Recent from talks
Nothing was collected or created yet.
| Wart | |
|---|---|
| Other names | Verrucae,[1] papillomas[2] |
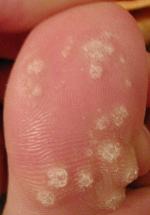 | |
| A large number of warts on the big toe | |
| Specialty | Dermatology |
| Symptoms | skin growth usually occurring on the hands, feet, or genitals[1][3] |
| Duration | Months to years[1] |
| Causes | Human papillomavirus[1] |
| Risk factors | Public showers and pools, eczema[3] |
| Differential diagnosis | Callus, seborrheic keratosis, squamous cell carcinoma[4] |
| Prevention | Avoiding skin contact with infected individual, not walking barefoot in public areas, having safe sex or sexual abstinence |
| Treatment | Salicylic acid, cryotherapy,[1] surgical removal |
| Frequency | Very common[2] |
Warts are non-cancerous viral growths usually occurring on the hands and feet but which can also affect other locations, such as the genitals or face.[1][3] One or many warts may appear.[3] They are distinguished from cancerous tumors as they are caused by a viral infection, such as a human papillomavirus, rather than a cancer growth.[3]
Factors that increase the risk include the use of public showers and pools, working with meat, eczema, and a weak immune system.[1][3] The virus is believed to infect the host through the entrance of a skin wound.[1] A number of types exist, including plantar warts, "filiform warts", and genital warts.[3] Genital warts are often sexually transmitted.[5]
Without treatment, most types of warts resolve in months to years.[1] Several treatments may speed resolution, including salicylic acid applied to the skin and cryotherapy.[1] In those who are otherwise healthy, they do not typically result in significant problems.[1] Treatment of genital warts differs from that of other types.[3] Infection with a virus, such as HIV, can cause warts. This is prevented through careful handling of needles or sharp objects that could infect the individual through physical trauma of the skin, plus the practice of safe sex using barrier methods such as condoms. Viruses that are not sexually transmitted, or are not transmitted in the case of a wart, can be prevented through several behaviors, such as wearing shoes outdoors and avoiding unsanitized areas without proper shoes or clothing, such as public restrooms or locker rooms.
Warts are very common, with most people being infected at some point in their lives.[2] The estimated current rate of non-genital warts among the general population is 1–13%.[1] They are more common among young people.[1] Before widespread adoption of the HPV vaccine, the estimated rate of genital warts in sexually active women was 12%.[5] Warts have been described as far back as 400 BC by Hippocrates.[4]
Types
[edit]
A range of types of warts have been identified, varying in shape and site affected, as well as the type of human papillomavirus involved.[6][7] These include:
- Common wart (verruca vulgaris),[8] a raised wart with a roughened surface, most common on hands, but can grow anywhere on the body. Sometimes known as a Palmer wart or junior wart.
- Flat wart (verruca plana), a small, smooth, flattened wart, flesh-coloured, which can occur in large numbers; most common on the face, neck, hands, wrists, and knees.
- Filiform or digitate wart, a thread- or finger-like wart, most common on the face, especially near the eyelids and lips.
- Genital wart (venereal wart, condyloma acuminatum, verruca acuminata), a wart that occurs on the genitalia.
- Periungual wart, a cauliflower-like cluster of warts that occurs around the nails.
- Plantar wart (verruca, verruca plantaris), a hard, sometimes painful lump, often with multiple black specks in the center; usually only found on pressure points on the soles of the feet and between toes.
- Mosaic wart, a group of tightly clustered plantar-type warts, commonly on the hands or soles of the feet.
Causes
[edit]Warts are caused by the human papillomavirus (HPV). There are about 130 known types of human papillomaviruses.[9] HPV infects the squamous epithelium, usually of the skin or genitals. Each HPV type is typically only able to infect a few specific areas of the body. Many HPV types can produce a benign growth, often called a "wart" or "papilloma", in the area they infect.[10] Many of the more common HPV and wart types are listed below.
- Common warts – HPV types 2 and 4 (most common); also types 1, 3, 26, 29, and 57, and others.
- Cancers and genital dysplasia – "high-risk" HPV types are associated with cancers, notably cervical cancer, and can also cause some vulvar, vaginal,[11] penile, anal[12] and some oropharyngeal cancers. "Low-risk" types are associated with warts or other conditions.[13][14]
- High-risk: 16, 18 (cause the most cervical cancer); also 31, 33, 35, 39, 45, 52, 58, 59, and others.
- Plantar warts (verruca) – HPV type 1 (most common); also types 2, 4, 27, 28, and others.
- Anogenital warts (condylomata acuminata or venereal warts) – HPV types 6 and 11 (most common); also types 42, 44, and others.[15]
- Low-risk: 6, 11 (most common); also 13, 44, 40, 43, 42, 54, 61, 72, 81, 89, and others.
- Verruca plana (flat warts) – HPV types 3, 10, and 28.
- Butcher's warts – HPV type 7.
- Heck's disease (focal epithelial hyperplasia) – HPV types 13 and 32.
Pathophysiology
[edit]Common warts have a characteristic appearance under the microscope. They have thickening of the stratum corneum (hyperkeratosis), thickening of the stratum spinosum (acanthosis), thickening of the stratum granulosum, rete ridge elongation, and large blood vessels at the dermoepidermal junction.[citation needed]
Diagnosis
[edit]
On dermatoscopic examination, warts will commonly have fingerlike or knoblike extensions.[16]
Prevention
[edit]Gardasil 6 is an HPV vaccine aimed at preventing cervical cancers and genital warts. Gardasil is designed to prevent infection with HPV types 16, 18, 6, and 11. HPV types 16 and 18 currently cause about 70% of cervical cancer cases,[13][14] and also cause some vulvar, vaginal,[11] penile and anal cancers.[12] HPV types 6 and 11 are responsible for 90% of documented cases of genital warts.[17]
Gardasil 9 protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.[18]
HPV vaccines do not currently protect against the virus strains responsible for plantar warts (verrucae).[19]
Disinfection
[edit]The virus is relatively hardy and immune to many common disinfectants. Exposure to 90% ethanol for at least 1 minute, 2% glutaraldehyde, 30% Chlorhexidine, and/or 1% sodium hypochlorite can disinfect the pathogen.[20]
The virus is resistant to drying and heat, but killed by 100 °C (212 °F) temperature and ultraviolet radiation.[20]
Treatment
[edit]There are many treatments and procedures associated with wart removal.[21] A review of various skin wart treatments concluded that topical treatments containing salicylic acid were more effective than placebo.[22] Cryotherapy appears to be as effective as salicylic acid, but there have been fewer trials.[22]
Medication
[edit]- Salicylic acid can be prescribed by a dermatologist in a higher concentration than that found in over-the-counter products. Several over-the-counter products are readily available at pharmacies and supermarkets of roughly two types: adhesive pads treated with salicylic acid, and bottled concentrated salicylic acid and lactic acid solution.
- Fluorouracil — Fluorouracil cream, a chemotherapy agent sometimes used to treat skin cancer, can be used on particularly resistant warts, by blocking viral DNA and RNA production and repair.[23]
- Imiquimod is a topical cream that helps the body's immune system fight the wart virus by encouraging interferon production. It has been approved by the U.S. Food and Drug Administration (FDA) for genital warts.[24]
- Cantharidin, found naturally in the bodies of many members of the beetle family Meloidae, causes dermal blistering. It is used either by itself or compounded with podophyllin. Not FDA approved, but available through Canada or select US compounding pharmacies.
