Recent from talks
Contribute something
Nothing was collected or created yet.
Shingles
View on Wikipedia
| Shingles | |
|---|---|
| Other names | Herpes zoster |
 | |
| Herpes zoster blisters on the neck and shoulder | |
| Specialty | Dermatology |
| Symptoms | Painful rash |
| Complications | Meningitis, facial nerve palsy, keratitis, postherpetic neuralgia[1] |
| Duration | 2–4 weeks[2] |
| Causes | Varicella zoster virus (VZV)[1] |
| Risk factors | Old age, poor immune function, having had chickenpox before 18 months of age[1] |
| Diagnostic method | Based on symptoms[3] |
| Differential diagnosis | Herpes simplex, chest pain, insect bites, cutaneous leishmaniasis[4] |
| Prevention | Shingles vaccine[1], chickenpox vaccine (before the child gets chickenpox)[5] |
| Medication | Aciclovir (if given early), pain medication[3] |
| Frequency | 33% (at some point)[1] |
| Deaths | 6,400 per year (including chickenpox deaths)[6] |
Shingles, also known as herpes zoster or zona,[7] is a viral disease characterized by a painful skin rash with blisters in a localized area.[2][8] Typically the rash occurs in a single, wide mark either on the left or right side of the body or face.[1] Two to four days before the rash occurs, there may be tingling or local pain in the area.[1][9] Other common symptoms are fever, headache, and tiredness.[1][10] The rash usually heals within two to four weeks,[2] but some people develop ongoing nerve pain which can last for months or years, a condition called postherpetic neuralgia (PHN).[1] In those with poor immune function the rash may occur widely.[1] If the rash involves the eye, vision loss may occur.[2][11]
Shingles is caused by the varicella zoster virus (VZV) that also causes chickenpox. In the case of chickenpox, also called varicella, the initial infection with the virus typically occurs during childhood or adolescence.[1] Once the chickenpox has resolved, the virus can remain dormant (inactive) in human nerve cells (dorsal root ganglia or cranial nerves)[12] for years or decades, after which it may reactivate and travel along nerve bodies to nerve endings in the skin, producing blisters.[1][9] During an outbreak of shingles, exposure to the varicella virus found in shingles blisters can cause chickenpox in someone who has not yet had chickenpox, although that person will not suffer from shingles, at least on the first infection.[13] How the virus remains dormant in nerve cells or subsequently re-activates is not well understood.[1][14]
The disease has been recognized since ancient times.[1] Risk factors for reactivation of the dormant virus include old age, poor immune function, and having contracted chickenpox before 18 months of age.[1] Diagnosis is typically based on the signs and symptoms presented.[3] Varicella zoster virus is not the same as herpes simplex virus, although they both belong to the alpha subfamily of herpesviruses.[15]
Shingles vaccines reduce the risk of shingles by 50 to 90%, depending on the vaccine used.[1][16] Vaccination also decreases rates of postherpetic neuralgia, and, if shingles occurs, its severity.[1] If shingles develops, antiviral medications such as aciclovir can reduce the severity and duration of disease if started within 72 hours of the appearance of the rash.[3] Evidence does not show a significant effect of antivirals or steroids on rates of postherpetic neuralgia.[17][18] Paracetamol, NSAIDs, or opioids may be used to help with acute pain.[3]
It is estimated that about a third of people develop shingles at some point in their lives.[1] While shingles is more common among older people, children may also get the disease.[15] According to the US National Institutes of Health, the number of new cases per year ranges from 1.2 to 3.4 per 1,000 person-years among healthy individuals to 3.9 to 11.8 per 1,000 person-years among those older than 65 years of age.[10][19] About half of those living to age 85 will have at least one attack, and fewer than 5% will have more than one attack.[1][20] Although symptoms can be severe, risk of death is very low: 0.28 to 0.69 deaths per million.[12]
Signs and symptoms
[edit]

The earliest symptoms of shingles, which include headache, fever, and malaise, are nonspecific, and may result in an incorrect diagnosis.[10][21] These symptoms are commonly followed by sensations of burning pain, itching, hyperesthesia (oversensitivity), or paresthesia ("pins and needles": tingling, pricking, or numbness).[22] Pain can be mild to severe in the affected dermatome, with sensations that are often described as stinging, tingling, aching, numbing or throbbing, and can be interspersed with quick stabs of agonizing pain.[23]
Shingles in children is often painless, but people are more likely to get shingles as they age, and the disease tends to be more severe.[24]
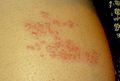
In most cases, after one to two days—but sometimes as long as three weeks—the initial phase is followed by the appearance of the characteristic skin rash. The pain and rash most commonly occur on the torso but can appear on the face, eyes, or other parts of the body. At first, the rash appears similar to the first appearance of hives; however, unlike hives, shingles causes skin changes limited to a dermatome, normally resulting in a stripe or belt-like pattern that is limited to one side of the body and does not cross the midline.[22] Zoster sine herpete ("zoster without herpes") describes a person who has all of the symptoms of shingles except this characteristic rash.[25]
Later, the rash becomes vesicular, forming small blisters filled with a serous exudate, as the fever and general malaise continue. The painful vesicles eventually become cloudy or darkened as they fill with blood, and crust over within seven to ten days; usually the crusts fall off and the skin heals, but sometimes, after severe blistering, scarring and discolored skin remain.[22] The blister fluid contains varicella zoster virus, which can be transmitted through contact or inhalation of fluid droplets until the lesions crust over, which may take up to four weeks.[26]
| Day 1 | Day 2 | Day 5 | Day 6 |
|---|---|---|---|

|
|

|

|
Face
[edit]Shingles may have additional symptoms, depending on the dermatome involved. The trigeminal nerve is the most commonly involved nerve,[27] of which the ophthalmic division is the most commonly involved branch.[28] When the virus is reactivated in this nerve branch it is termed zoster ophthalmicus. The skin of the forehead, upper eyelid and orbit of the eye may be involved. Zoster ophthalmicus occurs in approximately 10% to 25% of cases. In some people, symptoms may include conjunctivitis, keratitis, uveitis, and optic nerve palsies that can sometimes cause chronic ocular inflammation, loss of vision, and debilitating pain.[29]
Shingles oticus, also known as Ramsay Hunt syndrome type II, involves the ear. It is thought to result from the virus spreading from the facial nerve to the vestibulocochlear nerve. Symptoms include hearing loss and vertigo (rotational dizziness).[30]
Shingles may occur in the mouth if the maxillary or mandibular division of the trigeminal nerve is affected,[31] in which the rash may appear on the mucous membrane of the upper jaw (usually the palate, sometimes the gums of the upper teeth) or the lower jaw (tongue or gums of the lower teeth) respectively.[32] Oral involvement may occur alone or in combination with a rash on the skin over the cutaneous distribution of the same trigeminal branch.[31] As with shingles of the skin, the lesions tend to only involve one side, distinguishing it from other oral blistering conditions.[32] In the mouth, shingles appears initially as 1–4 mm opaque blisters (vesicles),[31] which break down quickly to leave ulcers that heal within 10–14 days.[32] The prodromal pain (before the rash) may be confused with toothache.[31] Sometimes this leads to unnecessary dental treatment.[32] Post-herpetic neuralgia is uncommonly associated with shingles in the mouth.[32] Unusual complications may occur with intra-oral shingles that are not seen elsewhere. Due to the close relationship of blood vessels to nerves, the virus can spread to involve the blood vessels and compromise the blood supply, sometimes causing ischemic necrosis.[31] In rare cases, oral involvement causes complications such as osteonecrosis, tooth loss, periodontitis (gum disease), pulp calcification, pulp necrosis, periapical lesions and tooth developmental anomalies.[27]
Disseminated shingles
[edit]In those with deficits in immune function, disseminated shingles may occur (wide rash).[1] It is defined as more than 20 skin lesions appearing outside either the primarily affected dermatome or dermatomes directly adjacent to it. Besides the skin, other organs, such as the liver or brain, may also be affected (causing hepatitis or encephalitis,[33][34] respectively), making the condition potentially lethal.[35]: 380
Pathophysiology
[edit]

The causative agent for shingles is the varicella zoster virus (VZV)—a double-stranded DNA virus related to the herpes simplex virus. Most individuals are infected with this virus as children, which causes an episode of chickenpox. The immune system eventually eliminates the virus from most locations, but it remains dormant (or latent) in the ganglia adjacent to the spinal cord (called the dorsal root ganglion) or the trigeminal ganglion in the base of the skull.[37]
Shingles occurs only in people who have been previously infected with VZV; although it can occur at any age, approximately half of the cases in the United States occur in those aged 50 years or older.[38] Shingles can recur.[39] In contrast to the frequent recurrence of herpes simplex symptoms, repeated attacks of shingles are unusual.[40] It is extremely rare for a person to have more than three recurrences.[37]
The disease results from virus particles in a single sensory ganglion switching from their latent phase to their active phase.[41] Due to difficulties in studying VZV reactivation directly in humans (leading to reliance on small-animal models), its latency is less well understood than that of the herpes simplex virus.[40] Virus-specific proteins continue to be made by the infected cells during the latent period, so true latency, as opposed to chronic, low-level, active infection, has not been proven to occur in VZV infections.[42][43] Although VZV has been detected in autopsies of nervous tissue,[44] there are no methods to find dormant virus in the ganglia of living people.
Unless the immune system is compromised, it suppresses reactivation of the virus and prevents shingles outbreaks. Why this suppression sometimes fails is poorly understood,[45] but shingles is more likely to occur in people whose immune systems are impaired due to aging, immunosuppressive therapy, psychological stress, or other factors.[46][47] Upon reactivation, the virus replicates in neuronal cell bodies, and virions are shed from the cells and carried down the axons to the area of skin innervated by that ganglion. In the skin, the virus causes local inflammation and blistering. The short- and long-term pain caused by shingles outbreaks originates from inflammation of affected nerves due to the widespread growth of the virus in those areas.[48]
As with chickenpox and other forms of alpha-herpesvirus infection, direct contact with an active rash can spread the virus to a person who lacks immunity to it. This newly infected individual may then develop chickenpox, but will not immediately develop shingles.[22]
The complete sequence of the viral genome was published in 1986.[49]
Diagnosis
[edit]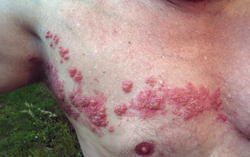
If the rash has appeared, identifying this disease (making a differential diagnosis) requires only a visual examination, since very few diseases produce a rash in a dermatomal pattern. However, herpes simplex virus (HSV) can occasionally produce a rash in such a pattern (zosteriform herpes simplex).[50][51]
When the rash is absent (early or late in the disease, or in the case of zoster sine herpete), shingles can be difficult to diagnose.[52] Apart from the rash, most symptoms can also occur in other conditions.
