Recent from talks
Nothing was collected or created yet.
Speciation
View on Wikipedia| Part of a series on |
| Evolutionary biology |
|---|
 |
Speciation is the evolutionary process by which populations evolve to become distinct species. The biologist Orator F. Cook coined the term in 1906 for cladogenesis, the splitting of lineages, as opposed to anagenesis, phyletic evolution within lineages.[1][2][3] Charles Darwin was the first to describe the role of natural selection in speciation in his 1859 book On the Origin of Species.[4] He also identified sexual selection as a likely mechanism, but found it problematic.
There are four geographic modes of speciation in nature, based on the extent to which speciating populations are isolated from one another: allopatric, peripatric, parapatric, and sympatric. Whether genetic drift is a minor or major contributor to speciation is the subject of much ongoing discussion.[5]
Rapid sympatric speciation can take place through polyploidy, such as by doubling of chromosome number; the result is progeny which are immediately reproductively isolated from the parent population. New species can also be created through hybridization, followed by reproductive isolation, if the hybrid is favoured by natural selection.[citation needed]
Historical background
[edit]In addressing the origin of species, there are two key issues:
- the evolutionary mechanisms of speciation
- how the separateness and individuality of species is maintained
Since Charles Darwin's time, efforts to understand the nature of species have primarily focused on the first aspect, and it is now widely agreed that the critical factor behind the origin of new species is reproductive isolation.[6]
Darwin's dilemma: why do species exist?
[edit]In On the Origin of Species (1859), Darwin interpreted biological evolution in terms of natural selection, but was perplexed by the clustering of organisms into species.[7] Chapter 6 of Darwin's book is entitled "Difficulties of the Theory". In discussing these "difficulties" he noted
Firstly, why, if species have descended from other species by insensibly fine gradations, do we not everywhere see innumerable transitional forms? Why is not all nature in confusion instead of the species being, as we see them, well defined?
— On the Origin of Species (1859), chapter 6[7]
This dilemma can be described as the absence or rarity of transitional varieties in habitat space.[8]
Another dilemma,[9] related to the first one, is the absence or rarity of transitional varieties in time. Darwin pointed out that by the theory of natural selection "innumerable transitional forms must have existed", and wondered "why do we not find them embedded in countless numbers in the crust of the earth". That clearly defined species actually do exist in nature in both space and time implies that some fundamental feature of natural selection operates to generate and maintain species.[7]
Effect of sexual reproduction on species formation
[edit]It has been argued that the resolution of Darwin's first dilemma lies in the fact that out-crossing sexual reproduction has an intrinsic cost of rarity.[10][11][12][13][14] The cost of rarity arises as follows. If, on a resource gradient, a large number of separate species evolve, each exquisitely adapted to a very narrow band on that gradient, each species will, of necessity, consist of very few members. Finding a mate under these circumstances may present difficulties when many of the individuals in the neighborhood belong to other species. Under these circumstances, if any species' population size happens, by chance, to increase (at the expense of one or other of its neighboring species, if the environment is saturated), this will immediately make it easier for its members to find sexual partners. The members of the neighboring species, whose population sizes have decreased, experience greater difficulty in finding mates, and therefore form pairs less frequently than the larger species. This has a snowball effect, with large species growing at the expense of the smaller, rarer species, eventually driving them to extinction. Eventually, only a few species remain, each distinctly different from the other.[10][11][13] Rarity not only imposes the risk of failure to find a mate, but it may also incur indirect costs, such as the resources expended or risks taken to seek out a partner at low population densities.[citation needed]

Rarity brings with it other costs. Rare and unusual features are very seldom advantageous. In most instances, they indicate a (non-silent) mutation, which is almost certain to be deleterious. It therefore behooves sexual creatures to avoid mates sporting rare or unusual features (koinophilia).[16][17] Sexual populations therefore rapidly shed rare or peripheral phenotypic features, thus canalizing the entire external appearance, as illustrated in the accompanying image of the African pygmy kingfisher, Ispidina picta. This uniformity of all the adult members of a sexual species has stimulated the proliferation of field guides on birds, mammals, reptiles, insects, and many other taxa, in which a species can be described with a single illustration (or two, in the case of sexual dimorphism). Once a population has become as homogeneous in appearance as is typical of most species (and is illustrated in the photograph of the African pygmy kingfisher), its members will avoid mating with members of other populations that look different from themselves.[18] Thus, the avoidance of mates displaying rare and unusual phenotypic features inevitably leads to reproductive isolation, one of the hallmarks of speciation.[19][20][21][22]
In the contrasting case of organisms that reproduce asexually, there is no cost of rarity; consequently, there are only benefits to fine-scale adaptation. Thus, asexual organisms very frequently show the continuous variation in form (often in many different directions) that Darwin expected evolution to produce, making their classification into "species" (more correctly, morphospecies) very difficult.[10][16][17][23][24][25]
Modes
[edit]
All forms of natural speciation have taken place over the course of evolution; however, debate persists as to the relative importance of each mechanism in driving biodiversity.[26]
One example of natural speciation is the diversity of the three-spined stickleback, a marine fish that, after the last glacial period, has undergone speciation into new freshwater colonies in isolated lakes and streams. Over an estimated 10,000 generations, the sticklebacks show structural differences that are greater than those seen between different genera of fish including variations in fins, changes in the number or size of their bony plates, variable jaw structure, and color differences.[27]
Allopatric
[edit]During allopatric (from the ancient Greek allos, "other" + patrā, "fatherland") speciation, a population splits into two geographically isolated populations (for example, by habitat fragmentation due to geographical change such as mountain formation). The isolated populations then undergo genotypic or phenotypic divergence as: (a) they become subjected to dissimilar selective pressures; (b) different mutations arise in the two populations. When the populations come back into contact, they have evolved such that they are reproductively isolated and are no longer capable of exchanging genes. Island genetics is the term associated with the tendency of small, isolated genetic pools to produce unusual traits. Examples include insular dwarfism and the radical changes among certain famous island chains, for example on Komodo. The Galápagos Islands are particularly famous for their influence on Charles Darwin. During his five weeks there he heard that Galápagos tortoises could be identified by island, and noticed that finches differed from one island to another, but it was only nine months later that he speculated that such facts could show that species were changeable. When he returned to England, his speculation on evolution deepened after experts informed him that these were separate species, not just varieties, and famously that other differing Galápagos birds were all species of finches. Though the finches were less important for Darwin, more recent research has shown the birds now known as Darwin's finches to be a classic case of adaptive evolutionary radiation.[28]
Peripatric
[edit]In peripatric speciation, a subform of allopatric speciation, new species are formed in isolated, smaller peripheral populations that are prevented from exchanging genes with the main population. It is related to the concept of a founder effect, since small populations often undergo bottlenecks. Genetic drift is often proposed to play a significant role in peripatric speciation.[29][30]
Case studies include Ernst Mayr's investigation of bird fauna;[31] the Australian bird Petroica multicolor;[32] and reproductive isolation in populations of Drosophila subject to population bottlenecking.[citation needed]
Parapatric
[edit]In parapatric speciation, there is only partial separation of the zones of two diverging populations afforded by geography; individuals of each species may come in contact or cross habitats from time to time, but reduced fitness of the heterozygote leads to selection for behaviours or mechanisms that prevent their interbreeding. Parapatric speciation is modelled on continuous variation within a "single", connected habitat acting as a source of natural selection rather than the effects of isolation of habitats produced in peripatric and allopatric speciation.[33]
Parapatric speciation may be associated with differential landscape-dependent selection. Even if there is a gene flow between two populations, strong differential selection may impede assimilation and different species may eventually develop.[34] Habitat differences may be more important in the development of reproductive isolation than the isolation time. Caucasian rock lizards Darevskia rudis, D. valentini and D. portschinskii all hybridize with each other in their hybrid zone; however, hybridization is stronger between D. portschinskii and D. rudis, which separated earlier but live in similar habitats than between D. valentini and two other species, which separated later but live in climatically different habitats.[35]
Ecologists refer to[clarification needed] parapatric and peripatric speciation in terms of ecological niches. A niche must be available in order for a new species to be successful. Ring species such as Larus gulls have been claimed to illustrate speciation in progress, though the situation may be more complex.[36] The grass Anthoxanthum odoratum may be starting parapatric speciation in areas of mine contamination.[37]
Sympatric
[edit]
Sympatric speciation is the formation of two or more descendant species from a single ancestral species all occupying the same geographic location.
Often-cited examples of sympatric speciation are found in insects that become dependent on different host plants in the same area.[38][39]
The best known example of sympatric speciation is that of the cichlids of East Africa inhabiting the Rift Valley lakes, particularly Lake Victoria, Lake Malawi and Lake Tanganyika. There are over 800 described species, and according to estimates, there could be well over 1,600 species in the region. Their evolution is cited as an example of both natural and sexual selection.[40][41] A 2008 study suggests that sympatric speciation has occurred in Tennessee cave salamanders.[42] Sympatric speciation driven by ecological factors may also account for the extraordinary diversity of crustaceans living in the depths of Siberia's Lake Baikal.[43]
Budding speciation has been proposed as a particular form of sympatric speciation, whereby small groups of individuals become progressively more isolated from the ancestral stock by breeding preferentially with one another. This type of speciation would be driven by the conjunction of various advantages of inbreeding such as the expression of advantageous recessive phenotypes, reducing the recombination load, and reducing the cost of sex.[44]

The hawthorn fly (Rhagoletis pomonella), also known as the apple maggot fly, appears to be undergoing sympatric speciation.[45] Different populations of hawthorn fly feed on different fruits. A distinct population emerged in North America in the 19th century some time after apples, a non-native species, were introduced. This apple-feeding population normally feeds only on apples and not on the historically preferred fruit of hawthorns. The current hawthorn feeding population does not normally feed on apples. Some evidence, such as that six out of thirteen allozyme loci are different, that hawthorn flies mature later in the season and take longer to mature than apple flies; and that there is little evidence of interbreeding (researchers have documented a 4–6% hybridization rate) suggests that sympatric speciation is occurring.[46]
Methods of selection
[edit]Reinforcement
[edit]
Reinforcement, also called the Wallace effect, is the process by which natural selection increases reproductive isolation.[19] It may occur after two populations of the same species are separated and then come back into contact. If their reproductive isolation was complete, then they will have already developed into two separate incompatible species. If their reproductive isolation is incomplete, then further mating between the populations will produce hybrids, which may or may not be fertile. If the hybrids are infertile, or fertile but less fit than their ancestors, then there will be further reproductive isolation and speciation has essentially occurred, as in horses and donkeys.[47]
One reasoning behind this is that if the parents of the hybrid offspring each have naturally selected traits for their own certain environments, the hybrid offspring will bear traits from both, therefore would not fit either ecological niche as well as either parent (ecological speciation). The low fitness of the hybrids would cause selection to favor assortative mating, which would control hybridization. This is sometimes called the Wallace effect after the evolutionary biologist Alfred Russel Wallace who suggested in the late 19th century that it might be an important factor in speciation.[48] Conversely, if the hybrid offspring are more fit than their ancestors, then the populations will merge back into the same species within the area they are in contact.[citation needed]
Another important theoretical mechanism is the arise of intrinsic genetic incompatibilities, addressed in the Bateson-Dobzhansky-Muller model.[49] Genes from allopatric populations will have different evolutionary backgrounds and are never tested together until hybridization at secondary contact, when negative epistatic interactions will be exposed. In other words, new alleles will emerge in a population and only pass through selection if they work well together with other genes in the same population, but it may not be compatible with genes in an allopatric population, be those other newly derived alleles or retained ancestral alleles. This is only revealed through new hybridization.[49][50] Such incompatibilities cause lower fitness in hybrids regardless of the ecological environment, and are thus intrinsic, although they can originate from the adaptation to different environments.[51] The accumulation of such incompatibilities increases faster and faster with time, creating a "snowball" effect.[52] There is a large amount of evidence supporting this theory, primarily from laboratory populations such as Drosophila and Mus, and some genes involved in incompatibilities have been identified.[50]
Reinforcement favoring reproductive isolation is required for both parapatric and sympatric speciation. Without reinforcement, the geographic area of contact between different forms of the same species, called their "hybrid zone", will not develop into a boundary between the different species. Hybrid zones are regions where diverged populations meet and interbreed. Hybrid offspring are common in these regions, which are usually created by diverged species coming into secondary contact. Without reinforcement, the two species would have uncontrollable inbreeding.[citation needed] Reinforcement may be induced in artificial selection experiments as described below.
