Recent from talks
Nothing was collected or created yet.
Myopia
View on Wikipedia
| Myopia | |
|---|---|
| Other names | near-sightedness, short-sightedness |
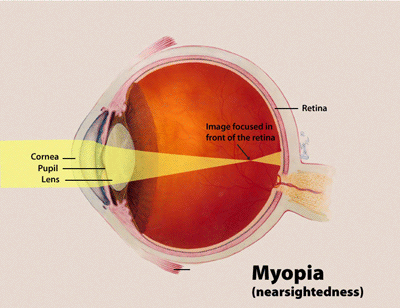
 | |
| Diagram showing changes in the eye with myopia | |
| Specialty | Ophthalmology, optometry |
| Symptoms | Distant objects appear blurry, headaches, eye strain[1] |
| Complications | Retinal detachment, cataracts, glaucoma[2] |
| Duration | Typically permanent once it develops |
| Causes | Combination of genetic and environmental factors[2] |
| Risk factors | Near work, greater time spent indoors, family history[2][3] |
| Diagnostic method | Eye examination[1] |
| Prevention | Unknown |
| Treatment | Eyeglasses, contact lenses, surgery[1] |
| Medication | Low-dose atropine eye drops[4] |
| Prognosis | Generally stable after it progresses in early adulthood[5] |
| Frequency | Approximately 30% of people around the world[6] |
| Deaths | Not deadly |
Myopia, also known as near-sightedness and short-sightedness,[7] is an eye condition[8][9] where light from distant objects focuses in front of, instead of on, the retina.[1][2][10] As a result, distant objects appear blurry, while close objects appear normal.[1] Other symptoms may include headaches and eye strain.[1][11] Severe myopia is associated with an increased risk of macular degeneration, retinal detachment, cataracts, and glaucoma.[2][12]
Myopia results from the length of the eyeball growing too long or less commonly the lens being too strong.[1][13] It is a type of refractive error.[1] Diagnosis is by the use of cycloplegics during eye examination.[14]
Myopia is less common in people who spent more time outside during childhood.[15][16] This lower risk may be due to greater exposure to sunlight.[17][18] Myopia can be corrected with eyeglasses, contact lenses, or by refractive surgery.[1][19] Eyeglasses are the simplest and safest method of correction.[1] Contact lenses can provide a relatively wider corrected field of vision, but are associated with an increased risk of infection.[1][20] Refractive surgeries such as LASIK and PRK permanently change the shape of the cornea. Other procedures include implantable collamer lens (ICL) placement inside the anterior chamber in front of the natural eye lens. ICL does not affect the cornea.[1][21]
Myopia is the most common eye problem and is estimated to affect 1.5 billion people (22% of the world population).[2][22] Rates vary significantly in different areas of the world.[2] Rates among adults are between 15% and 49%.[3][23] Among children, it affects 1% of rural Nepalese, 4% of South Africans, 12% of people in the US, and 37% in some large Chinese cities.[2][3] In China the proportion of girls is slightly higher than boys.[24] Rates have increased since the 1950s.[23] Uncorrected myopia is one of the most common causes of vision impairment globally along with cataracts, macular degeneration, and vitamin A deficiency.[23][25][26][27]
Signs and symptoms
[edit]A person with myopia can see clearly out to a certain distance (the far point of the eye), but objects placed beyond this distance appear blurred.[19][28] If the extent of the myopia is great enough, even standard reading distances can be affected. Upon routine examination of the eyes, the vast majority of myopic eyes appear structurally identical to nonmyopic eyes.[29][28]
Onset is often in school children, with worsening between the ages of 8 and 15.[30][31]
Myopic individuals have larger pupils than far-sighted (hypermetropic) and emmetropic individuals, likely due to requiring less accommodation (which results in pupil constriction).[32][33]
Causes
[edit]The underlying cause of myopia is believed to be a combination of genetic and environmental factors.[2][34][35] Risk factors include doing work that involves focusing on close objects, greater time spent indoors, urbanization, and a family history of the condition.[2][3][36][37] It is also associated with a high socioeconomic class and higher level of education.[2][37]
A 2012 review could not find strong evidence for any single cause, although many theories have been discredited.[38] Twin studies indicate that at least some genetic factors are involved.[30][39][40] Myopia has been increasing rapidly throughout the developed world, suggesting environmental factors are involved.[41]
The role of corrective lenses interfering with emmetropization has also been suggested.[42][43]
Genetics
[edit]A risk for myopia may be inherited from one's parents.[44] Genetic linkage studies have identified 18 possible loci on 15 different chromosomes that are associated with myopia, but none of these loci is part of the candidate genes that cause myopia. Instead of a simple one-gene locus controlling the onset of myopia, a complex interaction of many mutated proteins acting in concert may be the cause. Instead of myopia being caused by a defect in a structural protein, defects in the control of these structural proteins might be the actual cause of myopia.[45] A collaboration of all myopia studies worldwide identified 16 new loci for refractive error in individuals of European ancestry, of which 8 were shared with Asians. The new loci include candidate genes with functions in neurotransmission, ion transport, retinoic acid metabolism, extracellular matrix remodeling and eye development. The carriers of the high-risk genes have a tenfold increased risk of myopia.[46] Aberrant genetic recombination and gene splicing in the OPNLW1 and OPNMW1 genes that code for two retinal cone photopigment proteins can produce high myopia by interfering with refractive development of the eye.[47][48]
Human population studies suggest that contribution of genetic factors accounts for 60–90% of variance in refraction.[49][50][51][52] However, the currently identified variants account for only a small fraction of myopia cases, suggesting the existence of a large number of yet unidentified low-frequency or small-effect variants, which underlie the majority of myopia cases.[53]
Environmental factors
[edit]Environmental factors that increase the risk of myopia include insufficient light exposure, low physical activity, near work, and increased years of education.[30][43]
One hypothesis is that a lack of normal visual stimuli causes improper development of the eyeball. Under this hypothesis, "normal" refers to the environmental stimuli that the eyeball evolved to.[54] Modern humans who spend most of their time indoors, in dimly or fluorescently lit buildings may be at risk of development of myopia.[54]
People, and children especially, who spend more time doing physical exercise and outdoor play, have lower rates of myopia,[55][54][56][57][41] suggesting the increased magnitude and complexity of the visual stimuli encountered during these types of activities decrease myopic progression. There is preliminary evidence that the protective effect of outdoor activities on the development of myopia is due, at least in part, to the effect of long hours of exposure to daylight on the production and the release of retinal dopamine.[41][58][59][60]
Myopia can be induced with minus spherical lenses,[61] and overminus in prescription lenses can induce myopia progression.[62][63] Overminus during refraction can be avoided through various techniques and tests, such as fogging, plus to blur, and the duochrome test.[63]
The near work hypothesis, also referred to as the "use-abuse theory", states that spending time involved in near work strains the intraocular and extraocular muscles. Some studies support the hypothesis, while other studies do not.[3] While an association is present, it is not clearly causal.[3]
Myopia is also more common in children with diabetes, childhood arthritis, uveitis, and systemic lupus erythematosus.[30]
Other factors
[edit]Research indicates a relationship between body mass index (BMI) and myopia, with both low and high BMI associated with an increased risk of developing myopia. A nationwide study of 1.3 million Israeli adolescents found that individuals with underweight status had higher chances of mild-to-moderate and high myopia compared to those with low-normal BMI.[64]
Similarly, a study involving Korean young adult men reported that those who were of average or shorter height and lean had a higher prevalence of high myopia.[65][66]
Mechanism
[edit]Because myopia is a refractive error, the physical cause of myopia is comparable to any optical system that is out of focus. Borish and Duke-Elder classified myopia by these physical causes:[67][68]
- Axial myopia is attributed to an increase in the eye's axial length.[69]
- Refractive myopia is attributed to the condition of the refractive elements of the eye.[69] Borish further subclassified refractive myopia:[67]
- Curvature myopia is attributed to excessive, or increased, curvature of one or more of the refractive surfaces of the eye, especially the cornea.[69] In those with Cohen syndrome, myopia appears to result from high corneal and lenticular power.[70]
- Index myopia is attributed to variation in the index of refraction of one or more of the ocular media.[69]
As with any optical system experiencing a defocus aberration, the effect can be exaggerated or masked by changing the aperture size. In the case of the eye, a large pupil emphasizes refractive error and a small pupil masks it. This phenomenon can cause a condition in which an individual has a greater difficulty seeing in low-illumination areas, even though there are no symptoms in bright light, such as daylight.[71]
Under rare conditions, edema of the ciliary body can cause an anterior displacement of the lens, inducing a myopia shift in refractive error.[72]
Diagnosis
[edit]A diagnosis of myopia is typically made by an eye care professional, usually an optometrist or ophthalmologist. This is by refracting the eye with the use of cycloplegics such as atropine with responses recorded when accommodation is relaxed.[14] Diagnosis of progressive myopia requires regular eye examination using the same method.[14]
Types
[edit]Myopia can be classified into two major types; anatomical and clinical. The types of myopia based on anatomical features are axial, curvature, index and displacement of refractive element. Congenital, simple and pathological myopia are the clinical types of myopia.[7]
Various forms of myopia have been described by their clinical appearance:[68][73][74]
- Simple myopia: Myopia in an otherwise normal eye, typically less than 4.00 to 6.00 diopters.[75] This is the most common form of myopia.