Laboratory tests are available to diagnose shingles. The most popular test detects VZV-specific IgM antibody in blood; this appears only during chickenpox or shingles and not while the virus is dormant.[53] In larger laboratories, lymph collected from a blister is tested by polymerase chain reaction (PCR) for VZV DNA, or examined with an electron microscope for virus particles.[54] Molecular biology tests based on in vitro nucleic acid amplification (PCR tests) are currently considered the most reliable. Nested PCR test has high sensitivity, but is susceptible to contamination leading to false positive results. The latest real-time PCR tests are rapid, easy to perform, and as sensitive as nested PCR, and have a lower risk of contamination. They also have more sensitivity than viral cultures.[55]
Differential diagnosis
[edit]Shingles can be confused with herpes simplex, dermatitis herpetiformis and impetigo, and skin reactions caused by contact dermatitis, candidiasis, certain drugs and insect bites.[56]
Prevention
[edit]Shingles risk can be reduced in children by the chickenpox vaccine if the vaccine is administered before the individual gets chickenpox.[5] If primary infection has already occurred, there are shingles vaccines that reduce the risk of developing shingles or developing severe shingles if the disease occurs.[1][16] They include a live attenuated virus vaccine, Zostavax, and an adjuvanted subunit vaccine, Shingrix.[39][57][58]
A review by Cochrane concluded that Zostavax was useful for preventing shingles for at least three years.[9] This equates to about 50% relative risk reduction. The vaccine reduced rates of persistent, severe pain after shingles by 66% in people who contracted shingles despite vaccination.[59] Vaccine efficacy was maintained through four years of follow-up.[59] It has been recommended that people with primary or acquired immunodeficiency should not receive the live vaccine.[59]
Two doses of Shingrix are recommended, which provide about 90% protection at 3.5 years.[39][58] As of 2016, it had been studied only in people with an intact immune system.[16] It appears to also be effective in the very old.[16]
In the UK, shingles vaccination is offered by the National Health Service (NHS) to all people in their 70s. As of 2021[update], Zostavax is the usual vaccine, but the Shingrix vaccine is recommended if Zostavax is unsuitable, for example, for those with immune system issues. Vaccination is not available to people over 80 as "it seems to be less effective in this age group".[60][61] By August 2017, just under half of eligible 70–78 year olds had been vaccinated.[62] About 3% of those eligible by age have conditions that suppress their immune system, and should not receive Zostavax.[63] There had been 1,104 adverse reaction reports by April 2018.[63] In the US, it is recommended that healthy adults 50 years and older receive two doses of Shingrix, two to six months apart.[39][64]
Treatment
[edit]Treatment aims to limit the severity and duration of pain, shorten the duration of a shingles episode, and reduce complications. Symptomatic treatment is often needed for the complication of postherpetic neuralgia.[65] However, a study on untreated shingles shows that, once the rash has cleared, postherpetic neuralgia is very rare in people under 50 and wears off in time; in older people, the pain wore off more slowly, but even in people over 70, 85% were free from pain a year after their shingles outbreak.[66]
Analgesics
[edit]People with mild to moderate pain can be treated with over-the-counter pain medications. Topical lotions containing calamine can be used on the rash or blisters and may be soothing. Occasionally, severe pain may require an opioid medication, such as morphine. Once the lesions have crusted over, capsaicin cream (Zostrix) can be used. Topical lidocaine and nerve blocks may also reduce pain.[67] Administering gabapentin along with antivirals may offer relief of postherpetic neuralgia.[65]
Antivirals
[edit]Antiviral drugs may reduce the severity and duration of shingles;[68] however, they do not prevent postherpetic neuralgia.[69] Of these drugs, aciclovir has been the standard treatment, but the newer drugs valaciclovir and famciclovir demonstrate similar or superior efficacy and good safety and tolerability.[65] The drugs are used both for prevention (for example in people with HIV/AIDS) and as therapy during the acute phase. Complications in immunocompromised individuals with shingles may be reduced with intravenous aciclovir. In people who are at a high risk for repeated attacks of shingles, five daily oral doses of aciclovir are usually effective.[30]
Steroids
[edit]Corticosteroids do not appear to decrease the risk of long-term pain.[18] Side effects, however, appear to be minimal. Their use in Ramsay Hunt syndrome had not been properly studied as of 2008.[70]
Zoster ophthalmicus
[edit]
Treatment for zoster ophthalmicus is similar to standard treatment for shingles at other sites.[medical citation needed] A trial comparing aciclovir with its prodrug, valaciclovir, demonstrated similar efficacies in treating this form of the disease.[71]
Prognosis
[edit]The rash and pain usually subside within three to five weeks, but about one in five people develop a painful condition called postherpetic neuralgia, which is often difficult to manage. In some people, shingles can reactivate, presenting as zoster sine herpete: pain radiating along the path of a single spinal nerve (a dermatomal distribution), but without an accompanying rash. This condition may involve complications that affect several levels of the nervous system and cause many cranial neuropathies, polyneuritis, myelitis, or aseptic meningitis. Other serious effects that may occur in some cases include partial facial paralysis (usually temporary), ear damage, or encephalitis.[30] Although initial infections with VZV during pregnancy, causing chickenpox, may lead to infection of the fetus and complications in the newborn, chronic infection or reactivation in shingles are not associated with fetal infection.[72][73]
There is a slightly increased risk of developing cancer after a shingles episode. However, the mechanism is unclear, and mortality from cancer did not appear to increase as a direct result of the presence of the virus.[74] Instead, the increased risk may result from the immune suppression that allows the reactivation of the virus.[75]
Although shingles typically resolves within 3–5 weeks, certain complications may arise:
- Secondary bacterial infection.[11]
- Motor involvement,[11] including weakness especially in "motor herpes zoster".[76]
- Eye involvement: trigeminal nerve involvement (as seen in herpes ophthalmicus) should be treated early and aggressively as it may lead to blindness. Involvement of the tip of the nose in the zoster rash is a strong predictor of herpes ophthalmicus.[77]
- Postherpetic neuralgia, a condition of chronic pain following shingles.
Epidemiology
[edit]Varicella zoster virus (VZV) has a high level of infectivity and has a worldwide prevalence.[78] Shingles is a reactivation of latent VZV infection: zoster can only occur in someone who has previously had chickenpox (varicella).
Shingles has no relationship to season and does not occur in epidemics. There is, however, a strong relationship with increasing age.[24][46] The incidence rate of shingles ranges from 1.2 to 3.4 per 1,000 person‐years among younger healthy individuals, increasing to 3.9–11.8 per 1,000 person‐years among those older than 65 years,[10][24] and incidence rates worldwide are similar.[10][79] This relationship with age has been demonstrated in many countries,[10][79][80][81][82][83] and is attributed to the fact that cellular immunity declines as people grow older.
Another important risk factor is immunosuppression.[84][85][86] Other risk factors include psychological stress.[23][87][88] According to a study in North Carolina, "black subjects were significantly less likely to develop zoster than were white subjects."[89][90] It is unclear whether the risk is different by sex. Other potential risk factors include mechanical trauma and exposure to immunotoxins.[46][88]
There is no strong evidence for a genetic link or a link to family history. A 2008 study showed that people with close relatives who had shingles were twice as likely to develop it themselves,[91] but a 2010 study found no such link.[88]
Adults with latent VZV infection who are exposed intermittently to children with chickenpox receive an immune boost.[24][88] This periodic boost to the immune system helps to prevent shingles in older adults. When routine chickenpox vaccination was introduced in the United States, there was concern that, because older adults would no longer receive this natural periodic boost, there would be an increase in the incidence of shingles.
Multiple studies and surveillance data, at least when viewed superficially, demonstrate no consistent trends in incidence in the U.S. since the chickenpox vaccination program began in 1995.[92] However, upon closer inspection, the two studies that showed no increase in shingles incidence were conducted among populations where varicella vaccination was not as yet widespread in the community.[93][94] A later study by Patel et al. concluded that since the introduction of the chickenpox vaccine, hospitalization costs for complications of shingles increased by more than $700 million annually for those over age 60.[95] Another study by Yih et al. reported that as varicella vaccine coverage in children increased, the incidence of varicella decreased, and the occurrence of shingles among adults increased by 90%.[96] The results of a further study by Yawn et al. showed a 28% increase in shingles incidence from 1996 to 2001.[97] It is likely that incidence rate will change in the future, due to the aging of the population, changes in therapy for malignant and autoimmune diseases, and changes in chickenpox vaccination rates; a wide adoption of zoster vaccination could dramatically reduce the incidence rate.[10]
In one study, it was estimated that 26% of those who contract shingles eventually present complications. Postherpetic neuralgia arises in approximately 20% of people with shingles.[98] A study of 1994 California data found hospitalization rates of 2.1 per 100,000 person-years, rising to 9.3 per 100,000 person-years for ages 60 and up.[99] An earlier Connecticut study found a higher hospitalization rate; the difference may be due to the prevalence of HIV in the earlier study, or to the introduction of antivirals in California before 1994.[100]
History
[edit]Shingles has a long recorded history, although historical accounts fail to distinguish the blistering caused by VZV and those caused by smallpox,[38] ergotism, and erysipelas. Aulus Cornelius Celsus, around 25 BC to 50 AD, first used the term herpes zoster.[101] In the late 18th century William Heberden established a way to differentiate shingles and smallpox,[102] and in the late 19th century, shingles was differentiated from erysipelas. In 1831 Richard Bright hypothesized that the disease arose from the dorsal root ganglion, and an 1861 paper by Felix von Bärensprung confirmed this.[103]
Recognition that chickenpox and shingles were caused by the same virus came at the beginning of the 20th century. Physicians began to report that cases of shingles were often followed by chickenpox in younger people who lived with the person with shingles. The idea of an association between the two diseases gained strength when it was shown that lymph from a person with shingles could induce chickenpox in young volunteers. This was finally proved by the first isolation of the virus in cell cultures, by the Nobel laureate Thomas Huckle Weller, in 1953.[104] Some sources also attribute the first isolation of the herpes zoster virus to Evelyn Nicol.[105]
Until the 1940s, the disease was considered benign, and serious complications were thought to be very rare.[106] However, by 1942, it was recognized that shingles was a more serious disease in adults than in children and that it increased in frequency with advancing age. Further studies during the 1950s on immunosuppressed individuals showed that the disease was not as benign as once thought, and the search for various therapeutic and preventive measures began.[107] By the mid-1960s, several studies identified the gradual reduction in cellular immunity in old age, observing that in a cohort of 1,000 people who lived to the age of 85, approximately 500 (i.e., 50%) would have at least one attack of shingles, and 10 (i.e., 1%) would have at least two attacks.[108]
In historical shingles studies, shingles incidence generally increased with age. However, in his 1965 paper, Hope-Simpson suggested that the "peculiar age distribution of zoster may in part reflect the frequency with which the different age groups encounter cases of varicella and because of the ensuing boost to their antibody protection have their attacks of zoster postponed".[24] Lending support to this hypothesis that contact with children with chickenpox boosts adult cell-mediated immunity to help postpone or suppress shingles, a study by Thomas et al. reported that adults in households with children had lower rates of shingles than households without children.[109] Also, the study by Terada et al. indicated that pediatricians reflected incidence rates from 1/2 to 1/8 that of the general population their age.[110]
Etymology
[edit]The family name of all the herpesviruses derives from the Greek word έρπης herpēs,[111] from έρπω herpein ("to creep"),[112][113][114] referring to the latent, recurring infections typical of this group of viruses. Zoster comes from Greek ζωστήρ zōstēr,[115] meaning "belt" or "girdle", after the characteristic belt-like dermatomal rash.[116] The common name for the disease, shingles, derives from the Latin cingulus, a variant of Latin cingulum,[117] meaning "girdle".[118][119]
Research
[edit]Until the mid-1990s, infectious complications of the central nervous system (CNS) caused by VZV reactivation were regarded as rare. The presence of rash, as well as specific neurological symptoms, was required to diagnose a CNS infection caused by VZV. Since 2000, PCR testing has become more widely used, and the number of diagnosed cases of CNS infection has increased.[120]
Classic textbook descriptions state that VZV reactivation in the CNS is restricted to immunocompromised individuals and the elderly; however, studies have found that most participants are immunocompetent and younger than 60 years old. Historically, vesicular rash was considered a characteristic finding, but studies have found that rash is only present in 45% of cases.[120] In addition, systemic inflammation is not as reliable an indicator as previously thought: the mean level of C-reactive protein and mean white blood cell count are within the normal range in participants with VZV meningitis.[121] MRI and CT scans are usually normal in cases of VZV reactivation in the CNS. CSF pleocytosis, previously thought to be a strong indicator of VZV encephalitis, was absent in half of a group of people diagnosed with VZV encephalitis by PCR.[120]
The frequency of CNS infections presented at the emergency room of a community hospital is not negligible, so a means of diagnosing cases is needed. PCR is not a foolproof method of diagnosis, but because so many other indicators have turned out not to be reliable in diagnosing VZV infections in the CNS, PCR is the recommended method of testing for VZV. Negative PCR does not rule out VZV involvement, but a positive PCR can be used for diagnosis, and appropriate treatment started (for example, antivirals can be prescribed rather than antibiotics).[120]
The introduction of DNA analysis techniques has shown that some complications of varicella-zoster are more common than previously thought. For example, sporadic meningoencephalitis (ME) caused by varicella-zoster was regarded as a rare disease, mostly related to childhood chickenpox. However, meningoencephalitis caused by varicella-zoster is increasingly recognized as a predominant cause of ME among immunocompetent adults in non-epidemic circumstances.[122]
Diagnosis of complications of varicella-zoster, particularly in cases where the disease reactivates after years or decades of latency, is difficult. A rash (shingles) can be present or absent. Symptoms vary, and there is a significant overlap in symptoms with herpes simplex symptoms.[122]
Although DNA analysis techniques such as polymerase chain reaction (PCR) can be used to look for DNA of herpesviruses in spinal fluid or blood, the results may be negative, even in cases where other definitive symptoms exist.[123] Notwithstanding these limitations, the use of PCR has resulted in an advance in the state of the art in our understanding of herpesviruses, including VZV, during the 1990s and 2000s. For example, in the past, clinicians believed that encephalitis was caused by herpes simplex and that people always died or developed serious long-term functional problems. People were diagnosed at autopsy or by brain biopsy. Brain biopsy is not undertaken lightly: it is reserved only for serious cases that cannot be diagnosed by less invasive methods. For this reason, knowledge of these herpes virus conditions was limited to severe cases. DNA techniques have made it possible to diagnose "mild" cases, caused by VZV or HSV, in which the symptoms include fever, headache, and altered mental status. Mortality rates in treated people are decreasing.[122]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u Lopez A, Harrington T, Marin M (2015). "Chapter 22: Varicella". In Hamborsky J, Kroger A, Wolfe S (eds.). Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 30 December 2016. Retrieved 9 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d "Shingles (Herpes Zoster) Signs & Symptoms". Centers for Disease Control and Prevention (CDC). 1 May 2014. Archived from the original on 26 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e Cohen JI (July 2013). "Clinical practice: Herpes zoster". The New England Journal of Medicine. 369 (3): 255–263. doi:10.1056/NEJMcp1302674. PMC 4789101. PMID 23863052.