- Bleomycin — A more potent chemotherapy drug, can be injected into deep warts, destroying the viral DNA or RNA. Bleomycin is notably not US FDA approved for this purpose. Possible side effects include necrosis of the digits, nail loss, and Raynaud syndrome. The usual treatment is one or two injections.[25][26]
- Dinitrochlorobenzene (DNCB), like salicylic acid, is applied directly to the wart. Studies show this method is effective with a cure rate of 80%.[medical citation needed] But DNCB must be used much more cautiously than salicylic acid; the chemical is known to cause genetic mutations, so it must be administered by a physician. This drug induces an allergic immune response, resulting in inflammation that wards off the wart-causing virus.[27]
- Cidofovir is an antiviral drug which is injected into HPV lesions within the larynx (laryngeal papillomatosis) as an experimental treatment.[28]
- Verrutop verruca treatment is a topical solution made from a combination of organic acids, inorganic acids, and metal ions. This solution causes the production of nitrites, which act to denature viral proteins and mummify the wart tissue. The difference between Verrutop and other acid treatments is that it does not damage the surrounding skin.
- Another product available over-the-counter that can aid in wart removal is silver nitrate in the form of a caustic pencil, which is also available at drug stores. In a placebo-controlled study of 70 patients, silver nitrate given over nine days resulted in clearance of all warts in 43% and improvement in warts in 26% one month after treatment, compared to 11% and 14%, respectively, in the placebo group.[29] The instructions must be followed to minimize staining of skin and clothing. Occasionally, pigmented scars may develop.
- Trichloroacetic acid can be used to treat warts if salicylic acid or cryotherapy fail or are not available. It requires repeat treatments every week or so. Side effects are burning and stinging.[30]
-
Two viral warts on a middle finger, being treated with a mixture of acids (like salicylic acid) to remove them. A white precipitate forms on the area where the product was applied.
-
Throat warts before and after carbon dioxide laser treatment.
Procedures
[edit]
- Keratolysis, of dead surface skin cells usually using salicylic acid, blistering agents, immune system modifiers ("immunomodulators"), or formaldehyde, often with mechanical paring of the wart with a pumice stone, blade etc.[31]
- Electrodesiccation[32]
- Microwave Treatment[33][34][35][36][37][38]
- Cryosurgery or cryotherapy, which involves freezing the wart (generally with liquid nitrogen),[39] creating a blister between the wart and epidermal layer after which the wart and the surrounding dead skin fall off. An average of three to four treatments is required for warts on thin skin. Warts on calloused skin, like plantar warts, might take dozens or more treatments.[40]
- Surgical curettage of the wart
- Laser treatment – often with a pulse dye laser or carbon dioxide (CO2) laser. Pulse dye lasers (wavelength 582 nm) work by selective absorption by blood cells (specifically hemoglobin). CO2 lasers work by selective absorption by water molecules. Pulse dye lasers are less destructive and more likely to heal without scarring. CO2 laser works by vaporizing and destroying tissue and skin. Laser treatments can be painful, expensive (though covered by many insurance plans), and not extensively scarring when used appropriately. CO2 lasers will require local anaesthetic. Pulse dye laser treatment does not need conscious sedation or local anesthesia. It takes 2 to 4 treatments, but can be many more for extreme cases. Typically, 10–14 days are required between treatments. Preventive measures are important.[40]
- Infrared coagulator – an intense source of infrared light in a small beam like a laser. This works essentially on the same principle as laser treatment. It is less expensive. Like the laser, it can cause blistering, pain, and scarring.[41]
- Intralesional immunotherapy with purified candida, MMR, and tuberculin (PPD) protein appears safe and effective.[42][43]
- Duct tape occlusion therapy involves placing a piece of duct tape over the wart. The mechanism of action of this technique still remains unknown. Despite several trials, evidence for the efficacy of duct tape therapy is inconclusive.[44][45] Despite the mixed evidence for efficacy, the simplicity of the method and its limited side-effects lead some researchers to be reluctant to dismiss it.[46]
- No intervention. Spontaneous resolution within a few years can be recommended.[47]

Alternative medicine
[edit]
Daily application of the acrid yellow latex of Chelidonium majus (greater celandine) is a traditional treatment.[48][49]
A variety of traditional folk remedies and rituals claim to be able to remove warts. According to English folk belief, touching toads causes warts; according to a German folk belief, touching a toad under a full moon cures warts.[50] The most common Northern Hemisphere toads have glands that protrude from their skin that superficially resemble warts. Warts are caused by a virus, and toads do not harbor it.[51]
In The Adventures of Tom Sawyer, Mark Twain has his characters discuss a variety of such remedies. Tom Sawyer proposes "spunk-water" (or "stump-water", the water collecting in the hollow of a tree stump) as a remedy for warts on the hand. In his version, one puts one's hand into the water at midnight and says:
Barley-corn, barley-corn, injun-meal shorts,
Spunk-water, spunk-water, swaller these warts
One would then "walk away quick, eleven steps, with your eyes shut, and then turn around three times and walk home without speaking to anybody. Because if you speak the charm's busted." This is given as an example of Huckleberry Finn's planned remedy, which involves throwing a dead cat into a graveyard as a devil or devils comes to collect a recently buried wicked person. Another remedy involved splitting a bean, drawing blood from the wart and putting one of the bean halves against the wart, and burying that half at a crossroads at midnight. The theory of operation is that the blood on the buried bean will draw away the wart.[52] Twain is recognized as an early collector and recorder of genuine American folklore.[53]
Similar practices are recorded elsewhere. In Louisiana, one remedy for warts involves rubbing the wart with a potato, which is then buried; when the "buried potato dries up, the wart will be cured".[54] Another remedy similar to Twain's is reported from Northern Ireland, where water from a specific well on Rathlin Island is credited with the power to cure warts.[55]
History
[edit]
Surviving ancient medical texts show that warts were a documented disease since at least the time of Hippocrates, who lived c. 460 – c. 370 BC. In the book De Medecia by the Roman physician Aulus Cornelius Celsus, who lived c. 25 BC – c. 50 AD, different types of warts were described. Celsus described myrmecia, today recognized as plantar wart, and categorized the acrochordon (skin tag) as a wart. In the 13th century, warts were described in books published by the surgeons William of Saliceto and Lanfranc of Milan. The word verruca for a wart was introduced by the physician Daniel Sennert, who described them in his 1636 book Hypomnemata physicae.[56]
The cause of warts was initially disputed in the medical profession. In the early 18th century, the physician Daniel Turner, who published the first book on dermatology, suggested that warts were caused by damaged nerves close to the skin. In the mid-18th century, the surgeon John Hunter popularized the belief that warts were caused by a bacterial syphilis infection. The surgeon Benjamin Bell documented that warts were caused by a disease entirely unrelated to syphilis, and established a causal link between warts and cancer. In the 19th century, the chief physician of Verona Hospital established a link between warts and cervical cancer in particular. But in 1874, it was noted by the dermatologist Ferdinand Ritter von Hebra that while various theories were advanced by the medical profession, the "influences causing warts are still very obscure".[56]
In 1907, the physician Giuseppe Ciuffo first demonstrated that a viral infection causes warts. In 1976, the virologist Harald zur Hausen was the first to discover that warts were caused by the human papillomavirus (HPV). His continuous research established the evidence necessary to develop an HPV vaccine, which first became available in 2006.[56]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m Loo, SK; Tang, WY (12 June 2014). "Warts (non-genital)". BMJ Clinical Evidence. 2014. PMC 4054795. PMID 24921240.
