Recent from talks
Nothing was collected or created yet.
Oct-4
View on Wikipedia
Oct-4 (octamer-binding transcription factor 4), also known as POU5F1 (POU domain, class 5, transcription factor 1), is a protein that in humans is encoded by the POU5F1 gene.[5] Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells.[6] As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.[7]
Octamer-binding transcription factor 4, OCT-4, is a transcription factor protein that is encoded by the POU5F1 gene and is part of the POU (Pit-Oct-Unc) family.[8] OCT-4 consists of an octamer motif, a particular DNA sequence of AGTCAAAT that binds to their target genes and activates or deactivates certain expressions. These gene expressions then lead to phenotypic changes in stem cell differentiation during the development of a mammalian embryo.[9] It plays a vital role in determining the fates of both inner mass cells and embryonic stem cells and has the ability to maintain pluripotency throughout embryonic development.[10] Recently, it has been noted that OCT-4 not only maintains pluripotency in embryonic cells but also has the ability to regulate cancer cell proliferation and can be found in various cancers such as pancreatic, lung, liver and testicular germ cell tumors in adult germ cells.[11] Another defect this gene can have is dysplastic growth in epithelial tissues which are caused by a lack of OCT-4 within the epithelial cells.[12]
Expression and function
[edit]Oct-4 transcription factor is initially active as a maternal factor in the oocyte and remains active in embryos throughout the preimplantation period. Oct-4 expression is associated with an undifferentiated phenotype and tumors.[13] Gene knockdown of Oct-4 promotes differentiation, demonstrating a role for these factors in human embryonic stem cell self-renewal.[14] Oct-4 can form a heterodimer with Sox2, so that these two proteins bind DNA together.[15]
Mouse embryos that are Oct-4 deficient or have low expression levels of Oct-4 fail to form the inner cell mass, lose pluripotency, and differentiate into trophectoderm. Therefore, the level of Oct-4 expression in mice is vital for regulating pluripotency and early cell differentiation since one of its main functions is to keep the embryo from differentiating.
Orthologs
[edit]Orthologs of Oct-4 in humans and other species include:
| Species | Entrez GeneID | Chromosome | Location | RefSeq (mRNA) | RefSeq (protein) |
|---|---|---|---|---|---|
| Mus musculus (mouse) | 18999 | 17,17 B1; 17 19.23 cM | NC_000083.4, 35114104..35118822 (Plus Strand) | NM_013633.1 | NP_038661.1 |
| Homo sapiens (human) | 5460 | 6, 6p21.31 | NC_000006.10, 31246432-31240107 (Minus Strand) | NM_002701.3 | NP_002692.2 (full length isoform) NP_002692.1 (N-terminal truncated isoform) |
| Rattus norvegicus (rat) | 294562 | 20 | NW_001084776, 650467-655015 (Minus strand) | NM_001009178 | NP_001009178 |
| Danio rerio (zebrafish) | 303333 | 21 | NC_007127.1, 27995548-28000317 (Minus strand) | NM_131112 | NP_571187 |
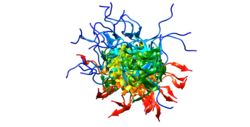
Structure
[edit]Oct-4 contains the following protein domains:
| Domain | Description | Length (AA) |
|---|---|---|
| POU domain | Found in Pit-Oct-Unc transcription factors | 75 |
| Homeodomain | DNA binding domains involved in the transcriptional regulation of key eukaryotic developmental processes; may bind to DNA as monomers or as homodimers and/or heterodimers in a sequence-specific manner. | 59 |
Implications in disease
[edit]Oct-4 has been implicated in tumorigenesis of adult germ cells. Ectopic expression of the factor in adult mice has been found to cause the formation of dysplastic lesions of the skin and intestine. The intestinal dysplasia resulted from an increase in progenitor cell population and the upregulation of β-catenin transcription through the inhibition of cellular differentiation.[16]
Pluripotency in embryo development
[edit]Animal model