- Degenerative myopia, also known as malignant, pathological, or progressive myopia, is characterized by marked fundus changes, such as posterior staphyloma, and associated with a high refractive error and subnormal visual acuity after correction.[69] This form of myopia gets progressively worse over time. Degenerative myopia has been reported as one of the main causes of visual impairment.[76]
- Pseudomyopia is the blurring of distance vision brought about by spasm of the accommodation system.[77]
- Nocturnal myopia: Without adequate stimulus for accurate accommodation, the accommodation system partially engages, pushing distance objects out of focus.[75]
- Nearwork-induced transient myopia (NITM): short-term myopic far point shift immediately following a sustained near visual task.[78] Some authors argue for a link between NITM and the development of permanent myopia.[79]
- Instrument myopia: over-accommodation when looking into an instrument such as a microscope.[74]
- Induced myopia, also known as acquired myopia, sometimes reversible myopic shift, results from various medications, increases in glucose levels, nuclear sclerosis, oxygen toxicity (e.g., from underwater diving or from oxygen and hyperbaric therapy) or other anomalous conditions.[80][75] Sulphonamide therapy can cause ciliary body edema, resulting in anterior displacement of the lens, pushing the eye out of focus.[72] Elevation of blood-glucose levels can also cause edema (swelling) of the crystalline lens as a result of sorbitol accumulating in the lens. This edema often causes temporary myopia. Scleral buckles, used in the repair of retinal detachments may induce myopia by increasing the axial length of the eye.[81]
- Index myopia is attributed to variation in the index of refraction of one or more of the ocular media.[69] Cataracts may lead to index myopia.[82]
- Form deprivation myopia occurs when the eyesight is deprived by limited illumination and vision range,[83] or the eye is modified with artificial lenses[84] or deprived of clear form vision.[85] In lower vertebrates, this kind of myopia seems to be reversible within short periods of time. Myopia is often induced this way in various animal models to study the pathogenesis and mechanism of myopia development.[86]
Degree
[edit]The degree of myopia is described in terms of the power of the ideal correction, which is measured in diopters:[87]
- Myopia between −0.00 and −0.50 diopters is usually classified as emmetropia.
- Low myopia usually describes myopia between −0.50 and −3.00 diopters.[69]
- Moderate myopia usually describes myopia between −3.00 and −6.00 diopters.[69] Those with moderate amounts of myopia are more likely to have pigment dispersion syndrome or pigmentary glaucoma.[88]
- High myopia usually describes myopia of −6.00 or more.[69][89] People with high myopia are more likely to have retinal detachments[90] and primary open angle glaucoma.[91] They are also more likely to experience floaters, shadow-like shapes which appear in the field of vision.[92] In addition to this, high myopia is linked to macular degeneration, cataracts, and significant visual impairment.[93][94][95][96]
The highest myopia ever recorded was −108 diopters by a Slovak, Jan Miskovic.[97]
Age at onset
[edit]Myopia is sometimes classified by the age at onset:[87]
- Congenital myopia, also known as infantile myopia, is present at birth and persists through infancy.[75]
- Youth onset myopia occurs in early childhood or teenage, and the ocular power can keep varying until the age of 21, before which any form of corrective surgery is usually not recommended by ophthalmic specialists around the world.[75]
- School myopia appears during childhood, particularly the school age years.[98] This form of myopia is attributed to the use of the eyes for close work during the school years.[69] A 2004–2015 Singapore–Sydney study of children of Chinese descent found that time spent on outdoor activities was a factor.[99]
- Adult onset myopia
Prevention and control
[edit]Various methods have been employed in an attempt to decrease the progression of myopia, although studies show mixed results.[100] Many myopia treatment studies have a number of design drawbacks: small numbers, lack of adequate control group, and failure to mask examiners from knowledge of treatments used. The best approach is to combine multiple prevention and control methods.[101] A test of repeated low-level red-light therapy (LLRL) on myopic Chinese children showed it to be a promising alternative treatment for myopia control in children.[102]
Spending time outdoors
[edit]Some studies have indicated that having children spend time outdoors reduces the incidence of myopia.[103] A 2017 study investigated the leading causal theory of association between greenspace exposure and spectacles use as a proxy for myopia, finding a 28% reduction in the likelihood of spectacles use per interquartile range increase in time spent in greenspace.[104] In Taiwan, government policies that require schools to send all children outdoors for a minimum amount of time have driven down the prevalence of myopia in children.[103][105]
Glasses and contacts
[edit]The use of reading glasses when doing close work may improve vision by reducing or eliminating the need to accommodate. Altering the use of eyeglasses between full-time, part-time, and not at all does not appear to alter myopia progression.[106][107] The American Optometric Association's Clinical Practice Guidelines found evidence of effectiveness of bifocal lenses and recommends it as the method for "myopia control".[75] In some studies, bifocal and progressive lenses have not shown differences in altering the progression of myopia compared to placebo.[100][108]
In the United States, the Food and Drug Administration (FDA) has approved myopia control contact lenses such as CooperVision's MiSight and Johnson & Johnson Vision's Acuvue Abiliti. Yet the agency has yet to approve any myopia control spectacle lenses.
Medication
[edit]Anti-muscarinic topical medications in children under 18 years of age may slow the worsening of myopia.[109][110] These treatments include pirenzepine gel, cyclopentolate eye drops, and atropine eye drops. While these treatments were shown to be effective in slowing the progression of myopia and reducing eyeball elongation associated with the condition, side effects included light sensitivity and near blur.[109][111]
Other methods
[edit]Scleral reinforcement surgery is aimed to cover the thinning posterior pole with a supportive material to withstand intraocular pressure and prevent further progression of the posterior staphyloma. The strain is reduced, although damage from the pathological process cannot be reversed. By stopping the progression of the disease, vision may be maintained or improved.[112] The use of orthoK can also slow down axial lens elongation.[113]
Treatment
[edit]
The National Institutes of Health says there is no known way of preventing myopia, and the use of glasses or contact lenses does not affect its progression, unless the glasses or contact lenses are too strong of a prescription.[114] There is no universally accepted method of preventing myopia and proposed methods need additional study to determine their effectiveness.[75] Optical correction using glasses or contact lenses is the most common treatment; other approaches include orthokeratology, and refractive surgery.[75]: 21–26 Medications (mostly atropine) and vision therapy can be effective in addressing the various forms of pseudomyopia.

Glasses and contacts
[edit]Corrective lenses bend the light entering the eye in a way that places a focused image accurately onto the retina. The power of any lens system can be expressed in diopters, the reciprocal of its focal length in meters. Corrective lenses for myopia have negative powers because a divergent lens is required to move the far point of focus out to the distance. More severe myopia needs lens powers further from zero (more negative). However, strong eyeglass prescriptions create distortions such as prismatic movement and chromatic aberration. Strongly myopic wearers of contact lenses do not experience these distortions because the lens moves with the cornea, keeping the optic axis in line with the visual axis and because the vertex distance has been reduced to zero.
Surgery
[edit]Refractive surgery includes procedures which alter the corneal curvature of some structure of the eye or which add additional refractive means inside the eye.
Photorefractive keratectomy
[edit]Photorefractive keratectomy (PRK) involves ablation of corneal tissue from the corneal surface using an excimer laser. The amount of tissue ablation corresponds to the amount of myopia. While PRK is a relatively safe procedure for up to 6 dioptres of myopia, the recovery phase post-surgery is usually painful.[115][116]
LASIK
[edit]In a LASIK pre-procedure, a corneal flap is cut into the cornea and lifted to allow the excimer laser beam access to the exposed corneal tissue. After that, the excimer laser ablates the tissue according to the required correction. When the flap again covers the cornea, the change in curvature generated by the laser ablation proceeds to the corneal surface. Though LASIK is usually painless and involves a short rehabilitation period post-surgery, it can potentially result in flap complications and loss of corneal stability (post-LASIK keratectasia).[117][118]
Phakic intra-ocular lens
[edit]Instead of modifying the corneal surface, as in laser vision correction (LVC), this procedure involves implanting an additional lens inside the eye (i.e., in addition to the already existing natural lens). While it usually results in good control of the refractive change, it can induce potential serious long-term complications such as glaucoma, cataract and endothelial decompensation.[119][120][121]
Orthokeratology
[edit]Orthokeratology or simply Ortho-K is a temporary corneal reshaping process using rigid gas permeable (RGP) contact lenses.[122] Overnight wearing of specially designed contact lenses will temporarily reshape cornea, so patients may see clearly without any lenses in daytime. Orthokeratology can correct myopia up to −6D.[123] Several studies shown that Ortho-K can reduce myopia progression also.[124][125] Risk factors of using Ortho-K lenses include microbial keratitis,[124] corneal edema,[126] etc. Other contact lens related complications such as corneal aberration, photophobia, pain, irritation, redness etc. are usually temporary conditions, which may be eliminated by proper usage of lenses.[126]
Intrastromal corneal ring segment
[edit]The Intrastromal corneal ring segment (ICRS), commonly used in keratoconus treatment now, was originally designed to correct mild to moderate myopia.[127] The thickness is directly related to flattening and the diameter of the ring is proportionally inverse to the flattening of cornea. So, if diameter is smaller or thickness is greater, resulting myopia correction will be greater.[128]
Alternative medicine
[edit]A number of alternative therapies have been claimed to improve myopia, including vision therapy, "behavioural optometry", various eye exercises and relaxation techniques, and the Bates method.[129] Scientific reviews have concluded that there was "no clear scientific evidence" that eye exercises are effective in treating myopia[130] and as such they "cannot be advocated".[131]
Epidemiology
[edit]Global refractive errors have been estimated to affect 800 million to 2.3 billion.[132] The incidence of myopia within sampled population often varies with age, country, sex, race, ethnicity, occupation, environment, and other factors.[133][134] Variability in testing and data collection methods makes comparisons of prevalence and progression difficult.[135]
The prevalence of myopia has been reported as high as 70–90% in some Asian countries, 30–40% in Europe and the United States, and 10–20% in Africa.[134] Myopia is about twice as common in Jewish people than in people of non-Jewish ethnicity.[136] Myopia is less common in African people and associated diaspora.[133] In Americans between the ages of 12 and 54, myopia has been found to affect African Americans less than Caucasians.[137]
A 2024 study published in the British Journal of Ophthalmology revealed that more than one-third of children worldwide were nearsighted in 2023, with this figure projected to rise to nearly 40% by 2050.[138] The prevalence of myopia among children and adolescents has increased significantly over the past 30 years, rising from 24% in 1990 to almost 36% in 2023, with researchers noting a sharp spike in cases following the COVID-19 pandemic and highlighting regional differences in myopia rates.[139]
A 2025 South Korean analysis of 45 studies, involving 335,524 participants and largely based on data from children, adolescents and young adults, that looked at the use of digital screen devices such as mobile phones, game consoles and television, revealed that an additional hour of daily screen time is, on average, associated with 21% higher odds of having myopia.[140]
Asia
[edit]
In some parts of Asia, myopia is very common.