- ^ a b c "Papillomas (Warts) – National Library of Medicine". PubMed Health. Archived from the original on 10 September 2017. Retrieved 6 November 2016.
- ^ a b c d e f g h "Warts: Overview". U.S. National Library of Medicine. 30 July 2014. Archived from the original on 10 September 2017.
- ^ a b Bope, Edward T.; Kellerman, Rick D. (2012). Conn's Current Therapy 2012. Elsevier Health Sciences. p. 275. ISBN 978-1-4557-3305-7. Archived from the original on 7 November 2016.
- ^ a b Buck, Henry W. Jr. (13 August 2010). "Warts (genital)". BMJ Clinical Evidence. 2010. PMC 3217761. PMID 21418685.
- ^ Anderson, Keith; Keith, Jeff; Novak, Patricia D.; Elliot, Michelle A. (2005). Mosby's Medical, Nursing & Allied Health Dictionary (5th ed.). C. V. Mosby. ISBN 978-0-323-03736-5. Archived from the original on 7 January 2017.
- ^ "MedlinePlus: Warts". 2010. Archived from the original on 16 May 2013.
- ^ Adigun, Chris G. "Verruca Vulgaris". Merck Manuals. Retrieved 16 December 2022.
- ^ De Villiers, E. M.; Fauquet, C.; Broker, T. R.; Bernard, H. U.; Zur Hausen, H. (June 2004). "Classification of papillomaviruses". Virology. 324 (1): 17–27. doi:10.1016/j.virol.2004.03.033. PMID 15183049.
- ^ Syrjänen, Stina (1 August 2003). "Human papillomavirus infections and oral tumors". Medical Microbiology and Immunology. 192 (3): 123–128. doi:10.1007/s00430-002-0173-7. ISSN 1432-1831. PMID 12920585. S2CID 2768273.
- ^ a b "FDA Approves Expanded Uses for Gardasil to Include Preventing Certain Vulvar and Vaginal Cancers". FDA. 12 September 2008. Archived from the original on 6 March 2010.
- ^ a b Cortez, Michelle Fay; Pettypiece, Shannon (13 November 2008). "Merck Cancer Shot Cuts Genital Warts, Lesions in Men". Bloomberg News. Retrieved 17 May 2013.
- ^ a b Lowy, D. R.; Schiller, J. T/ (2006). "Prophylactic human papillomavirus vaccines". J. Clin. Invest. 116 (5): 1167–73. doi:10.1172/JCI28607. PMC 1451224. PMID 16670757.
- ^ a b Muñoz, N.; Bosch, F. X.; Castellsagué, X.; Díaz, M.; de Sanjose, S.; Hammouda, D.; Shah, K. V.; Meijer, C. J. (20 August 2004). "Against which human papillomavirus types shall we vaccinate and screen? The international perspective". International Journal of Cancer. 111 (2): 278–85. doi:10.1002/ijc.20244. PMID 15197783. S2CID 20679802.
- ^ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Mitchell, Richard (2007). "Chapter 19: The Female Genital System and Breast". Robbins Basic Pathology (8 ed.). Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
- ^ Dong, Huiting (2011). "Dermatoscopy of genital warts". Journal of the American Academy of Dermatology. 64 (5): 859–864. doi:10.1016/j.jaad.2010.03.028. PMID 21429619. S2CID 33381261 – via Elsevier Science Direct.
- ^ Steinbrook, Robert (2006). "The Potential of Human Papillomavirus Vaccines". New England Journal of Medicine. 354 (11): 1109–12. doi:10.1056/NEJMp058305. PMID 16540608.
- ^ "Prescribing information Gardasil 9" (PDF). Food and Drug Administration. 2015. Archived from the original (PDF) on 10 October 2016.
- ^ Bossart, Simon; Imstepf, Valentina; Hunger, Robert E.; Seyed Jafari, S. Morteza (2020). "Nonavalent Human Papillomavirus Vaccination as a Treatment for Skin Warts in Immunosuppressed Adults: A Case Series". Acta Dermato Venereologica. 100 (6): adv00078-2. doi:10.2340/00015555-3437. PMC 9128913. PMID 32115668.
- ^ a b Human Papillomavirus Archived 23 August 2015 at the Wayback Machine. Public Health Agency of Canada
- ^ Lipke, M. M. (2006). "An armamentarium of wart treatments". Clinical Medicine & Research. 4 (4): 273–93. doi:10.3121/cmr.4.4.273. PMC 1764803. PMID 17210977.
- ^ a b Kwok, C. S.; Gibbs, Sam; Bennett, C.; Holland, R.; Abbott, R. (12 September 2012). "Topical treatments for cutaneous warts". The Cochrane Database of Systematic Reviews. 9 (9) CD001781. doi:10.1002/14651858.CD001781.pub3. PMC 8101088. PMID 22972052. Archived from the original on 4 July 2013.
- ^ Salk, RS; Grogan, KA; Chang, TJ (May 2006). "Topical 5% 5-fluorouracil cream in the treatment of plantar warts: a prospective, randomized, and controlled clinical study". Journal of Drugs in Dermatology. 5 (5): 418–24. PMID 16703777.
- ^ Barclay, L. (4 June 2011). "Short-acting Imiquimod Cream Approved for Genital Warts". Medscape. Archived from the original on 18 August 2011. Retrieved 10 August 2011.
- ^ Soni, Prasoon; Khandelwal, Kanika; Aara, Naushin; Ghiya, Bhikam C; Mehta, Rajesh D; Bumb, Ram A (2011). "Efficacy of Intralesional Bleomycin in Palmo-plantar and Periungual Warts". Journal of Cutaneous and Aesthetic Surgery. 4 (3): 188–191. doi:10.4103/0974-2077.91250. PMC 3263129. PMID 22279384.
- ^ Champion, R. H.; et al. (1998). Rook's Textbook of Dermatology. Blackwell Science. p. 1044. ISBN 0-632-06429-3.
- ^ "Treating Warts". British Medical Journal. 31 August 2002. Archived from the original on 3 November 2010. Retrieved 17 May 2013.
- ^ Soma, Marlene A.; Albert, David M. (2008). "Cidofovir: To use or not to use?". Current Opinion in Otolaryngology & Head and Neck Surgery. 16 (1): 86–90. doi:10.1097/MOO.0b013e3282f43408. PMID 18197029. S2CID 22895067.
- ^ Sterling, J. C.; Handfield-Jones, S.; Hudson, P. M. (2001). "Guidelines for the management of cutaneous warts" (PDF). British Journal of Dermatology. 144 (1): 4–11. doi:10.1046/j.1365-2133.2001.04066.x. PMID 11167676. S2CID 20179474. Archived from the original (PDF) on 3 March 2012.
- ^ "Common warts – Diagnosis and treatment". MayoClinic.org. Retrieved 6 November 2022.