[edit]In 2000, Niwa et al. used conditional expression and repression in murine embryonic stem cells to determine requirements for Oct-4 in the maintenance of developmental potency.[7] Although transcriptional determination has often been considered as a binary on-off control system, they found that the precise level of Oct-4 governs 3 distinct fates of ES cells. An increase in expression of less than 2-fold causes differentiation into primitive endoderm and mesoderm. In contrast, repression of Oct-4 induces loss of pluripotency and dedifferentiation to trophectoderm. Thus, a critical amount of Oct-4 is required to sustain stem cell self-renewal, and up- or down-regulation induces divergent developmental programs. Changes to Oct-4 levels do not independently promote differentiation, but are also controlled by levels of Sox2. A decrease in Sox2 accompanies increased levels of Oct-4 to promote a mesendodermal fate, with Oct-4 actively inhibiting ectodermal differentiation. Repressed Oct-4 levels that lead to ectodermal differentiation are accompanied by an increase in Sox2, which effectively inhibits mesendodermal differentiation.[17] Niwa et al. suggested that their findings established a role for Oct-4 as a master regulator of pluripotency that controls lineage commitment and illustrated the sophistication of critical transcriptional regulators and the consequent importance of quantitative analyzes.
The transcription factors Oct-4, Sox2, and Nanog are part of a complex regulatory network, with Oct-4 and Sox2 being capable of directly regulating Nanog by binding to its promoter, and are essential for maintaining the self-renewing undifferentiated state of the inner cell mass of the blastocyst, embryonic stem cell lines[18] (which are cell lines derived from the inner cell mass), and induced pluripotent stem cells.[15] While differential up- and down-regulation of Oct-4 and Sox2 has been shown to promote differentiation, down-regulation of Nanog must occur for differentiation to proceed.[17]
Role in reprogramming
[edit]Oct-4 is one of the transcription factors that is used to create induced pluripotent stem cells (iPSCs), together with Sox2, Klf4, and often c-Myc (OSKM) in mice,[19][20][21] demonstrating its capacity to induce an embryonic stem-cell-like state. These factors are often referred to as "Yamanaka reprogramming factors". This reprogramming effect has also been seen with the Thomson reprogramming factors, reverting human fibroblast cells to iPSCs via Oct-4, along with Sox2, Nanog, and Lin28. The use of Thomson reprogramming factors avoids the need to overexpress c-Myc, an oncogene.[22] It was later determined that only two of these four factors, namely Oct4 and Klf4, are sufficient to reprogram mouse adult neural stem cells.[23] Finally it was shown that a single factor, Oct-4 was sufficient for this transformation.[24] Moreover, while Sox2, Klf4, and cMyc could be replaced by their respective family members, Oct4's closer relatives, Oct1 and Oct6, fail to induce pluripotency, thus demonstrating the exclusiveness of Oct4 among POU transcription factors.[25] However, later it was shown that Oct4 could be completely omitted from the Yamanaka cocktail, and the remaining three factors, Sox2, Klf4, and cMyc (SKM) could generate mouse iPSCs with dramatically enhanced developmental potential.[26] This suggests that Oct4 increases the efficiency of reprogramming, but decreases the quality of resulting iPSCs. This is possibly due to a mutated OCT4 DBD cysteine residue (Cys48) that has been identified as a central reprogramming determinant.[27] The serine residue in OCT1 is mutated in the presence of the OCT4 N terminus, which conversely causes OCT4's serine (converted from cysteine) to reduce reprogramming efficiency by 60%.[27]
In embryonic stem cells
[edit]- In in vitro experiments of mouse embryonic stem cells, Oct-4 has often been used as a marker of stemness, as differentiated cells show reduced expression of this marker.