Ecological
[edit]Ecological selection is "the interaction of individuals with their environment during resource acquisition".[53] Natural selection is inherently involved in the process of speciation, whereby, "under ecological speciation, populations in different environments, or populations exploiting different resources, experience contrasting natural selection pressures on the traits that directly or indirectly bring about the evolution of reproductive isolation".[54] Evidence for the role ecology plays in the process of speciation exists. Studies of stickleback populations support ecologically linked speciation arising as a by-product,[55] alongside numerous studies of parallel speciation, where isolation evolves between independent populations of species adapting to contrasting environments than between independent populations adapting to similar environments.[56] Ecological speciation occurs with much of the evidence, "...accumulated from top-down studies of adaptation and reproductive isolation".[56]
Sexual selection
[edit]Sexual selection can drive speciation in a clade, independently of natural selection.[57] However the term "speciation", in this context, tends to be used in two different, but not mutually exclusive senses. The first and most commonly used sense refers to the "birth" of new species. That is, the splitting of an existing species into two separate species, or the budding off of a new species from a parent species, both driven by a biological "fashion fad" (a preference for a feature, or features, in one or both sexes, that do not necessarily have any adaptive qualities).[57][58][59][60] In the second sense, "speciation" refers to the wide-spread tendency of sexual creatures to be grouped into clearly defined species,[61][20] rather than forming a continuum of phenotypes both in time and space – which would be the more obvious or logical consequence of natural selection. This was indeed recognized by Darwin as problematic, and included in his On the Origin of Species (1859), under the heading "Difficulties with the Theory".[7] There are several suggestions as to how mate choice might play a significant role in resolving Darwin's dilemma.[20][10][16][17][18][62] If speciation takes place in the absence of natural selection, it might be referred to as nonecological speciation.[63][64]
Artificial speciation
[edit]

New species have been created by animal husbandry, but the dates and methods of the initiation of such species are not clear. Often, the domestic counterpart can still interbreed and produce fertile offspring with its wild ancestor. This is the case with domestic cattle, which can be considered the same species as several varieties of wild ox, gaur, and yak; and with domestic sheep that can interbreed with the mouflon.[65][66]
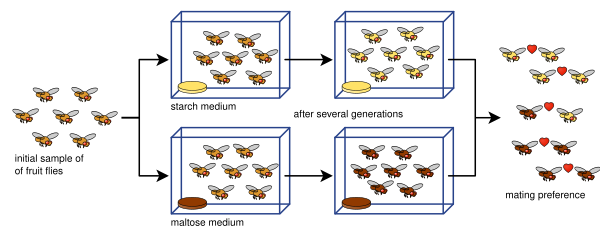
The best-documented creations of new species in the laboratory were performed in the late 1980s. William R. Rice and George W. Salt bred Drosophila melanogaster fruit flies using a maze with three different choices of habitat such as light/dark and wet/dry. Each generation was placed into the maze, and the groups of flies that came out of two of the eight exits were set apart to breed with each other in their respective groups. After thirty-five generations, the two groups and their offspring were isolated reproductively because of their strong habitat preferences: they mated only within the areas they preferred, and so did not mate with flies that preferred the other areas.[67] The history of such attempts is described by Rice and Elen E. Hostert (1993).[68][69] Diane Dodd used a laboratory experiment to show how reproductive isolation can develop in Drosophila pseudoobscura fruit flies after several generations by placing them in different media, starch- and maltose-based media.[70]
Dodd's experiment has been replicated many times, including with other kinds of fruit flies and foods.[71] Such rapid evolution of reproductive isolation may sometimes be a relic of infection by Wolbachia bacteria.[72]
An alternative explanation is that these observations are consistent with sexually-reproducing animals being inherently reluctant to mate with individuals whose appearance or behavior is different from the norm. The risk that such deviations are due to heritable maladaptations is high. Thus, if an animal, unable to predict natural selection's future direction, is conditioned to produce the fittest offspring possible, it will avoid mates with unusual habits or features.[73][74][16][17][18] Sexual creatures then inevitably group themselves into reproductively isolated species.[17]
Genetics
[edit]Species barriers
[edit]In evolutionary biology, a species barrier is any genetic difference that reduces gene flow between diverging lineages. In Darwin's framework, natural selection acting in heterogeneous environments drives phenotypic divergence; hybrids with intermediate phenotypes may suffer reduced fitness where parental populations are locally adapted.[75][page needed] The genetic basis of such barriers was first mapped using crosses between closely related species; for example, Dobzhansky showed in Drosophila that some chromosomal regions contribute disproportionately to hybrid sterility, establishing that reproductive isolation has a genomic basis rather than a single-locus cause.[76]
These findings motivated the Dobzhansky–Muller model: hybrid dysfunction arises from negative epistasis between derived alleles that evolved separately in each lineage, without either lineage having to cross a low-fitness state.[77] Subsequent theory formalized how selected loci impede introgression at nearby neutral loci, quantifying the "barrier to gene flow" and showing that many loci are typically required to strongly reduce exchange across most of the genome.[78] In parallel, the "genic view" of speciation argued that differentiation and isolation often begin at a subset of loci under divergent or sexual selection, while the remainder of the genome can remain permeable for long periods.[79]
Accumulation of species barriers
[edit]Experimental crosses across clades show that overall postzygotic isolation increases with genetic divergence, but the architecture is typically polygenic, asymmetric, and often involves complex (≥3-locus) Dobzhansky–Muller incompatibilities.[80][page needed][81] Theory predicts different accumulation dynamics: under the classic "snowball" model, the number of pairwise incompatibilities grows roughly with the square of substitutions, whereas alternative models (e.g. Fisher's geometric model) can yield more linear behavior depending on trait architecture and selection.[82]
Because barrier loci impede nearby introgression, genomes of diverging lineages often become mosaics with semipermeable regions during "semi-isolated" stages; linkage disequilibria and parallel clines in hybrid zones provide estimates of selection and dispersal maintaining such barriers.[83] Population-genomic inference now makes it possible to quantify how gene flow declines with molecular divergence and to identify when genomic heterogeneity of introgression arises, thereby enabling cross-taxon comparisons along the speciation continuum to uncover the factors driving the accumulation of species barriers.[84]
Few speciation genes have been found. They usually involve the reinforcement process of late stages of speciation. In 2008, a speciation gene causing reproductive isolation was reported.[85] It causes hybrid sterility between related subspecies. The order of speciation of three groups from a common ancestor may be unclear or unknown; a collection of three such species is referred to as a "trichotomy".[citation needed]
Speciation via polyploidy
[edit]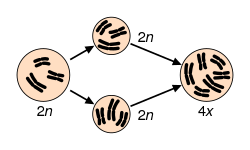
Polyploidy is a mechanism that has caused many rapid speciation events in sympatry because offspring of, for example, tetraploid x diploid matings often result in triploid sterile progeny.[86] However, among plants, not all polyploids are reproductively isolated from their parents, and gene flow may still occur, such as through triploid hybrid x diploid matings that produce tetraploids, or matings between meiotically unreduced gametes from diploids and gametes from tetraploids (see also hybrid speciation).[citation needed]
It has been suggested that many of the existing plant and most animal species have undergone an event of polyploidization in their evolutionary history.[87][88] Reproduction of successful polyploid species is sometimes asexual, by parthenogenesis or apomixis, as for unknown reasons many asexual organisms are polyploid. Rare instances of polyploid mammals are known, but most often result in prenatal death.[89]
Hybrid speciation
[edit]Hybridization between two different species sometimes leads to a distinct phenotype. This phenotype can also be fitter than the parental lineage and as such natural selection may then favor these individuals. Eventually, if reproductive isolation is achieved, it may lead to a separate species. However, reproductive isolation between hybrids and their parents is particularly difficult to achieve and thus hybrid speciation is considered an extremely rare event. The Mariana mallard is thought to have arisen from hybrid speciation.[citation needed]
Hybridization is an important means of speciation in plants, since polyploidy (having more than two copies of each chromosome) is tolerated in plants more readily than in animals.[90][91] Polyploidy is important in hybrids as it allows reproduction, with the two different sets of chromosomes each being able to pair with an identical partner during meiosis.[88] Polyploids also have more genetic diversity, which allows them to avoid inbreeding depression in small populations.[92]
Hybridization without change in chromosome number is called homoploid hybrid speciation. It is considered very rare but has been shown in Heliconius butterflies[93] and sunflowers. Polyploid speciation, which involves changes in chromosome number, is a more common phenomenon, especially in plant species. [citation needed]
Gene transposition
[edit]Theodosius Dobzhansky, who studied fruit flies in the early days of genetic research in 1930s, speculated that parts of chromosomes that switch from one location to another might cause a species to split into two different species. He mapped out how it might be possible for sections of chromosomes to relocate themselves in a genome. Those mobile sections can cause sterility in inter-species hybrids, which can act as a speciation pressure. In theory, his idea was sound, but scientists long debated whether it actually happened in nature. Eventually a competing theory involving the gradual accumulation of mutations was shown to occur in nature so often that geneticists largely dismissed the moving gene hypothesis.[94] However, 2006 research shows that jumping of a gene from one chromosome to another can contribute to the birth of new species.[95] This validates the reproductive isolation mechanism, a key component of speciation.[96]
Rates
[edit]
There is debate as to the rate at which speciation events occur over geologic time. While some evolutionary biologists claim that speciation events have remained relatively constant and gradual over time (known as "Phyletic gradualism" – see diagram), some palaeontologists such as Niles Eldredge and Stephen Jay Gould[97] have argued that species usually remain unchanged over long stretches of time, and that speciation occurs only over relatively brief intervals, a view known as punctuated equilibrium. (See diagram, and Darwin's dilemma.) [citation needed]
Punctuated evolution
[edit]Evolution can be extremely rapid, as shown in the creation of domesticated animals and plants in a very short geological space of time, spanning only a few tens of thousands of years. Maize (Zea mays), for instance, was created in Mexico in only a few thousand years, starting about 7,000 to 12,000 years ago.[98] This raises the question of why the long term rate of evolution is far slower than is theoretically possible.[99][100][101][102]
Evolution is imposed on species or groups. It is not planned or striven for in some Lamarckist way.[103] The mutations on which the process depends are random events, and, except for the "silent mutations" which do not affect the functionality or appearance of the carrier, are thus usually disadvantageous, and their chance of proving to be useful in the future is vanishingly small. Therefore, while a species or group might benefit from being able to adapt to a new environment by accumulating a wide range of genetic variation, this is to the detriment of the individuals who have to carry these mutations until a small, unpredictable minority of them ultimately contributes to such an adaptation. Thus, the capability to evolve would require group selection, a concept discredited by (for example) George C. Williams,[104] John Maynard Smith[105] and Richard Dawkins[106][107][108][109] as selectively disadvantageous to the individual.