- ^ Warts Archived 17 May 2008 at the Wayback Machine at About.com
- ^ Stone, K. M.; Becker, T. M.; Hadgu, A.; Kraus, S. J. (1990). "Treatment of external genital warts: A randomized clinical trial comparing podophyllin, cryotherapy, and electrodesiccation". Genitourinary Medicine. 66 (1): 16–19. doi:10.1136/sti.66.1.16. PMC 1194434. PMID 2179111.
- ^ Solomon, Katie; Yip, Vincent (December 2023). "The novel treatment of children with viral warts using microwave technology". Skin Health and Disease. 3 (6) e291. doi:10.1002/ski2.291. ISSN 2690-442X. PMC 10690651. PMID 38047251.
- ^ Hagon, Wendy; Hagon, Jonathan; Noble, Greer; Brenton-Rule, Angela; Stewart, Sarah; Bristow, Ivan (January 2023). "Microwave therapy for the treatment of plantar warts". Journal of Foot and Ankle Research. 16 (1): 37. doi:10.1186/s13047-023-00638-8. ISSN 1757-1146. PMC 10268531. PMID 37322512.
- ^ Bristow, Ivan Robert; Webb, Christopher; Ardern-Jones, Michael Roger (27 July 2017). "The Successful Use of a Novel Microwave Device in the Treatment of a Plantar Wart". Case Reports in Dermatology. 9 (2): 102–107. doi:10.1159/000477377. ISSN 1662-6567. PMC 5624246. PMID 29033812.
- ^ Gupta, Aditya K.; Wang, Tong; Cooper, Elizabeth A.; Conenello, Robert M.; Bristow, Ivan R. (October 2023). "The treatment of plantar warts using microwave—A review of 85 consecutive cases in the United States". Journal of Cosmetic Dermatology. 22 (10): 2729–2736. doi:10.1111/jocd.15802. ISSN 1473-2130. PMID 37340590.
- ^ Dhinsa, Arpreet; Philip, Gladis; Daknish, Fatima; Amin, Sahil; Vlahovic, Tracey (September 2023). "42057 Microwave Therapy for Plantar Warts: A Chart Review". Journal of the American Academy of Dermatology. 89 (3): AB64. doi:10.1016/j.jaad.2023.07.259. ISSN 0190-9622.
- ^ "The Royal College of Podiatry". College of Podiatry. Retrieved 22 March 2024.
- ^ "Cryotherapy for Warts". WebMD. Archived from the original on 9 July 2016.
- ^ a b Bacelieri, R.; Johnson, S. M. (2005). "Cutaneous warts: An evidence-based approach to therapy". American Family Physician. 72 (4): 647–52. PMID 16127954. Archived from the original on 21 April 2014.
- ^ Halasz, C. L. (1994). "Treatment of common warts using the infrared coagulator". The Journal of Dermatologic Surgery and Oncology. 20 (4): 252–56. doi:10.1111/j.1524-4725.1994.tb01620.x. PMID 8163746.
- ^ Aldahan, A. S.; Mlacker, S.; Shah, V. V.; Kamath, P.; Alsaidan, M.; Samarkandy, S.; Nouri, K. (May 2016). "Efficacy of intralesional immunotherapy for the treatment of warts: A review of the literature". Dermatologic Therapy. 29 (3): 197–207. doi:10.1111/dth.12352. PMID 26991521. S2CID 40536366.
- ^ Salman, Samer (2019). "Intralesional Immunotherapy for the Treatment of Warts: A Network Meta-analysis". Journal of the American Academy of Dermatology. 80 (4): 922–930.e4. doi:10.1016/j.jaad.2018.07.003. PMID 30003983. S2CID 51617793 – via Elsevier Science Direct.
- ^ Loo, S. K.; Tang, W. Y. (12 June 2014). "Warts (non-genital)". BMJ Clinical Evidence. 2014. PMC 4054795. PMID 24921240.
- ^ Kwok, C. S.; Gibbs, S.; Bennett, C.; Holland, R.; Abbott, R. (12 September 2012). "Topical treatments for cutaneous warts". Cochrane Database Syst Rev. 9 (9) CD001781. doi:10.1002/14651858.CD001781.pub3. PMC 8101088. PMID 22972052.
- ^ Stubbings, A.; Wacogne, I. (September 2011). "Question 3: What is the efficacy of duct tape as a treatment for verruca vulgaris?". Archives of Disease in Childhood. 96 (9): 897–99. doi:10.1136/archdischild-2011-300533. PMID 21836182. S2CID 206853952.
- ^ Goldman, Ran D. (May 2019). "Duct tape for warts in children: Should nature take its course?". Canadian Family Physician. 65 (5): 337–338. ISSN 1715-5258. PMC 6516695. PMID 31088871.
- ^ Gilca, Marilena; Gaman, Laura; Panait, Elena; Stoian, Irina; Atanasiu, Valeriu (8 October 2010). "Chelidonium majus – An Integrative Review: Traditional Knowledge Versus Modern Findings" (PDF). Forschende Komplementärmedizin. 17 (5): 241–248. doi:10.1159/000321397. Archived from the original (PDF) on 5 December 2020 – via MedicinaBiomolecular.com.br.
- ^ "Greater Celandine for Warts". Botanical-Online.com. Archived from the original on 17 July 2014.
- ^ Ley, Willy (December 1963). "The Names of the Constellations". For Your Information. Galaxy Science Fiction. pp. 90–99.
- ^ Clark, Josh (2 March 2009). "Do toads cause warts?". Science.HowStuffWorks.com. p. 2. Archived from the original on 16 October 2012. Retrieved 20 October 2012.
- ^ Twain, Mark. "Chapter VI". The Adventures of Tom Sawyer.
- ^ LeMaster, J. R. (1993) The Mark Twain Encyclopedia (Taylor and Francis, pp. 293–94 Archived 7 January 2017 at the Wayback Machine, ISBN 0-8240-7212-X.
- ^ Webb, Julie Yvonne (1971). "Louisiana Voodoo and Superstitions Relating to Health". HSMHA Health Reports. 86 (4): 291, 296–97. doi:10.2307/4594154. JSTOR 4594154. PMC 1937133. PMID 4324337.
- ^ Ballard, L. M. (2009). "An approach to traditional cures in Ulster". The Ulster Medical Journal. 78 (1): 26–33. PMC 2629017. PMID 19252727.
- ^ a b c Karamanou, Marianna; Agapitos, Emmanovil; Kousoulis, Antonis; Androutsos, George (17 August 2010). "From the humble wart to HPV: A fascinating story throughout centuries". Oncology Reviews. 4 (3): 133–135. doi:10.1007/s12156-010-0060-1. S2CID 72238300.