- ^ "Herpes Zoster Diagnosis, Testing, Lab Methods". Centers for Disease Control and Prevention (CDC). April 2022. Archived from the original on 3 June 2022. Retrieved 10 June 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Weinmann S, Naleway AL, Koppolu P, Baxter R, Belongia EA, Hambidge SJ, et al. (July 2019). "Incidence of Herpes Zoster Among Children: 2003–2014". Pediatrics. 144 (1): e20182917. doi:10.1542/peds.2018-2917. PMC 7748320. PMID 31182552. S2CID 184486904.*Lay summary in: "Two-for-One: Chickenpox Vaccine Lowers Shingles Risk in Children". Scientific American. 11 June 2019.
- ^ GBD 2015 Mortality and Causes of Death Collaborators (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ "Herpes zoster | Shingles, Varicella-Zoster, Pain Relief | Britannica". www.britannica.com. 18 September 2024. Retrieved 26 October 2024.
- ^ Sivapathasundharam B, Gururaj N, Ranganathan K (2014). "Viral Infections of the Oral Cavity". In Rajendran A, Sivapathasundharam B (eds.). Shafer's textbook of oral pathology (Seventh ed.). Elsevier Health Sciences ]. p. 351. ISBN 978-8131238004. Archived from the original on 17 December 2019. Retrieved 11 September 2017.
- ^ a b c de Oliveira Gomes, Juliana; Gagliardi, Anna Mz; Andriolo, Brenda Ng; Torloni, Maria Regina; Andriolo, Regis B.; Puga, Maria Eduarda Dos Santos; Canteiro Cruz, Eduardo (2 October 2023). "Vaccines for preventing herpes zoster in older adults". The Cochrane Database of Systematic Reviews. 2023 (10) CD008858. doi:10.1002/14651858.CD008858.pub5. ISSN 1469-493X. PMC 10542961. PMID 37781954.
- ^ a b c d e f g Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. (2007). "Recommendations for the management of herpes zoster". Clin. Infect. Dis. 44 (Suppl 1): S1–26. doi:10.1086/510206. PMID 17143845.
- ^ a b c Johnson RW, Alvarez-Pasquin MJ, Bijl M, Franco E, Gaillat J, Clara JG, et al. (July 2015). "Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective". Therapeutic Advances in Vaccines. 3 (4): 109–120. doi:10.1177/2051013615599151. PMC 4591524. PMID 26478818.
- ^ a b Pan CX, Lee MS, Nambudiri VE (2022). "Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review". Therapeutic Advances in Vaccines and Immunotherapy. 10. doi:10.1177/25151355221084535. PMC 8941701. PMID 35340552.
- ^ "Shingles (Herpes Zoster) Transmission". Centers for Disease Control and Prevention (CDC). 17 September 2014. Archived from the original on 6 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Researchers discover how chickenpox and shingles virus remains dormant". UCLH Biomedical Research Centre. 20 April 2018. Retrieved 25 April 2023.
- ^ a b "Overview". Centers for Disease Control and Prevention (CDC). 17 September 2014. Archived from the original on 16 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d Cunningham AL (2016). "The herpes zoster subunit vaccine". Expert Opinion on Biological Therapy. 16 (2): 265–271. doi:10.1517/14712598.2016.1134481. PMID 26865048. S2CID 46480440.
- ^ Chen N, Li Q, Yang J, Zhou M, Zhou D, He L (February 2014). "Antiviral treatment for preventing postherpetic neuralgia". The Cochrane Database of Systematic Reviews. 2014 (2) CD006866. doi:10.1002/14651858.CD006866.pub3. PMC 10583132. PMID 24500927.
- ^ a b Jiang X, Li Y, Chen N, Zhou M, He L (December 2023). "Corticosteroids for preventing postherpetic neuralgia". The Cochrane Database of Systematic Reviews. 2023 (12) CD005582. doi:10.1002/14651858.CD005582.pub5. PMC 10696631. PMID 38050854.
- ^ Nair PA, Patel BC (2 November 2021). "Herpes zoster". StatPearls. PMID 28722854. Archived from the original on 10 June 2022. Retrieved 10 June 2022 – via NCBI Bookshelf.
- ^ Schmader KE, Dworkin RH (2011). "Herpes Zoster and Postherpetic Neuralgia". In Benzon HT (ed.). Essentials of Pain Medicine (3rd ed.). London: Elsevier Health Sciences. p. 358. ISBN 978-1437735932. Archived from the original on 17 December 2019. Retrieved 11 September 2017.
- ^ Zamula E (May–June 2001). "Shingles: an unwelcome encore". FDA Consumer. 35 (3): 21–25. PMID 11458545. Archived from the original on 3 November 2009. Retrieved 5 January 2010. Revised June 2005.
- ^ a b c d Stankus SJ, Dlugopolski M, Packer D (2000). "Management of herpes zoster (shingles) and postherpetic neuralgia". Am. Fam. Physician. 61 (8): 2437–2444, 2447–2448. PMID 10794584. Archived from the original on 29 September 2007.
- ^ a b Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH (2004). "Acute pain in herpes zoster and its impact on health-related quality of life". Clin. Infect. Dis. 39 (3): 342–348. doi:10.1086/421942. PMID 15307000.
- ^ a b c d e Hope-Simpson RE (1965). "The nature of herpes zoster: a long-term study and a new hypothesis". Proceedings of the Royal Society of Medicine. 58 (1): 9–20. doi:10.1177/003591576505800106. PMC 1898279. PMID 14267505.
- ^ Furuta Y, Ohtani F, Mesuda Y, Fukuda S, Inuyama Y (2000). "Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy". Neurology. 55 (5): 708–710. doi:10.1212/WNL.55.5.708. PMID 10980741. S2CID 29270135.
- ^ "Shingles (herpes zoster)". Department of Health. New York State. January 2023. Retrieved 9 March 2023.
- ^ a b Gupta S, Sreenivasan V, Patil PB (2015). "Dental complications of herpes zoster: Two case reports and review of literature". Indian Journal of Dental Research. 26 (2): 214–219. doi:10.4103/0970-9290.159175. PMID 26096121.
- ^ Samaranayake L (2011). Essential Microbiology for Dentistry (4th ed.). Elsevier Health Sciences. pp. 638–642. ISBN 978-0702046957. Archived from the original on 8 September 2017.
- ^ Shaikh S, Ta CN (2002). "Evaluation and management of herpes zoster ophthalmicus". Am. Fam. Physician. 66 (9): 1723–1730. PMID 12449270. Archived from the original on 14 May 2008.
- ^ a b c Johnson RW, Dworkin RH (2003). "Clinical review: Treatment of herpes zoster and postherpetic neuralgia". BMJ. 326 (7392): 748–750. doi:10.1136/bmj.326.7392.748. PMC 1125653. PMID 12676845.
- ^ a b c d e Chi AC, Damm DD, Neville BW, Allen CM, Bouquot J (2008). "Viral Infections". Oral and Maxillofacial Pathology. Elsevier Health Sciences. pp. 250–253. ISBN 978-1437721973. Archived from the original on 8 September 2017.
- ^ a b c d e Glick M (2015). Burket's oral medicine (12th ed.). coco. pp. 62–65. ISBN 978-1607951889.
- ^ Chai W, Ho MG (November 2014). "Disseminated varicella zoster virus encephalitis". Lancet. 384 (9955): 1698. doi:10.1016/S0140-6736(14)60755-8. PMID 24999086.
- ^ Grahn A, Studahl M (September 2015). "Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment". Journal of Infection. 71 (3): 281–293. doi:10.1016/j.jinf.2015.06.004. PMID 26073188.
- ^ Elston DM, Berger TG, James WD (2006). Andrews' Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. ISBN 978-0721629216.
- ^ "Pathogen Safety Data Sheets: Infectious Substances – Varicella-zoster virus". Public Health Agency of Canada. 30 April 2012. Archived from the original on 22 March 2020. Retrieved 3 June 2022.
- ^ a b Steiner I, Kennedy PG, Pachner AR (2007). "The neurotropic herpes viruses: herpes simplex and varicella-zoster". The Lancet Neurology. 6 (11): 1015–1028. doi:10.1016/S1474-4422(07)70267-3. PMID 17945155. S2CID 6691444.
- ^ a b Weinberg JM (2007). "Herpes zoster: epidemiology, natural history, and common complications". Journal of the American Academy of Dermatology. 57 (6 Suppl): S130 – S135. doi:10.1016/j.jaad.2007.08.046. PMID 18021864.
- ^ a b c d Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, et al. (January 2018). "Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines" (PDF). MMWR Morb. Mortal. Wkly. Rep. 67 (3): 103–108. doi:10.15585/mmwr.mm6703a5. PMC 5812314. PMID 29370152. Archived (PDF) from the original on 29 August 2021. Retrieved 9 January 2020.
- ^ a b Kennedy PG, Rovnak J, Badani H, Cohrs RJ (2015). "A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation". The Journal of General Virology. 96 (Pt 7): 1581–1602. doi:10.1099/vir.0.000128. PMC 4635449. PMID 25794504.
- ^ Gilden DH, Cohrs RJ, Mahalingam R (2003). "Clinical and molecular pathogenesis of varicella virus infection". Viral Immunology. 16 (3): 243–258. doi:10.1089/088282403322396073. PMID 14583142.
- ^ Kennedy PG (2002). "Varicella-zoster virus latency in human ganglia". Reviews in Medical Virology. 12 (5): 327–334. doi:10.1002/rmv.362. PMID 12211045. S2CID 34582060.
- ^ Kennedy PG (2002). "Key issues in varicella-zoster virus latency". Journal of Neurovirology. 8 (Suppl 2): 80–84. CiteSeerX 10.1.1.415.2755. doi:10.1080/13550280290101058. PMID 12491156.
- ^ Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG (2003). "Herpes simplex virus-1 and varicella-zoster virus latency in ganglia" (PDF). Journal of Neurovirology. 9 (2): 194–204. doi:10.1080/13550280390194000. PMID 12707850. S2CID 5964582. Archived (PDF) from the original on 17 May 2008.
- ^ Donahue JG, Choo PW, Manson JE, Platt R (1995). "The incidence of herpes zoster". Archives of Internal Medicine. 155 (15): 1605–1609. doi:10.1001/archinte.155.15.1605. PMID 7618983.
- ^ a b c Thomas SL, Hall AJ (2004). "What does epidemiology tell us about risk factors for herpes zoster?". The Lancet Infectious Diseases. 4 (1): 26–33. doi:10.1016/S1473-3099(03)00857-0. PMID 14720565.
- ^ "Shingles". NHS.UK. Archived from the original on 26 September 2017. Retrieved 25 September 2017.
- ^ Schmader K (2007). "Herpes zoster and postherpetic neuralgia in older adults". Clinics in Geriatric Medicine. 23 (3): 615–632, vii–viii. doi:10.1016/j.cger.2007.03.003. PMC 4859150. PMID 17631237.
- ^ Davison, AJ, Scott, JE (1986). "The complete DNA sequence of varicella-zoster virus". Journal of General Virology. 67 (9): 1759–1816. doi:10.1099/0022-1317-67-9-1759. PMID 3018124.
- ^ Koh MJ, Seah PP, Teo RY (February 2008). "Zosteriform herpes simplex" (PDF). Singapore Med. J. 49 (2): e59–60. PMID 18301829. Archived (PDF) from the original on 2 June 2014.
- ^ Kalman CM, Laskin OL (November 1986). "Herpes zoster and zosteriform herpes simplex virus infections in immunocompetent adults". Am. J. Med. 81 (5): 775–778. doi:10.1016/0002-9343(86)90343-8. PMID 3022586.