The resolution to Darwin's second dilemma might thus come about as follows:
If sexual individuals are disadvantaged by passing mutations on to their offspring, they will avoid mutant mates with strange or unusual characteristics.[74][16][17][62] Mutations that affect the external appearance of their carriers will then rarely be passed on to the next and subsequent generations. They would therefore seldom be tested by natural selection. Evolution is, therefore, effectively halted or slowed down considerably. The only mutations that can accumulate in a population, on this punctuated equilibrium view, are ones that have no noticeable effect on the outward appearance and functionality of their bearers (i.e., they are "silent" or "neutral mutations", which can be, and are, used to trace the relatedness and age of populations and species.[16][110])
This argument implies that evolution can only occur if mutant mates cannot be avoided, as a result of a severe scarcity of potential mates. This is most likely to occur in small, isolated communities. These occur most commonly on small islands, in remote valleys, lakes, river systems, or caves,[111] or during the aftermath of a mass extinction.[110] Under these circumstances, not only is the choice of mates severely restricted but population bottlenecks, founder effects, genetic drift and inbreeding cause rapid, random changes in the isolated population's genetic composition.[111] Furthermore, hybridization with a related species trapped in the same isolate might introduce additional genetic changes. If an isolated population such as this survives its genetic upheavals, and subsequently expands into an unoccupied niche, or into a niche in which it has an advantage over its competitors, a new species, or subspecies, will have come into being. In geological terms, this will be an abrupt event. A resumption of avoiding mutant mates will thereafter result, once again, in evolutionary stagnation.[97][100]
In apparent confirmation of this punctuated equilibrium view of evolution, the fossil record of an evolutionary progression typically consists of species that suddenly appear, and ultimately disappear, hundreds of thousands or millions of years later, without any change in external appearance.[97][110][112] Graphically, these fossil species are represented by lines parallel with the time axis, whose lengths depict how long each of them existed. The fact that the lines remain parallel with the time axis illustrates the unchanging appearance of each of the fossil species depicted on the graph. During each species' existence new species appear at random intervals, each also lasting many hundreds of thousands of years before disappearing without a change in appearance. The exact relatedness of these concurrent species is generally impossible to determine. This is illustrated in the diagram depicting the distribution of hominin species through time since the hominins separated from the line that led to the evolution of their closest living primate relatives, the chimpanzees.[112]
For similar evolutionary time lines see, for instance, the paleontological list of African dinosaurs, Asian dinosaurs, the Lampriformes and Amiiformes. [citation needed]
See also
[edit]References
[edit]- ^ Berlocher 1998, p. 3
- ^ Cook, Orator F. (March 30, 1906). "Factors of species-formation". Science. 23 (587): 506–507. Bibcode:1906Sci....23..506C. doi:10.1126/science.23.587.506. PMID 17789700.
- ^ Cook, Orator F. (November 1908). "Evolution Without Isolation". The American Naturalist. 42 (503): 727–731. Bibcode:1908ANat...42..727C. doi:10.1086/279001. S2CID 84565616.
- ^ Via, Sara (June 16, 2009). "Natural selection in action during speciation". PNAS. 106 (Suppl 1): 9939–9946. Bibcode:2009PNAS..106.9939V. doi:10.1073/pnas.0901397106. PMC 2702801. PMID 19528641.
- ^ Schneider, Christopher J. (31 October 2000). "Natural selection and speciation". Proceedings of the National Academy of Sciences. 97 (23): 12398–12399. Bibcode:2000PNAS...9712398S. doi:10.1073/pnas.240463297. PMC 34057. PMID 11058173.
- ^ Mayr 1982, p. 273
- ^ a b c d Darwin 1859
- ^ Sepkoski, David (2012). "1. Darwin's Dilemma: Paleontology, the Fossil Record, and Evolutionary Theory". Rereading the Fossil Record: The Growth of Paleobiology as an Evolutionary Discipline. University of Chicago Press. pp. 9–50. ISBN 978-0-226-74858-0.
One of his greatest anxieties was that the "incompleteness" of the fossil record would be used to criticize his theory: that the apparent "gaps" in fossil succession could be cited as negative evidence, at the very least, for his proposal that all organisms have descended by minute and gradual modifications from a common ancestor.
- ^ Stower, Hannah (2013). "Resolving Darwin's Dilemma". Nature Reviews Genetics. 14 (747): 747. doi:10.1038/nrg3614. S2CID 45302603.
The near-simultaneous appearance of most modern animal body plans in the Cambrian explosion suggests a brief interval of rapid phenotypic and genetic evolution, which Darwin believed were too fast to be explained by natural selection.
- ^ a b c d Bernstein, Harris; Byerly, Henry C.; Hopf, Frederic A.; et al. (December 21, 1985). "Sex and the emergence of species". Journal of Theoretical Biology. 117 (4): 665–690. Bibcode:1985JThBi.117..665B. doi:10.1016/S0022-5193(85)80246-0. PMID 4094459.
- ^ a b Hopf, Frederic A.; Hopf, F. W. (February 1985). "The role of the Allee effect in species packing". Theoretical Population Biology. 27 (1): 27–50. Bibcode:1985TPBio..27...27H. doi:10.1016/0040-5809(85)90014-0.
- ^ Bernstein & Bernstein 1991
- ^ a b Michod 1995
- ^ Michod 1999
- ^ Hockey, Dean & Ryan 2005, pp. 176, 193
- ^ a b c d e f Koeslag, Johan H. (May 10, 1990). "Koinophilia groups sexual creatures into species, promotes stasis, and stabilizes social behaviour". Journal of Theoretical Biology. 144 (1): 15–35. Bibcode:1990JThBi.144...15K. doi:10.1016/s0022-5193(05)80297-8. ISSN 0022-5193. PMID 2200930.
- ^ a b c d e f Koeslag, Johan H. (December 21, 1995). "On the Engine of Speciation". Journal of Theoretical Biology. 177 (4): 401–409. Bibcode:1995JThBi.177..401K. doi:10.1006/jtbi.1995.0256. ISSN 0022-5193.
- ^ a b c Poelstra, Jelmer W.; Vijay, Nagarjun; Bossu, Christen M.; et al. (June 20, 2014). "The genomic landscape underlying phenotypic integrity in the face of gene flow in crows". Science. 344 (6190): 1410–1414. Bibcode:2014Sci...344.1410P. doi:10.1126/science.1253226. PMID 24948738. S2CID 14431499.
The Phenotypic Differences between Carrion and Hooded Crows across the Hybridization Zone in Europe are Unlikely to be due to Assortative Mating.
— Commentary by Mazhuvancherry K. Unnikrishnan and H. S. Akhila - ^ a b Ridley, Mark. "Speciation - What is the role of reinforcement in speciation?". Retrieved 2015-09-07. Adapted from Evolution (2004), 3rd edition (Malden, MA: Blackwell Publishing), ISBN 978-1-4051-0345-9.
- ^ a b c Maynard Smith 1989, pp. 275–280
- ^ Mayr 1988
- ^ Williams 1992, p. 118
- ^ Maynard Smith, John (December 1983). "The Genetics of Stasis and Punctuation" (PDF). Annual Review of Genetics. 17: 11–25. doi:10.1146/annurev.ge.17.120183.000303. PMID 6364957. S2CID 3901837. Archived from the original (PDF) on 2019-03-05.
- ^ Clapham, Tutin & Warburg 1952
- ^ Grant 1971
- ^ Baker, Jason M. (June 2005). "Adaptive speciation: The role of natural selection in mechanisms of geographic and non-geographic speciation" (PDF). Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 36 (2): 303–326. doi:10.1016/j.shpsc.2005.03.005. PMID 19260194. S2CID 3555049.
- ^ Kingsley, David M. (January 2009). "Diversity Revealed: From Atoms to Traits". Scientific American. 300 (1): 52–59. doi:10.1038/scientificamerican0109-52. PMID 19186749.
- ^ Sulloway, Frank J. (September 30, 1982). "The Beagle collections of Darwin's finches (Geospizinae)". Bulletin of the British Museum (Natural History), Zoology. 43 (2): 49–58.
- ^ Coyne & Orr 2004, p. 105.
- ^ Lawson, Lucinda P.; Bates, John M.; Menegon, Michele; Loader, Simon P. (2015). "Divergence at the edges: peripatric isolation in the montane spiny throated reed frog complex". BMC Evolutionary Biology. 15 (128): 128. Bibcode:2015BMCEE..15..128L. doi:10.1186/s12862-015-0384-3. PMC 4487588. PMID 26126573.
- ^ Mayr 1992, pp. 21–53
- ^ Tokeshi, M. (1999). Species coexistence: ecological and evolutionary perspectives. Oxford: Blackwell Science. ISBN 0-632-06146-4. OCLC 47011551.
- ^ "Speciation: The Origin of New Species | Learn Science at Scitable". Nature. Retrieved 2020-02-16.
- ^ Endler 1977
- ^ Tarkhnishvili, David; Murtskhvaladze, Marine; Gavashelishvili, Alexander (August 2013). "Speciation in Caucasian lizards: climatic dissimilarity of the habitats is more important than isolation time". Biological Journal of the Linnean Society. 109 (4): 876–892. doi:10.1111/bij.12092.
- ^ Liebers, Dorit; Knijff, Peter de; Helbig, Andreas J. (2004). "The herring gull complex is not a ring species". Proc Biol Sci. 271 (1542): 893–901. doi:10.1098/rspb.2004.2679. PMC 1691675. PMID 15255043.
- ^ "Parapatric speciation". University of California Berkeley. Retrieved 3 April 2017.
- ^ Feder, Jeffrey L.; Xianfa Xie; Rull, Juan; et al. (May 3, 2005). "Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis". PNAS. 102 (Suppl 1): 6573–6580. Bibcode:2005PNAS..102.6573F. doi:10.1073/pnas.0502099102. PMC 1131876. PMID 15851672.
- ^ Berlocher, Stewart H.; Feder, Jeffrey L. (January 2002). "Sympatric Speciation in Phytophagous Insects: Moving Beyond Controversy?". Annual Review of Entomology. 47: 773–815. doi:10.1146/annurev.ento.47.091201.145312. PMID 11729091. S2CID 9677456.
- ^ Machado, Heather E.; Pollen, Alexander A.; Hofmann, Hans A.; et al. (December 2009). "Interspecific profiling of gene expression informed by comparative genomic hybridization: A review and a novel approach in African cichlid fishes". Integrative and Comparative Biology. 49 (6): 644–659. doi:10.1093/icb/icp080. PMID 21665847.
- ^ Fan, Shaohua; Elmer, Kathryn R.; Meyer, Axel (February 5, 2012). "Genomics of adaptation and speciation in cichlid fishes: recent advances and analyses in African and Neotropical lineages". Philosophical Transactions of the Royal Society B. 367 (1587): 385–394. doi:10.1098/rstb.2011.0247. PMC 3233715. PMID 22201168.
- ^ Niemiller, Matthew L.; Fitzpatrick, Benjamin M.; Miller, Brian T. (May 2008). "Recent divergence with gene flow in Tennessee cave salamanders (Plethodontidae: Gyrinophilus) inferred from gene genealogies". Molecular Ecology. 17 (9): 2258–2275. Bibcode:2008MolEc..17.2258N. doi:10.1111/j.1365-294X.2008.03750.x. PMID 18410292. S2CID 20761880.
