—over 90% in early mammalian attempts like Dolly the sheep in 1996—and associated health defects, limiting near-term feasibility.[6] Widespread adoption, if achieved, could challenge notions of individuality, introducing cloned siblings with identical genetics and prompting legal redefinitions of kinship and inheritance, as genetic duplicates might complicate identity-based rights.[278] Agriculturally, cloning technologies could standardize livestock production, yielding uniform herds of high-milk or disease-resistant animals, as seen in cloned cattle and mules since the 1990s, potentially enhancing global food security amid projected population growth to 9.7 billion by 2050.[205] In conservation, cloning offers a mechanism for biodiversity restoration by propagating endangered species from preserved cells—evidenced by the 2003 cloning of a banteng or efforts toward de-extinct species like the woolly mammoth—countering habitat loss that has driven over 1 million species toward extinction per recent assessments.[205] [278] These applications might foster economic shifts toward biotech-driven industries, but realization depends on overcoming epigenetic abnormalities observed in clones, which cause enlarged organs and premature aging in up to 50% of cases.[6]Policy Recommendations for Balanced Regulation
Balanced regulation of cloning technologies requires distinguishing between reproductive cloning, which aims to create genetically identical humans and remains unsafe based on animal data showing failure rates exceeding 97% and common defects like organ enlargement and immune deficiencies, and therapeutic cloning via somatic cell nuclear transfer (SCNT) for stem cell production, which offers potential for disease modeling without gestation.[6][279] Policies should enforce a global moratorium on human reproductive cloning until empirical evidence demonstrates viability without significant health risks, as evidenced by the 2020 Chinese cloning of a rhesus monkey that survived only seven months due to developmental failures.[280] This approach prioritizes causal risks over speculative benefits, given that over 50 countries, including the United States and members of the European Union, already prohibit it through national laws or UN-guided declarations.[175][281] For therapeutic applications, governments should permit SCNT research under rigorous oversight, including mandatory ethical review boards, limits on embryo culture to 14 days to avoid sentience concerns, and prohibitions on transfer to uteri, aligning with guidelines from bodies like the International Society for Stem Cell Research that emphasize safety and non-reproductive intent.[282][9] Such frameworks, as proposed in U.S. analyses, would enable advances in regenerative medicine—such as patient-specific cells for Parkinson's treatment—while mitigating ethical issues like embryo destruction through alternatives like induced pluripotent stem cells when feasible.[283] Funding restrictions, as in the U.S. federal policy since 1997 barring support for human embryo cloning leading to viable offspring, should persist to prevent escalation toward reproduction.[157] Animal cloning for agriculture or conservation warrants product-specific safety assessments rather than outright bans, with the U.S. FDA affirming since 2008 that meat and milk from clones pose no unique risks after evaluating health data from over 100 cattle and swine clones.[284] For de-extinction efforts, regulations should integrate biodiversity laws, requiring environmental impact studies and genetic diversity safeguards to avoid ecological disruptions, as current projects like mammoth revival demonstrate technical feasibility but unproven long-term viability.[8] International coordination via updated UNESCO bioethics protocols could harmonize standards, countering regulatory arbitrage where lax jurisdictions like certain Asian nations enable unchecked experiments.[174]- Licensing and Enforcement: Mandate pre-approval for all cloning protocols by independent agencies, with penalties including license revocation for violations, as modeled in the UK's Human Fertilisation and Embryology Authority framework.[285]
- Public Transparency: Require disclosure of success rates, adverse outcomes, and funding sources to counter hype, informed by historical overstatements in early cloning claims.[8]
- Equity Measures: Prioritize research addressing unmet needs over enhancement, prohibiting commercial reproductive cloning to prevent access disparities based on wealth.