- ^ Chan J, Bergstrom RT, Lanza DC, Oas JG (2004). "Lateral sinus thrombosis associated with zoster sine herpete". Am. J. Otolaryngol. 25 (5): 357–360. doi:10.1016/j.amjoto.2004.03.007. PMID 15334402.
- ^ Arvin AM (1996). "Varicella-zoster virus" (PDF). Clin. Microbiol. Rev. 9 (3): 361–381. doi:10.1128/CMR.9.3.361. PMC 172899. PMID 8809466. Archived (PDF) from the original on 25 June 2008.
- ^ Beards G, Graham C, Pillay D (1998). "Investigation of vesicular rashes for HSV and VZV by PCR". J. Med. Virol. 54 (3): 155–157. doi:10.1002/(SICI)1096-9071(199803)54:3<155::AID-JMV1>3.0.CO;2-4. PMID 9515761. S2CID 24215093.
- ^ De Paschale M, Clerici P (2016). "Microbiology laboratory and the management of mother-child varicella-zoster virus infection". World J Virol (Review). 5 (3): 97–124. doi:10.5501/wjv.v5.i3.97. PMC 4981827. PMID 27563537.
- ^ Sampathkumar P, Drage LA, Martin DP (2009). "Herpes zoster (shingles) and postherpetic neuralgia". Mayo Clin Proc (Review). 84 (3): 274–280. doi:10.4065/84.3.274. PMC 2664599. PMID 19252116.
- ^ Harpaz R, Ortega-Sanchez IR, Seward JF (6 June 2008). "Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm. Rep. 57 (RR–5): 1–30, quiz CE2–4. PMID 18528318. Archived from the original on 17 November 2009. Retrieved 4 January 2010.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, et al. (September 2016). "Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older". N. Engl. J. Med. 375 (11): 1019–1032. doi:10.1056/NEJMoa1603800. hdl:10536/DRO/DU:30086550. PMID 27626517.
- ^ a b c Shapiro M, Kvern B, Watson P, Guenther L, McElhaney J, McGeer A (October 2011). "Update on herpes zoster vaccination: a family practitioner's guide". Can. Fam. Physician. 57 (10): 1127–1131. PMC 3192074. PMID 21998225.
- ^ "Shingles vaccine overview". NHS (UK). 31 August 2021. Archived from the original on 2 June 2014. Retrieved 9 October 2021. Overview to be reviewed 31 August 2024.
- ^ "The shingles immunisation programme: evaluation of the programme and implementation in 2018" (PDF). Public Health England (PHE). 9 April 2018. Archived (PDF) from the original on 9 January 2020. Retrieved 9 January 2020.
- ^ "Herpes zoster (shingles) immunisation programme 2016 to 2017: evaluation report". GOV.UK. 15 December 2017. Archived from the original on 9 January 2020. Retrieved 9 January 2020.
- ^ a b Shaun Linter (18 April 2018). "NHS England warning as vaccine programme extended". Health Service Journal. Archived from the original on 15 November 2019. Retrieved 10 June 2018.
- ^ "Shingles (Herpes Zoster) Vaccination". Centers for Disease Control and Prevention (CDC). 25 October 2018. Archived from the original on 16 January 2020. Retrieved 18 January 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c Tyring SK (2007). "Management of herpes zoster and postherpetic neuralgia". J. Am. Acad. Dermatol. 57 (6 Suppl): S136 – S142. doi:10.1016/j.jaad.2007.09.016. PMID 18021865.
- ^ Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA (2000). "Prevalence of postherpetic neuralgia after a single episode of herpes zoster: prospective study with long term follow up". British Medical Journal. 321 (7264): 794–796. doi:10.1136/bmj.321.7264.794. PMC 27491. PMID 11009518.
- ^ Baron R (2004). "Post-herpetic neuralgia case study: optimizing pain control". Eur. J. Neurol. 11 (Suppl 1): 3–11. doi:10.1111/j.1471-0552.2004.00794.x. PMID 15061819. S2CID 24555396.
- ^ Bader MS (September 2013). "Herpes zoster: diagnostic, therapeutic, and preventive approaches". Postgraduate Medicine. 125 (5): 78–91. doi:10.3810/pgm.2013.09.2703. PMID 24113666. S2CID 5296437.
- ^ Chen N, Li Q, Yang J, Zhou M, Zhou D, He (2014). He L (ed.). "Antiviral treatment for preventing postherpetic neuralgia". Cochrane Database of Systematic Reviews. 2014 (2) CD006866. doi:10.1002/14651858.CD006866.pub3. PMC 10583132. PMID 24500927.
- ^ Uscategui T, Doree C, Chamberlain IJ, Burton MJ (16 July 2008). "Corticosteroids as adjuvant to antiviral treatment in Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults". Cochrane Database of Systematic Reviews (3) CD006852. doi:10.1002/14651858.CD006852.pub2. PMID 18646170.
- ^ Colin J, Prisant O, Cochener B, Lescale O, Rolland B, Hoang-Xuan T (2000). "Comparison of the Efficacy and Safety of Valaciclovir and Acyclovir for the Treatment of Herpes zoster Ophthalmicus". Ophthalmology. 107 (8): 1507–1511. doi:10.1016/S0161-6420(00)00222-0. PMID 10919899.
- ^ Paryani SG, Arvin AM (1986). "Intrauterine infection with varicella-zoster virus after maternal varicella". The New England Journal of Medicine. 314 (24): 1542–1546. doi:10.1056/NEJM198606123142403. PMID 3012334.
- ^ Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M (1994). "Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases". The Lancet. 343 (8912): 1548–1551. doi:10.1016/S0140-6736(94)92943-2. PMID 7802767. S2CID 476280.
- ^ Sørensen HT, Olsen JH, Jepsen P, Johnsen SP, Schønheyder HC, Mellemkjaer (2004). "The risk and prognosis of cancer after hospitalisation for herpes zoster: a population-based follow-up study". Br. J. Cancer. 91 (7): 1275–1279. doi:10.1038/sj.bjc.6602120. PMC 2409892. PMID 15328522.
- ^ Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO (1982). "Risk of cancer after herpes zoster: a population-based study". The New England Journal of Medicine. 307 (7): 393–397. doi:10.1056/NEJM198208123070701. PMID 6979711.
- ^ Ismail A, Rao DG, Sharrack B (2009). "Pure motor Herpes Zoster induced brachial plexopathy". Journal of Neurology. 256 (8): 1343–1345. doi:10.1007/s00415-009-5149-8. PMID 19434442. S2CID 26443976.
- ^ Roat MI (September 2014). "Herpes Zoster Ophthalmicus". Merck Manual. Archived from the original on 12 August 2016. Retrieved 14 August 2016.
- ^ Apisarnthanarak A, Kitphati R, Tawatsupha P, Thongphubeth K, Apisarnthanarak P, Mundy LM (2007). "Outbreak of varicella-zoster virus infection among Thai healthcare workers". Infect. Control Hosp. Epidemiol. 28 (4): 430–434. doi:10.1086/512639. PMID 17385149. S2CID 20844136.
- ^ a b Araújo LQ, Macintyre CR, Vujacich C (2007). "Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America" (PDF). Herpes. 14 (Suppl 2): 40A – 44A. PMID 17939895. Archived from the original (PDF) on 5 December 2019. Retrieved 16 December 2007.
- ^ Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. (2001). "Epidemiology of varicella zoster virus infection in Canada and the United Kingdom". Epidemiol. Infect. 127 (2): 305–314. doi:10.1017/S0950268801005921. PMC 2869750. PMID 11693508.
- ^ Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA (2005). "The incidence of herpes zoster in a United States administrative database". J. Gen. Intern. Med. 20 (8): 748–753. doi:10.1111/j.1525-1497.2005.0150.x. PMC 1490195. PMID 16050886.
- ^ Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS (2007). "A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction". Mayo Clin. Proc. 82 (11): 1341–1349. doi:10.4065/82.11.1341. PMID 17976353.
- ^ de Melker H, Berbers G, Hahné S, Rümke H, van den Hof S, de Wit A, et al. (2006). "The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination" (PDF). Vaccine. 24 (18): 3946–3952. doi:10.1016/j.vaccine.2006.02.017. hdl:10029/5604. PMID 16564115. Archived (PDF) from the original on 8 August 2017. Retrieved 3 September 2019.
- ^ Colebunders R, Mann JM, Francis H, Bila K, Izaley L, Ilwaya M, et al. (1988). "Herpes zoster in African patients: a clinical predictor of human immunodeficiency virus infection". J. Infect. Dis. 157 (2): 314–318. doi:10.1093/infdis/157.2.314. PMID 3335810.
- ^ Buchbinder SP, Katz MH, Hessol NA, Liu JY, O'Malley PM, Underwood R, et al. (1992). "Herpes zoster and human immunodeficiency virus infection". J. Infect. Dis. 166 (5): 1153–1156. doi:10.1093/infdis/166.5.1153. PMID 1308664.
- ^ Tsai SY, Chen HJ, Lio CF, Ho HP, Kuo CF, Jia X, et al. (22 August 2017). "Increased risk of herpes zoster in patients with psoriasis: A population-based retrospective cohort study". PLOS ONE. 12 (8) e0179447. Bibcode:2017PLoSO..1279447T. doi:10.1371/journal.pone.0179447. ISSN 1932-6203. PMC 5567491. PMID 28829784.
- ^ Livengood JM (2000). "The role of stress in the development of herpes zoster and postherpetic neuralgia". Curr. Rev. Pain. 4 (1): 7–11. doi:10.1007/s11916-000-0003-9. PMID 10998709. S2CID 37086354.
- ^ a b c d Gatti A, Pica F, Boccia MT, De Antoni F, Sabato AF, Volpi A (2010). "No evidence of family history as a risk factor for herpes zoster in patients with post-herpetic neuralgia". J. Med. Virol. 82 (6): 1007–1011. doi:10.1002/jmv.21748. hdl:2108/15842. PMID 20419815. S2CID 31667542.
- ^ Schmader K, George LK, Burchett BM, Pieper CF (1998). "Racial and psychosocial risk factors for herpes zoster in the elderly". J. Infect. Dis. 178 (Suppl 1): S67 – S70. doi:10.1086/514254. PMID 9852978.
- ^ Schmader K, George LK, Burchett BM, Hamilton JD, Pieper CF (1998). "Race and stress in the incidence of herpes zoster in older adults". J. Am. Geriatr. Soc. 46 (8): 973–977. doi:10.1111/j.1532-5415.1998.tb02751.x. PMID 9706885. S2CID 7583608.
- ^ Hicks LD, Cook-Norris RH, Mendoza N, Madkan V, Arora A, Tyring SK (May 2008). "Family history as a risk factor for herpes zoster: a case-control study". Arch. Dermatol. 144 (5): 603–608. doi:10.1001/archderm.144.5.603. PMID 18490586.
- ^ Marin M, Güris D, Chaves SS, Schmid S, Seward JF, et al. (Advisory Committee On Immunization Practices) (22 June 2007). "Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm. Rep. 56 (RR–4): 1–40. PMID 17585291. Archived from the original on 4 September 2011.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF (2005). "Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002". J. Infect. Dis. 191 (12): 2002–2007. doi:10.1086/430325. PMID 15897984.
- ^ Whitley RJ (2005). "Changing dynamics of varicella-zoster virus infections in the 21st century: the impact of vaccination". J. Infect. Dis. 191 (12): 1999–2001. doi:10.1086/430328. PMID 15897983.
- ^ Patel MS, Gebremariam A, Davis MM (December 2008). "Herpes zoster-related hospitalizations and expenditures before and after introduction of the varicella vaccine in the United States". Infect. Control Hosp. Epidemiol. 29 (12): 1157–1163. doi:10.1086/591975. PMID 18999945. S2CID 21934553.
- ^ Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, et al. (2005). "The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003". BMC Public Health. 5: 68. doi:10.1186/1471-2458-5-68. PMC 1177968. PMID 15960856.
- ^ Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS (2007). "A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction". Mayo Clin. Proc. 82 (11): 1341–1349. doi:10.4065/82.11.1341. PMID 17976353.
- ^ Volpi A (2007). "Severe complications of herpes zoster" (PDF). Herpes. 14 (Suppl 2): 35A – 39A. PMID 17939894. Archived (PDF) from the original on 27 January 2019. Retrieved 18 December 2007.
- ^ Coplan P, Black S, Rojas C, Shinefield H, Ray P, Lewis E, et al. (2001). "Incidence and hospitalization rates of varicella and herpes zoster before varicella vaccine introduction: a baseline assessment of the shifting epidemiology of varicella disease". Pediatr. Infect. Dis. J. 20 (7): 641–645. doi:10.1097/00006454-200107000-00002. PMID 11465834. S2CID 25626718.