- ^ Martens, Koen (May 1997). "Speciation in ancient lakes". Trends in Ecology & Evolution. 12 (5): 177–182. Bibcode:1997TEcoE..12..177M. doi:10.1016/S0169-5347(97)01039-2. PMID 21238028.
- ^ Joly, E. (9 December 2011). "The existence of species rests on a metastable equilibrium between inbreeding and outbreeding. An essay on the close relationship between speciation, inbreeding and recessive mutations". Biology Direct. 6: 62. doi:10.1186/1745-6150-6-62. PMC 3275546. PMID 22152499.
- ^ Feder, Jeffrey L.; Roethele, Joseph B.; Filchak, Kenneth; et al. (March 2003). "Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly, Rhagoletis pomonella". Genetics. 163 (3): 939–953. doi:10.1093/genetics/163.3.939. PMC 1462491. PMID 12663534. Retrieved 2015-09-07.
- ^ Berlocher, Stewart H.; Bush, Guy L. (June 1982). "An electrophoretic analysis of Rhagoletis (Diptera: Tephritidae) phylogeny". Systematic Zoology. 31 (2): 136–155. doi:10.2307/2413033. JSTOR 2413033.
- ^ Sætre, Glenn-Peter (2012). "Reinforcement". eLS. doi:10.1002/9780470015902.a0001754.pub3. ISBN 978-0-470-01617-6.
- ^ Ollerton, Jeff (September 2005). "Speciation: Flowering time and the Wallace Effect" (PDF). Heredity. 95 (3): 181–182. Bibcode:2005Hered..95..181O. doi:10.1038/sj.hdy.6800718. PMID 16077739. S2CID 13300641. Archived from the original (PDF) on 2007-06-05. Retrieved 2015-09-07.
- ^ a b Orr, H. A. (December 1996). "Dobzhansky, Bateson, and the Genetics of Speciation". Genetics. 144 (4): 1331–1335. doi:10.1093/genetics/144.4.1331. ISSN 0016-6731. PMC 1207686. PMID 8978022.
- ^ a b Presgraves, Daven C. (December 2010). "Darwin and the Origin of Interspecific Genetic Incompatibilities". The American Naturalist. 176 (S1): S45 – S60. Bibcode:2010ANat..176S..45P. doi:10.1086/657058. ISSN 0003-0147. PMID 21043780. S2CID 5592958.
- ^ Kulmuni, J.; Westram, A. M. (2017). "Intrinsic incompatibilities evolving as a by-product of divergent ecological selection: Considering them in empirical studies on divergence with gene flow". Molecular Ecology. 26 (12): 3093–3103. Bibcode:2017MolEc..26.3093K. doi:10.1111/mec.14147. ISSN 1365-294X. PMID 28423210. S2CID 41904934.
- ^ Orr, H A (1995-04-01). "The population genetics of speciation: the evolution of hybrid incompatibilities". Genetics. 139 (4): 1805–1813. doi:10.1093/genetics/139.4.1805. ISSN 1943-2631. PMC 1206504. PMID 7789779.
- ^ Howard D. Rundle and Patrik Nosil (2005), "Ecological speciation", Ecology Letters, 8 (3): 336–352, Bibcode:2005EcolL...8..336R, doi:10.1111/j.1461-0248.2004.00715.x
- ^ Dolph Schluter (2001), "Ecology and the origin of species", Trends in Ecology and Evolution, 16 (7): 372–380, doi:10.1016/S0169-5347(01)02198-X, PMID 11403870, S2CID 9845298
- ^ Jeffrey S. McKinnon; et al. (2004), "Evidence for ecology's role in speciation", Nature, 429 (6989): 294–298, Bibcode:2004Natur.429..294M, doi:10.1038/nature02556, PMID 15152252, S2CID 2744267
- ^ a b Dolph Schluter (2009), "Evidence for Ecological Speciation and Its Alternative", Science, 326 (5915): 737–740, Bibcode:2009Sci...323..737S, doi:10.1126/science.1160006, PMID 19197053, S2CID 307207
- ^ a b Panhuis, Tami M.; Butlin, Roger; Zuk, Marlene; et al. (July 2001). "Sexual selection and speciation" (PDF). Trends in Ecology & Evolution. 16 (7): 364–371. doi:10.1016/s0169-5347(01)02160-7. PMID 11403869.
- ^ Darwin, Charles; A. R. Wallace (1858). "On the Tendency of Species to form Varieties; and on the Perpetuation of Varieties and Species by Natural Means of Selection" (PDF). Journal of the Proceedings of the Linnean Society of London. Zoology. 3 (9): 46–50. doi:10.1111/j.1096-3642.1858.tb02500.x.
- ^ Darwin 1859, p. 89, "IV. Natural Selection".
- ^ Eberhard, W. G. (1985). Sexual Selection and Animal Genitalia. Harvard University Press, Cambridge, Massachusetts
- ^ Gould 1980, pp. 204–213, "A Quahog is a Quahog".
- ^ a b Miller 2013, pp. 177, 395–396
- ^ Rundell, Rebecca J.; Price, Trevor D. (2009-07-01). "Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation". Trends in Ecology & Evolution. 24 (7): 394–399. Bibcode:2009TEcoE..24..394R. doi:10.1016/j.tree.2009.02.007. ISSN 0169-5347. PMID 19409647.
- ^ Czekanski-Moir, Jesse E.; Rundell, Rebecca J. (2019-05-01). "The Ecology of Nonecological Speciation and Nonadaptive Radiations". Trends in Ecology & Evolution. 34 (5): 400–415. Bibcode:2019TEcoE..34..400C. doi:10.1016/j.tree.2019.01.012. ISSN 0169-5347. PMID 30824193. S2CID 73494468.
- ^ Nowak 1999
- ^ Hiendleder, Stefan; Kaupe, Bernhard; Wassmuth, Rudolf; et al. (May 7, 2002). "Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies". Proceedings of the Royal Society B. 269 (1494): 893–904. doi:10.1098/rspb.2002.1975. PMC 1690972. PMID 12028771.
- ^ Rice, William R.; Salt, George W. (June 1988). "Speciation Via Disruptive Selection on Habitat Preference: Experimental Evidence". The American Naturalist. 131 (6): 911–917. Bibcode:1988ANat..131..911R. doi:10.1086/284831. S2CID 84876223.
- ^ Rice, William R.; Hostert, Ellen E. (December 1993). "Laboratory Experiments on Speciation: What Have We Learned in 40 Years?". Evolution. 47 (6): 1637–1653. doi:10.2307/2410209. JSTOR 2410209. PMID 28568007.
- ^ Gavrilets, Sergey (October 2003). "Perspective: Models of Speciation: What Have We Learned in 40 Years?". Evolution. 57 (10): 2197–2215. doi:10.1554/02-727. PMID 14628909. S2CID 198158082.
- ^ Dodd, Diane M. B. (September 1989). "Reproductive Isolation as a Consequence of Adaptive Divergence in Drosophila pseudoobscura". Evolution. 43 (6): 1308–1311. doi:10.2307/2409365. JSTOR 2409365. PMID 28564510.
- ^ Kirkpatrick, Mark; Ravigné, Virginie (March 2002). "Speciation by Natural and Sexual Selection: Models and Experiments". The American Naturalist. 159 (S3): S22 – S35. Bibcode:2002ANat..159S..22K. doi:10.1086/338370. ISSN 0003-0147. PMID 18707367. S2CID 16516804.
- ^ Koukou, Katerina; Pavlikaki, Haris; Kilias, George; et al. (January 2006). "Influence of Antibiotic Treatment and Wolbachia Curing on Sexual Isolation Among Drosophila melanogaster Cage Populations". Evolution. 60 (1): 87–96. doi:10.1554/05-374.1. PMID 16568634. S2CID 198153238.
- ^ Symons 1979
- ^ a b Langlois, Judith H.; Roggman, Lori A. (March 1990). "Attractive Faces Are Only Average". Psychological Science. 1 (2): 115–121. doi:10.1111/j.1467-9280.1990.tb00079.x. S2CID 18557871.
- ^ Darwin 1859.
- ^ Dobzhansky, Theodosius (1936). "Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids". Genetics. 21 (2): 113–135. doi:10.1093/genetics/21.2.113. PMC 1208664. PMID 17246786.
- ^ Orr, H. Allen (1995). "The population genetics of speciation: the evolution of hybrid incompatibilities". Genetics. 139 (4): 1805–1813. doi:10.1093/genetics/139.4.1805. PMC 1206504. PMID 7789779.
- ^ Barton, N. H.; Bengtsson, B. O. (1986). "The barrier to genetic exchange between hybridising populations". Heredity. 56 (3): 357–376. Bibcode:1986Hered..57..357B. doi:10.1038/hdy.1986.135.
- ^ Wu, Chung-I. (2001). "The genic view of the process of speciation". Journal of Evolutionary Biology. 14 (6): 851–865. doi:10.1046/j.1420-9101.2001.00335.x.
- ^ Coyne & Orr 2004.
- ^ Fraïsse, C.; Elderfield, J. A. D.; Welch, J. J. (2014). "The genetics of speciation: are complex incompatibilities easier to evolve?". Journal of Evolutionary Biology. 27 (4): 688–699. doi:10.1111/jeb.12339. PMID 24581268.
- ^ Orr, H. Allen (1995). "The population genetics of speciation: the evolution of hybrid incompatibilities". Genetics. 139 (4): 1805–1813. doi:10.1093/genetics/139.4.1805. PMC 1206504. PMID 7789779.
- ^ Barton, N. H.; Bengtsson, B. O. (1986). "The barrier to genetic exchange between hybridising populations". Heredity. 56 (3): 357–376. Bibcode:1986Hered..57..357B. doi:10.1038/hdy.1986.135.
- ^ Monnet, F.; Postel, Z.; Touzet, P.; Fraïsse, C.; Van de Peer, Y.; Vekemans, X.; Roux, C. (2025). "Rapid establishment of species barriers in plants compared with that in animals". Science. 389 (6765): 1147–1150. Bibcode:2025Sci...389.1147M. doi:10.1126/science.adl2356. PMID 40934327.
- ^ Phadnis, Nitin; Orr, H. Allen (January 16, 2009). "A Single Gene Causes Both Male Sterility and Segregation Distortion in Drosophila Hybrids". Science. 323 (5912): 376–379. Bibcode:2009Sci...323..376P. doi:10.1126/science.1163934. PMC 2628965. PMID 19074311.
- ^ Ramsey, Justin; Schemske, Douglas W. (November 1998). "Pathways, Mechanisms, and Rates of Polyploid Formation in Flowering Plants" (PDF). Annual Review of Ecology and Systematics. 29 (1): 467–501. Bibcode:1998AnRES..29..467R. doi:10.1146/annurev.ecolsys.29.1.467. S2CID 31637733. Archived from the original (PDF) on 2020-06-08.
- ^ Otto, Sarah P.; Whitton, Jeannette (December 2000). "Polyploid Incidence and Evolution" (PDF). Annual Review of Genetics. 34: 401–437. CiteSeerX 10.1.1.323.1059. doi:10.1146/annurev.genet.34.1.401. PMID 11092833.
- ^ a b Comai, Luca (November 2005). "The advantages and disadvantages of being polyploid". Nature Reviews Genetics. 6 (11): 836–846. doi:10.1038/nrg1711. PMID 16304599. S2CID 3329282.
- ^ Mezzasalma, Marcello; Brunelli, Elvira; Odierna, Gaetano; Guarino, Fabio Maria (2023-03-12). "Evolutionary and Genomic Diversity of True Polyploidy in Tetrapods". Animals. 13 (6): 1033. doi:10.3390/ani13061033. ISSN 2076-2615. PMC 10044425. PMID 36978574.