Types
Common Warts
Common warts, also known as verruca vulgaris, present as rough, dome-shaped, hyperkeratotic papules that typically measure 1-10 mm in diameter. These growths often exhibit a grainy or verrucous surface texture and are commonly flesh-colored or grayish in hue, sometimes featuring small black dots within the lesion that correspond to thrombosed capillaries.[3][8][1][9] They are primarily caused by human papillomavirus (HPV) types 2 and 4, with self-inoculation through minor skin trauma leading to the formation of clusters on the hands.[1][10] Common locations include the hands, fingers, knees, and elbows, where the lesions tend to develop on exposed or frequently traumatized skin.[3][8] These warts are more prevalent among children and individuals with immunocompromised states.[1] Distinguishing common warts from calluses or corns relies on clinical examination, as warts disrupt the normal continuity of skin lines (dermatoglyphics), whereas calluses and corns preserve these lines within the lesion.[11][12] Common warts are often painful when squeezed laterally from the sides due to their structure and involvement of nerve endings, whereas calluses are typically tender only when direct pressure is applied. The presence of small black dots (thrombosed capillaries) further supports a diagnosis of warts, as calluses lack these features and appear as thick, hardened, dry patches of skin. On the fingers, common warts may be confused with blood blisters, which present as raised, fluid-filled pockets that are red, purple, or black from pooled blood, caused by acute pinching or injury, and generally heal spontaneously in about a week. Black dots suggest a wart, solid hardened skin without dots suggests a callus, and a fluid-filled dark lesion suggests a blood blister. For detailed differential diagnosis and confirmatory tests (such as paring the lesion to reveal pinpoint bleeding in warts), refer to the Diagnosis section. This feature, along with the presence of thrombosed capillaries, aids in accurate diagnosis without invasive procedures.Plantar Warts
Plantar warts, also known as verruca plantaris, are benign skin growths that develop on the weight-bearing surfaces of the feet, characterized by their hard, thickened appearance due to hyperkeratosis from mechanical pressure. These warts typically present as rough, callus-like lesions with disrupted skin lines, often featuring small black dots in the center that represent thrombosed capillaries. Unlike other wart types, their location on the soles exposes them to constant friction and compression, leading to inward growth and a flattened profile surrounded by a thickened rim of hardened skin.[13][14] These growths commonly occur on the soles, heels, and balls of the toes, where pressure is greatest during walking or standing, and they may cluster into mosaic warts—tight groupings of multiple small, flat warts that cover a larger area. The painful nature of plantar warts stems from this mechanical stress, as the lesions press against underlying nerves and tissues, causing discomfort that intensifies with each step; in severe cases, they can lead to limping or bleeding if traumatized. Single, deeper lesions known as myrmecia are particularly tender due to their conical shape penetrating the skin.[15][16] Plantar warts are caused by specific strains of the human papillomavirus (HPV), primarily types 1, 2, and 4, which infect the skin through minor cuts or abrasions. These viruses thrive in warm, moist environments, resulting in higher incidence around public swimming pools, locker rooms, and communal showers, where barefoot exposure facilitates transmission. Preventive measures, such as wearing protective footwear in these settings, can reduce risk, though the infection often remains dormant until triggered by pressure or trauma.[13][17] A key distinction from corns or calluses lies in their vascular nature: paring down a plantar wart reveals pinpoint bleeding and the characteristic black dots, whereas corns show uniform, dry hyperkeratosis without vascular elements. This diagnostic feature, confirmed through gentle scraping, helps differentiate the viral etiology of warts from the frictional response seen in corns.[14][18]Flat Warts
Flat warts, also known as plane warts, are characterized by their smooth, flat-topped papules that measure 1-5 mm in diameter and are only slightly elevated above the surrounding skin.[19] These lesions typically exhibit a skin-colored, pink, light brown, or yellowish hue, blending subtly with the skin tone, which often leads to them being overlooked until they multiply.[19][20] They frequently appear in clusters numbering from 20 to 100, forming widespread but minimally raised groups that distinguish them from the more prominent, bulky elevations seen in common warts on the hands.[19][21] These warts commonly affect the face, especially in children, where they may cluster due to minor facial trauma or scratching.[7] In adults, they often emerge on the legs or arms, particularly following shaving or abrasion that facilitates autoinoculation and spread along the skin.[13] Unlike genital warts, flat warts occur on non-mucosal, cutaneous sites without sexual transmission.[22] Flat warts are primarily caused by human papillomavirus (HPV) types 3, 10, and 28, which induce epidermal hyperplasia in a manner that results in their subtle profile.[1][23] They are more prevalent among adolescents and young adults, potentially linked to variations in immune response that allow for lesion multiplicity.[7] A variation of the Koebner phenomenon can lead to linear arrangements of these warts along lines of skin trauma, such as from scratching or shaving.[21][24]Filiform Warts
Filiform warts, also known as digitate or facial warts, are distinguished by their elongated, thread-like or finger-like projections that protrude from the skin in a frond-like manner. These growths typically range from 1 to 2 mm in length but can extend up to 1 cm, featuring a narrow, stalk-like base with multiple fine extensions. They often appear flesh-colored but may be pigmented, presenting as brown, pink, yellow, or grayish tones that align with the surrounding skin. Their rapid development sets them apart, allowing them to emerge and elongate quickly after infection.[25][26][27] These warts preferentially affect facial areas, most commonly the eyelids, lips, neck, and nose, where the skin is thinner and more exposed. They are more prevalent in adults, particularly men, compared to children, though they can occur on other sites like the fingers or legs through direct spread. Due to their prominent and visible locations, filiform warts frequently cause cosmetic distress, leading many affected individuals to pursue removal for appearance rather than medical necessity.[26][28][24] Filiform warts result from infection with specific human papillomavirus (HPV) strains, primarily types 1, 2, 4, 27, and 29, which target keratinocytes in the epidermis. These non-enveloped DNA viruses gain entry through minor skin abrasions, initiating localized hyperplasia. The condition is more frequently observed in warm, humid climates, where perspiration and moisture promote viral replication and transmission via skin-to-skin contact.[25][26][1]Genital Warts
Genital warts, also known as condyloma acuminata, are soft, moist growths that typically appear as small, skin-colored or flesh-colored bumps resembling the texture of a cauliflower when clustered, or as flat plaques in some cases; their color can vary from skin-toned to reddish-brown depending on the site and individual skin type.[29][30] These lesions are distinct from cutaneous warts due to their occurrence on mucosal surfaces and association with sexual transmission, often presenting as papular or pedunculated growths on the genital and perianal mucosa.[30] They commonly develop on the vulva, vagina, cervix, penis (including the shaft and under the foreskin), scrotum, anus, perineum, and perianal skin; internal locations such as the urethra, anal canal, or vagina are possible, and oral cavity involvement can occur following orogenital contact.[29][30] In women, vulvar and vaginal sites are frequent, while in men, the penile shaft and scrotum are typical; perianal warts are more common in individuals engaging in receptive anal intercourse.[30] Rarely, they may appear in the mouth or throat from oral-genital contact.[29] Genital warts are primarily caused by low-risk human papillomavirus (HPV) types 6 and 11, which account for approximately 90% of cases, though high-risk types such as HPV 16 or 18 may occasionally be involved, potentially linking to precursors of cervical or anal cancers if undetected.[30] These infections are sexually transmitted and carry implications for sexual health, including the need for partner notification and screening for co-infections or oncogenic strains.[30][31] Most genital warts are asymptomatic, but they can cause itching, discomfort, or bleeding, particularly during sexual activity or if located in sensitive areas like the anus or vagina.[29][31] Recurrence is common, often within three months of treatment, due to viral latency and persistence of subclinical infections rather than new exposures, highlighting the chronic nature of HPV in the anogenital region.[30]Epidemiology