- Singapore is believed to have the highest prevalence of myopia in the world; up to 80% of people there have myopia, but the accurate figure is unknown.[142]
- China's myopia rate is 31%: 400 million of its 1.3 billion people are myopic. The prevalence of myopia in high school in China is 77%, and in college is more than 80%.[143]
- In some areas, such as China and Malaysia, up to 41% of the adult population is myopic to 1.00 dpt,[144] and up to 80% to 0.5 dpt.[145]
- A study of Jordanian adults aged 17 to 40 found more than half (54%) were myopic.[146]
- A study indicated that the prevalence of myopia among urban children in India of aged 5 to 15 increased from 4.44% in 1999 to 21.15% in 2019. Projections suggest that by 2050, this figure could reach 48.14%.[147]
- Some research suggests the prevalence of myopia in Indian children is less than 15%.[148]
- In South Korea among the general population, national data indicates that 70.6% of the adult population has myopia, with 8.0% affected by high myopia. The prevalence decreases with age, from 81.3% in individuals aged 19 to 24 years to 55.2% in those aged 45 to 49 years.[149]
- Up to 90% of young people in Taiwan have myopia.[150]
Europe
[edit]
- In first-year undergraduate students in the United Kingdom 50% of British whites and 53% of British Asians were myopic.[152]
- A recent review found 27% of Western Europeans aged 40 or older have at least −1.00 diopters of myopia and 5% have at least −5.00 diopters.[153]
North America
[edit]Myopia is common in the United States, with research suggesting this condition has increased dramatically in recent decades. In 1971–1972, the National Health and Nutrition Examination Survey provided the earliest nationally representative estimates for myopia prevalence in the U.S., and found the prevalence in persons aged 12–54 was 25%. Using the same method, in 1999–2004, myopia prevalence was estimated to have climbed to 42%.[154]
A study of 2,523 children in grades 1 to 8 (age, 5–17 years) found nearly one in 10 (9%) have at least −0.75 diopters of myopia.[155] In this study, 13% had at least +1.25 D hyperopia (farsightedness), and 28% had at least 1.00-D difference between the two principal meridians (cycloplegic autorefraction) of astigmatism. For myopia, Asians had the highest prevalence (19%), followed by Hispanics (13%). Caucasian children had the lowest prevalence of myopia (4%), which was not significantly different from African Americans (7%).[155]
A recent review found 25% of Americans aged 40 or older have at least −1.00 diopters of myopia and 5% have at least −5.00 diopters.[153]
Australia
[edit]In Australia, the overall prevalence of myopia (worse than −0.50 diopters) has been estimated to be 17%.[156] In one recent study, less than one in 10 (8%) Australian children between the ages of four and 12 were found to have myopia greater than −0.50 diopters.[157] A recent review found 16% of Australians aged 40 or older have at least −1.00 diopters of myopia and 3% have at least −5.00 diopters.[153]
South America
[edit]In Brazil, a 2005 study estimated 6% of Brazilians between the ages of 12 and 59 had −1.00 diopter of myopia or more, compared with 3% of the indigenous people in northwestern Brazil.[158] Another found nearly 1 in 8 (13%) of the students in the city of Natal were myopic.[159]
History
[edit]The difference between the near-sighted and far-sighted people was noted already by Aristotle.[160] Graeco-Roman physician Galen first used the term "myopia" (from Greek words "myein" meaning "to close or shut" and "ops" (gen. opos) meaning "eye") for near-sightedness.[160] The first spectacles for correcting myopia were invented by a German cardinal in the year 1451.[161] Johannes Kepler in his Clarification of Ophthalmic Dioptrics (1604) first demonstrated that myopia was due to the incident light focusing in front of the retina. Kepler also showed that myopia could be corrected by concave lenses.[160] In 1632, Vopiscus Fortunatus Plempius examined a myopic eye and confirmed that myopia was due to a lengthening of its axial diameter.[162]
The idea that myopia was caused by the eye strain involved in reading or doing other work close to the eyes was a consistent theme for several centuries.[105] In Taiwan, faced with a staggering rise in the number of young military recruits needing glasses, the schools were told to give students' eyes a 10-minute break after every half-hour of reading; however, the rate of myopia continued to climb.[105][163] The policy that reversed the epidemic of myopia was the government ordering all schools to have the children outside for a minimum of 80 minutes every day.[163]
Society and culture
[edit]The terms "myopia" and "myopic" (or the common terms "short-sightedness" or "short-sighted", respectively) have been used metaphorically to refer to cognitive thinking and decision making that is narrow in scope or lacking in foresight or in concern for wider interests or for longer-term consequences.[164] It is often used to describe a decision that may be beneficial in the present, but detrimental in the future, or a viewpoint that fails to consider anything outside a very narrow and limited range. Hyperopia, the biological opposite of myopia, may also be used metaphorically for a value system or motivation that exhibits "farsighted" or possibly visionary thinking and behavior; that is, emphasizing long-term interests at the apparent expense of near-term benefit.[165]
Keeping children indoors, whether to promote early academic activities, because urban development choices leave no place for children to play outside, or because people avoid sunlight because of a preference for lighter skin color, causes myopia.[105] Taiwan has developed an aggressive program to identify pre-school-age children with pre-myopia and treat them with atropine, and to have schools send students outdoors every day.[105] The Tian-tian 120 program ("Every day 120") encourages 120 minutes of outdoor time each day.[105] Compared to the cost of lifelong treatment for myopia with glasses, and in some cases, preventable blindness, the US$13 spent on screening young children for pre-myopia is considered a good investment in public health.[105]
Because myopia can be mitigated through lifestyle choices, it is possible that being myopic will become a marker of an impoverished or neglected childhood, with wealthy families ensuring that their children spend enough time outdoors to prevent or at least reduce it, and poor families, who rely on lower-quality childcare arrangements or not having access to a safe outdoor space, being unable to provide the same benefits to their children.[105]
Correlations
[edit]Numerous studies have found correlations between myopia, on the one hand, and intelligence and academic achievement, on the other;[166] it is not clear whether there is a causal relationship.[167] Myopia is also correlated with increased microsaccade amplitude, suggesting that blurred vision from myopia might cause instability in fixational eye movements.[168][169]
Etymology
[edit]The term myopia is of Koine Greek origin: μυωπία myōpia 'short-sight' and μυωπίασις (myōpiasis) 'short-sight-ness'. It is derived from the ancient Greek μύωψ (myōps) 'short-sighted' (man), from μύειν (myein) 'to shut the eyes' and ὤψ (ōps) 'eye, look, sight' (GEN ὠπός (ōpos)).[170][171][172][173][174] The opposite of myopia in English is hypermetropia, or far-sightedness.[175]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l "Facts About Refractive Errors". NEI. October 2010. Archived from the original on 28 July 2016. Retrieved 30 July 2016.
- ^ a b c d e f g h i j k Foster PJ, Jiang Y (February 2014). "Epidemiology of myopia". Eye. 28 (2): 202–8. doi:10.1038/eye.2013.280. PMC 3930282. PMID 24406412.
- ^ a b c d e f Pan CW, Ramamurthy D, Saw SM (January 2012). "Worldwide prevalence and risk factors for myopia". Ophthalmic & Physiological Optics. 32 (1): 3–16. doi:10.1111/j.1475-1313.2011.00884.x. PMID 22150586. S2CID 32397628.
- ^ "Myopia Control Clinic". University of Alabama at Birmingham. Retrieved 28 April 2025.
- ^ Jackson TL, Vote B, Knight BC, El-Amir A, Stanford MR (2004). "Safety Testing of Infracyanine Green Using Retinal Pigment Epithelium and Glial Cell Cultures". Investigative Ophthalmology & Visual Science. 45 (10): 3588–3593. doi:10.1167/iovs.04-0304 (inactive 18 July 2025).
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ Sankaridurg P, Tahhan N, Kandel H, Naduvilath T, Zou H, Frick KD, Marmamula S, Friedman DS, Lamoureux E, Keeffe J, Walline JJ, Fricke TR, Kovai V, Resnikoff S (2021). "IMI Impact of Myopia". Investigative Ophthalmology & Visual Science. 62 (5): 2. doi:10.1167/iovs.62.5.2. PMC 8083082. PMID 33909036. Retrieved 28 April 2025.
- ^ a b Bikas B (2009). Textbook of Visual Science and Clinical Optometry (First ed.). New Delhi, India: Jaypee Brothers Medical Publisher. p. 143. ISBN 978-81-8448-599-8.
- ^ "Myopia: Symptoms, Causes, Treatment and Complications".
- ^ "Myopia | Specsavers UK". Archived from the original on 20 May 2020. Retrieved 29 July 2025.
- ^ "Nearsightedness: What Is Myopia?". American Academy of Ophthalmology. 22 September 2022. Retrieved 3 October 2023.
- ^ Whitney S. "Eye Health and Nearsightedness in Children and Adults". WebMD. Retrieved 3 October 2023.
- ^ Haarman AE, Enthoven CA, Tideman JW, Tedja MS, Verhoeven VJ, Klaver CC (29 April 2020). "The Complications of Myopia: A Review and Meta-Analysis". Investigative Ophthalmology & Visual Science. 61 (4): 49. doi:10.1167/iovs.61.4.49. ISSN 0146-0404. PMC 7401976. PMID 32347918.
- ^ Ledford A, Nemeth SC, Ledford JK (2008). Ocular anatomy and physiology (2nd ed.). Thorofare, NJ: SLACK. p. 158. ISBN 978-1-55642-792-3. Archived from the original on 8 September 2017.
- ^ a b c Ajay Kumar B (2014). Clinical Refraction Guide (First ed.). New Delhi, India: Jaypee Brothers Medical Publishers (P) LTD. p. 63. ISBN 978-93-5152-063-4.