- ^ Wendel, Jonathan F. (January 2000). "Genome evolution in polyploids". Plant Molecular Biology. 42 (1): 225–249. Bibcode:2000PMolB..42..225W. doi:10.1023/A:1006392424384. PMID 10688139. S2CID 14856314.
- ^ Sémon, Marie; Wolfe, Kenneth H. (December 2007). "Consequences of genome duplication". Current Opinion in Genetics & Development. 17 (6): 505–512. doi:10.1016/j.gde.2007.09.007. PMID 18006297.
- ^ Soltis, Pamela S.; Soltis, Douglas E. (June 20, 2000). "The role of genetic and genomic attributes in the success of polyploids". PNAS. 97 (13): 7051–7057. Bibcode:2000PNAS...97.7051S. doi:10.1073/pnas.97.13.7051. PMC 34383. PMID 10860970.
- ^ Mavarez, Jesús; Salazar, Camilo A.; Bermingham, Eldredge; et al. (June 15, 2006). "Speciation by hybridization in Heliconius butterflies". Nature. 441 (7095): 868–871. Bibcode:2006Natur.441..868M. doi:10.1038/nature04738. PMID 16778888. S2CID 2457445.
- ^ Sherwood, Jonathan (September 8, 2006). "Genetic Surprise Confirms Neglected 70-Year-Old Evolutionary Theory" (Press release). University of Rochester. Retrieved 2015-09-10.
- ^ Masly, John P.; Jones, Corbin D.; Mohamed, A. F. Noor; et al. (September 8, 2006). "Gene Transposition as a Cause of Hybrid Sterility in Drosophila". Science. 313 (5792): 1448–1450. Bibcode:2006Sci...313.1448M. doi:10.1126/science.1128721. PMID 16960009. S2CID 23462115.
- ^ Minkel, J. R. (September 8, 2006). "Wandering Fly Gene Supports New Model of Speciation". Scientific American. Retrieved 2015-09-11.
- ^ a b c Gould, Stephen Jay; Eldredge, Niles (Spring 1977). "Punctuated equilibria: the tempo and mode of evolution reconsidered" (PDF). Paleobiology. 3 (2): 115–151. Bibcode:1977Pbio....3..115G. doi:10.1017/s0094837300005224. JSTOR 2400177. S2CID 83492071. Archived from the original (PDF) on 2014-06-24. Retrieved 2015-09-15.
- ^ Laws 2010, pp. 210–215
- ^ Williams 1992, chpt. 9
- ^ a b Eldredge & Gould 1972, chpt. 5
- ^ Mayr 1954, pp. 157–180
- ^ Maynard Smith 1989, p. 281
- ^ Gould 1980, pt. 4, chpt. 18
- ^ Williams 1974
- ^ Maynard Smith, John (March 14, 1964). "Group Selection and Kin Selection". Nature. 201 (4924): 1145–1147. Bibcode:1964Natur.201.1145S. doi:10.1038/2011145a0. S2CID 4177102.
- ^ Dawkins 1995, chpt. 4
- ^ Dawkins, Richard (December 1994). "Burying the Vehicle". Behavioral and Brain Sciences. 17 (4): 616–617. doi:10.1017/S0140525X00036207. ISSN 0140-525X. S2CID 143378724. Archived from the original on 2006-09-15. Retrieved 2015-09-15. "Remarks on an earlier article by [Elliot] Sober [sic] and David Sloan Wilson, who made a more extended argument in their recent book Unto Others : The Evolution and Psychology of Unselfish Behavior"
- ^ Dennett, Daniel C. (December 1994). "E Pluribus Unum?". Behavioral and Brain Sciences. 17 (4): 617–618. doi:10.1017/S0140525X00036219. S2CID 146359497. Archived from the original on 2007-12-27. "Commentary on Wilson & Sober: Group Selection."
- ^ Pinker, Steven (June 18, 2012). "The False Allure of Group Selection". edge.org. Edge Foundation, Inc. Retrieved 2015-09-15.
- ^ a b c Campbell 1990, pp. 450–451, 487–490, 499–501
- ^ a b Ayala 1982, pp. 73–83, 182–190, 198–215
- ^ a b McCarthy & Rubidge 2005
Bibliography
[edit]- Ayala, Francisco J. (1982). Population and Evolutionary Genetics. Benjamin/Cummings Series in the Life Sciences. Menlo Park, CA: Benjamin/Cummings Pub. Co. ISBN 978-0-8053-0315-5. LCCN 81021623. OCLC 8034790.
- Berlocher, Stewart H. (1998). "Origins: A Brief History of Research on Speciation". In Howard, Daniel J.; Berlocher, Stewart H. (eds.). Endless Forms: Species and Speciation. New York: Oxford University Press. ISBN 978-0-19-510901-6. LCCN 97031461. OCLC 37545522.
- Bernstein, Carol; Bernstein, Harris (1991). Aging, Sex, and DNA Repair. San Diego, CA: Academic Press. ISBN 978-0-12-092860-6. LCCN 90014467. OCLC 22542921.
- Campbell, Neil A. (1990). Biology (2nd ed.). Redwood City, CA: Benjamin/Cummings Pub. Co. ISBN 978-0-8053-1800-5. LCCN 89017952. OCLC 20352649.
- Clapham, Arthur Roy; Tutin, Thomas G.; Warburg, Edmund F. (1952). Flora of the British Isles. Cambridge, UK: Cambridge University Press. LCCN 52008880. OCLC 1084058.
- Coyne, Jerry A.; Orr, H. Allen (2004). Speciation. Sunderlands, MA: Sinauer Associates. ISBN 978-0-87893-089-0. LCCN 2004009505. OCLC 55078441.
- Darwin, Charles (1859). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life (1st ed.). London: John Murray. LCCN 06017473. OCLC 741260650. The book is available from John van Wyhe, ed. (2002), The Complete Work of Charles Darwin Online, retrieved 2015-09-12.
- Dawkins, Richard (1995). River Out of Eden: A Darwinian View of Life. Science Masters Series. New York: Basic Books. ISBN 978-0-465-01606-8. LCCN 94037146. OCLC 31376584.
- Eldredge, Niles; Gould, Stephen Jay (1972). "Punctuated Equilibria: An Alternative to Phyletic Gradualism". In Schopf, Thomas J. M. (ed.). Models in Paleobiology. San Francisco, CA: Freeman Cooper & Co. ISBN 978-0-87735-325-6. LCCN 72078387. OCLC 572084. Reprinted in Eldredge 1985, pp. 193–223
- Eldredge, Niles (1985). Time Frames: The Rethinking of Darwinian Evolution and the Theory of Punctuated Equilibria. New York: Simon & Schuster. ISBN 978-0-671-49555-8. LCCN 84023632. OCLC 11443805.
- Endler, John A. (1977). Geographic Variation, Speciation, and Clines. Monographs in Population Biology. Vol. 10. Princeton, NJ: Princeton University Press. pp. 1–246. ISBN 978-0-691-08187-8. LCCN 76045896. OCLC 2645720. PMID 409931.
- Gould, Stephen Jay (1980). The Panda's Thumb: More Reflections in Natural History (1st ed.). New York: W. W. Norton & Company. ISBN 978-0-393-01380-1. LCCN 80015952. OCLC 6331415. 1982 edition via Internet Archive.
- Grant, Verne (1971). Plant Speciation. New York: Columbia University Press. ISBN 978-0-231-03208-7. LCCN 75125620. OCLC 139834.
- Hockey, Phil A. R.; Dean, W. Richard J.; Ryan, Peter G., eds. (2005). Roberts Birds of Southern Africa (7th ed.). Cape Town, South Africa: Trustees of the J. Voelcker Bird Book Fund. ISBN 978-0-620-34053-3. LCCN 2006376728. OCLC 65978899.
- Laws, Bill (2010). Fifty Plants that Changed the Course of History. Buffalo, NY: Firefly Books. ISBN 978-1-55407-798-4. LCCN 2011414731. OCLC 711609823.
- Maynard Smith, John (1989). Evolutionary Genetics. Oxford; New York: Oxford University Press. ISBN 978-0-19-854215-5. LCCN 88017041. OCLC 18069049.
- Mayr, Ernst (1954). "Change of Genetic Environment and Evolution". In Huxley, Julian; Hardy, Alister C.; Ford, Edmund B. (eds.). Evolution as a Process. London: Allen & Unwin. LCCN 54001781. OCLC 974739.
- Mayr, Ernst (1982). The Growth of Biological Thought: Diversity, Evolution, and Inheritance. Cambridge, Massachusetts: Belknap Press of Harvard University Press. ISBN 978-0-674-36445-5. LCCN 81013204. OCLC 7875904.
- Mayr, Ernst (1988). Toward a New Philosophy of Biology: Observations of an Evolutionist. Cambridge, Massachusetts: Belknap Press of Harvard University Press. ISBN 978-0-674-89665-9. LCCN 87031892. OCLC 17108004.
- Mayr, Ernst (1992). "Speciational Evolution or Punctuated Equilibrium". In Somit, Albert; Peterson, Steven A. (eds.). Dynamics of Evolution: The Punctuated Equilibrium Debate in the Natural and Social Sciences. Ithaca, NY: Cornell University Press. ISBN 978-0-8014-9763-6. LCCN 91055569. OCLC 24374091.
- McCarthy, Terence; Rubidge, Bruce (2005). The Story of Earth & Life: A Southern African Perspective on a 4.6-Billion-Year Journey. Cape Town, South Africa: Struik Publishers. ISBN 978-1-77007-148-3. LCCN 2006376206. OCLC 62098231.
- Michod, Richard E. (1995). Eros and Evolution: A Natural Philosophy of Sex. Helix Books. Reading, MA: Addison-Wesley. ISBN 978-0-201-40754-9. LCCN 94013158. OCLC 30625193.
- Michod, Richard E. (1999). Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality. Princeton, NJ: Princeton University Press. ISBN 978-0-691-02699-2. LCCN 98004166. OCLC 38948118.
- Miller, William B. Jr. (2013). The Microcosm Within: Evolution and Extinction in the Hologenome. Boca Raton, FL: Universal-Publishers. ISBN 978-1-61233-277-2. LCCN 2013033832. OCLC 859168474.
- Nowak, Ronald M. (1999). Walker's Mammals of the World (6th ed.). Baltimore, MD: Johns Hopkins University Press. ISBN 978-0-8018-5789-8. LCCN 98023686. OCLC 39045218.
- Symons, Donald (1979). The Evolution of Human Sexuality. New York: Oxford University Press. ISBN 978-0-19-502535-4. LCCN 78023361. OCLC 4494283.
- Williams, George C. (1974) [Originally published 1966]. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought. Princeton Science Library. Princeton, NJ: Princeton University Press. ISBN 978-0-691-02357-1. LCCN 65017164. OCLC 8500898.
- Williams, George C. (1992). Natural Selection: Domains, Levels, and Challenges. Oxford Series in Ecology and Evolution. New York: Oxford University Press. ISBN 978-0-19-506933-4. LCCN 91038938. OCLC 228136567.
Further reading
[edit]- Gavrilets, S. (2004). Fitness Landscapes and the Origin of Species. Princeton University Press. ISBN 978-0-691-11983-0.
- Grant, Verne (1981). Plant Speciation (2nd ed.). New York: Columbia University Press. ISBN 978-0-231-05112-5. LCCN 81006159. OCLC 7552165.