- ^ Weaver BA (1 March 2007). "The burden of herpes zoster and postherpetic neuralgia in the United States". J. Am. Osteopath. Assoc. 107 (3 Suppl): S2–57. PMID 17488884. Archived from the original on 13 January 2008.
- ^ Leung, Jessica; Harrington, Theresa; Dooling, Kathleen (2024), "Chapter 23: Zoster", Epidemiology and Prevention of Vaccine-Preventable Diseases, Center for Disease Control, retrieved 31 March 2025
- ^ Weller TH (2000). "Chapter 1. Historical perspective". In Arvin AM, Gershon AA (eds.). Varicella-Zoster Virus: Virology and Clinical Management. Cambridge University Press. ISBN 978-0521660242.
- ^ Oaklander AL (October 1999). "The pathology of shingles: Head and Campbell's 1900 monograph". Arch. Neurol. 56 (10): 1292–1294. doi:10.1001/archneur.56.10.1292. PMID 10520948.
- ^ Weller TH (1953). "Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster". Proc. Soc. Exp. Biol. Med. 83 (2): 340–346. doi:10.3181/00379727-83-20354. PMID 13064265. S2CID 28771357.
- ^ "Evelyn Nicol 1930 - 2020 - Obituary". legacy.com. Archived from the original on 27 May 2022. Retrieved 28 August 2020.
- ^ Holt LE, McIntosh R (1936). Holt's Diseases of Infancy and Childhood. D Appleton Century Company. pp. 931–933.
- ^ Weller TH (1997). "Varicella-herpes zoster virus". In Evans AS, Kaslow RA (eds.). Viral Infections of Humans: Epidemiology and Control. Plenum Press. pp. 865–892. ISBN 978-0306448553.
- ^ Hope-Simpson RE (1965). "The nature of herpes zoster; a long-term study and a new hypothesis". Proceedings of the Royal Society of Medicine. 58 (1): 9–20. doi:10.1177/003591576505800106. PMC 1898279. PMID 14267505.
- ^ Thomas SL, Wheeler JG, Hall AJ (2002). "Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study". The Lancet. 360 (9334): 678–682. doi:10.1016/S0140-6736(02)09837-9. PMID 12241874. S2CID 28385365.
- ^ Terada K, Hiraga Y, Kawano S, Kataoka N (1995). "Incidence of herpes zoster in pediatricians and history of reexposure to varicella-zoster virus in patients with herpes zoster". Kansenshogaku Zasshi. 69 (8): 908–912. doi:10.11150/kansenshogakuzasshi1970.69.908. ISSN 0387-5911. PMID 7594784.
- ^ ἕρπης. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ^ ἕρπειν in Liddell and Scott.
- ^ Harper, Douglas. "herpes". Online Etymology Dictionary.
- ^ "Herpes | Define Herpes at Dictionary.com". Archived from the original on 25 February 2011. Retrieved 14 March 2011.
- ^ ζωστήρ in Liddell and Scott.
- ^ Harper, Douglas. "zoster". Online Etymology Dictionary.
- ^ cingulus, cingulum. Charlton T. Lewis and Charles Short. A Latin Dictionary on Perseus Project.
- ^ Harper, Douglas. "shingles". Online Etymology Dictionary.
- ^ Yawn BP, Gilden D (3 September 2013). "The global epidemiology of herpes zoster". Neurology. 81 (10): 928–930. doi:10.1212/wnl.0b013e3182a3516e. PMC 3885217. PMID 23999562.
- ^ a b c d Becerra JC, Sieber R, Martinetti G, Costa ST, Meylan P, Bernasconi E (July 2013). "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. 17 (7): e529–534. doi:10.1016/j.ijid.2013.01.031. PMID 23566589. Archived from the original on 17 December 2019. Retrieved 2 March 2016.
- ^ Ihekwaba UK, Kudesia G, McKendrick MW (15 September 2008). "Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections". Clinical Infectious Diseases. 47 (6): 783–789. doi:10.1086/591129. ISSN 1058-4838. PMID 18680414.
- ^ a b c Pollak L, Dovrat S, Book M, Mendelson E, Weinberger M (August 2011). "Varicella zoster vs. herpes simplex meningoencephalitis in the PCR era. A single center study". Journal of the Neurological Sciences. 314 (1–2): 29–36. doi:10.1016/j.jns.2011.11.004. PMID 22138027. S2CID 3321888.
- ^ Kojima Y, Hashiguchi H, Hashimoto T, Tsuji S, Shoji H, Kazuyama Y (15 September 2008). "Recurrent Herpes Simplex Virus Type 2 Meningitis: A Case Report of Mollaret's Meningitis" (PDF). Japanese Journal of Infectious Diseases. 55 (3): 85–88. ISSN 1344-6304. PMID 12195049. Archived (PDF) from the original on 22 January 2013.
Further reading
[edit]- Saguil A (November 2017). "Herpes Zoster and Postherpetic Neuralgia: Prevention and Management". American Family Physician. 96 (10): 656–663. PMID 29431387.
External links
[edit]Shingles
View on GrokipediaIntroduction
Definition
Shingles, also known as herpes zoster, is a viral infection resulting from the reactivation of the varicella-zoster virus (VZV), the same virus that causes chickenpox (varicella).[1][3] After an initial episode of chickenpox, VZV establishes latency in the sensory dorsal root ganglia of the nervous system, where it can remain dormant for decades before reactivating under certain conditions.[6] The typical presentation of shingles involves a unilateral rash confined to one or more adjacent dermatomes, often accompanied by pain and the formation of fluid-filled blisters (vesicles).[7] This dermatomal distribution reflects the virus's reactivation along specific sensory nerves, most commonly affecting the trunk in a thoracic dermatome.[7] Shingles differs from primary VZV infection, or chickenpox, which produces a widespread, pruritic vesicular rash across the body rather than a localized dermatomal pattern.[2] It is also distinct from infections caused by other herpesviruses, such as herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), which typically cause recurrent oral or genital lesions in clustered groups without the characteristic unilateral dermatomal involvement of shingles.[2][6]Overview
Shingles, medically known as herpes zoster, results from the reactivation of the varicella-zoster virus (VZV), the same virus responsible for chickenpox, within the body of individuals who previously contracted that infection. This condition predominantly impacts adults aged 50 years and older, as well as those who are immunocompromised due to factors such as HIV, cancer treatments, or organ transplants.[1][4] Unlike chickenpox, shingles is not transmitted directly from person to person; however, the active rash can spread VZV to susceptible contacts who lack immunity to chickenpox, potentially causing varicella in them via direct contact with blister fluid or airborne particles from the lesions.[1][4] The typical course of shingles includes a prodromal phase of 1 to 5 days marked by localized pain or tingling, followed by rash eruption that scabs over in 7 to 10 days and generally resolves within 2 to 4 weeks, although postherpetic neuralgia—a chronic pain condition—may develop and persist for months or longer in some cases. The rash itself is often intensely painful, following a dermatomal pattern on one side of the body.[8][9] In the United States, an estimated 1 million cases of shingles occur each year, underscoring its significant public health burden and the critical role of vaccination in reducing incidence and complications among at-risk populations.[1][10]Clinical Presentation
Signs and Symptoms
Shingles typically begins with a prodromal phase lasting 2 to 3 days, characterized by flu-like symptoms such as fever and malaise, along with localized pain, itching, or tingling in the affected dermatome.[6][8] This early warning often precedes the rash and can mimic other conditions due to its nonspecific nature.[2] The hallmark rash of shingles appears unilaterally in a band-like distribution along a sensory nerve dermatome, most commonly thoracic or lumbar.[6] It starts as red macules or papules that rapidly evolve into clusters of fluid-filled vesicles within 1 to 5 days, progressing to pustules and then crusting over 7 to 10 days as the lesions heal.[11] The rash is typically confined to one or a few adjacent dermatomes and does not cross the midline.[2] Pain is a prominent feature, often fluctuating in intensity and character as intermittent acute neuropathic discomfort that varies from mild aching to severe burning, stabbing, or electric-shock-like sensations due to early virus activation; it can be influenced by external factors such as daily activities or stress, and may temporarily improve but recur if the virus is not fully cleared.[12][2][13] The intensity ranges from mild to severe and often intensifies with the rash's onset.[6] This pain arises from inflammation of the affected nerve and sensory ganglia.[11] A rare variant known as zoster sine herpete is characterized by the typical dermatomal pain without the development of a rash or blisters.[7][14] Due to the absence of blister fluid for viral shedding, the contagiousness of this form is negligible.[1] The trunk is the most frequent site, accounting for 50-60% of cases, followed by the face or cranium in about 20%, while limbs are less commonly involved.[6] In immunocompromised individuals, the rash may disseminate to multiple dermatomes or distant sites.[7]Complications
One of the most common complications of shingles is postherpetic neuralgia (PHN), defined as persistent neuropathic pain lasting more than three months after the rash has resolved, often characterized by burning or lancinating sensations in the affected dermatome.[15] PHN affects 10-18% of shingles patients overall, with incidence rising significantly in older adults—reaching approximately 60% in those aged 60 years and 75% in those aged 70 years—due to age-related declines in immune function and slower viral clearance.[16] The underlying mechanisms involve varicella-zoster virus-induced neuroinflammation leading to peripheral nerve damage, including myelin and axon deficiencies, dorsal root ganglion atrophy, and central sensitization in the spinal cord, which perpetuate ectopic nerve firing even after viral replication ceases.[15] In immunocompromised individuals, shingles can progress to disseminated zoster, characterized by widespread cutaneous lesions exceeding 20 outside the primary dermatome, occurring in up to 37% of untreated cases and 10-30% overall in this population, such as those with malignancies or transplants.[16] This dissemination heightens the risk of visceral organ involvement, including pneumonia (incidence 2.5% in immunocompromised patients) and hepatitis (0.8%), potentially leading to severe systemic illness like acute respiratory distress syndrome if untreated.[17] Zoster ophthalmicus arises when the virus reactivates in the ophthalmic division of the trigeminal nerve, accounting for 10-25% of all shingles cases, particularly in older or immunocompromised patients.[18] Ocular complications are frequent, with keratitis affecting up to 65% of cases through epithelial, stromal, or neurotrophic forms that cause corneal inflammation, scarring, or ulceration; untreated progression can result in uveitis, retinal necrosis, optic neuritis, or permanent vision loss in severe instances.[18] Other complications include bacterial superinfection of skin lesions, which can occur due to disrupted epidermal barriers and lead to cellulitis or impetigo requiring antibiotic intervention.[6] Ramsay Hunt syndrome, a rare cranial neuropathy variant, involves varicella-zoster virus spread to the geniculate ganglion, manifesting as ipsilateral facial palsy, ear pain, vesicles in the auditory canal, hearing loss, vertigo, and tinnitus, with incomplete recovery in many patients.[16] Neurological sequelae such as transverse myelitis, encephalitis, or aseptic meningitis are uncommon but can arise from direct central nervous system invasion or immune-mediated damage, potentially causing motor deficits, sensory loss, or autonomic dysfunction.[6]Pathophysiology
Viral Mechanism
Varicella-zoster virus (VZV), a member of the alphaherpesvirus family, establishes infection through primary exposure, typically manifesting as chickenpox (varicella), during which the virus disseminates via the bloodstream to sensory ganglia. Following acute replication in epithelial cells and T lymphocytes, VZV enters sensory neurons and travels retrogradely along axons to dorsal root ganglia and cranial nerve ganglia, where it establishes lifelong latency.[6] In this latent state, the virus persists as episomal DNA in neuronal nuclei with minimal viral gene expression, primarily limited to immediate-early genes such as open reading frame 63 (ORF63), which helps maintain dormancy without producing infectious virions.[19] This latency can endure for decades, evading host immune surveillance.[20] Reactivation of latent VZV, leading to shingles (herpes zoster), is primarily triggered by waning cell-mediated immunity, often associated with advancing age or immunosuppressive conditions.[6] Upon reactivation, viral gene expression shifts from restricted latency-associated transcripts to a full lytic cycle: immediate-early genes (e.g., ORF4 and ORF62) initiate transcription, followed by early genes involved in DNA replication (e.g., ORF28 and ORF29), and culminating in late genes for structural proteins and virion assembly (e.g., glycoprotein genes).[21] This cascade enables viral replication within neuronal cell bodies, followed by anterograde axonal transport of newly formed virions to the skin, where they infect keratinocytes and produce the characteristic dermatomal rash.[22] The pathological effects of VZV reactivation center on sensory neurons and ganglia, inducing acute inflammation through mononuclear cell infiltration and cytokine release, which contributes to severe pain.[20] The pain in herpes zoster often fluctuates in intensity and character, ranging from mild aching to severe burning or electric shock-like sensations, due to early virus activation in sensory nerves leading to ongoing inflammatory responses and nerve damage.[12][13] External factors, such as stress or physical activity, can influence these fluctuations, and symptoms may temporarily improve but recur if the virus is not fully cleared by the immune system or antiviral treatment.[2][12] This inflammatory response can lead to demyelination in affected nerve segments, neuronal fibrosis, and loss of sensory neurons, exacerbating nerve damage and potentially resulting in prolonged hypersensitivity.[20] T-cell immunity, particularly CD4+ and CD8+ T cells specific to VZV antigens, plays a crucial role in suppressing latency and preventing reactivation by surveilling infected neurons and limiting viral spread; decline in these responses, as seen in aging, permits the virus to overcome immune control.[23]Risk Factors