- ^ Ramamurthy D, Lin Chua SY, Saw SM (November 2015). "A review of environmental risk factors for myopia during early life, childhood and adolescence". Clinical & Experimental Optometry (Review). 98 (6): 497–506. doi:10.1111/cxo.12346. PMID 26497977.
- ^ Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, et al. (September 2017). "Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review". Acta Ophthalmologica. 95 (6): 551–566. doi:10.1111/aos.13403. PMC 5599950. PMID 28251836.
- ^ Hobday R (January 2016). "Myopia and daylight in schools: a neglected aspect of public health?". Perspectives in Public Health. 136 (1): 50–5. doi:10.1177/1757913915576679. PMID 25800796. S2CID 19400451.
- ^ Sims C (29 March 2025). "Simulating the outdoors inside schools seems to slow myopia". New Scientist. 265 (3536): 10. doi:10.1016/S0262-4079(25)00487-7.
- ^ a b "Short-sightedness (myopia)". nhs.uk. 23 October 2017. Retrieved 25 October 2023.
- ^ "Benefits of Vision Correction with Contact Lenses | Contact Lenses | CDC". www.cdc.gov. 30 November 2022. Retrieved 25 October 2023.
- ^ Chen X, Wang XY, Zhang X, Chen Z, Zhou XT (18 October 2016). "Implantable collamer lens for residual refractive error after corneal refractive surgery". International Journal of Ophthalmology. 9 (10): 1421–1426. doi:10.18240/ijo.2016.10.09. ISSN 2222-3959. PMC 5075656. PMID 27803858.
- ^ Holden B, Sankaridurg P, Smith E, Aller T, Jong M, He M (February 2014). "Myopia, an underrated global challenge to vision: where the current data takes us on myopia control". Eye. 28 (2): 142–6. doi:10.1038/eye.2013.256. PMC 3930268. PMID 24357836.
- ^ a b c Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM (March 2015). "The age-specific prevalence of myopia in Asia: a meta-analysis". Optometry and Vision Science. 92 (3): 258–66. doi:10.1097/opx.0000000000000516. PMID 25611765. S2CID 42359341.
- ^ Dong L, Kang YK, Li Y, Wei WB, Jonas JB (March 2020). "Prevalence And Time Trends Of Myopia In Children And Adolescents In China: A Systemic Review and Meta-Analysis". Retina. 40 (3): 399–411. doi:10.1097/IAE.0000000000002590. PMID 31259808. S2CID 195756787.
- ^ Fredrick DR (18 May 2002). "Myopia". BMJ: British Medical Journal. 324 (7347): 1195–1199. doi:10.1136/bmj.324.7347.1195. ISSN 0959-8138. PMC 1123161. PMID 12016188.
- ^ "Common Eye Disorders and Diseases | CDC". www.cdc.gov. 29 September 2023. Retrieved 25 October 2023.
- ^ "Vision impairment and blindness". www.who.int. Retrieved 25 October 2023.
- ^ a b Carr BJ, Stell WK (1995), Kolb H, Fernandez E, Nelson R (eds.), "The Science Behind Myopia", Webvision: The Organization of the Retina and Visual System, Salt Lake City (UT): University of Utah Health Sciences Center, PMID 29266913, retrieved 25 October 2023
- ^ Hennelly ML (2019). "How to detect myopia in the eye clinic". Community Eye Health. 32 (105): 15–16. ISSN 0953-6833. PMC 6688402. PMID 31409949.
- ^ a b c d Coviltir V, Burcel M, Cherecheanu AP, Ionescu C, Dascalescu D, Potop V, Burcea M (2019). "Update on Myopia Risk Factors and Microenvironmental Changes". Journal of Ophthalmology. 2019 4960852. doi:10.1155/2019/4960852. PMC 6875023. PMID 31781378.
- ^ Recko M, Stahl ED (2015). "Childhood Myopia: Epidemiology, Risk Factors, and Prevention". Missouri Medicine. 112 (2): 116–121. ISSN 0026-6620. PMC 6170055. PMID 25958656.
- ^ Cakmak HB, Cagil N, Simavlı H, Duzen B, Simsek S (February 2010). "Refractive Error May Influence Mesopic Pupil Size". Current Eye Research. 35 (2): 130–136. doi:10.3109/02713680903447892. ISSN 0271-3683. PMID 20136423. S2CID 27407880.
- ^ Zhu X, Ye H, Yang J, Lu Y (2015). "Effect of pupil size on higher-order aberrations in high-myopic pseudophakic eyes with posterior staphyloma". Eye. 29 (1): 98–105. doi:10.1038/eye.2014.242. ISSN 0950-222X. PMC 4289834. PMID 25323850.
- ^ Wang YM, Lu SY, Zhang XJ, Chen LJ, Pang CP, Yam JC (9 March 2022). "Myopia Genetics and Heredity". Children. 9 (3): 382. doi:10.3390/children9030382. ISSN 2227-9067. PMC 8947159. PMID 35327754.
- ^ Li J, Zhang Q (31 December 2017). "Insight into the molecular genetics of myopia". Molecular Vision. 23: 1048–1080. ISSN 1090-0535. PMC 5757860. PMID 29386878.
- ^ Huang HM, Chang DS, Wu PC (2015). "The Association between Near Work Activities and Myopia in Children-A Systematic Review and Meta-Analysis". PLOS ONE. 10 (10) e0140419. Bibcode:2015PLoSO..1040419H. doi:10.1371/journal.pone.0140419. PMC 4618477. PMID 26485393.
- ^ a b Shapira Y, Mimouni M, Machluf Y, Chaiter Y, Saab H, Mezer E (December 2019). "The Increasing Burden of Myopia in Israel among Young Adults over a Generation: Analysis of Predisposing Factors". Ophthalmology. 126 (12): 1617–1626. doi:10.1016/j.ophtha.2019.06.025. PMID 31474440. S2CID 198380872.
- ^ Sivak J (November 2012). "The cause(s) of myopia and the efforts that have been made to prevent it". Clinical & Experimental Optometry. 95 (6): 572–82. doi:10.1111/j.1444-0938.2012.00781.x. PMID 22845416. S2CID 32003286.
- ^ Cai XB, Shen SR, Chen DF, Zhang Q, Jin ZB (1 November 2019). "An overview of myopia genetics". Experimental Eye Research. 188 107778. doi:10.1016/j.exer.2019.107778. ISSN 0014-4835. PMID 31472110. S2CID 201700595.
- ^ Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D (1 July 2015). "Meta-analysis of the heritability of human traits based on fifty years of twin studies". Nature Genetics. 47 (7): 702–709. doi:10.1038/ng.3285. ISSN 1546-1718. PMID 25985137. S2CID 205349969.
- ^ a b c Dolgin E (March 2015). "The myopia boom". Nature. 519 (7543): 276–8. Bibcode:2015Natur.519..276D. doi:10.1038/519276a. PMID 25788077.
- ^ Medina A (June 2021). "The cause of myopia development and progression: Theory, evidence, and treatment". Survey of Ophthalmology. 67 (2): 488–509. doi:10.1016/j.survophthal.2021.06.005. PMID 34181975.
- ^ a b Biswas S, El Kareh A, Qureshi M, Lee DM, Sun CH, Lam JS, Saw SM, Najjar RP (31 January 2024). "The influence of the environment and lifestyle on myopia". Journal of Physiological Anthropology. 43 (1): 7. doi:10.1186/s40101-024-00354-7. ISSN 1880-6805. PMC 10829372. PMID 38297353.
- ^ "Myopia (Nearsightedness)". www.aoa.org. Retrieved 25 December 2019.
- ^ Jacobi FK, Pusch CM (January 2010). "A decade in search of myopia genes". Frontiers in Bioscience. 15 (1): 359–72. doi:10.2741/3625. PMID 20036825.
- ^ Verhoeven VJ, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Höhn R, et al. (March 2013). "Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia". Nature Genetics. 45 (3): 314–8. doi:10.1038/ng.2554. PMC 3740568. PMID 23396134.
- ^ Neitz M, Neitz J (August 2021). "Intermixing the OPN1LW and OPN1MW Genes Disrupts the Exonic Splicing Code Causing an Array of Vision Disorders". Genes. 12 (8): 1180. doi:10.3390/genes12081180. PMC 8391646. PMID 34440353.
- ^ Li J, Gao B, Guan L, Xiao X, Zhang J, Li S, et al. (June 2015). "Unique Variants in OPN1LW Cause Both Syndromic and Nonsyndromic X-Linked High Myopia Mapped to MYP1". Investigative Ophthalmology & Visual Science. 56 (6): 4150–4155. doi:10.1167/iovs.14-16356. PMID 26114493.
- ^ Dirani M, Chamberlain M, Shekar SN, Islam AF, Garoufalis P, Chen CY, et al. (November 2006). "Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study". Investigative Ophthalmology & Visual Science. 47 (11): 4756–61. doi:10.1167/iovs.06-0270. PMID 17065484.
- ^ Lopes MC, Andrew T, Carbonaro F, Spector TD, Hammond CJ (January 2009). "Estimating heritability and shared environmental effects for refractive error in twin and family studies". Investigative Ophthalmology & Visual Science. 50 (1): 126–31. doi:10.1167/iovs.08-2385. PMID 18757506.
- ^ Peet JA, Cotch MF, Wojciechowski R, Bailey-Wilson JE, Stambolian D (September 2007). "Heritability and familial aggregation of refractive error in the Old Order Amish". Investigative Ophthalmology & Visual Science. 48 (9): 4002–6. doi:10.1167/iovs.06-1388. PMC 1995233. PMID 17724179.
- ^ Tkatchenko AV, Tkatchenko TV, Guggenheim JA, Verhoeven VJ, Hysi PG, Wojciechowski R, et al. (August 2015). "APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans". PLOS Genetics. 11 (8) e1005432. doi:10.1371/journal.pgen.1005432. PMC 4551475. PMID 26313004.