- Marko, Peter B. (2008). "Allopatry". In Jørgensen, Sven Erik; Fath, Brian (eds.). Encyclopedia of Ecology. Vol. 1, A–C (1st ed.). Oxford, UK: Elsevier. pp. 131–138. ISBN 978-0-444-52033-3. LCCN 2008923435. OCLC 173240026.
- Mayr, Ernst (1963). Animal Species and Evolution. Cambridge, Massachusetts: Belknap Press of Harvard University Press. ISBN 978-0-674-03750-2. LCCN 63009552. OCLC 899044868.
{{cite book}}: ISBN / Date incompatibility (help) - Schilthuizen, Menno (2001). Frogs, Flies, and Dandelions: The Making of Species. Oxford; New York: Oxford University Press. ISBN 978-0-19-850393-4. LCCN 2001270180. OCLC 46729094.
- Shapiro, J. B.; Leducq, J-B.; Mallet, J. (2016). "What is Speciation?". PLOS Genetics. 12 (3) e1005860. doi:10.1371/journal.pgen.1005860. PMC 4816541. PMID 27030977.
- White, Michael J. D. (1978). Modes of Speciation. A Series of Books in Biology. San Francisco, CA: W. H. Freeman and Company. ISBN 978-0-7167-0284-9. LCCN 77010955. OCLC 3203453.
External links
[edit]- Boxhorn, Joseph (September 1, 1995). "Observed Instances of Speciation". TalkOrigins Archive. Houston, TX: The TalkOrigins Foundation, Inc.
- Hawks, John D. (February 9, 2005). "Speciation". John Hawks Weblog.
- "Speciation". University of California, Berkeley. 13 March 2021.
Speciation
View on GrokipediaConceptual Foundations
Definition and Process Overview
Speciation is the evolutionary process by which populations diverge to form distinct biological species, primarily through the development of reproductive barriers that prevent interbreeding and gene flow between them.[10] This lineage-splitting event results in two or more separately evolving groups, where accumulated genetic differences—driven by mechanisms such as natural selection, genetic drift, and mutation—render populations incompatible for successful reproduction.[11] The core outcome is reproductive isolation, which maintains the integrity of each emerging species against hybridization.[5] The process typically initiates when gene flow between populations is restricted, either by physical separation, ecological divergence, or behavioral changes, allowing independent evolutionary trajectories.[7] Divergence proceeds as populations adapt to different selective pressures or accumulate neutral variations, leading to prezygotic barriers (e.g., habitat isolation, temporal mismatches in breeding, or mate recognition failures) that prevent mating attempts, or postzygotic barriers (e.g., hybrid inviability or sterility) that reduce fitness of any offspring produced.[5] Over time, these barriers strengthen, solidifying species boundaries; empirical studies, such as those on Darwin's finches, demonstrate how morphological and genetic shifts in beak structure correlate with reduced hybrid viability, illustrating causal links between environmental adaptation and isolation.[12] In causal terms, speciation reflects the interplay of reduced gene flow and differential fitness landscapes, where barriers evolve as byproducts of local adaptations rather than direct selection for isolation in most cases, though reinforcement—selection against maladaptive hybrids—can accelerate the process in secondary contact zones.[7] This framework aligns with observations across taxa, from insects to vertebrates, where genomic analyses reveal speciation genes underlying isolation, such as those affecting gamete compatibility or developmental viability.[13] While the timeline varies—from rapid events in polyploid plants (occurring in a single generation) to gradual divergence over thousands of generations in animals—the endpoint is consistently the cessation of effective gene exchange, enabling independent evolution.[5]Species Concepts and Their Implications
The biological species concept, formalized by Ernst Mayr in 1942, defines a species as a group of actually or potentially interbreeding natural populations that are reproductively isolated from other such groups.[14] This concept emphasizes intrinsic barriers to gene flow, such as prezygotic (e.g., behavioral or temporal isolation) or postzygotic (e.g., hybrid inviability) mechanisms, positioning speciation as the evolution of reproductive isolation that prevents interbreeding between diverging populations.[5] Under this framework, speciation often requires geographic separation to allow genetic divergence without homogenizing gene flow, though sympatric isolation is theoretically possible if strong disruptive selection overcomes panmixia.[15] In contrast, the morphological species concept classifies organisms based on shared physical traits distinguishable from other groups, a method historically dominant in taxonomy due to its reliance on observable phenotypes without requiring breeding data.[15] Its limitations include vulnerability to convergent evolution, where unrelated lineages develop similar morphologies under analogous selective pressures (e.g., streamlined bodies in sharks and dolphins), potentially lumping distinct evolutionary lineages or splitting variable populations artifactually.[16] For speciation studies, this concept implies that phenotypic divergence signals species formation but fails to verify underlying genetic cohesion or isolation, often overemphasizing stasis over dynamic evolutionary processes. The phylogenetic species concept identifies species as the smallest monophyletic clusters of organisms sharing a common ancestor and diagnosably distinct from others via fixed heritable traits, often assessed through molecular markers or cladistic analysis.[17] Unlike the biological concept, it applies to asexual reproducers, fossils, and allopatric populations where breeding tests are infeasible, revealing cryptic species hidden by morphological similarity; however, it risks proliferating species counts by treating minor diagnosable variants as separate, potentially inflating perceived speciation rates without evidence of ecological or reproductive independence.[18] Implications for speciation include a focus on lineage splitting via historical contingency and diagnosable divergence, facilitating integration with phylogenetic trees to trace branching events but challenging causal inference about barriers to gene flow.| Species Concept | Core Definition | Key Proponent(s) | Strengths for Speciation Analysis | Limitations and Implications |
|---|---|---|---|---|
| Biological | Interbreeding populations reproductively isolated from others | Ernst Mayr (1942) | Directly links speciation to evolution of isolation mechanisms; predicts testable outcomes like hybrid sterility | Inapplicable to asexuals or fossils; assumes isolation equates to adaptive divergence, overlooking ecological convergence without gene flow barriers[14][5] |
| Morphological | Groups sharing distinctive physical traits | Historical taxonomy (pre-20th century) | Practical for field identification and fossil records; highlights phenotypic signals of divergence | Prone to errors from phenotypic plasticity or mimicry; underestimates speciation in cryptic taxa, implying over-reliance on observable traits biases toward gradualism over saltational change[15][16] |
| Phylogenetic | Smallest diagnosable monophyletic cluster | Joel Cracraft (1980s onward) | Reveals hidden diversity via ancestry; aligns with cladogenesis models of speciation | May fragment continua into excessive units; emphasizes historical diagnosis over causal processes like selection or drift driving splits[17][18] |
| Ecological | Lineages occupying distinct adaptive zones or niches with minimal competitive overlap | Leigh van Valen (1976) | Ties speciation to niche partitioning and resource use; explains parapatric or sympatric divergence via disruptive selection | Vague on "minimal difference" thresholds; risks circularity if niches are defined post-hoc, implying speciation as ecological opportunism rather than isolation per se[19][20] |
Historical Development
Pre-Darwinian Perspectives
Prior to the mid-19th century, the predominant perspective in Western natural philosophy held that species were fixed and immutable entities, originating through separate acts of divine creation or as eternal archetypes inherent to the natural order. Ancient thinkers such as Plato (c. 428–348 BCE) conceived species as manifestations of unchanging ideal forms crafted by a divine intelligence, while Aristotle (384–322 BCE) described them as eternal kinds within a teleological hierarchy, reproducing true-to-type without transformation into novel forms.[23] This fixity was reinforced in medieval scholasticism through integration with Christian theology, viewing species as distinct "kinds" established at creation and preserved across generations, with variations dismissed as minor deviations insufficient to generate new species.[24] In the 17th and 18th centuries, systematists advanced classification schemes that presupposed species stability. John Ray (1627–1705) defined species in his Historia Plantarum (1686) as lineages descending from a common seed stock, where reproductive limits confined variations to within-type changes, explicitly rejecting origins from distinct parental stocks.[15] Carl Linnaeus (1707–1778) formalized this in Systema Naturae (1735) and Species Plantarum (1753), employing typological criteria—such as reproductive morphology—to delineate fixed species boundaries, attributing their origins to divine fiat rather than natural processes of divergence.[23] Georges Cuvier (1769–1832) further supported fixity through catastrophism, positing that mass extinctions necessitated successive divine recreations of species assemblages, with no mechanism for gradual emergence of new forms from ancestral ones.[23] Emerging challenges to strict fixity appeared in limited transformist hypotheses, though these did not articulate speciation as branching diversification. Georges-Louis Leclerc, Comte de Buffon (1707–1788), in Histoire Naturelle (1749–1788), suggested environmental degeneration could alter forms but stopped short of endorsing transmutation into reproductively isolated lineages.[23] Jean-Baptiste Lamarck (1744–1829) proposed a more systematic transformism in Philosophie Zoologique (1809), arguing that species progressively adapted via inheritance of acquired traits driven by use or disuse of organs in response to environmental needs, with simpler forms arising spontaneously; however, this envisioned linear chains of transformation rather than isolated speciation events, and it faced rejection for lacking empirical support on heritability.[23] Such ideas remained marginal, as the era's empirical focus on morphology and distribution upheld species as discrete, non-originating units without causal pathways for novel reproductive barriers.[24]Darwinian Foundations and Early Challenges
Charles Darwin's On the Origin of Species by Means of Natural Selection, published on November 24, 1859, established the foundational framework for understanding speciation as a process of descent with modification from common ancestors, driven by natural selection. Darwin posited that populations diverge over time as advantageous variations accumulate in response to differing environmental pressures, eventually leading to the formation of new species incapable of interbreeding with ancestral forms. While he avoided a rigid definition of species—describing them instead as clusters of varieties that maintain distinctions under natural conditions—Darwin emphasized that speciation occurs through gradual divergence, often facilitated by geographic isolation, which reduces gene flow and allows isolated populations to adapt independently.[25][26] Illustrative examples drawn from Darwin's observations bolstered his arguments. During his voyage on the HMS Beagle, which visited the Galápagos Islands in 1835, Darwin collected specimens of finches that exhibited variations in beak morphology suited to exploiting different food sources, such as seeds, insects, and cacti. Although Darwin initially underestimated their significance, ornithologist John Gould's post-voyage classification in 1837–1841 revealed these as distinct species derived from a common South American ancestor, exemplifying adaptive radiation—a burst of speciation following colonization of new habitats. In Origin, Darwin extended such observations to domesticated pigeons, demonstrating artificial selection's capacity to produce varieties approaching species-level differences, analogizing this to natural processes that multiply lineages over geological time.[27][28] Early scientific challenges to Darwin's speciation mechanism arose primarily from uncertainties in inheritance and reproductive barriers. Darwin's adherence to blending inheritance—where offspring traits average parental ones—implied that novel variations would dilute rapidly in populations, hindering the sustained divergence required for speciation; mathematician Fleeming Jenkin articulated this critique in 1867, arguing a beneficial mutation in one individual would blend out without isolation. Critics also noted Darwin's relative neglect of reproductive isolation as a prerequisite, with adaptation alone insufficient to prevent gene swamping via interbreeding in continuous populations. Additionally, the fossil record's paucity of transitional forms, which Darwin attributed to its imperfection and the rarity of preservable intermediates, fueled skepticism, as expected gradual transitions were scarce despite extensive 19th-century paleontological efforts. These issues contributed to the "eclipse of Darwinism" by the 1890s, when natural selection's role in speciation was questioned in favor of alternatives like orthogenesis, though empirical support for Darwin's core divergence principle persisted among naturalists.[29][30]Integration into Modern Evolutionary Synthesis