- Oct3/4 can both repress and activate the promoter of Rex1. In cells that already express high level of Oct3/4, exogenously transfected Oct3/4 will lead to the repression of Rex1.[28] However, in cells that are not actively expressing Oct3/4, exogenous transfection of Oct3/4 will lead to the activation of Rex1.[28] This implies a dual regulatory ability of Oct3/4 on Rex1. At low levels of the Oct3/4 protein, the Rex1 promoter is activated, while at high levels of the Oct3/4 protein, the Rex1 promoter is repressed.
- Oct4 contributes to the rapid cell cycle of ESCs by promoting progression through the G1 phase, specifically through transcriptional inhibition of cyclin-dependent kinase inhibitors such as p21.[29]
- CRISPR-Cas9 knockout of the gene in human embryonic stem cells demonstrated that Oct-4 is essential for the development after fertilisation.[30]
- Oct3/4 represses Suv39h1 expression through the activation of an antisense long non-coding RNA. Suv39h1 inhibition maintains low level of H3K9me3 in pluripotent cells limiting the formation of heterochromatin.[31]
In adult stem cells
[edit]Several studies suggest a role for Oct-4 in sustaining self-renewal capacity of adult somatic stem cells (i.e. stem cells from epithelium, bone marrow, liver, etc.).[32] Other scientists have produced evidence to the contrary,[33] and dismiss those studies as artifacts of in vitro culture, or interpreting background noise as signal,[34] and warn about Oct-4 pseudogenes giving false detection of Oct-4 expression.[35] Oct-4 has also been implicated as a marker of cancer stem cells.[36][37] A majority of Oct4 cancer stem-like cell studies (CSC) report a positive association between expression of OCT4 and chemoresistance.[38] Chemotherapy resulting in the enrichment of CSCs showed changes in the phenotypes and increased stem cell markers of OCT4.[39] Various cancers such as lung cancer, bladder cancer, and mesothelioma cells with high OCT4 expressions showed resistance to cisplatin, general drug resistance, and tumor recurrence.[38] Breast cancer patients had tamoxifen resistance and poor clinical outcomes associated with OCT4.[40] OCT4 knock-downs increase sensitivity to cisplatin and irradiation in lung cells, retained tumorigenicity in glioma-initiating cells, and metastasis mediation in ovarian cancer.[38] In in vitro studies looking at cisplatin, knock-down of OCT4 increased their sensitivity and reduced cell proliferation.[38] Further investigation is needed due to the sparsity of stem cells within tumors and their heterogeneity.
See also
[edit]References
[edit]- ^ a b c ENSG00000206454, ENSG00000204531, ENSG00000237582, ENSG00000229094, ENSG00000233911, ENSG00000235068 GRCh38: Ensembl release 89: ENSG00000230336, ENSG00000206454, ENSG00000204531, ENSG00000237582, ENSG00000229094, ENSG00000233911, ENSG00000235068 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024406 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Takeda J, Seino S, Bell GI (September 1992). "Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues". Nucleic Acids Research. 20 (17): 4613–20. doi:10.1093/nar/20.17.4613. PMC 334192. PMID 1408763.
- ^ Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. (September 2005). "Core transcriptional regulatory circuitry in human embryonic stem cells". Cell. 122 (6). Elsevier BV: 947–956. doi:10.1016/j.cell.2005.08.020. PMC 3006442. PMID 16153702.
- ^ a b Niwa H, Miyazaki J, Smith AG (April 2000). "Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells". Nature Genetics. 24 (4): 372–6. doi:10.1038/74199. PMID 10742100. S2CID 33012290.
- ^ Zeineddine, Dana et al. "The Oct4 protein: more than a magic stemness marker." American journal of stem cells vol. 3,2 74-82. 5 Sep. 2014
- ^ Pan GJ, Chang ZY, Schöler HR, Pei D (December 2002). "Stem cell pluripotency and transcription factor Oct4". Cell Research. 12 (5–6). Springer Science and Business Media LLC: 321–329. doi:10.1038/sj.cr.7290134. PMID 12528890. S2CID 2982527.