Prevalence and Distribution
Cutaneous warts, caused by various human papillomavirus (HPV) types, affect approximately 7–12% of the general population at any given time.[22] Prevalence is notably higher among children and adolescents, reaching up to 10–33% in school-aged groups, with incidence peaking between ages 12 and 16 years.[32][33] These patterns reflect the virus's transmission dynamics in communal settings like schools, where close contact facilitates spread.[1] Geographically, warts occur worldwide but show variations in distribution, with higher incidence reported in urban areas compared to rural ones, likely due to denser populations and increased opportunities for transmission.[34][35] Seasonal fluctuations are evident, particularly in temperate regions, where outpatient visits for warts increase during winter months, possibly linked to drier skin and indoor crowding.[36][37] For genital warts specifically, the point prevalence among sexually active adults is around 1%, representing a visible manifestation of HPV infection.[38] Overall, approximately 80% of sexually active individuals acquire at least one HPV infection over their lifetime, though the majority remain subclinical and resolve without warts.[39] Since the introduction of HPV vaccines targeting low-risk types like HPV-6 and HPV-11, genital wart prevalence has declined substantially, with reductions of 50–90% observed in vaccinated cohorts by the 2020s; as of 2024, studies show continued reductions exceeding 80% in some populations, including decreased hospitalizations among young adults.[40][41][42]Risk Factors and Demographics
Warts are most prevalent among children and adolescents, particularly those aged 10 to 14 years, due to frequent skin trauma from play and shared environments such as schools and pools that facilitate viral exposure.[43] In school-aged children, the prevalence can reach 10-33%.[44] For genital warts specifically, incidence rates are slightly higher in males, with studies reporting medians of 137 per 100,000 in men compared to 120 per 100,000 in women, and one cohort showing 73% of cases in males.[45][46] Key risk factors include immunosuppression, which substantially elevates susceptibility; for instance, in solid organ transplant recipients on long-term therapy, wart prevalence rises to 50-92% after five years, representing a 50-100% higher incidence than in the general population.[47] Similarly, individuals with HIV or other immunosuppressive conditions experience more extensive and persistent warts due to impaired cell-mediated immunity.[48] Skin breaks from habits like nail biting or shaving provide entry points for the virus, increasing infection risk in affected areas such as the hands or face.[3][49] Occupational exposure heightens risk for certain groups, notably meat handlers who develop "butchers' warts" from frequent contact with raw meat contaminated by HPV type 7, with prevalence historically ranging from 8.5% to 23.8% in this profession.[50] Genetic predispositions, such as the rare syndrome epidermodysplasia verruciformis caused by mutations in EVER1 or EVER2 genes, lead to lifelong susceptibility to widespread HPV-induced warts and skin cancers.[51] In the elderly, age-related immune decline (immunosenescence) contributes to higher wart persistence and recurrence by reducing the body's ability to clear infections.[52] Behavioral factors further modulate risk; poor hygiene practices, such as inadequate handwashing or sharing personal items, promote viral spread in communal settings.[53] For genital warts, multiple sexual partners significantly increase exposure, as the virus transmits primarily through intimate contact.[54]Etiology
Viral Causation
Warts are exclusively caused by infection with human papillomavirus (HPV), a group of small, non-enveloped DNA viruses belonging to the Papillomaviridae family.[55] Over 200 distinct HPV types have been identified, classified into low-risk and high-risk groups based on their potential to cause disease; benign cutaneous and mucosal warts are primarily induced by low-risk types that do not typically lead to malignancy.[54] These viruses specifically target keratinocytes, the primary cells of the epidermis, where they replicate and induce hyperproliferation leading to wart formation.[1] Cutaneous warts on non-genital skin are most commonly associated with HPV types 1, 2, 4, 27, and 57, while mucosal warts, such as those in the anogenital region, are predominantly caused by types 6 and 11.[56] In contrast, high-risk HPV types like 16 and 18 possess oncogenic potential and are linked to cancers of the cervix, anus, and oropharynx, but they rarely cause benign warts.[55] No bacterial, fungal, or other non-viral pathogens are known to cause warts; all verified cases trace back to HPV infection.[57] HPV infections can establish latency, persisting asymptomatically in basal keratinocytes for months to years without visible lesions, and may reactivate under conditions such as immune suppression or psychological stress.[58] During latency, the viral genome remains episomal and transcriptionally quiescent, evading host detection until triggered, which can result in recurrent or new wart outbreaks.[59] This latent phase underscores the virus's ability to maintain long-term carriage in the host population.[60]Transmission Modes
Warts, caused by various strains of the human papillomavirus (HPV), are primarily transmitted through direct skin-to-skin contact, allowing the virus to enter the body via minor cuts, abrasions, or disrupted epithelial barriers. This mode facilitates both interpersonal spread and autoinoculation, where an individual spreads the virus to other parts of their own body through activities like scratching or shaving affected areas. For common warts, transmission often occurs in close-contact settings such as schools or households, where children may unknowingly share the virus through play or shared objects, leading to outbreaks among primary schoolchildren with higher incidence linked to family and peer exposure.[1][61][62] Plantar warts, affecting the soles of the feet, are particularly associated with environments like communal showers, locker rooms, and swimming pool decks, where barefoot contact with contaminated moist surfaces increases risk; studies show shower users have up to 27% prevalence compared to non-users. Genital warts, in contrast, spread mainly through sexual activity, including vaginal, anal, or oral sex, as well as intimate skin-to-skin contact, with transmission rates estimated at around 4-5 per 100 person-months in heterosexual couples, though cumulative risk to partners can reach 60% over multiple encounters. The incubation period for wart development typically ranges from 3 weeks to 8 months, with most appearing 2-3 months after infection.[63][64][65][66][67] Indirect transmission via fomites, such as towels, razors, or floors, is possible but less common, as HPV can survive on moist surfaces for up to several days, retaining infectivity in laboratory models for at least 7 days under damp conditions. However, the virus has no known animal reservoir, with humans serving as the sole natural host, limiting zoonotic spread.[68][69][70][54][71]Pathophysiology
Viral Infection Process
The human papillomavirus (HPV) initiates infection in the basal layer of stratified squamous epithelium, targeting undifferentiated keratinocytes. Entry occurs primarily through micro-abrasions or wounds that expose the basement membrane, allowing the viral capsid—composed mainly of the major L1 protein—to bind to heparan sulfate proteoglycans on the extracellular matrix or cell surface.[72] This attachment facilitates endocytosis into the basal keratinocyte, where the viral genome is uncoated and transported to the nucleus, establishing infection without immediate cell lysis.[73] Upon nuclear entry, the circular double-stranded DNA genome persists as an extrachromosomal episome, typically at 20–100 copies per cell in the basal layer. Early viral genes, including E6 and E7, are transcribed from the early promoter, with E6 promoting degradation of p53 to inhibit apoptosis and E7 inactivating retinoblastoma protein (pRb) to drive cell cycle progression into S-phase, enabling initial low-level replication of the viral genome in synchrony with host DNA.[74] As infected keratinocytes divide and migrate upward during epithelial differentiation, a switch to the late promoter amplifies genome copies to thousands per cell; late genes L1 and L2 are then expressed in suprabasal layers to assemble new icosahedral virions within the nucleus.[73] Unlike lytic viruses, HPV does not destroy host cells; instead, mature virions accumulate in the granular layer and are released passively through desquamation of the uppermost cornified cells, completing the productive cycle over the ~28-day epithelial turnover without triggering overt cell death.[74] This replication strategy induces hyperproliferation of infected keratinocytes, resulting in acanthosis (epidermal thickening due to expanded spinous layer) and hyperkeratosis (excessive keratin production in the stratum corneum), which manifest as the visible wart lesion.[73] The full infectious cycle from entry to virion shedding aligns with host differentiation, typically spanning weeks to months for lesion development, though episomal genomes can maintain long-term persistence in basal cells for years to decades by replicating only in dividing progeny.[74]Host Immune Response