- ^ Gusev A, Bhatia G, Zaitlen N, Vilhjalmsson BJ, Diogo D, Stahl EA, et al. (2013). "Quantifying missing heritability at known GWAS loci". PLOS Genetics. 9 (12) e1003993. doi:10.1371/journal.pgen.1003993. PMC 3873246. PMID 24385918.
- ^ a b c Lieberman, Daniel E. (2013) The Story of the Human Body: Evolution, Health, and Disease. New York: Pantheon Books.[page needed]
- ^ Sherwin J (25 October 2011). "Lack of outdoor play linked to short-sighted children". BBC News. Archived from the original on 25 October 2011. Retrieved 25 October 2011.
- ^ Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, et al. (August 2009). "Outdoor activity and myopia in Singapore teenage children". The British Journal of Ophthalmology. 93 (8): 997–1000. doi:10.1136/bjo.2008.150979. PMID 19211608. S2CID 30301026.
- ^ Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P (August 2008). "Outdoor activity reduces the prevalence of myopia in children". Ophthalmology. 115 (8): 1279–85. doi:10.1016/j.ophtha.2007.12.019. PMID 18294691.
- ^ Cui D, Trier K, Munk Ribel-Madsen S (May 2013). "Effect of day length on eye growth, myopia progression, and change of corneal power in myopic children". Ophthalmology. 120 (5): 1074–9. doi:10.1016/j.ophtha.2012.10.022. PMID 23380471.
- ^ Feldkaemper M, Schaeffel F (September 2013). "An updated view on the role of dopamine in myopia". Experimental Eye Research (review). 114: 106–19. doi:10.1016/j.exer.2013.02.007. PMID 23434455. S2CID 35493712.
- ^ Nickla DL (September 2013). "Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber". Experimental Eye Research (Review). 114: 25–34. doi:10.1016/j.exer.2012.12.013. PMC 3742730. PMID 23298452.
- ^ Nickla DL, Jordan K, Yang J, Totonelly K (1 August 2017). "Brief hyperopic defocus or form deprivation have varying effects on eye growth and ocular rhythms depending on the time-of-day of exposure". Experimental Eye Research. 161: 132–142. doi:10.1016/j.exer.2017.06.003. PMC 5557081. PMID 28596085.
- ^ "Overminus Lenses Associated with Myopia Progression".
- ^ a b https://www.optometrystudents.com/pearl/over-minus-you-probably-do-itstop-it/ Archived 2 December 2022 at the Wayback Machine[unreliable medical source?][full citation needed]
- ^ Peled A, Nitzan I, Megreli J, Derazne E, Tzur D, Pinhas-Hamiel O, Afek A, Twig G (August 2022). "Myopia and BMI: a nationwide study of 1.3 million adolescents". Obesity. 30 (8): 1691–1698. doi:10.1002/oby.23482. ISSN 1930-739X. PMID 35894082.
- ^ Qu Y, Huang H, Zhang H (5 December 2023). "Association between body mass index and myopia in the United States population in the National Health and Nutrition Examination Surveys 1999 to 2008: a cross-sectional study". European Journal of Medical Research. 28 (1): 561. doi:10.1186/s40001-023-01542-4. ISSN 2047-783X. PMC 10696841. PMID 38049883.
- ^ Lee DC, Lee SY, Kim YC (2018). "An epidemiological study of the risk factors associated with myopia in young adult men in Korea". Scientific Reports. 8 (1) 511. Bibcode:2018NatSR...8..511L. doi:10.1038/s41598-017-18926-2. PMC 5764954. PMID 29323203.
- ^ a b Borish, Irvin M. (1949). Clinical Refraction. Chicago: The Professional Press.
- ^ a b Duke-Elder, Sir Stewart (1969). The Practice of Refraction (8th ed.). St. Louis: The C.V. Mosby Company. ISBN 0-7000-1410-1.
- ^ a b c d e f g h i j Cline D, Hofstetter HW, Griffin JR (1997). Dictionary of Visual Science (4th ed.). Boston: Butterworth-Heinemann. ISBN 978-0-7506-9895-5.
- ^ Summanen P, Kivitie-Kallio S, Norio R, Raitta C, Kivelä T (May 2002). "Mechanisms of myopia in Cohen syndrome mapped to chromosome 8q22". Investigative Ophthalmology & Visual Science. 43 (5): 1686–93. PMID 11980891.
- ^ The Eyecare Trust. Night Driving – The Facts. OR Eye care advice for driving in the dark Archived 20 March 2012 at the Wayback Machine 26 January 2005.'
- ^ a b Panday VA, Rhee DJ (September 2007). "Review of sulfonamide-induced acute myopia and acute bilateral angle-closure glaucoma". Comprehensive Ophthalmology Update (Review). 8 (5): 271–6. PMID 18201514.
- ^ Goss DA, Eskridge JB (1988). "Myopia". In Amos JB (ed.). Diagnosis and management in vision care. Boston: Butterworths. p. 445. ISBN 978-0-409-95082-3. OCLC 14967262.
- ^ a b Richards OW (October 1976). "Instrument myopia--microscopy". American Journal of Optometry and Physiological Optics. 53 (10): 658–63. doi:10.1097/00006324-197610000-00003. PMID 1015520. S2CID 37513722.
- ^ a b c d e f g h i j American Optometric Association (1997). Optometric Clinical Practice Guideline: Care of the Patient with Myopia (PDF) (Report). Archived from the original (PDF) on 22 January 2015. Retrieved 17 February 2015.
- ^ Li CY, Lin KK, Lin YC, Lee JS (March 2002). "Low vision and methods of rehabilitation: a comparison between the past and present". Chang Gung Medical Journal. 25 (3): 153–61. PMID 12022735.
- ^ Cassin, B. and Solomon, S. (2001) Dictionary of Eye Terminology. Gainesville, Florida: Triad Publishing Company. ISBN 0937404632.
- ^ Ong E, Ciuffreda KJ (1995). "Nearwork-induced transient myopia: a critical review". Documenta Ophthalmologica. Advances in Ophthalmology. 91 (1): 57–85. doi:10.1007/BF01204624. PMID 8861637. S2CID 2065074.
- ^ Ciuffreda KJ, Vasudevan B (March 2008). "Nearwork-induced transient myopia (NITM) and permanent myopia—is there a link?". Ophthalmic & Physiological Optics. 28 (2): 103–14. doi:10.1111/j.1475-1313.2008.00550.x. PMID 18339041. S2CID 28700508.
- ^ Bennett MH, Cooper JS (21 June 2022). "Hyperbaric Cataracts". www.ncbi.nlm.nih.gov. StatPearls Publishing LLC. PMID 29261974. Retrieved 30 July 2022.
- ^ Vukojević N, Šikić J, Ćurković T, Juratovac Z, Katušić D, Šarić B, Jukić T (20 June 2005). "Axial Eye Length after Retinal Detachment Surgery". Collegium Antropologicum. 29 – Supplement 1 (1): 25–27. PMID 16193671.
- ^ Metge P, Donnadieu M (September 1993). "Myopie et cataracte" [Myopia and cataract]. La Revue du Praticien (in French). 43 (14): 1784–6. OCLC 116851621. PMID 8310218.
- ^ Young FA (February 1962). "The effect of nearwork illumination level on monkey refraction". American Journal of Optometry & Archives of American Academy of Optometry. 39 (2): 60–7. doi:10.1097/00006324-196202000-00002. PMID 14009334.
- ^ Zhu X, Park TW, Winawer J, Wallman J (July 2005). "In a matter of minutes, the eye can know which way to grow". Investigative Ophthalmology & Visual Science. 46 (7): 2238–41. doi:10.1167/iovs.04-0956. PMID 15980206.
- ^ Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA (July 1987). "Local retinal regions control local eye growth and myopia". Science. 237 (4810): 73–7. Bibcode:1987Sci...237...73W. doi:10.1126/science.3603011. JSTOR 1699607. PMID 3603011. S2CID 31790023.
- ^ Shen W, Vijayan M, Sivak JG (May 2005). "Inducing form-deprivation myopia in fish". Investigative Ophthalmology & Visual Science. 46 (5): 1797–803. doi:10.1167/iovs.04-1318. PMID 15851585.
- ^ a b Grosvenor T (July 1987). "A review and a suggested classification system for myopia on the basis of age-related prevalence and age of onset". American Journal of Optometry and Physiological Optics. 64 (7): 545–54. doi:10.1097/00006324-198707000-00012. PMID 3307441.
- ^ "Glaucoma." Archived 19 August 2006 at the Wayback Machine EyeMDLink.com. Retrieved 27 August 2006.
- ^ Zejmo M, Formińska-Kapuścik M, Pieczara E, Filipek E, Mrukwa-Kominek E, Samochowiec-Donocik E, Leszczyński R, Smuzyńska M (September 2009). "Etiopathogenesis and management of high-degree myopia. Part I". Medical Science Monitor. 15 (9): RA199-202. PMID 19721411. INIST 21992936.
- ^ Retinal Detachment at eMedicine
- ^ "More Information on Glaucoma." AgingEye Times. Retrieved 27 August 2006.
- ^ Messmer DE (May 1992). "[Retinal detachment]". Schweizerische Rundschau für Medizin Praxis = Revue Suisse de Médecine Praxis (in German). 81 (19): 622–5. PMID 1589678.
- ^ Banerjee S, Horton J. Lenses and Spectacles to Prevent Myopia Worsening in Children [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 Apr. Available from:[1]
- ^ Walline JJ, Walker MK, Mutti DO, et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA. 2020;324(6):571–580.
- ^ Ruiz-Pomeda A, Perez-Sanchez B, Valls I, Prieto-Garrido FL, Gutierrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS). A 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1011–1021.
- ^ Garcia-Del Valle AM, Blazquez V, Gros-Otero J, et al. Efficacy and safety of a soft contact lens to control myopia progression. Clin Exp Optom.2021;104(1):14–21
- ^ Fitoussi S (23 May 2023). "The World Record of Myopia".
- ^ Morgan I, Rose K (January 2005). "How genetic is school myopia?". Progress in Retinal and Eye Research. 24 (1): 1–38. doi:10.1016/j.preteyeres.2004.06.004. PMID 15555525. S2CID 18045281.