- ^ Wu G, Schöler HR (2014). "Role of Oct4 in the early embryo development". Cell Regeneration. 3 (1). Springer Science and Business Media LLC: 7. doi:10.1186/2045-9769-3-7. PMC 4230828. PMID 25408886.
- ^ Saha SK, Jeong Y, Cho S, Cho SG (October 2018). "Systematic expression alteration analysis of master reprogramming factor OCT4 and its three pseudogenes in human cancer and their prognostic outcomes". Scientific Reports. 8 (1). Springer Science and Business Media LLC: 14806. Bibcode:2018NatSR...814806S. doi:10.1038/s41598-018-33094-7. PMC 6172215. PMID 30287838.
- ^ Hochedlinger K, Yamada Y, Beard C, Jaenisch R (May 2005). "Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues". Cell. 121 (3). Elsevier BV: 465–477. doi:10.1016/j.cell.2005.02.018. PMID 15882627. S2CID 1913872.
- ^ Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, et al. (May 2003). "POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors". Cancer Research. 63 (9): 2244–50. PMID 12727846.
- ^ Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ (March 2005). "High-efficiency RNA interference in human embryonic stem cells". Stem Cells. 23 (3): 299–305. doi:10.1634/stemcells.2004-0252. PMID 15749924. S2CID 1395518.
- ^ a b Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. (July 2005). "Transcriptional regulation of nanog by OCT4 and SOX2". The Journal of Biological Chemistry. 280 (26): 24731–7. doi:10.1074/jbc.M502573200. PMID 15860457.
- ^ Hochedlinger K, Yamada Y, Beard C, Jaenisch R (May 2005). "Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues". Cell. 121 (3): 465–77. doi:10.1016/j.cell.2005.02.018. PMID 15882627. S2CID 1913872.
- ^ a b Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S (June 2011). "Pluripotency factors in embryonic stem cells regulate differentiation into germ layers". Cell. 145 (6): 875–89. doi:10.1016/j.cell.2011.05.017. PMC 5603300. PMID 21663792.
- ^ Heurtier, V., Owens, N., Gonzalez, I. et al. The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat Commun 10, 1109 (2019). https://doi.org/10.1038/s41467-019-09041-z
- ^ Okita K, Ichisaka T, Yamanaka S (July 2007). "Generation of germline-competent induced pluripotent stem cells". Nature. 448 (7151): 313–7. Bibcode:2007Natur.448..313O. doi:10.1038/nature05934. PMID 17554338. S2CID 459050.
- ^ Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. (July 2007). "In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state". Nature. 448 (7151): 318–24. Bibcode:2007Natur.448..318W. doi:10.1038/nature05944. PMID 17554336. S2CID 4377572.
- ^ Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. (June 2007). "Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution". Cell Stem Cell. 1 (1): 55–70. doi:10.1016/j.stem.2007.05.014. PMID 18371336.
- ^ Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. (December 2007). "Induced pluripotent stem cell lines derived from human somatic cells". Science. 318 (5858): 1917–20. Bibcode:2007Sci...318.1917Y. doi:10.1126/science.1151526. PMID 18029452. S2CID 86129154.
- ^ Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, et al. (July 2008). "Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors". Nature. 454 (7204): 646–50. Bibcode:2008Natur.454..646K. doi:10.1038/nature07061. PMID 18594515. S2CID 4318637.
- ^ Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, et al. (February 2009). "Oct4-induced pluripotency in adult neural stem cells". Cell. 136 (3): 411–9. doi:10.1016/j.cell.2009.01.023. PMID 19203577. S2CID 1630949.
- ^ Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. (January 2008). "Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts". Nature Biotechnology. 26 (1): 101–6. doi:10.1038/nbt1374. PMID 18059259. S2CID 1705950.