The Modern Evolutionary Synthesis, formalized between the 1930s and 1950s, reconciled Charles Darwin's theory of natural selection with Gregor Mendel's principles of inheritance through advances in population genetics, providing a mechanistic framework for speciation as the accumulation of genetic differences leading to reproductive isolation.[31] Key contributors, including Ronald Fisher, J.B.S. Haldane, and Sewall Wright, developed mathematical models demonstrating how allele frequencies shift under mutation, selection, genetic drift, and gene flow, with speciation arising when gene flow between populations is sufficiently restricted to permit divergent evolution.[32] In this synthesis, speciation was not viewed as a saltational process but as an extension of microevolutionary changes, where barriers to gene flow—geographic, behavioral, or genetic—allow drift and selection to fix isolating mechanisms without requiring adaptive peaks for every divergence.[33] Theodosius Dobzhansky's 1937 book Genetics and the Origin of Species marked a foundational integration by applying Drosophila experiments to show how chromosomal inversions and genic mutations disrupt meiosis in hybrids, establishing reproductive isolation as a genetic outcome of population divergence.[34] Dobzhansky emphasized isolating mechanisms, such as hybrid sterility and inviability, as evolved barriers that maintain species integrity, bridging Mendelian genetics with Darwinian gradualism by quantifying how small genetic changes accumulate under local selection or drift.[35] His work highlighted that speciation often involves polygenic traits and epistatic interactions, where gene flow's cessation enables fixation of incompatible alleles, as evidenced by laboratory crosses revealing Dobzhansky-Muller incompatibilities.[36] Ernst Mayr's 1942 Systematics and the Origin of Species further embedded speciation within the synthesis by advocating the biological species concept—groups of interbreeding populations reproductively isolated from others—and prioritizing allopatric speciation, where geographic separation halts gene flow, fostering divergence via founder effects or local adaptation.[37] Mayr argued that peripheral isolates undergo rapid genetic revolution due to drift in small populations, contrasting with central stability, and integrated field observations from birds and insects to support that most speciation events require spatial isolation to overcome homogenizing gene flow.[38] This synthesis clarified speciation's causal realism: not merely morphological divergence but the evolution of barriers preventing gene exchange, with empirical support from ring species and island radiations demonstrating stepwise isolation.[5]Primary Modes of Speciation
Allopatric and Peripatric Speciation
Allopatric speciation occurs when populations of a species become geographically isolated by extrinsic barriers such as mountains, rivers, or oceans, preventing gene flow and allowing independent evolution through genetic drift, mutation, and natural selection until reproductive isolation develops.[39] This mode requires physical separation that splits a continuous population into distinct groups, leading to divergence over time, often measured in thousands to millions of years depending on generation time and selective pressures.[40] Classic examples include the diversification of Darwin's finches on the Galápagos Islands, where ancestral populations colonized isolated islands, resulting in 18 species with distinct beak morphologies adapted to local food sources; genetic analyses indicate divergence from a South American ancestor approximately 2 to 2.5 million years ago.[6][41] Peripatric speciation represents a specific variant of allopatric speciation, characterized by the isolation of a small peripheral population from the main continental or central group, often via founder events where few individuals establish a new colony at the range's edge.[42] This process, first conceptualized by Ernst Mayr in 1954, emphasizes the founder effect, whereby the limited genetic diversity in the small founding group triggers rapid genetic restructuring, inbreeding, and shifts in developmental or mating systems, potentially accelerating speciation compared to vicariant allopatry involving larger populations.[43] Unlike standard allopatric speciation, where both isolated groups may be sizable and diverge gradually, peripatric events rely on the periphery for innovation, with the small isolate facing novel environments that amplify divergence through strong selection or drift.[44] Empirical support for allopatric and peripatric mechanisms draws from island biogeography and phylogenetic reconstructions; for instance, secondary contact zones in Darwin's finches on islands like Daphne Major demonstrate persistent reproductive isolation post-divergence, with genetic markers confirming allopatric origins despite occasional hybridization.[45] In peripatric cases, theoretical models validate the plausibility of founder-induced speciation under certain parameters, though empirical examples remain debated due to challenges in distinguishing from other modes; Hawaiian Drosophila species flocks illustrate rapid radiation from peripheral colonizations, aligning with Mayr's predictions of genetic revolutions in isolates.[46] Overall, these modes underscore geographic isolation as a primary driver of biodiversity, with barriers reducing gene flow to enable causal accumulation of incompatibilities.[47]Parapatric and Sympatric Speciation
Parapatric speciation involves the divergence of populations inhabiting contiguous but distinct habitats, where gene flow occurs primarily across a narrow contact zone but is limited by distance and local adaptation to environmental gradients.[48] Divergence proceeds through strong divergent selection along ecological clines, such as soil type or altitude, which generates barriers to gene flow despite spatial adjacency, though complete reproductive isolation requires additional mechanisms like reinforcement to counter residual hybridization.[49] Empirical examples remain debated due to challenges in distinguishing parapatry from cryptic allopatry or ongoing gene flow, with proposed cases including alpine shrubs like Rosa sericea and Rosa omeiensis, where population genetic analyses reveal boundaries correlating with habitat transitions.[50] Theoretical models indicate that parapatric speciation demands exceptionally steep selection gradients to overcome gene swamping, limiting its prevalence compared to allopatric modes.[51] Sympatric speciation arises within a single, overlapping geographic range without physical barriers, necessitating mechanisms that promote assortative mating and disrupt panmixia, such as host shifts, polyploidy, or ecological niche partitioning coupled with sexual selection.[52] In animals, it often involves temporal or behavioral isolation; for instance, the apple maggot fly Rhagoletis pomonella shifted from native hawthorn to introduced apple hosts around 1864, leading to host-specific races with divergent host preference, phenology, and reduced gene flow via assortative mating, supported by genetic markers showing FST values up to 0.2 between races.[53] [54] Similarly, in Lake Victoria cichlids of the Pundamilia genus, sympatric sister species diverge on male nuptial coloration (red vs. blue) linked to depth-related visual adaptation and female mate choice, with genomic studies identifying pleiotropic loci under selection and hybridization rates below 1% in clear water.[55] [56] Recent genomic evidence confirms sympatric origins in these systems but highlights ongoing debates over whether micro-scale spatial structuring (micro-parapatry) contributes, as pure panmixia proves rare without secondary contact.[57] Plant examples, like Howea palms on Lord Howe Island, demonstrate sympatric divergence via soil preference and flowering time shifts, with divergence times estimated at 2 million years ago via coalescent analyses.[58] Overall, sympatric speciation's feasibility hinges on high disruptive selection strength, empirically validated in fewer than 10 robust animal cases as of 2023.[59]Genetic and Molecular Underpinnings
Role of Genetic Drift, Mutations, and Gene Flow Barriers
Mutations serve as the ultimate source of novel genetic variation underlying speciation, providing alleles that can disrupt reproductive compatibility when fixed in diverging populations. In mutation-order speciation, populations adapting to similar selective pressures independently accumulate incompatible mutations at different loci, leading to post-zygotic isolation without requiring divergent ecological niches.[60] This process contrasts with ecological speciation, where mutations align with habitat-specific adaptations, but underscores mutations' role in generating the raw material for barriers regardless of selection's direction. Empirical studies, such as those on Drosophila and plants, demonstrate that single-locus mutations rarely suffice for complete isolation due to their deleterious effects in heterozygotes, necessitating polygenic accumulation over time.[61] Genetic drift amplifies the fixation of such mutations in small or isolated populations, where random allele frequency changes dominate over selection, promoting divergence through mechanisms like founder effects and bottlenecks. In peripatric speciation, peripheral isolates experience intensified drift, rapidly shifting genotypic compositions and reducing hybrid fitness via "system drift," where coordinated gene networks evolve incompatibly across populations.[62] Theoretical models indicate drift accelerates speciation rates in low-recombination genomic regions by hindering the restoration of favorable allele combinations, while empirical genomic analyses post-whole-genome duplication reveal drift-driven regulatory evolution dominating over selection in paralog divergence.[63] Although drift alone may not drive adaptive divergence, it synergizes with mutations to establish intrinsic incompatibilities, particularly in stochastic environments where population size fluctuations are common.[64] Barriers to gene flow are essential for preserving these drift- and mutation-induced differences, as ongoing migration homogenizes allele frequencies and erodes divergence. Pre-zygotic barriers, such as temporal or behavioral isolation, reduce initial hybridization, while post-zygotic barriers—like Dobzhansky-Muller incompatibilities arising from epistatic interactions between diverged loci—lower hybrid viability or fertility, with plants exhibiting faster establishment of such intrinsic barriers than animals.[65] Speciation fundamentally requires curtailing gene flow to allow stochastic processes to accumulate, as evidenced in island systems where even low-level exchange impedes divergence unless countered by selection or drift in low-dispersal taxa.[66] In cases of incipient speciation with residual gene flow, barriers strengthen asymmetrically, often via chromosomal rearrangements or pleiotropic mutations that simultaneously enhance adaptation and isolation.[7] These mechanisms collectively ensure that neutral or mildly deleterious variants, fixed by drift, evolve into reproductive isolating factors without reliance on divergent selection.Polyploidy and Whole-Genome Duplication
Polyploidy refers to the condition in which an organism possesses more than two complete sets of chromosomes, typically arising through whole-genome duplication (WGD) events.[67] In the context of speciation, polyploidy facilitates rapid reproductive isolation, often leading to sympatric speciation without geographic barriers, as polyploid individuals are generally sterile when mating with diploid progenitors due to the formation of inviable triploid offspring.[68] This mechanism is particularly prevalent in plants, where chromosome doubling can occur via errors in meiosis, mitosis, or fertilization, instantly creating a new lineage incapable of gene flow with the parental population.[69] Autopolyploidy involves WGD within a single species, resulting in multiple chromosome sets from the same genome, which can lead to challenges such as polysomic inheritance and reduced fertility from multivalent formations during meiosis.[70] In contrast, allopolyploidy arises from hybridization between distinct species followed by genome duplication, yielding a stable tetraploid with fixed heterozygosity that enhances fertility and adaptability.[71] Allopolyploids often exhibit hybrid vigor and novel gene interactions, contributing to their evolutionary success, though autopolyploids may face higher extinction risks due to establishment difficulties in mixed populations.[72] Empirical estimates indicate that polyploidy accompanies approximately 15% of speciation events in angiosperms and 31% in ferns, underscoring its role as a major driver of plant diversity, with all extant angiosperms descending from ancient polyploid ancestors.[69] In animals, polyploid speciation is rarer, constrained by sex chromosome complications and dosage sensitivities, but documented in groups like fish (e.g., Carassius gibelio) and amphibians, where it can restore fertility in sterile hybrids.[73] Recent examples include the allopolyploid grass Spartina anglica, formed around 1870 through hybridization and duplication, which rapidly invaded coastal habitats.[74] Despite initial minority cytotype disadvantages, polyploids can establish via self-fertilization, clonal propagation, or spatial clustering, with WGD providing raw material for sub- and neofunctionalization that bolsters long-term persistence.[75]Hybrid Speciation and Chromosomal Rearrangements