- ^ "School based program". Archived from the original on 10 November 2022. Retrieved 10 November 2022.
- ^ a b Saw SM, Gazzard G, Au Eong KG, Tan DT (November 2002). "Myopia: attempts to arrest progression". The British Journal of Ophthalmology. 86 (11): 1306–1311. doi:10.1136/bjo.86.11.1306. PMC 1771373. PMID 12386095.
- ^ Zhang G, Jiang J, Qu C (27 April 2023). "Myopia prevention and control in children: a systematic review and network meta-analysis". Eye. 37 (16): 3461–3469. doi:10.1038/s41433-023-02534-8. ISSN 1476-5454. PMC 10630522. PMID 37106147. S2CID 258376819.
- ^ Jiang M.D., Yu, et al., Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children, Ophthalmology, American Academy of Ophthalmology, Volume 129, Issue 5P509-519, May 2022.
- ^ a b Lawrenson JG, Huntjens B, Virgili G, Ng S, Dhakal R, Downie LE, Verkicharla PK, Kernohan A, Li T, Walline JJ (13 February 2025). "Interventions for myopia control in children: a living systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews. 2025 (2) CD014758. doi:10.1002/14651858.CD014758.pub3. ISSN 1469-493X. PMC 11822883. PMID 39945354.
- ^ Sprague NL, Bancalari P, Karim W, Siddiq S (September 2022). "Growing up green: a systematic review of the influence of greenspace on youth development and health outcomes". Journal of Exposure Science & Environmental Epidemiology. 32 (5): 660–681. Bibcode:2022JESEE..32..660S. doi:10.1038/s41370-022-00445-6. ISSN 1559-064X. PMC 9482936. PMID 35614136.
- ^ a b c d e f g h Katwala A. "The World Is Going Blind. Taiwan Offers a Warning, and a Cure". Wired. ISSN 1059-1028. Retrieved 1 September 2023.
- ^ Ong E, Grice K, Held R, Thorn F, Gwiazda J (June 1999). "Effects of spectacle intervention on the progression of myopia in children". Optometry and Vision Science. 76 (6): 363–9. doi:10.1097/00006324-199906000-00015. PMID 10416930.
- ^ Pärssinen O, Hemminki E, Klemetti A (July 1989). "Effect of spectacle use and accommodation on myopic progression: final results of a three-year randomised clinical trial among schoolchildren". The British Journal of Ophthalmology. 73 (7): 547–51. doi:10.1136/bjo.73.7.547. PMC 1041798. PMID 2667638.
- ^ Wolffsohn JS, Flitcroft DI, Gifford KL, Jong M, Jones L, Klaver CC, Logan NS, Naidoo K, Resnikoff S, Sankaridurg P, Smith EL, Troilo D, Wildsoet CF (2019). "IMI – Myopia Control Reports Overview and Introduction". Investigative Ophthalmology & Visual Science. 60 (3): M1 – M19. doi:10.1167/iovs.18-25980. ISSN 0146-0404. PMC 6735780. PMID 30817825.
- ^ a b Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD (2020). "Interventions to slow progression of myopia in children". The Cochrane Database of Systematic Reviews. 1 (1): CD0O6460. doi:10.1002/14651858.CD004916.pub4. PMC 6984636. PMID 31930781.
- ^ Smith MJ, Walline JJ (2015). "Controlling myopia progression in children and adolescents". Adolescent Health, Medicine and Therapeutics. 6: 133–40. doi:10.2147/AHMT.S55834. PMC 4542412. PMID 26316834.
- ^ Li FF, Yam JC (4 October 2019). "Low-Concentration Atropine Eye Drops for Myopia Progression". Asia-Pacific Journal of Ophthalmology. 8 (5): 360–365. doi:10.1097/APO.0000000000000256. ISSN 2162-0989. PMC 6784858. PMID 31478936.
- ^ Ward B, Tarutta EP, Mayer MJ (December 2009). "The efficacy and safety of posterior pole buckles in the control of progressive high myopia". Eye. 23 (12): 2169–74. doi:10.1038/eye.2008.433. PMID 19229272.
- ^ Wolffsohn JS, Flitcroft DI, Gifford KL, Jong M, Jones L, Klaver CC, Logan NS, Naidoo K, Resnikoff S, Sankaridurg P, Smith EL, Troilo D, Wildsoet CF (2019). "IMI – Myopia Control Reports Overview and Introduction". Investigative Ophthalmology & Visual Science. 60 (3): 19. doi:10.1167/iovs.18-25980. ISSN 0146-0404. PMC 6735780. PMID 30817825.
- ^ Near-sightedness . National Institutes of Health. 2010.
- ^ Trokel SL, Srinivasan R, Braren B (December 1983). "Excimer laser surgery of the cornea". American Journal of Ophthalmology. 96 (6): 710–5. doi:10.1016/s0002-9394(14)71911-7. PMID 6660257.
- ^ Seiler T, Bende T, Wollensak J, Trokel S (February 1988). "Excimer laser keratectomy for correction of astigmatism". American Journal of Ophthalmology. 105 (2): 117–24. doi:10.1016/0002-9394(88)90173-0. PMID 3341427.
- ^ Pallikaris IG, Siganos DS (1997). "Laser in situ keratomileusis to treat myopia: early experience". Journal of Cataract and Refractive Surgery. 23 (1): 39–49. doi:10.1016/s0886-3350(97)80149-6. PMID 9100106. S2CID 38655546.
- ^ Pallikaris IG, Kymionis GD, Astyrakakis NI (November 2001). "Corneal ectasia induced by laser in situ keratomileusis". Journal of Cataract and Refractive Surgery. 27 (11): 1796–802. doi:10.1016/s0886-3350(01)01090-2. PMID 11709254. S2CID 2333450.
- ^ Menezo JL, Peris-Martínez C, Cisneros-Lanuza AL, Martínez-Costa R (2004). "Rate of cataract formation in 343 highly myopic eyes after implantation of three types of phakic intraocular lenses". Journal of Refractive Surgery. 20 (4): 317–24. doi:10.3928/1081-597X-20040701-03. PMID 15307392.
- ^ Torun N, Bertelmann E, Klamann MK, Maier AK, Liekfeld A, Gonnermann J (July 2013). "Posterior chamber phakic intraocular lens to correct myopia: long-term follow-up". Journal of Cataract and Refractive Surgery. 39 (7): 1023–8. doi:10.1016/j.jcrs.2013.01.041. PMID 23664355. S2CID 31750663.
- ^ Moshirfar M, Imbornoni LM, Ostler EM, Muthappan V (2014). "Incidence rate and occurrence of visually significant cataract formation and corneal decompensation after implantation of Verisyse/Artisan phakic intraocular lens". Clinical Ophthalmology. 8: 711–6. doi:10.2147/OPTH.S59878. PMC 3986296. PMID 24748765.
- ^ "Orthokeratology (Ortho-k) – Corneal Reshaping with GP Contacts". www.contactlenses.org.
- ^ "Orthokeratology: A Heated Debate Continues". www.ophthalmologyweb.com.
- ^ a b "Orthokeratology slows myopic progression in young patients". American Academy of Ophthalmology. 17 April 2019.
- ^ "Orthokeratology (Ortho-K) treatment for Myopia Prevention and Control". www.myopiaprevention.org. Archived from the original on 6 February 2020. Retrieved 4 June 2020.
- ^ a b Daniels K. "Consider Ortho-K For Myopia Control". www.reviewofoptometry.com.
- ^ Vega-Estrada A, Alio JL (15 March 2016). "The use of intracorneal ring segments in keratoconus". Eye and Vision. 3 8. doi:10.1186/s40662-016-0040-z. PMC 4791885. PMID 26981548.
- ^ Pathak AK, Villarreal Gonzalez AJ, Karacal H. "ICRS: Corneal biomechanics effects".
- ^ Bates, Wm H (1920) Sight Without Glasses Archived 20 December 2016 at the Wayback Machine. Ch. 10, p. 106. ISBN 1479118540.
- ^ Rawstron JA, Burley CD, Elder MJ (2005). "A systematic review of the applicability and efficacy of eye exercises". Journal of Pediatric Ophthalmology and Strabismus. 42 (2): 82–8. doi:10.3928/01913913-20050301-02. PMID 15825744.
- ^ Barrett BT (January 2009). "A critical evaluation of the evidence supporting the practice of behavioural vision therapy". Ophthalmic & Physiological Optics. 29 (1): 4–25. doi:10.1111/j.1475-1313.2008.00607.x. PMID 19154276. S2CID 13588501.
- ^ Dunaway D, Berger I. "Worldwide Distribution of Visual Refractive Errors and What to Expect at a Particular Location" Archived 29 January 2007 at the Wayback Machine. infocusonline.org.
- ^ a b Phakic Intraocular Lens (IOL) for Myopia Correction at eMedicine
- ^ a b Fredrick DR (May 2002). "Myopia". BMJ. 324 (7347): 1195–9. doi:10.1136/bmj.324.7347.1195. PMC 1123161. PMID 12016188.
- ^ National Research Council Commission (1989). Myopia: Prevalence and Progression Archived 6 January 2014 at the Wayback Machine, Washington, D.C.: National Academy Press, ISBN 0-309-04081-7
- ^ Jensen, A.R. (1998) The g Factor. Westport, Connecticut: Praeger Publishers, ISBN 0275961036
- ^ Sperduto RD, Seigel D, Roberts J, Rowland M (March 1983). "Prevalence of myopia in the United States". Archives of Ophthalmology. 101 (3): 405–7. doi:10.1001/archopht.1983.01040010405011. PMID 6830491.
- ^ Liang J, Pu Y, Chen J, Liu M, Ouyang B, Jin Z, Ge W, Wu Z, Yang X, Qin C, Wang C, Huang S, Jiang N, Hu L, Zhang Y (14 August 2024). "Global prevalence, trend and projection of myopia in children and adolescents from 1990 to 2050: a comprehensive systematic review and meta-analysis". British Journal of Ophthalmology. 109 (3): 362–371. doi:10.1136/bjo-2024-325427. ISSN 0007-1161. PMID 39317432.