- ^ Velychko S, Adachi K, Kim KP, Hou Y, MacCarthy CM, Wu G, et al. (December 2019). "Excluding Oct4 from Yamanaka Cocktail Unleashes the Developmental Potential of iPSCs". Cell Stem Cell. 25 (6): 737–753.e4. doi:10.1016/j.stem.2019.10.002. PMC 6900749. PMID 31708402.
- ^ a b Shen Z, Wu Y, Manna A, Yi C, Cairns BR, Evason KJ, et al. (May 2024). "Oct4 redox sensitivity potentiates reprogramming and differentiation". Genes & Development. 38 (7–8): 308–321. doi:10.1101/gad.351411.123. PMC 11146590. PMID 38719541.
- ^ a b Ben-Shushan E, Thompson JR, Gudas LJ, Bergman Y (April 1998). "Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site". Molecular and Cellular Biology. 18 (4): 1866–78. doi:10.1128/mcb.18.4.1866. PMC 121416. PMID 9528758.
- ^ Lee J, Go Y, Kang I, Han YM, Kim J (February 2010). "Oct-4 controls cell-cycle progression of embryonic stem cells". The Biochemical Journal. 426 (2): 171–81. doi:10.1042/BJ20091439. PMC 2825734. PMID 19968627.
- ^ Fogarty NM, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, et al. (October 2017). "Genome editing reveals a role for OCT4 in human embryogenesis". Nature. 550 (7674): 67–73. Bibcode:2017Natur.550...67F. doi:10.1038/nature24033. PMC 5815497. PMID 28953884.
- ^ Bernard LD, Dubois A, Heurtier V, Fischer V, Gonzalez I, Chervova A, et al. (July 2022). "OCT4 activates a Suv39h1-repressive antisense lncRNA to couple histone H3 Lysine 9 methylation to pluripotency". Nucleic Acids Research. 50 (13): 7367–7379. doi:10.1093/nar/gkac550. PMC 9303268. PMID 35762231.
- ^ For example:
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE (February 2005). "Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis". Carcinogenesis. 26 (2): 495–502. doi:10.1093/carcin/bgh321. PMID 15513931.
- Kim JH, Jee MK, Lee SY, Han TH, Kim BS, Kang KS, et al. (September 2009). Mei L (ed.). "Regulation of adipose tissue stromal cells behaviors by endogenic Oct4 expression control". PLOS ONE. 4 (9) e7166. Bibcode:2009PLoSO...4.7166K. doi:10.1371/journal.pone.0007166. PMC 2747014. PMID 19777066.
- ^ Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, et al. (October 2007). "Oct4 expression is not required for mouse somatic stem cell self-renewal". Cell Stem Cell. 1 (4): 403–15. doi:10.1016/j.stem.2007.07.020. PMC 2151746. PMID 18159219.
- ^ Lengner CJ, Welstead GG, Jaenisch R (March 2008). "The pluripotency regulator Oct4: a role in somatic stem cells?". Cell Cycle. 7 (6): 725–8. doi:10.4161/cc.7.6.5573. PMID 18239456.
- ^ Zangrossi S, Marabese M, Broggini M, Giordano R, D'Erasmo M, Montelatici E, et al. (July 2007). "Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker". Stem Cells. 25 (7): 1675–80. doi:10.1634/stemcells.2006-0611. PMID 17379765. S2CID 23662657.
- ^ Kim RJ, Nam JS (June 2011). "OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer". Laboratory Animal Research. 27 (2): 147–52. doi:10.5625/lar.2011.27.2.147. PMC 3145994. PMID 21826175.
- ^ Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR (April 2007). "OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer". International Journal of Cancer. 120 (7): 1598–602. doi:10.1002/ijc.22508. PMID 17205510. S2CID 23516214.
- ^ a b c d Mohiuddin IS, Wei SJ, Kang MH (April 2020). "Role of OCT4 in cancer stem-like cells and chemotherapy resistance". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1866 (4): 165432. doi:10.1016/j.bbadis.2019.03.005. PMC 6754810. PMID 30904611.