The primary risk factor for developing shingles, or herpes zoster, is a prior infection with the varicella-zoster virus (VZV) that causes chickenpox, as the virus establishes lifelong latency in sensory ganglia following primary infection.[1] In populations born before widespread chickenpox vaccination, such as those in the United States prior to 1980, nearly all adults (approximately 99.5%) have had chickenpox, making them susceptible to reactivation.[4] Age is the most significant non-modifiable risk factor, with incidence rising sharply after age 50 due to immunosenescence, or age-related decline in cell-mediated immunity.[24] The annual incidence increases from about 4 cases per 1,000 persons overall to approximately 10 per 1,000 (1%) in those over age 80.[10] By age 85, the cumulative lifetime risk approaches 50%. Immunosuppression substantially elevates risk, often by 10- to 100-fold depending on the underlying condition or treatment.[25] Conditions such as HIV/AIDS (relative risk [RR] 3.22), malignancies like leukemia or lymphoma (RR 2.17), organ transplantation, and therapies including chemotherapy or prolonged corticosteroid use impair VZV-specific T-cell immunity, facilitating viral reactivation.[24] For instance, solid organ transplant recipients experience a 2- to 10-fold increase in risk compared to immunocompetent individuals.[26] Other factors include a slight increase in risk among females (pooled adjusted RR 1.31).[27] Psychological stress has been associated with elevated risk (RR approximately 2.0), potentially through suppression of cellular immunity, while physical trauma to the affected dermatome may trigger reactivation (RR 2.01).[24] Genetic factors, including family history (RR 2.48), increase susceptibility, though specific genes remain to be firmly identified.[28][24] Individuals with a history of chickenpox vaccination have a reduced risk of shingles compared to those who experienced natural infection, though the risk is not entirely eliminated due to possible vaccine-strain latency. Widespread varicella vaccination since 1995 has contributed to lower overall HZ incidence in vaccinated cohorts as of 2025.[10][29] For example, vaccinated children show a 78% lower incidence of herpes zoster than unvaccinated children who had breakthrough chickenpox.Diagnosis
Clinical Diagnosis
The clinical diagnosis of shingles, or herpes zoster, is primarily based on a patient's medical history and physical examination, as the condition often presents with characteristic features that allow for recognition without laboratory testing in typical cases.[30] During history taking, clinicians focus on prodromal symptoms such as localized pain, tingling, or itching in a specific area, which may precede the rash by several days and often follows a unilateral dermatomal distribution; a history of recent chickenpox exposure is unnecessary, as shingles results from reactivation of latent varicella-zoster virus rather than new infection.[7] Additional prodromal features can include headache, malaise, or photophobia, helping to distinguish the condition from other causes of unilateral pain.[6] On physical examination, the key finding is a unilateral vesicular rash confined to one or two adjacent dermatomes, most commonly on the trunk (thoracic dermatomes) or face, without crossing the midline.[7] The rash typically evolves from erythematous macules to grouped vesicles on an erythematous base over 3 to 5 days, eventually crusting and healing within 2 to 4 weeks, sometimes leaving scars.[6] A notable sign during examination is Hutchinson's sign, characterized by herpetic lesions on the nasal tip or side, indicating involvement of the nasociliary branch of the trigeminal nerve and raising concern for ocular complications such as herpes zoster ophthalmicus.[6] The standard clinical criteria for diagnosing shingles include the presence of a unilateral vesicular rash accompanied by pain or sensory changes in one or more contiguous dermatomes.[30] This presentation is highly suggestive in immunocompetent individuals, though zoster sine herpete—a rare variant involving dermatomal pain without rash, as detailed in the Signs and Symptoms section—may occur and significantly complicates bedside diagnosis, often necessitating laboratory confirmation to rule out other causes of neuropathic pain.[6] In atypical cases, particularly among immunocompromised patients, the rash may appear multifocal, disseminated (involving more than 20 lesions outside the primary dermatome), or even absent, potentially leading to visceral involvement such as pneumonitis or hepatitis; in these scenarios, a thorough history of immunosuppression is crucial for suspicion.[7][6]Laboratory Confirmation
Laboratory confirmation of herpes zoster (shingles) is typically pursued when the clinical presentation is atypical or uncertain, such as in immunocompromised individuals or cases lacking a classic dermatomal rash, to verify varicella-zoster virus (VZV) infection through objective testing.[31] These tests are adjunctive to clinical evaluation and are not routinely required for straightforward diagnoses, as the characteristic unilateral vesicular eruption often suffices.[32] Common specimen sources include vesicular fluid, lesion swabs, scabs, or cerebrospinal fluid (CSF) in cases of neurological involvement.[6] Polymerase chain reaction (PCR) testing represents the gold standard for laboratory confirmation, detecting VZV DNA with high sensitivity exceeding 95% and specificity approaching 100% in vesicular fluid, lesion swabs, or CSF.[33] The procedure involves collecting a sample from an unroofed vesicle or swabbed lesion base, followed by amplification and detection of viral genetic material, often using real-time PCR for rapid results within hours to one day.[31] This method is particularly valuable for early diagnosis before vesicle formation or in disseminated disease.[32] Viral culture, once a historical mainstay, isolates live VZV from lesion specimens but has lower sensitivity (typically under 70%) and requires 1-2 weeks for growth, rendering it less practical today.[6] The Tzanck smear, performed by scraping a fresh vesicle and staining for microscopic examination, reveals multinucleated giant cells indicative of herpetic infection but lacks specificity, as it cannot differentiate VZV from herpes simplex virus, and its sensitivity is limited to around 60%.[32] Serologic testing measures VZV-specific IgM (for acute infection) or IgG (for immunity) antibodies in serum, but it is unreliable for acute shingles diagnosis due to widespread prior varicella exposure conferring baseline IgG in most adults; a four-fold IgG rise in paired acute-convalescent samples may confirm recent reactivation, though sensitivity remains low compared to PCR.[31] Indications for laboratory confirmation include atypical rashes, immunocompromised patients at risk for dissemination, and zoster sine herpete—a rashless form presenting with dermatomal pain—where PCR from CSF or plasma is especially useful for early detection.[32] Additionally, in suspected neurological complications like encephalitis or myelitis, magnetic resonance imaging (MRI) of the brain or spine may be employed alongside CSF PCR to assess involvement and exclude mimics, though MRI findings are supportive rather than confirmatory of VZV.[6]Prevention
Vaccination
Vaccination against shingles, caused by reactivation of the varicella-zoster virus, primarily involves two vaccines: the live attenuated Zostavax and the recombinant Shingrix. Zostavax, approved in 2006, demonstrated approximately 51% efficacy in preventing shingles in adults aged 60 years and older, but its protection waned over time and it is no longer preferred or available in many regions, having been discontinued in the United States in 2020 and in other countries by 2023-2025.[34][35] Shingrix, a recombinant zoster vaccine approved in 2017, has become the standard for prevention due to its superior efficacy and safety profile. It consists of recombinant varicella-zoster virus glycoprotein E (gE) antigen combined with the AS01B adjuvant system, which enhances T-cell mediated immunity by stimulating strong CD4+ T-cell responses essential for controlling viral reactivation.[36][37][34] The vaccine is administered as a two-dose intramuscular series, with doses spaced 2 to 6 months apart. The Centers for Disease Control and Prevention (CDC) recommends Shingrix for all adults aged 50 years and older, regardless of prior shingles history or vaccination, and for immunocompromised adults aged 19 years and older who are at increased risk. In clinical trials, Shingrix showed 97% efficacy against shingles in adults over 50 years and 90% efficacy in those over 70 years, with effectiveness ranging from 68% to 91% in immunocompromised individuals depending on the underlying condition.[38][39] Shingrix also reduces the risk of postherpetic neuralgia (PHN), a common complication, by approximately 90% in adults aged 50 years and older. Recent studies in 2025 have further indicated that vaccination is associated with a 20-25% lower risk of dementia and cardiovascular events, such as heart attacks and strokes, potentially due to reduced viral-induced inflammation.[40][41][42] Contraindications include a history of severe allergic reaction to any vaccine component or active shingles infection at the time of vaccination. Shingrix is generally safe for most immunocompromised individuals, though consultation with a healthcare provider is advised for those with severe immunosuppression.[36][39]Lifestyle and Risk Reduction
Maintaining a healthy immune system through lifestyle practices is essential for reducing the risk of shingles, as the varicella-zoster virus (VZV) reactivation depends on robust T-cell mediated immunity.[1] A balanced diet rich in vitamins C and E, zinc, and antioxidants supports overall immune function, including T-cell responses that help keep VZV latent.[43] Regular physical activity, such as moderate aerobic exercise or practices like tai chi, has been shown to enhance varicella-zoster virus-specific immunity in older adults by improving cellular immune responses.[44] Adequate sleep, aiming for 7-9 hours per night, is crucial for immune regulation, as sleep deprivation can impair T-cell proliferation and increase susceptibility to viral reactivations like shingles.[45] Chronic stress can suppress cellular immunity, potentially triggering VZV reactivation by reducing the activity of virus-specific T-cells.[46] Stress management techniques, such as mindfulness meditation, yoga, or cognitive behavioral therapy, help mitigate this by lowering cortisol levels and preserving immune competence.[47] Individuals with high perceived stress levels may face up to a twofold increased risk of herpes zoster compared to those with low stress.[46] To avoid unnecessary immunosuppression, healthcare providers should use corticosteroids judiciously, as systemic use increases shingles risk by approximately 59%, with higher doses correlating to greater hazard.[48] Smoking cessation is recommended, as former smokers exhibit a 17% higher risk of herpes zoster compared to never smokers, likely due to lingering inflammatory effects on immunity.[49] Receiving the chickenpox vaccine in childhood prevents primary VZV infection, thereby eliminating the possibility of latent virus establishment and subsequent shingles in adulthood.[50] Shingles itself is not directly contagious and does not cause shingles in others; however, the varicella-zoster virus in its blisters can infect people who have never had chickenpox or the vaccine, causing chickenpox in them.[1][13] Transmission occurs through direct contact with fluid from the rash blisters and lasts until the blisters dry and crust over, typically 7 to 10 days, after which there is no risk.[1][51] The risk is low for those previously exposed to chickenpox or vaccinated. For those with active shingles, practicing good hygiene and isolating from susceptible contacts—such as unvaccinated children, pregnant women, newborns, or immunocompromised individuals—prevents transmission of VZV, which can cause chickenpox in non-immune people and indirectly contribute to future shingles risk in the population.[52] Covering the rash and avoiding direct contact until lesions crust over is key to this strategy.[1]Treatment
Antiviral Medications
Antiviral medications are the cornerstone of treatment for acute shingles (herpes zoster), aiming to inhibit varicella-zoster virus (VZV) replication, thereby shortening the duration and severity of the illness.[1] These drugs are most effective when initiated promptly, ideally within 72 hours of rash onset, as this window allows for maximal reduction in viral shedding and lesion formation.[53] The primary agents include acyclovir, valacyclovir, and famciclovir, all of which are nucleoside analogs that selectively inhibit viral DNA polymerase without significantly affecting host cell replication.[53] Valacyclovir is often preferred as a first-line oral therapy due to its superior bioavailability compared to acyclovir, requiring less frequent dosing.[53] Standard regimens for immunocompetent adults include valacyclovir at 1 g three times daily for 7 days, famciclovir at 500 mg three times daily for 7 days, or acyclovir at 800 mg five times daily for 7 to 10 days.[53] For severe or disseminated cases, particularly in immunocompromised patients, intravenous acyclovir is recommended at 10 mg/kg every 8 hours, with subsequent transition to oral therapy once clinically stable.[53] Renal function monitoring is essential during high-dose therapy to prevent potential nephrotoxicity, especially in older adults.[53] Clinical trials have demonstrated that these antivirals reduce the duration of rash healing and acute pain by approximately 1 to 2 days and can lower the risk of postherpetic neuralgia (PHN) by 20% to 50% when started early, with greater benefits observed in patients over 50 years old.[53] However, acute antiviral treatment does not prevent future reactivations of the varicella-zoster virus or recurrent episodes of shingles, as it does not eradicate the latent virus from the sensory ganglia; the frequency of recurrence remains similar in treated and untreated cases.[53] The benefit diminishes significantly if treatment begins after 72 hours, though it may still be considered for select cases with ongoing lesion formation.[3] Common side effects are mild and include nausea, headache, and diarrhea, occurring in less than 10% of patients; these agents are generally well-tolerated.[53] As of 2025, no major new antivirals have emerged for standard acute shingles treatment, but evidence from the Zoster Eye Disease Study (ZEDS) supports long-term low-dose valacyclovir (1 g daily for 1 year) to prevent ocular complications in high-risk patients with herpes zoster ophthalmicus, reducing recurrence risk by 26% at 18 months compared to placebo.[54] This approach targets persistent viral activity in the ophthalmic division of the trigeminal nerve.[55]Pain Management