The human papillomavirus (HPV), responsible for cutaneous warts, employs several strategies to evade the host immune response during initial infection. Notably, HPV lacks pathogen-associated molecular patterns (PAMPs) that typically trigger innate immune detection, allowing it to infect keratinocytes without eliciting strong danger signals.[75] Additionally, the viral E7 oncoprotein downregulates major histocompatibility complex (MHC) class I expression on infected cells, reducing their visibility to cytotoxic T cells and facilitating persistent infection.[76] Clearance of warts primarily relies on cell-mediated immunity, where CD4+ helper T cells, CD8+ cytotoxic T cells, and natural killer (NK) cells infiltrate the lesion to target HPV-infected keratinocytes. Cytokines such as interferon-gamma (IFN-γ), produced by these cells, play a crucial role in inducing apoptosis and limiting viral replication, leading to spontaneous regression in approximately two-thirds of cases within two years.[77] In contrast, humoral immunity, involving B cells and antibodies, contributes minimally to wart clearance, as HPV primarily resides intracellularly and does not provoke robust antibody responses sufficient for resolution.[78] Signs of impending regression often include localized inflammation within the wart, characterized by erythema, swelling, and lymphocytic infiltration, which signals effective immune activation.[79] However, in about one-third of cases, immune failure results in persistent warts due to inadequate T-cell responses or viral persistence.[80] Recurrence of warts frequently stems from latent HPV reservoirs in basal keratinocytes, where the virus maintains low-level persistence without active replication. This risk is markedly heightened in states of immunosuppression, such as HIV infection or organ transplantation, where impaired T-cell function allows reactivation and proliferation of latent virus.[81][82]Diagnosis
Clinical Examination
Clinical examination of warts primarily involves visual inspection and simple physical maneuvers to identify characteristic features suggestive of human papillomavirus (HPV) infection, often sufficient for diagnosis in typical cases.[6] Warts appear as well-demarcated, hyperkeratotic papules with a rough, verrucous surface, typically measuring 2-10 mm in diameter and exhibiting colors ranging from skin-toned to gray, yellow, brown, or black.[13] A hallmark finding during inspection is the presence of small black dots within the lesion, representing thrombosed capillaries in the papillary dermis.[83] Dermoscopy enhances visualization, revealing dotted or linear vessels, hairpin-like structures, and bleeding spots amid papillomatous growth patterns; in some cases, a mosaic pattern of grouped vessels surrounded by white reticular networks may be observed, particularly in genital warts.[84][85] Physical tests further aid in confirming the diagnosis at the bedside. Paring the lesion's surface with a scalpel or curette exposes the black dots as pinpoint bleeding points from thrombosed vessels, distinguishing warts from similar hyperkeratotic lesions.[6][13] For plantar warts, applying lateral pressure with a tongue blade elicits sharp pain due to involvement of deeper dermal structures, unlike the diffuse discomfort of calluses.[83][13] Similar distinctions apply to common warts on the fingers and hands, where lateral compression often elicits pain, in contrast to calluses. Common warts present as small, rough, grainy bumps that are fleshy or raised, frequently featuring tiny black dots (clotted blood vessels), caused by HPV infection, and contagious.[3][28] Calluses appear as thick, hardened, dry patches of skin that are flat or slightly raised, without black dots, resulting from repeated friction or pressure, and are usually painless or tender only when pressed directly. Blood blisters are raised, fluid-filled pockets appearing red, purple, or black from pooled blood, caused by acute pinching or injury, initially painful, and typically resolving on their own within about a week.[86] Key distinguishing features include black dots suggesting a wart, solid hardened skin without dots indicating a callus, and fluid-filled dark appearance pointing to a blood blister. Consultation with a healthcare professional is recommended if the diagnosis remains uncertain, the lesion is painful, or persistent. Additionally, disruption of normal dermatoglyphic patterns (skin ridges) on weight-bearing surfaces serves as a cardinal sign.[13] Clinicians routinely inquire about the patient's history of skin trauma or exposure to shared surfaces, as warts often arise at sites of minor injury via the Koebner phenomenon.[83] Site-specific clues include linear arrangements of lesions along scratch lines or shave paths, such as on the beard area in men due to autoinoculation during grooming.[15] Warts are most prevalent in children and young adults, where common types frequently affect the hands and knees following playground injuries.[15] In sexually active adults, genital warts may present as softer, coalescing papules in the anogenital region, prompting consideration of sexual history alongside physical findings.[85]Confirmatory Tests
Confirmatory tests for warts are typically reserved for cases where clinical examination yields inconclusive results, such as atypical growths, persistent lesions despite treatment, or patients with immunosuppression, to rule out differentials like seborrheic keratosis or malignancy.[87][88] Biopsy serves as the gold standard for histopathological confirmation, involving either shave or punch techniques to obtain tissue samples for microscopic analysis. Shave biopsy is often preferred for superficial lesions, while punch biopsy allows deeper sampling for plantar or thicker warts.[89][90] Histopathological examination reveals characteristic features including koilocytes—keratinocytes with perinuclear halos and pyknotic nuclei—along with acanthosis (epidermal thickening), parakeratosis (retained nuclei in the stratum corneum), hyperkeratosis, and papillomatosis.[89][91] Polymerase chain reaction (PCR) testing for HPV DNA typing provides molecular confirmation, particularly useful for genital warts or in research settings to identify specific high-risk HPV strains. This sensitive technique amplifies viral DNA from lesion swabs or biopsies, aiding in cases of subclinical infection or treatment monitoring.[91][87] Acetic acid whitening, involving application of 3-5% acetic acid for 5-10 minutes, highlights subclinical or flat lesions by turning HPV-infected epithelium white, enhancing visibility in genital areas but prone to false positives from conditions like inflammation.[87][88] Imaging modalities are rarely employed but ultrasound can assess the depth and extent of plantar warts, appearing as hypoechoic subdermal lesions with possible surrounding hyperkeratosis, guiding treatment for deeply invasive cases.[92][93]Prevention
Hygiene Practices