- ^ Lee S, Wottrich S, Bonavida B (April 2017). "Crosstalks between Raf-kinase inhibitor protein and cancer stem cell transcription factors (Oct4, KLF4, Sox2, Nanog)". Tumour Biology. 39 (4) 1010428317692253. doi:10.1177/1010428317692253. PMID 28378634.
- ^ Gwak JM, Kim M, Kim HJ, Jang MH, Park SY (May 2017). "Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance". Oncotarget. 8 (22): 36305–36318. doi:10.18632/oncotarget.16750. PMC 5482656. PMID 28422735.
Further reading
[edit]- Lamoury FM, Croitoru-Lamoury J, Brew BJ (2006). "Undifferentiated mouse mesenchymal stem cells spontaneously express neural and stem cell markers Oct-4 and Rex-1". Cytotherapy. 8 (3): 228–42. doi:10.1080/14653240600735875. PMID 16793732.
- Hough SR, Clements I, Welch PJ, Wiederholt KA (June 2006). "Differentiation of mouse embryonic stem cells after RNA interference-mediated silencing of OCT4 and Nanog". Stem Cells. 24 (6): 1467–75. doi:10.1634/stemcells.2005-0475. PMID 16456133. S2CID 27609337.
- Feldman N, Gerson A, Fang J, Li E, Zhang Y, Shinkai Y, et al. (February 2006). "G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis". Nature Cell Biology. 8 (2): 188–94. doi:10.1038/ncb1353. PMID 16415856. S2CID 23740530.
- Gerrard L, Zhao D, Clark AJ, Cui W (2005). "Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency". Stem Cells. 23 (1): 124–33. doi:10.1634/stemcells.2004-0102. PMID 15625129. S2CID 21603127.
- Reményi A, Lins K, Nissen LJ, Reinbold R, Schöler HR, Wilmanns M (August 2003). "Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers". Genes & Development. 17 (16): 2048–59. doi:10.1101/gad.269303. PMC 196258. PMID 12923055.
- Schoorlemmer J, Kruijer W (December 1991). "Octamer-dependent regulation of the kFGF gene in embryonal carcinoma and embryonic stem cells". Mechanisms of Development. 36 (1–2): 75–86. doi:10.1016/0925-4773(91)90074-G. PMID 1723621. S2CID 8353907.
- Wey E, Lyons GE, Schäfer BW (March 1994). "A human POU domain gene, mPOU, is expressed in developing brain and specific adult tissues". European Journal of Biochemistry. 220 (3): 753–62. doi:10.1111/j.1432-1033.1994.tb18676.x. PMID 7908264.
- Crouau-Roy B, Amadou C, Bouissou C, Clayton J, Vernet C, Ribouchon MT, et al. (May 1994). "Localization of the OTF3 gene within the human MHC class I region by physical and meiotic mapping". Genomics. 21 (1): 241–3. doi:10.1006/geno.1994.1249. PMID 8088794.
- Guillaudeux T, Mattei MG, Depetris D, Le Bouteiller P, Pontarotti P (1993). "In situ hybridization localizes the human OTF3 to chromosome 6p21.3→p22 and OTF3L to 12p13". Cytogenetics and Cell Genetics. 63 (4): 212–4. doi:10.1159/000133537. PMID 8500351.
- Abdel-Rahman B, Fiddler M, Rappolee D, Pergament E (October 1995). "Expression of transcription regulating genes in human preimplantation embryos". Human Reproduction. 10 (10): 2787–92. doi:10.1093/oxfordjournals.humrep.a135792. PMID 8567814.
- Hillier LD, Lennon G, Becker M, Bonaldo MF, Chiapelli B, Chissoe S, et al. (September 1996). "Generation and analysis of 280,000 human expressed sequence tags". Genome Research. 6 (9): 807–28. doi:10.1101/gr.6.9.807. PMID 8889549.