Pain management in acute shingles (herpes zoster) focuses on alleviating the characteristic neuropathic and nociceptive pain, which can range from mild discomfort to severe, burning sensations along the affected dermatome. Effective strategies combine pharmacological interventions with supportive measures to improve patient comfort and daily functioning during the typical 2-4 week rash duration. Early pain control is crucial, as uncontrolled acute pain may increase the risk of transitioning to postherpetic neuralgia (PHN).[56] For mild to moderate acute pain, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen (400 mg every 4-6 hours, maximum 2,400 mg/day) or acetaminophen (325-1,000 mg every 4-6 hours, maximum 4,000 mg/day) are first-line options, providing relief for nociceptive components without significant side effects in most patients.[57][56] In cases of severe pain, short-term use of opioids like oxycodone or morphine is recommended, titrated to efficacy while monitoring for constipation and sedation; these should follow the principles of the WHO analgesic ladder, adapted for the neuropathic elements of shingles pain.[56][58] Neuropathic pain, often described as burning or shooting, responds to agents like gabapentin (starting at 100-300 mg daily, titrated up to 1,800-3,600 mg/day in divided doses) or pregabalin (150-300 mg/day initially, up to 600 mg/day), which modulate nerve excitability and are effective for acute zoster pain when initiated early.[56][30] Tricyclic antidepressants such as amitriptyline (10-25 mg at bedtime, titrated to 75 mg/day) or nortriptyline are also useful for burning-type pain, offering dual benefits through serotonin and norepinephrine reuptake inhibition, though they require caution in elderly patients due to anticholinergic effects.[30][56] Topical therapies provide localized relief with minimal systemic absorption. Lidocaine patches (5% applied up to 12 hours daily) numb the affected area and are suitable for intact skin over the rash, reducing hypersensitivity.[30][56] Capsaicin cream (0.025-0.075%) or high-dose patches (8% applied under supervision) can deplete substance P in sensory nerves, offering relief after initial burning, applied at least 3-5 times daily.[30][56] Supportive non-pharmacological approaches include cool, wet compresses or baths to soothe itching and inflammation, applied several times daily for 10-15 minutes.[30] Calamine lotion serves as an astringent to dry weeping blisters and reduce pruritus, while wet dressings with 5% aluminum acetate (Burow's solution) for 30-60 minutes, 4-6 times daily, help manage oozing lesions.[56] For intractable pain unresponsive to standard measures, a multidisciplinary approach involving nerve blocks or referral to pain specialists is advised, aligning with adapted WHO ladder escalation for combined nociceptive and neuropathic pain.[57][58]Management of Complications
Postherpetic neuralgia (PHN), defined as pain persisting beyond three months after the shingles rash resolves, requires multimodal management tailored to severity. First-line pharmacological options include high-dose gabapentinoids such as gabapentin (up to 3600 mg/day) or pregabalin (up to 600 mg/day), which target neuropathic pain through calcium channel modulation, alongside serotonin-norepinephrine reuptake inhibitors like duloxetine (60-120 mg/day).[59][15] For refractory PHN unresponsive to oral therapies, intravenous lidocaine infusions (1-5 mg/kg over 30-60 minutes) provide short-term relief by blocking sodium channels in nociceptive fibers.[60] In severe, intractable cases, interventional procedures such as peripheral nerve blocks or epidural injections offer targeted analgesia, while spinal cord stimulation via implanted devices modulates pain signals for long-term control, with success rates exceeding 50% in selected patients.[15][61] Herpes zoster ophthalmicus (HZO), involving the ophthalmic division of the trigeminal nerve, demands prompt ophthalmologic evaluation to mitigate risks of uveitis, keratitis, or secondary glaucoma. Standard treatment combines oral antivirals—such as valacyclovir (1000 mg three times daily for 7-10 days)—with topical corticosteroids (e.g., prednisolone acetate 1% eyedrops tapered over weeks) to reduce inflammation and prevent corneal scarring.[18][62] Immediate referral to an ophthalmologist is essential for slit-lamp examination and intraocular pressure monitoring, as untreated uveitis can lead to vision loss in up to 10% of cases.[63][64] Disseminated zoster, characterized by more than 20 lesions beyond the primary dermatome, particularly in immunocompromised individuals, necessitates hospitalization for intravenous acyclovir (10-15 mg/kg every 8 hours for 7-14 days) to halt viral dissemination and prevent visceral involvement.[6] Close monitoring for organ failure, including hepatic or pulmonary complications, involves serial imaging and laboratory assessments, with dose adjustments for renal impairment to avoid toxicity.[11] Secondary bacterial superinfections of skin lesions require empiric antibiotics such as cephalexin or clindamycin, guided by culture results. Ramsay Hunt syndrome, resulting from varicella-zoster virus reactivation affecting the geniculate ganglion, is managed with combined antiviral and corticosteroid therapy to improve facial nerve recovery. Oral or intravenous acyclovir (800 mg five times daily) or valacyclovir (1000 mg three times daily) for 7-10 days, paired with high-dose prednisone (1 mg/kg/day tapered over 2-3 weeks), enhances viral suppression and reduces nerve edema, potentially improving outcomes if initiated within 72 hours.[65][66] For associated facial weakness, physical therapy including facial muscle exercises and neuromuscular re-education is recommended to prevent synkinesis and promote symmetry, with evidence showing better recovery rates when started early.[67] A 2025 advancement from the Zoster Eye Disease Study demonstrates that long-term valacyclovir (1000 mg daily for 12 months) reduces the risk of new or worsening ocular disease in HZO by approximately 26% at 18 months compared to placebo, with 32% affected in the treated group versus 40% in placebo (recurrence-free rates of 68% versus 60%), alongside shorter pain duration and reduced need for neuropathic pain medications.[68][69]Prognosis
Short-Term Outcomes
In uncomplicated cases of shingles (herpes zoster), the rash typically progresses from red patches to fluid-filled blisters that begin to crust over within 7 to 10 days of onset.[2] Full resolution of the rash, including scab shedding and skin healing, generally occurs within 2 to 4 weeks, though mild scarring or discoloration may persist temporarily.[4] This timeline applies to most immunocompetent individuals without dissemination, allowing return to normal activities as the lesions dry and heal.[70] Acute pain associated with shingles, often described as burning or stabbing, typically subsides as the rash resolves, particularly in younger patients.[71] Initiation of antiviral therapy within 72 hours of rash appearance accelerates pain relief and reduces its duration by promoting faster viral clearance and inflammation control.[4] Without treatment, pain may linger longer during the acute phase, but early intervention improves overall short-term comfort and recovery speed.[56] Hospitalization is required in fewer than 5% of shingles cases, primarily among immunocompromised patients or elderly individuals experiencing disseminated infection or severe complications such as bacterial superinfection.[10] Younger age and prompt antiviral treatment are key factors favoring favorable short-term outcomes, with reduced risk of prolonged acute symptoms and quicker return to baseline function.[57]Long-Term Effects
The most significant long-term complication of shingles is postherpetic neuralgia (PHN), a chronic neuropathic pain syndrome that persists or emerges after the rash has resolved, affecting 10% to 18% of patients overall.[10] The prevalence increases with age, occurring in 10% to 20% of individuals over 50 years and reaching up to 21% in those aged 80 to 84 years.[72] PHN can substantially impair quality of life, leading to depression, sleep disturbances, reduced mobility, chronic fatigue, and social isolation, with pain often described as burning, stabbing, or electric-shock-like and persisting for months or years.[72] Recurrence of shingles, though uncommon, carries a lifetime risk of 1% to 6% in the general population, rising significantly to 0% to 18% in immunocompromised individuals due to factors such as organ transplantation or HIV.[73] Recurrent episodes typically involve the same dermatome as the initial infection or an adjacent one, driven by persistent varicella-zoster virus latency and waning immunity.[74] Scarring from shingles is generally minimal and resolves without intervention in most cases, though hyperpigmentation or minor discoloration may linger, particularly in individuals with darker skin tones.[75] In ophthalmic shingles (herpes zoster ophthalmicus), which affects about 10% to 20% of cases, there is a 5% to 10% risk of permanent vision impairment, often due to corneal scarring, uveitis, or secondary glaucoma, with higher rates linked to older age and immunosuppression.[76] Recent 2025 research has established a link between shingles and increased dementia risk, with unvaccinated individuals facing approximately 20% higher odds of developing dementia compared to those vaccinated, an effect attributed to varicella-zoster virus reactivation contributing to neuroinflammation; vaccination mitigates this by reducing both shingles incidence and subsequent cognitive decline.[41]Epidemiology
Incidence and Prevalence
In the United States, an estimated 1 million cases of shingles (herpes zoster) occur annually, with a lifetime risk of approximately 30% for the general population.[1] The annual incidence rate overall ranges from 2 to 9 cases per 1,000 person-years, increasing significantly with age.[4] Shingles is rare in individuals under 20 years of age, with incidence rates of approximately 0.6 to 0.7 cases per 1,000 person-years in this group.[77] In contrast, rates rise sharply in older adults; for those over 50 years, the annual incidence is approximately 8 to 10 cases per 1,000 person-years, escalating to about 12 cases per 1,000 person-years among those over 80.[78][79] Following the rollout of the Shingrix vaccine in 2017, incidence rates have shown a decline in vaccinated cohorts, with effectiveness estimates of 70% to 90% against shingles in adults aged 50 and older by 2025, depending on immune status and follow-up duration.[80] Population-level trends indicate a modest overall reduction of around 16% in herpes zoster incidence among older adults through 2023, attributed to increasing vaccine uptake, with continued declines observed into 2025 due to higher vaccination rates.[81][10] Postherpetic neuralgia (PHN), a common complication, affects approximately 12% of shingles cases overall, with rates rising to 20% or higher in those over 60 and decreasing with prompt antiviral treatment initiation within 72 hours of rash onset.[82][15]Global Distribution
Shingles, or herpes zoster, exhibits significant geographical variations in incidence and burden, largely influenced by population demographics, healthcare infrastructure, and underlying health conditions. In regions with aging populations such as Europe and Japan, the disease imposes a higher burden, with incidence rates among individuals over 60 years old ranging from 5 to 10 per 1,000 person-years.[83] These elevated rates reflect the increased risk of varicella-zoster virus reactivation in older adults, exacerbated by longer life expectancies and better diagnostic reporting in high-income settings. In contrast, regions like sub-Saharan Africa and parts of Asia report lower overall incidence due to younger demographic profiles, where fewer individuals reach the age groups most susceptible to shingles; however, substantial underreporting occurs owing to limited surveillance systems and prioritization of other infectious diseases.[84] Socioeconomic factors further accentuate disparities, particularly in low-resource settings where access to varicella vaccination remains limited, leading to higher primary chickenpox exposure and a potential future surge in shingles cases as populations age.[84] In areas with high HIV prevalence, such as sub-Saharan Africa, immunosuppression drives notably higher rates, with incidence among untreated HIV patients estimated at 35 to 54 per 1,000 person-years, compared to general populations.[85] This elevated burden underscores the interplay between infectious comorbidities and shingles distribution in resource-constrained environments. Vaccination programs are expanding in low- and middle-income countries (LMICs) to mitigate this, with initiatives focusing on integrating shingles vaccines into national immunization schedules to address inequities in access and reduce future incidence.[86]History
Historical Recognition