Maintaining personal hygiene is essential for reducing the risk of human papillomavirus (HPV) transmission that causes warts, including preventing autoinoculation where the virus spreads from one part of the body to another. Regular handwashing with soap and water after touching warts or potentially contaminated surfaces helps remove viral particles and lowers the likelihood of spread, as HPV can persist on skin and fomites. [53] Keeping skin clean and dry, particularly in moist areas prone to infection like hands and feet, minimizes the virus's ability to enter through minor cuts or abrasions. [28] Individuals with existing warts should cover them with bandages or waterproof plasters, especially during activities like swimming, to limit direct contact and shedding of viral particles. [8] Avoiding the sharing of personal items such as razors, towels, nail clippers, and socks is a key behavioral measure to prevent indirect transmission of HPV, as these items can harbor the virus even without visible blood or fluids. [94] For periungual warts around the nails, proper nail care practices like keeping nails trimmed short and clean reduce skin breaks that serve as entry points for the virus, thereby preventing spread to adjacent areas. [95] Biting or picking at cuticles should be avoided, as this habit facilitates autoinoculation in the nail region. [96] In shared environments like pools, locker rooms, and gyms, wearing flip-flops or protective footwear prevents direct contact with contaminated wet surfaces where HPV thrives. [94] Disinfecting high-touch surfaces, such as floors or benches, with a 1:10 dilution of household bleach (sodium hypochlorite) effectively inactivates HPV, as the virus is susceptible to hypochlorite-based solutions when applied for sufficient contact time. [97] For genital warts caused by low-risk HPV types, consistent condom use during sexual activity provides partial protection by covering areas of potential transmission, though it does not eliminate risk due to skin-to-skin contact beyond the covered region. Avoiding sexual contact during active outbreaks further reduces partner transmission, emphasizing communication and abstinence until lesions resolve. [30]Vaccination Strategies
Vaccination strategies for preventing warts primarily involve prophylactic human papillomavirus (HPV) vaccines that target specific viral strains responsible for genital warts, with limited applicability to cutaneous warts. The primary vaccine used is Gardasil 9, a nonavalent formulation that protects against nine HPV types, including 6 and 11, which cause approximately 90% of genital warts, as well as high-risk types 16 and 18 associated with cervical cancer and other malignancies.[98] Another option, Cervarix, is a bivalent vaccine targeting high-risk HPV types 16 and 18 for cancer prevention but offers no direct protection against wart-causing low-risk types like 6 and 11.[98] These vaccines generate antibodies that prevent initial HPV infection but do not treat existing warts or infections.[99] Clinical trials have demonstrated high efficacy for Gardasil 9, with nearly 100% effectiveness in preventing genital warts caused by HPV types 6 and 11 when administered before exposure.[98] Population-level data indicate 90-100% reductions in vaccine-targeted HPV infections and associated genital warts among vaccinated individuals.[100] Additionally, widespread vaccination has led to indirect herd immunity, reducing genital wart incidence by up to 90% in unvaccinated populations through decreased transmission.[101] However, herd effects are limited for cutaneous warts, as current vaccines do not cover the diverse HPV types (e.g., HPV-2) responsible for common and plantar warts on non-genital skin. Emerging research as of 2025 suggests that HPV vaccines like Gardasil 9 may offer off-label benefits in preventing recurrence of cutaneous warts, with complete response rates of 44-62% in some studies, possibly due to cross-reactive immune responses, though this is not yet standard prophylaxis and requires further validation.[102][103] The U.S. Centers for Disease Control and Prevention (CDC) recommends routine HPV vaccination at ages 11-12 years, with initiation possible as early as age 9, and catch-up vaccination through age 26 for those not adequately vaccinated earlier.[104] The schedule consists of two doses, 6-12 months apart, for individuals starting before age 15; three doses (at 0, 1-2, and 6 months) are required for those initiating at ages 15-26 or with immunocompromising conditions.[99] For ages 27-45, vaccination is based on shared clinical decision-making due to potentially lower benefits from prior exposures.[104] Post-vaccination surveillance data from the 2020s show an 88% decline in HPV types causing genital warts among U.S. adolescent girls since vaccine introduction in 2006, highlighting the public health impact.[99] Despite these advances, limitations persist: HPV vaccines do not protect against all wart-causing strains, particularly those affecting cutaneous sites like HPV-2, which is prevalent in common hand and foot warts.[103] Ongoing research explores broader-spectrum vaccines, but current formulations remain focused on anogenital protection.[105]Treatment
Topical Medications
Topical medications for warts primarily involve keratolytic agents that chemically destroy wart tissue or immune modulators that stimulate the host's antiviral response. Salicylic acid, available in concentrations of 17% to 40% as plasters, solutions, or soaks, acts as a keratolytic by softening and peeling away the hyperkeratotic layers of the wart, allowing penetration to infected keratinocytes.[106] Daily application is recommended, often after soaking the wart in warm water to enhance efficacy, with treatment durations typically spanning 12 weeks. Pooled data from placebo-controlled trials indicate cure rates of approximately 75% for salicylic acid compared to 48% for placebo, though overall efficacy ranges from 50% to 70% depending on wart location and patient compliance.[107] Over-the-counter formulations are suitable for common warts on hands and feet, while higher concentrations or combined preparations may require prescription.[108] Other topical agents target specific wart types, particularly genital warts caused by low-risk human papillomavirus strains. Imiquimod, a prescription immune response modifier in 5% cream, enhances local cytokine production to promote viral clearance, achieving complete wart resolution in about 50% of patients after 8 to 16 weeks of thrice-weekly application.[109] Podophyllotoxin, an antimitotic resin derivative available as a 0.5% solution or gel, disrupts cell division in rapidly proliferating wart tissue and is applied twice daily for three days followed by four days off, yielding clearance rates of around 72% in genital warts.[110] Topical 5-fluorouracil (5-FU), a cytotoxic pyrimidine analog in 5% cream, inhibits DNA synthesis in HPV-infected cells and has shown high efficacy, with 95% complete eradication of plantar warts after 12 weeks when used with occlusion.[111] These agents are prescription-only for genital applications due to potential systemic absorption risks.[112] Common side effects across these medications include local skin irritation, erythema, burning, and desquamation, with salicylic acid occasionally causing hyperpigmentation or ulceration in sensitive areas.[108] Imiquimod may induce flu-like symptoms or severe inflammation in 10-20% of users, while podophyllotoxin and 5-FU can lead to blistering or necrosis if overapplied.[113] Recent studies have explored microwave therapy as an adjunct to topical treatments like salicylic acid for recalcitrant plantar warts, reporting 80-83% resolution rates after 2-3 sessions combined with daily applications.[114]Procedural Methods
Procedural methods for treating warts involve clinician-administered techniques that physically destroy or excise the affected tissue, typically reserved for cases where topical treatments have failed or for multiple, large, or recalcitrant lesions. These office-based procedures aim to ablate the wart and stimulate an immune response against the human papillomavirus (HPV), though recurrence remains possible due to viral persistence.[115] Cryotherapy, one of the most common procedural approaches, uses liquid nitrogen at -196°C to freeze the wart tissue, causing cell death through ice crystal formation and vascular damage. It typically requires 1 to 4 sessions spaced at 2-week intervals to allow the full therapeutic effect to develop (typically observable after 10-14 days), facilitate healing of blisters or wounds, and reduce risks including severe pain, infection, scarring, or pigment changes associated with premature re-treatment, with each application lasting 10 to 20 seconds, and achieves a cure rate of 70-80% for common warts.[116][117] For a finger wart (a type of common wart), the healing stages after successful cryotherapy typically progress as follows:- Immediately after freezing: The wart turns white or pale (frosted appearance), and the surrounding skin becomes red and swollen.
- Within 24-48 hours: A blister often forms under or around the wart, which may be painful, clear, or blood-filled.
- Over the next 1-2 weeks: The blister dries, the dead wart tissue darkens (often black), forms a scab or crust, and eventually falls off.
- After the scab falls off: New pink or normal-colored skin appears underneath, with no wart remaining.
- Full healing: The skin returns to its normal appearance, usually within a few weeks, with possible mild temporary discoloration or scarring that fades.