Hybrid speciation refers to the formation of a new species arising from hybridization between two divergent parental lineages, resulting in reproductive isolation from both parents. This process contrasts with standard modes of speciation by relying on interspecific gene combinations rather than solely within-lineage divergence. Two primary forms exist: allopolyploid hybrid speciation, involving chromosome doubling to restore fertility in sterile hybrids, and homoploid hybrid speciation, which maintains the parental chromosome number but requires mechanisms to overcome hybrid sterility or inviability.[76][77] Chromosomal rearrangements, including inversions, translocations, and fusions, are pivotal in facilitating hybrid speciation, particularly the homoploid variant, by altering meiotic pairing and suppressing recombination in hybrid zones. In F1 hybrids, mismatched chromosomes from divergent parents often lead to reduced fertility due to improper segregation; however, recombinant progeny inheriting complementary rearrangements from each parent can exhibit restored fertility, as these configurations minimize unbalanced gametes. Such rearrangements act as barriers to gene flow by linking adaptive alleles into non-recombining blocks, preserving transgressive phenotypes suited to novel ecological niches while isolating the hybrid lineage. Theoretical models indicate that parental species differing by multiple rearrangements increase the likelihood of viable hybrid recombinants, with inversion heterozygotes showing reduced fertility that selects for homozygotes matching the hybrid karyotype.[78][79][79] In plants, homoploid hybrid speciation exemplifies this mechanism, as seen in the sunflower species Helianthus anomalus and H. deserticola, which originated within the last 50,000–100,000 years from hybridization between H. annuus and H. petiolaris. Genomic analyses reveal that these hybrids possess 20–30 chromosomal rearrangements, including inversions, relative to parents, which suppress recombination and stabilize dune and sand-slope adapted traits; experimental recreations confirm hybrid origin and isolation via these structural changes. Similarly, in Louisiana irises (Iris fulva × I. brevicaulis hybrids), translocations and inversions contribute to mosaic genomes where spatial sorting and selection favor distinct hybrid forms. Animal examples are rarer but documented, such as in European spined loaches (Cobitis), where homoploid hybrids exhibit fixed rearrangements enhancing hybrid viability in disturbed habitats.[80][80][81] Recent genomic studies underscore that post-speciation, chromosomal rearrangements continue to evolve under selection and incompatibilities; for instance, in a 2024 analysis of hybrid Spartina grasses, both polyploid and homoploid pathways involved rearrangements that resolved meiotic instability, with natural selection purging deleterious alleles. While polyploid hybrids often rely on genome duplication for initial isolation, subsequent rearrangements refine chromosome pairing, as evidenced in Tragopogon allopolyploids where intergenomic translocations occurred within generations. Critically, the prevalence of rearrangements in hybrid speciation highlights their causal role in reducing introgression, though empirical rates remain low due to pervasive hybrid sterility—estimated at <1% success for homoploid cases—necessitating ecological divergence for establishment.[82][82][83]Selective Pressures Driving Divergence
Ecological and Natural Selection
Ecological speciation arises when divergent natural selection imposed by heterogeneous environments promotes reproductive isolation between populations, often without geographic barriers to gene flow.[84] In this process, natural selection favors adaptations to specific ecological niches, such as resource availability or habitat conditions, leading to phenotypic divergence in traits like morphology, behavior, or physiology.[85] Traits under selection may pleiotropically influence mating preferences or hybrid viability, thereby reducing gene flow and facilitating speciation.[86] Empirical evidence indicates that this mechanism operates across taxa, with selection intensities varying by environmental heterogeneity and population connectivity.[87] A classic example involves Darwin's finches on the Galápagos Islands, where Peter and Rosemary Grant documented natural selection driving beak morphology divergence in response to seed size and availability fluctuations.[88] On Daphne Major, medium ground finches (Geospiza fortis) experienced directional selection on beak depth during droughts, favoring deeper beaks for harder seeds, with heritability estimates around 0.7, leading to rapid evolutionary shifts.[89] Hybridization occurs but is limited by ecological trait mismatches, contributing to partial reproductive isolation; a novel lineage emerged in 1981 from a hybrid pair, developing into a reproductively isolated population within two generations by 2017, adapting to available food sources.[90] This demonstrates how episodic selection in variable environments can accelerate ecological divergence.[91] In threespine stickleback fish (Gasterosteus aculeatus), post-glacial colonization of freshwater lakes from marine ancestors has produced benthic (bottom-dwelling) and limnetic (open-water) ecotypes under divergent selection for foraging efficiency.[92] Benthic forms evolve deeper bodies and stronger armor against littoral predators, while limnetic forms develop streamlined shapes for zooplankton capture, with genetic basis involving fewer than 100 loci under selection.[93] Hybrid fitness is reduced in non-native habitats due to maladaptive intermediates, enforcing ecological barriers to gene flow even in sympatry.[94] Laboratory crosses confirm environment-dependent selection, with F1 hybrids showing 20-50% lower survival in parental habitats compared to controls. The apple maggot fly (Rhagoletis pomonella) exemplifies sympatric ecological speciation via host-plant shift, with a hawthorn-feeding race diverging after colonizing apples around 1864 in North America.[53] Selection favors earlier diapause and fruit odor preferences in apple populations to match host phenology, resulting in 4-6 week reproductive asynchrony and reduced hybridization to under 5%.[95] Genomic scans reveal allele frequency clines at >100 loci linked to host adaptation, supporting causal roles for ecological selection in barrier evolution.[96] These cases underscore that ecological speciation rates can span decades to millennia, contingent on selection strength and migration rates.[3]Sexual Selection and Reinforcement
Sexual selection contributes to speciation by promoting divergence in mating signals, preferences, and behaviors that reduce interbreeding between populations, often independently of ecological factors. This process favors traits conferring advantages in mate acquisition or competition, such as elaborate ornaments or displays, which can evolve rapidly and establish prezygotic barriers. Empirical studies indicate a positive correlation between the intensity of sexual selection—measured by metrics like sexual size dimorphism or ornamentation—and speciation rates across taxa, including insects, birds, and fish.[97][98] For instance, in lineages with strong sexual selection, signal evolution accelerates, facilitating reproductive isolation even in sympatric conditions.[99] Reinforcement specifically enhances this role by driving the strengthening of assortative mating in zones of secondary contact, where hybridization produces low-fitness offspring, imposing selection for discrimination against heterospecific mates. Under reinforcement, sexual selection targets female preferences or male traits to minimize costly matings, often resulting in exaggerated divergence of sexual signals. This mechanism is predicted to be most evident in sympatric populations compared to allopatric ones, with genetic underpinnings involving loci under pleiotropic effects for both viability and mating traits. Evidence from comparative analyses supports reinforcement's efficacy, particularly when hybridization rates are high and hybrid fitness is reduced.[100][101] Laboratory and field studies provide concrete examples of these processes. In Drosophila pseudoobscura, reinforcement has strengthened sexual isolation through selection against interspecific courtship, as demonstrated in experiments showing reduced hybridization in sympatric versus allopatric strains.[100] Similarly, in barn swallows (Hirundo rustica), ongoing speciation involves sexual selection on plumage and tail traits, where genomic analyses reveal loci linked to sexual signals that correlate with reproductive isolation between Eurasian and American subspecies, with reinforcement evident in hybrid zones.[102] In African cichlid fishes of the genus Pundamilia, female mate choice based on male nuptial coloration has driven sympatric divergence and reinforcement, with ecological gradients amplifying selection against hybrids.[103] Challenges in detecting reinforcement include distinguishing it from other divergence processes, such as sensory drive, but replicated patterns across taxa affirm its role in completing speciation. Recent genomic insights highlight how sexual selection can interact with genetic drift or natural selection to accelerate barrier formation, underscoring its causal importance in biodiversity generation.[104][105]Evidence from Observations and Experiments
Fossil Record and Phylogenetic Evidence
The fossil record reveals speciation through the origination of distinct morphospecies and branching cladogenetic patterns, often characterized by prolonged stasis punctuated by geologically brief episodes of morphological innovation, as articulated in the punctuated equilibrium hypothesis by Niles Eldredge and Stephen Jay Gould in 1972. This pattern, observed in taxa such as Devonian trilobites and Cenozoic bryozoans, aligns with allopatric speciation in small, isolated populations where rapid divergence occurs but leaves sparse transitional fossils due to limited geographic range and low population sizes. For example, bryozoan colonies in the fossil record, dating back to the Early Ordovician around 480 million years ago, exhibit sudden appearances of novel forms in stratigraphic sequences, with cheilostome bryozoans diversifying markedly from 160 million years ago onward.[106][107][108] In marine microfossils like planktonic foraminifera, the record documents both gradual speciation, with morphological differentiation spanning up to 500,000 years, and abrupt cladogenetic splits, challenging uniform expectations of phyletic gradualism and supporting context-dependent speciation dynamics. The equid (horse) lineage, spanning 55 million years from Eocene dog-sized ancestors to modern forms, displays a combination of anagenetic trends and branching speciation events, evidenced by transitional fossils like Mesohippus and Merychippus that mark divergences into grazing-adapted clades around 20-15 million years ago. These patterns underscore the fossil record's role in quantifying speciation rates via stratigraphic ranges, though incompleteness—estimated at 10% for some echinoid branches totaling 360 million years—necessitates integration with other data.[109][110][111] Phylogenetic reconstructions from molecular data provide independent corroboration of speciation by depicting bifurcation nodes as divergence events, with calibrated divergence times often aligning with fossil benchmarks to infer timing and tempo. In echinoids, molecular clock analyses using three genes across 28 families yield divergence estimates concordant with paleontological data at 65-70% of internal nodes, such as the Cidaroidea split around 255 million years ago, validating speciation chronologies from the Permian. Similarly, cetacean phylogenies and fossils reconcile apparent rate discrepancies—phylogenetic data suggesting rising diversity over 12 million years versus fossil-indicated declines—through models accounting for budding speciation modes, yielding equivalent net diversification rates across eight of nine clades examined. This synergy highlights phylogenetics' utility in resolving fossil gaps, such as extinct lineages, while affirming branching speciation as a pervasive evolutionary process.[111][112][112]Contemporary and Laboratory Examples
The apple maggot fly (Rhagoletis pomonella), native to hawthorn trees in North America, underwent a host shift to domesticated apples following their introduction in the mid-19th century, marking an incipient case of sympatric speciation.[53] This shift, first documented around the 1860s, has led to the formation of distinct host races with differences in adult emergence timing (allochronic isolation), host preference, and assortative mating, reducing gene flow between populations to approximately 4-6% per generation.[113] Genomic analyses reveal allele frequency differences at quantitative trait loci for diapause and odor discrimination, supporting divergence driven by natural selection on host-specific traits despite ongoing gene flow.[114] Laboratory experiments with Drosophila pseudoobscura have demonstrated the evolution of reproductive isolation under controlled conditions. In a 1989 study by Diane Dodd, a single population was divided into groups reared on diets of maltose or starch for eight generations, after which flies showed significant assortative mating preferences based on rearing medium, with up to 72% non-random mating observed in choice tests.[115] This experiment illustrates how divergent ecological selection can rapidly generate prezygotic barriers, though the isolation was partial and reversed under certain conditions.[116] Subsequent founder-flush experiments with the same species confirmed speciation-like divergence through cycles of bottlenecks and expansions, yielding reproductively isolated lines after multiple generations.[117] More recent laboratory work has replicated reproductive isolation in adapted populations. A 2024 experiment adapting populations to novel hot environments resulted in strong pre- and post-zygotic barriers within dozens of generations, with hybrid fitness reduced by over 50% due to Dobzhansky-Muller incompatibilities.[118] Meta-analyses of such arthropod and yeast studies indicate that divergent selection consistently promotes isolation faster than neutral processes, though phenotypic plasticity can confound interpretations of genetic divergence.[119] These findings underscore the feasibility of speciation under strong, directional pressures but highlight that complete isolation often requires multiple barriers.[116]