- Inamoto S, Segil N, Pan ZQ, Kimura M, Roeder RG (November 1997). "The cyclin-dependent kinase-activating kinase (CAK) assembly factor, MAT1, targets and enhances CAK activity on the POU domains of octamer transcription factors". The Journal of Biological Chemistry. 272 (47): 29852–8. doi:10.1074/jbc.272.47.29852. PMID 9368058.
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. (October 1998). "Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4". Cell. 95 (3): 379–91. doi:10.1016/S0092-8674(00)81769-9. PMID 9814708. S2CID 12892299.
- Gonzalez MI, Robins DM (March 2001). "Oct-1 preferentially interacts with androgen receptor in a DNA-dependent manner that facilitates recruitment of SRC-1". The Journal of Biological Chemistry. 276 (9): 6420–8. doi:10.1074/jbc.M008689200. PMID 11096094.
- Butteroni C, De Felici M, Schöler HR, Pesce M (December 2000). "Phage display screening reveals an association between germline-specific transcription factor Oct-4 and multiple cellular proteins". Journal of Molecular Biology. 304 (4): 529–40. doi:10.1006/jmbi.2000.4238. PMID 11099378.
- Ezashi T, Ghosh D, Roberts RM (December 2001). "Repression of Ets-2-induced transactivation of the tau interferon promoter by Oct-4". Molecular and Cellular Biology. 21 (23): 7883–91. doi:10.1128/MCB.21.23.7883-7891.2001. PMC 99954. PMID 11689681.
- Guo Y, Costa R, Ramsey H, Starnes T, Vance G, Robertson K, et al. (March 2002). "The embryonic stem cell transcription factors Oct-4 and FoxD3 interact to regulate endodermal-specific promoter expression". Proceedings of the National Academy of Sciences of the United States of America. 99 (6): 3663–7. Bibcode:2002PNAS...99.3663G. doi:10.1073/pnas.062041099. PMC 122580. PMID 11891324.
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, et al. (May 2003). "POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors". Cancer Research. 63 (9): 2244–50. PMID 12727846.
- Wang P, Branch DR, Bali M, Schultz GA, Goss PE, Jin T (October 2003). "The POU homeodomain protein OCT3 as a potential transcriptional activator for fibroblast growth factor-4 (FGF-4) in human breast cancer cells". The Biochemical Journal. 375 (Pt 1): 199–205. doi:10.1042/BJ20030579. PMC 1223663. PMID 12841847.
- Reményi A, Lins K, Nissen LJ, Reinbold R, Schöler HR, Wilmanns M (August 2003). "Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers". Genes & Development. 17 (16): 2048–59. doi:10.1101/gad.269303. PMC 196258. PMID 12923055.
- Rajpert-De Meyts E, Hanstein R, Jørgensen N, Graem N, Vogt PH, Skakkebaek NE (June 2004). "Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads". Human Reproduction. 19 (6): 1338–44. doi:10.1093/humrep/deh265. PMID 15105401.
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, et al. (2005). "Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells". Stem Cells. 22 (5): 659–68. doi:10.1634/stemcells.22-5-659. PMID 15342930. S2CID 35018708.
- Baal N, Reisinger K, Jahr H, Bohle RM, Liang O, Münstedt K, et al. (October 2004). "Expression of transcription factor Oct-4 and other embryonic genes in CD133 positive cells from human umbilical cord blood". Thrombosis and Haemostasis. 92 (4): 767–75. doi:10.1160/TH04-02-0079. PMID 15467907. S2CID 4646923.
External links
[edit]- Oct-4+Transcription+Factor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- FactorBook POU5F1
- Generating iPS Cells from MEFS through Forced Expression of Sox-2, Oct-4, c-Myc, and Klf4 Archived 2008-04-09 at the Wayback Machine