- ^ Guy J (25 September 2024). "1 in 3 children worldwide is now nearsighted, study shows". CNN. Retrieved 29 September 2024.
- ^ Davis N (20 February 2025). "Every hour children spend on screens raises chance of myopia, study finds". The Guardian. Retrieved 20 February 2025.
- ^ Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, Rose KA (January 2018). "The epidemics of myopia: Aetiology and prevention". Progress in Retinal and Eye Research. 62: 134–149. doi:10.1016/j.preteyeres.2017.09.004. hdl:1885/139488. PMID 28951126. S2CID 9323449.
- ^ "Discovery of Gene May Provide Treatment for Near-sightedness". Disabled-world.com. 12 September 2010. Retrieved 2 August 2012.[permanent dead link]
- ^ 全国近视眼人数近4亿 近视已影响国人健康 Archived 27 October 2012 at the Wayback Machine. Xinhua News Agency. Retrieved on 21 April 2013.
- ^ Chandran S (June 1972). "Comparative study of refractive errors in West Malaysia". The British Journal of Ophthalmology. 56 (6): 492–5. doi:10.1136/bjo.56.6.492. PMC 1208824. PMID 5069190.
- ^ Wu HM, Seet B, Yap EP, Saw SM, Lim TH, Chia KS (April 2001). "Does education explain ethnic differences in myopia prevalence? A population-based study of young adult males in Singapore". Optometry and Vision Science. 78 (4): 234–9. doi:10.1097/00006324-200104000-00012. PMID 11349931. S2CID 46445087.
- ^ Mallen EA, Gammoh Y, Al-Bdour M, Sayegh FN (July 2005). "Refractive error and ocular biometry in Jordanian adults". Ophthalmic & Physiological Optics. 25 (4): 302–9. doi:10.1111/j.1475-1313.2005.00306.x. PMID 15953114. S2CID 24694696.
- ^ Priscilla JJ, Verkicharla PK (May 2021). "Time trends on the prevalence of myopia in India – A prediction model for 2050". Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists). 41 (3): 466–474. doi:10.1111/opo.12806. ISSN 1475-1313. PMID 33860952.
- ^ Saxena R, Vashist P, Tandon R, Pandey RM, Bhardawaj A, Gupta V, Menon V (2017). "Incidence and progression of myopia and associated factors in urban school children in Delhi: The North India Myopia Study (NIM Study)". PLOS ONE. 12 (12) e0189774. Bibcode:2017PLoSO..1289774S. doi:10.1371/journal.pone.0189774. PMC 5734754. PMID 29253002.
- ^ Han SB, Jang J, Yang HK, Hwang J, Park SK (2019). "Prevalence and risk factors of myopia in adult Korean population: Korea national health and nutrition examination survey 2013–2014 (KNHANES VI)". PLOS ONE. 14 (1) e0211204. Bibcode:2019PLoSO..1411204H. doi:10.1371/journal.pone.0211204. PMC 6345425. PMID 30677087.
- ^ Davidson H (28 February 2025). "Shortsighted Taiwan may have lessons for the world as a preventable disease skyrockets". The Guardian. Retrieved 10 March 2025.
- ^ Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E, et al. (July 2015). "Increasing Prevalence of Myopia in Europe and the Impact of Education". Ophthalmology. 122 (7): 1489–97. doi:10.1016/j.ophtha.2015.03.018. PMC 4504030. PMID 25983215.
- ^ Logan NS, Davies LN, Mallen EA, Gilmartin B (April 2005). "Ametropia and ocular biometry in a U.K. university student population". Optometry and Vision Science. 82 (4): 261–6. doi:10.1097/01.OPX.0000159358.71125.95. PMID 15829853. S2CID 25384178.
- ^ a b c Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, et al. (April 2004). "The prevalence of refractive errors among adults in the United States, Western Europe, and Australia". Archives of Ophthalmology. 122 (4): 495–505. doi:10.1001/archopht.122.4.495. PMID 15078666.
- ^ Vitale S, Sperduto RD, Ferris FL (December 2009). "Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004". Archives of Ophthalmology. 127 (12): 1632–9. doi:10.1001/archophthalmol.2009.303. PMID 20008719.
- ^ a b Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, et al. (August 2003). "Refractive error and ethnicity in children". Archives of Ophthalmology. 121 (8): 1141–7. doi:10.1001/archopht.121.8.1141. PMID 12912692.
- ^ Wensor M, McCarty CA, Taylor HR (May 1999). "Prevalence and risk factors of myopia in Victoria, Australia". Archives of Ophthalmology. 117 (5): 658–63. doi:10.1001/archopht.117.5.658. PMID 10326965.
- ^ Junghans BM, Crewther SG (February 2005). "Little evidence for an epidemic of myopia in Australian primary school children over the last 30 years". BMC Ophthalmology. 5 1. doi:10.1186/1471-2415-5-1. PMC 552307. PMID 15705207.
- ^ Thorn F, Cruz AA, Machado AJ, Carvalho RA (April 2005). "Refractive status of indigenous people in the northwestern Amazon region of Brazil". Optometry and Vision Science. 82 (4): 267–72. doi:10.1097/01.OPX.0000159371.25986.67. PMID 15829854. S2CID 38979284.
- ^ Garcia CA, Oréfice F, Nobre GF, Souza D, Rocha ML, Vianna RN (June 2005). "[Prevalence of refractive errors in students in Northeastern Brazil]". Arquivos Brasileiros de Oftalmologia. 68 (3): 321–5. doi:10.1590/S0004-27492005000300009. PMID 16059562.
- ^ a b c Spaide RF, Ohno-Matsui KM, Yannuzzi LA, eds. (2013). Pathologic Myopia. Springer Science & Business Media. p. 2. ISBN 978-1-4614-8338-0.
- ^ "Myopia – Birth Story". Archived from the original on 20 April 2016. Retrieved 1 June 2020.
- ^ Dunphy EB (October 1970). "The biology of myopia". The New England Journal of Medicine. 283 (15): 796–800. doi:10.1056/NEJM197010082831507. PMID 4917270.
- ^ a b Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, Logan NS, Liu M, Morgan I, Ohno-Matsui K, Pärssinen O, Resnikoff S, Sankaridurg P, Saw SM, Smith EL (28 April 2021). "IMI Prevention of Myopia and Its Progression". Investigative Ophthalmology & Visual Science. 62 (5): 6. doi:10.1167/iovs.62.5.6. ISSN 1552-5783. PMC 8083117. PMID 33909032.
- ^ Brooks, David (19 March 2009). Perverse Cosmic Myopia Archived 7 November 2015 at the Wayback Machine. New York Times.
- ^ Thompson C (17 September 2009). "Don't Work All the Time". Wired. Vol. 17, no. 8. Archived from the original on 17 August 2009. Retrieved 14 August 2009.
- ^ Williams KM, Hysi PG, Yonova-Doing E, Mahroo OA, Snieder H, Hammond CJ (April 2017). "Phenotypic and genotypic correlation between myopia and intelligence". Scientific Reports. 7 (1) 45977. Bibcode:2017NatSR...745977W. doi:10.1038/srep45977. PMC 5382686. PMID 28383074.
- ^ Verma A, Verma A (2015). "A novel review of the evidence linking myopia and high intelligence". Journal of Ophthalmology. 2015 271746. doi:10.1155/2015/271746. PMC 4306218. PMID 25653868.
- ^ Ghasia FF, Shaikh AG (April 2015). "Uncorrected Myopic Refractive Error Increases Microsaccade Amplitude". Investigative Ophthalmology & Visual Science. 56 (4): 2531–5. doi:10.1167/iovs.14-15882. PMID 25678690.
- ^ Alexander RG, Macknik SL, Martinez-Conde S (2018). "Microsaccade Characteristics in Neurological and Ophthalmic Disease". Frontiers in Neurology. 9 144. doi:10.3389/fneur.2018.00144. PMC 5859063. PMID 29593642.
- ^ μυωπία, μυωπίασις, μύωψ, μύειν, ὤψ. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ^ Robert B (2010). Etymological Dictionary of Greek. Leiden Indo-European Etymological Dictionary Series. Vol. 2. With the assistance of Lucien van Beek. Leiden, Boston: Brill. pp. 988–9. ISBN 978-90-04-17418-4.
- ^ "μυωπία". Dictionary of Standard Modern Greek. Institute for Modern Greek Studies of the Artistotle University of Thessaloniki (in Greek). Retrieved 19 February 2016.
- ^ "myopia". Oxford English Dictionary (2nd ed.). Oxford University Press. 1989.
- ^ Harper D. "myopia". Online Etymology Dictionary.
- ^ WebMD E. "Hyperopia (Farsightedness)". WebMD. Retrieved 25 October 2023.