The earliest descriptions of what is now known as shingles date back to ancient times. Hippocrates in the 5th century BCE used the term "herpes" (Greek: "to creep") to describe ulcerative skin lesions that appeared to crawl along the skin, thus encompassing conditions including herpes simplex and herpes zoster.[87] These observations captured the characteristic unilateral, girdle-like eruption and associated neuralgia, distinguishing it from other skin afflictions of the era.[88] Ancient physicians, including the Roman encyclopedist Aulus Cornelius Celsus (c. 25 BCE–50 CE), recognized the disease's distinctive belt-shaped ("zoster" from Greek for girdle) manifestation along nerve pathways, using the term "herpes zoster" for the first time.[87][89] In the 18th century, British physician William Heberden advanced the understanding of the disease with a detailed clinical description in 1768, highlighting the vesicular eruption confined to a single dermatome, often with severe pain, and its tendency to occur in older individuals, setting it apart from more generalized herpes infections or smallpox.[90] This characterization laid the groundwork for recognizing shingles as a distinct entity and influenced subsequent medical literature. By the 19th century, connections began to emerge between shingles and chickenpox, with Hungarian physician János von Bókay proposing in 1909 that the two were manifestations of the same infectious agent, based on epidemiological observations of varicella outbreaks following zoster cases in households.[91] Von Bókay's work, building on his earlier 1892 suggestions, documented instances where susceptible children developed chickenpox after exposure to adults with shingles, prefiguring the viral etiology and challenging the view of zoster as merely a degenerative nerve condition.[87] This linkage shifted perceptions toward an infectious origin, though the causative agent remained unidentified until the mid-20th century.[92] The definitive confirmation of shingles' cause came in 1953, when American virologist Thomas H. Weller successfully isolated the varicella-zoster virus (VZV) from vesicular fluid of patients with both varicella and herpes zoster, demonstrating that the same pathogen was responsible for primary chickenpox infection and its later reactivation as shingles.[93] Weller's breakthrough, achieved through human tissue cell culture techniques, not only verified von Bókay's hypothesis but also enabled serological and virological studies that solidified the understanding of VZV latency in dorsal root ganglia.[94] This isolation marked a pivotal advancement, transforming shingles from a clinically observed syndrome into a well-defined viral disease.[95]Vaccine Development
The development of vaccines for shingles, resulting from varicella-zoster virus (VZV) reactivation, originated in the mid-20th century following the virus's initial isolation. In the 1950s, VZV was first successfully cultured in human embryonic lung cells, enabling foundational virological research. Specifically, in 1953, Thomas Weller isolated VZV from vesicular fluid of patients with varicella or zoster using cell culture techniques, marking a critical step toward vaccine development. These efforts laid the groundwork for attenuated vaccines targeting VZV. In the 1970s, live attenuated vaccines were developed primarily for preventing primary VZV infection (chickenpox), which indirectly informed shingles prevention strategies. Japanese researcher Michiaki Takahashi isolated the Oka strain from a child with varicella in 1971 and attenuated it through serial passage in human and guinea pig cells by 1974, creating the first viable live varicella vaccine.[96] This Oka strain became the basis for subsequent shingles vaccines, as it demonstrated the potential to stimulate cell-mediated immunity against VZV latency and reactivation. The first dedicated shingles vaccine, Zostavax, received FDA approval in 2006 as a live attenuated formulation using a higher potency (14 times that of the varicella vaccine) of the Oka strain. Administered as a single intramuscular dose to adults aged 60 and older, Zostavax aimed to reduce the incidence of herpes zoster by approximately 51% and postherpetic neuralgia by 67% in clinical trials.[97] Subsequent innovations addressed Zostavax's limitations, such as waning efficacy over time and contraindications in immunocompromised individuals, leading to Shingrix. Approved by the FDA in October 2017 and by the EMA in 2018, Shingrix is a non-live recombinant vaccine comprising VZV glycoprotein E (gE) antigen combined with the AS01B liposome-based adjuvant to enhance T-cell and antibody responses. Pivotal phase 3 trials—ZOE-50 in adults aged 50 and older, and ZOE-70 in those aged 70 and older—showed vaccine efficacy of 97.2% and 89.8% against shingles, respectively, with pooled efficacy exceeding 90% and sustained reduction in postherpetic neuralgia by over 88%.[34][98][99] By 2025, Shingrix has become the standard, with universal CDC recommendations for two doses in immunocompetent adults aged 50 and older, and in immunocompromised adults aged 19 and older, following Zostavax's discontinuation in 2020 due to superior alternatives. The WHO recommends Shingrix for adults ≥50 years in high-income settings as of 2023.[100] Long-term follow-up data from the ZOE-LTFU study indicate Shingrix retains 79.7% efficacy against shingles 6 to 11 years after vaccination in adults aged 50 and older.[101] This durability supports current guidelines without routine boosters, though ongoing research explores additional dosing to extend protection beyond 10 years, particularly in high-risk populations.[102]Etymology
Origin of the Term
The English term "shingles" for the disease emerged in the late 14th century, derived from Medieval Latin cingulus, a variant of cingulum meaning "girdle" or "belt." This etymology stems from the Latin verb cingere, "to gird," capturing the rash's typical band-like distribution around the torso or other body areas.[103] The word entered Middle English through medical texts, reflecting the observable pattern of the skin eruption that resembles a fastened belt.[104] The medical nomenclature herpes zoster originates from ancient Greek roots. Herpes comes from herpein (ἕρπω), meaning "to creep," which describes the progressive, spreading character of the vesicular lesions along nerve paths. Zoster derives from zōstēr (ζωστήρ), denoting a "girdle" or "warrior's belt," again emphasizing the encircling, dermatomal rash.[105] The term "herpes zoster" was first used by the Roman encyclopedist Aulus Cornelius Celsus (c. 25 BC – c. 50 AD), based on earlier classical Greek descriptions of similar skin conditions. Another historical name is "zona," borrowed from Latin zona, itself from Greek zōnē (ζώνη), signifying a "belt," "zone," or "girdle." The term gained medical usage in the 1st century AD when Roman physician Scribonius Largus linked it to herpes-like conditions, highlighting the zonal eruption pattern.[106] In French, the disease is termed zona, preserving this classical root to denote the belt-shaped affliction.[107]Research
Current Studies
Recent clinical trials have extended understanding of the Shingrix vaccine's long-term efficacy, demonstrating sustained protection against herpes zoster (shingles) for over a decade. An interim analysis of long-term follow-up data from phase 3 trials indicated that Shingrix maintains efficacy of 84.0% against shingles over approximately 5.1–7.1 years of follow-up, with data extending to a mean of 5.6 to 9.6 years in adults aged 50 and older.[108] Final analyses of the ZOE-LTFU study further confirmed efficacy of 79.7% six to 11 years after vaccination in this population.[109] Additionally, 2025 observational data have linked Shingrix to a reduction in dementia risk, with a natural experiment showing a 3.5 percentage point absolute decrease in the probability of a new dementia diagnosis over seven years among vaccinated individuals.[41] Innovations in antiviral therapies for shingles focus on addressing varicella-zoster virus (VZV) reactivation, particularly in complicated cases. Low-dose, long-term oral valacyclovir has shown benefits in reducing ocular complications and persistent pain in patients with herpes zoster ophthalmicus, a severe form of shingles affecting the eye, by lowering recurrence rates and improving outcomes in a 2025 study.[55] For resistant or refractory VZV infections, emerging research explores novel formulations like GS-1, a compound of undecylenic acid and L-arginine, which inhibits viral entry and reduces symptoms and contagiousness in preclinical and early clinical evaluations as of 2025.[110] These approaches aim to provide longer-acting options beyond standard acyclovir, though specific trials for highly resistant VZV strains remain limited. Phase 3 trials of monoclonal antibodies targeting nerve growth factor (NGF) continue to investigate their potential for postherpetic neuralgia (PHN), the chronic pain complication following shingles. Anti-NGF agents like tanezumab have demonstrated efficacy in reducing neuropathic pain in broader chronic pain studies, with pooled analyses from over 5,000 patients showing significant improvements in pain scores and function, though primarily evaluated in osteoarthritis contexts.[111] For PHN specifically, investigational anti-NGF therapies, such as fulranumab, have been evaluated in phase 2 trials, showing potential in pain relief without FDA approval.[112] Longitudinal population studies in 2025 have established connections between shingles vaccination and reduced cardiovascular risks. A May 2025 analysis reported a 23% relative risk reduction in major cardiovascular events, including heart attacks and strokes, among vaccinated adults compared to unvaccinated controls, based on large cohort data.[113] Systematic reviews and meta-analyses further support this, finding 18% and 16% reductions in composite cardiovascular outcomes for recombinant zoster vaccine (RZV) and zoster vaccine live (ZVL) recipients, respectively, with stronger effects in higher-risk groups like older males.[114] These findings underscore the vaccine's broader protective role beyond shingles prevention.Emerging Therapies
Recent advancements in shingles research have focused on next-generation vaccines utilizing mRNA technology to enhance prevention strategies. mRNA-based vaccines targeting varicella-zoster virus (VZV) glycoproteins, such as glycoprotein E (gE), are currently in phase I/II clinical trials as of 2025, with candidates like those developed by Pfizer/BioNTech demonstrating robust immunogenicity in preclinical models.[115] These vaccines aim to provide single-dose efficacy and broader cellular immunity, including stronger CD8+ T cell responses compared to existing recombinant zoster vaccines, potentially offering longer-lasting protection against reactivation.[116] In mouse studies, low doses (1 µg) of lyophilized mRNA constructs elicited IgG levels and T cell activation superior to benchmarks, with stability maintained for up to 24 months under refrigeration, addressing logistical challenges in vaccine deployment.[115] Gene editing technologies, particularly CRISPR/Cas9, represent a preclinical frontier for eradicating latent VZV reservoirs in sensory ganglia to prevent reactivation. Approaches using Staphylococcus aureus Cas9 (saCas9) delivered via adeno-associated virus (AAV2) vectors target duplicated VZV open reading frames (ORF62/71), essential for viral replication and latency maintenance.[117] In human embryonic stem cell-derived neuron models simulating ganglionic latency, CRISPR editing reduced infectious virus production by over 100-fold upon induced reactivation and limited cell-to-cell spread, without evidence of off-target effects in neuronal cultures.[117] Although in vivo animal models for VZV latency remain limited due to species specificity, these findings suggest potential for disrupting persistent viral genomes, paving the way for therapies that could eliminate the risk of shingles in latently infected individuals.[118] Neuroprotective agents, including small-molecule modulators of inflammatory pathways, are under investigation to mitigate postherpetic neuralgia (PHN) by preserving neuronal integrity during acute VZV infection. Compounds like olodanrigan (EMA401), an angiotensin II type 2 receptor (AT2R) antagonist, have shown promise in phase II trials by reducing neuroinflammation and pain signaling in PHN models, though development was paused due to preclinical hepatotoxicity concerns.[112] Similarly, LANCL2 activators such as LAT8881 target anti-inflammatory cascades to dampen glial activation and cytokine release, with early-phase studies indicating safety but mixed efficacy in preventing chronic pain progression. These agents operate via pathways that inhibit pro-inflammatory mediators like TNF-α and IL-6, offering a complementary approach to antivirals by addressing the neuropathic sequelae of shingles at the molecular level.[112] Prophylactic long-term suppressive antiviral therapies are being evaluated in clinical trials for high-risk immunocompromised populations to suppress VZV reactivation beyond acute treatment durations. In hematopoietic cell transplantation (HCT) recipients, one-year regimens of acyclovir or valacyclovir have demonstrated significant reductions in herpes zoster incidence, with rates dropping below 1% during prophylaxis and no rebound effect observed post-discontinuation.[119] Extended protocols, lasting until immunosuppression resolves, further lower disease occurrence but highlight the need for personalized durations, as approximately 6% of patients experienced breakthrough events in the second year.[119] Ongoing trials emphasize low-dose oral formulations to balance efficacy with tolerability in groups like solid organ transplant recipients, aiming to integrate these with vaccination for sustained prevention.[120] Looking toward the 2025 horizon, AI-driven predictive models are emerging to forecast shingles reactivation risk by analyzing immune biomarkers, enabling targeted interventions. Machine learning algorithms integrating multi-omics data, such as CD8+ T cell frequencies and cytokine profiles, have shown high accuracy in stratifying reactivation probabilities in herpesvirus models, with applications extending to VZV in immunocompromised cohorts.[121] For instance, predictive scoring systems like AIRMET, validated in post-allogeneic HCT patients, incorporate biomarkers of immune reconstitution to identify late-onset risks, achieving reliable stratification for prophylactic decisions.[122] These tools leverage temporal data on immune surveillance to simulate reactivation triggers, potentially reducing shingles burden through precision medicine.[121]References
- https://en.wiktionary.org/wiki/shingles#Etymology_1