Myopia
View on GrokipediaClinical Presentation
Signs and Symptoms
Myopia, or nearsightedness, is characterized by difficulty seeing distant objects clearly while near vision remains intact.[11][12] This refractive error results in images focusing in front of the retina, leading to blurred distance vision.[7] Common symptoms include eye strain, headaches, and squinting to sharpen focus on faraway targets.[13][12] Patients may report fatigue after tasks requiring prolonged distance viewing, such as driving or watching television.[7] In children, subtle behavioral indicators often precede formal diagnosis, such as holding books or screens excessively close to the face or complaints about not seeing classroom blackboards or sports details from afar.[14][15] These signs reflect compensatory habits to overcome visual deficits.[7] High myopia, defined as greater than -6 diopters, may present with additional risks like floaters from vitreous changes or early cataracts, though the primary symptom remains uncorrected distance blur.[7][16] Nocturnal myopia can cause exacerbated blur in low-light conditions due to shifts in accommodation.[17] Without correction, chronic symptoms can impair daily activities and quality of life.[12]Classification by Type and Severity
Myopia is classified into several types based on anatomical, etiological, and clinical features. Anatomically, it is categorized by the primary optical mechanism causing the refractive error: axial myopia, resulting from excessive elongation of the eye's axial length (with each 1 mm increase typically producing a 3 diopter myopic shift); curvature myopia, due to excessive corneal or lenticular curvature; and index myopia, arising from alterations in the refractive index of the ocular media, such as in diabetes or nuclear cataracts (though rare).[7][18] Etiologically, myopia includes simple or physiologic forms, which are non-pathologic and often school-age onset without structural damage; pathologic or degenerative myopia, characterized by high refractive errors (typically > -6 diopters) accompanied by posterior staphyloma, lacquer cracks, or myopic maculopathy leading to potential vision loss; and secondary or induced types, such as those from drugs (e.g., sulfonamides), accommodative spasm, or postoperative changes.[17][18] Transient variants, including pseudomyopia from ciliary muscle spasm or nocturnal myopia in low light, are temporary and reversible upon addressing the trigger.[17] Severity is primarily graded by the spherical equivalent refractive error under cycloplegia to minimize accommodation effects. The International Myopia Institute proposes standardized thresholds: myopia as ≤ -0.50 diopters (D), low myopia as > -6.00 D to ≤ -0.50 D, and high myopia as ≤ -6.00 D, with pathologic myopia distinguished not solely by degree but by structural ocular changes conferring risks like retinal detachment.[18] Alternative clinical categorizations include mild (-0.50 D to -4.00 D), moderate (-4.00 D to -8.00 D), and severe (> -8.00 D), though thresholds vary across guidelines, with high myopia often starting at > -6.00 D and associated with elevated complication risks. Severe myopia (e.g., -13 to -14 diopters) is typically not classified as a disability if correctable to good visual acuity (≥ 0.8); disability recognition generally applies only if permanent complications, such as retinal degeneration, result in uncorrectable vision loss.[19][20][7][17]| Severity Category | Diopter Range (Spherical Equivalent) | Key Characteristics |
|---|---|---|
| Low/Mild | ≤ -0.50 D to > -6.00 D | Generally physiologic; low risk of pathology; correctable with standard optics.[18][17] |
| High/Severe | ≤ -6.00 D | Increased axial length; higher incidence of complications like choroidal neovascularization.[7][18] |
| Pathologic | Often > -8.00 D with structural changes | Degenerative retinal/choroidal alterations; requires monitoring beyond refraction.[7] |
Pathogenesis
Ocular Mechanisms
Myopia arises primarily from axial elongation of the eyeball, which shifts the retinal plane posterior to the focal point of incoming light rays, resulting in blurred distance vision. This elongation disrupts emmetropization, the developmental process that normally calibrates ocular growth to achieve refractive neutrality. In emmetropic eyes, axial length approximates 23-24 mm in adults, but myopic eyes exceed this, with each millimeter increase corresponding to roughly 3 diopters of myopia.[21][22] The sclera plays a central role in accommodating this elongation through biomechanical remodeling, transitioning from a rigid structure to one capable of expansion. During myopia progression, scleral thickness decreases, particularly posteriorly, while extracellular matrix (ECM) components like collagen fibrils exhibit reduced diameter and packing density, diminishing tensile strength. This is accompanied by upregulated matrix metalloproteinases (MMP-2 and MMP-3) and decreased tissue inhibitors of metalloproteinases (TIMPs), yielding net ECM degradation and scleral thinning.[23][24] Proteoglycan alterations, including reduced lumican and biglycan, further weaken the scleral matrix, facilitating passive distension under intraocular pressure.[23] Retinal and choroidal tissues contribute via growth-regulating signals in response to optical defocus. Hyperopic defocus—where the image plane falls behind the retina—triggers local retinal pathways that promote elongation, potentially through dopamine and glucagon signaling deficits. The choroid thins rapidly in early myopia, reflecting vascular and stromal changes that may modulate scleral perfusion and metabolite delivery. Retinal pigment epithelium (RPE) ion transport and growth factor release, such as those involving vascular endothelial growth factor (VEGF), influence adjacent choroidal and scleral remodeling.[25][26] Corneal and lenticular changes are minor contributors, with corneal power typically flattening slightly (0.2-0.5 diopters) in myopes, insufficient to explain refractive shifts. In high myopia, the globe assumes a prolate ellipsoid shape, stretching photoreceptor arrays and thinning the retina, which elevates risks for complications like macular degeneration. Axial elongation persists into adulthood in high myopes at rates of about 0.03 mm/year, decelerating with age and baseline length.[27][28]Genetic Factors
Heritability estimates for myopia, derived from twin and family studies, indicate a substantial genetic component, with narrow-sense heritability ranging from 0.60 to 0.94 for refractive error and axial length in various populations.[29] Monozygotic twins exhibit concordance rates for myopia significantly higher than dizygotic twins, supporting additive genetic influences over shared environment alone.[30] These studies consistently demonstrate that genetic factors account for 70-90% of variance in myopia susceptibility, particularly in low to moderate cases, though estimates vary by age, ethnicity, and myopia severity.[31] Genome-wide association studies (GWAS) have identified over 500 common genetic variants associated with refractive error and myopia, primarily through large-scale meta-analyses involving hundreds of thousands of participants.[32] These loci, often near genes involved in eye development, scleral remodeling, and neuronal signaling (e.g., those regulating extracellular matrix or dopamine pathways), each confer small effect sizes but collectively explain up to 15-20% of phenotypic variance.[33] High myopia shows enrichment for rare variants, with exome sequencing revealing pathogenic mutations in genes like those implicated in syndromic forms (e.g., collagen-related genes), contributing to severe axial elongation.[34] Polygenic risk scores (PRS), aggregating effects from GWAS-derived variants, predict myopia onset and progression with moderate accuracy, achieving area under the receiver operating characteristic curve (AUROC) values of 0.65-0.70 in independent cohorts.[35] Recent PRS models, refined for specific ancestries such as East Asian populations, enhance detection of high myopia risk in children, though predictive power remains limited without integration of non-genetic factors.[36] Overall, myopia's genetic architecture reflects a polygenic threshold model, where cumulative liability from common and rare variants predisposes individuals, underscoring the absence of single-gene determinism except in rare familial cases.[37]Environmental Factors
Increased time spent on near work activities, such as reading or using digital screens, is associated with higher odds of myopia development and progression, with meta-analyses reporting an odds ratio of 1.14 (95% CI: 1.08-1.20) for additional near work time.[38] This association holds across cohort and cross-sectional studies, though causation remains debated due to potential confounding by factors like education intensity.[39] Prolonged near work may induce accommodative stress or alter retinal signaling, contributing to axial elongation of the eye.[40] Greater time outdoors, particularly exposure to natural sunlight, consistently shows a protective effect against myopia onset, with multiple reviews finding reduced incidence and slower progression in children spending more than 2 hours daily (~14 hours per week) outside.[41] For instance, interventions increasing outdoor time by 1-2 hours per day lowered myopia risk by up to 50% in randomized trials, independent of ethnicity or baseline refractive error.[42] The mechanism likely involves higher-intensity light (typically >1,000–2,000 lux outdoors) stimulating retinal dopamine release, which inhibits scleral remodeling and axial growth.[43] Even short bursts of continuous sunlight exposure (≥15 minutes at ≥2,000 lux) correlate with less myopic shift and slower progression, as measured by wearable devices.[44] Higher educational attainment and intensive schooling environments correlate strongly with elevated myopia prevalence, with studies showing odds ratios up to 2-3 times higher in populations with prolonged indoor academic demands.[45] This link persists after adjusting for genetics, as evidenced by birth month analyses where later school entry reduces myopia risk due to more pre-school outdoor exposure.[46] Urbanization amplifies these effects through reduced green space access and increased near work, with rural-urban prevalence gaps exceeding 20% in multiple cohorts.[47] Digital screen time, a modern near work variant, shows dose-dependent risks, though evidence is stronger for total near work duration than device type alone.[48]Gene-Environment Interactions and Debates
Twin and family studies consistently demonstrate high heritability for myopia, with estimates ranging from 0.60 to 0.90, indicating that genetic factors account for a substantial portion of refractive error variation within populations.[49] However, the explosive rise in myopia prevalence—such as from approximately 10-20% in European cohorts born in the 1950s to over 50% in those born after 1990—occurs too rapidly to be attributable to shifts in gene frequencies, underscoring the necessity of environmental modifiers acting on genetic predispositions.[47] Polygenic risk scores derived from genome-wide association studies (GWAS), encompassing over 450 loci associated with myopia, interact with environmental exposures; for instance, individuals with elevated genetic risk show amplified refractive progression when exposed to high levels of near work or education, as evidenced by longitudinal cohort data.[50][51] These interactions likely operate through pathways where genetic variants influence scleral remodeling or retinal signaling, modulated by environmental cues like reduced natural light exposure, which may suppress dopamine release and alter emmetropization. Mendelian randomization analyses, leveraging genetic variants as instrumental variables, provide causal evidence that prolonged education—a proxy for intensive near work—elevates myopia risk independently of confounding socioeconomic factors.[37] Conversely, outdoor time exerts a protective effect, with randomized trials showing 2-3 hours daily reducing incidence by up to 50% in high-risk groups, suggesting gene-dependent responsiveness to light-mediated mechanisms.[37] Longitudinal studies have identified a positive association between the pubertal growth spurt, measured by peak height velocity, and myopia progression in teenagers. Earlier or more pronounced peak height velocity is linked to earlier myopia onset, earlier peak axial length elongation, and faster myopia progression. In the Singapore Cohort Study of the Risk Factors for Myopia (SCORM), children with earlier peak height velocity experienced earlier myopia onset and peak axial length velocity, with similar patterns in both genders but occurring earlier in girls.[52] Recent research further shows that myopia control treatments blunt axial elongation during these growth periods but do not fully decouple it from systemic growth, with the association persisting and appearing particularly strong in girls aged 10-12 years.[53] Debates center on the relative primacy of genetic versus environmental drivers: proponents of a predominantly genetic etiology argue that heritability metrics and stable familial patterns indicate environment primarily accelerates progression in genetically susceptible individuals, rather than initiating de novo cases.[54] Critics counter that population-level epidemics, particularly in urbanizing East Asia where prevalence exceeds 80% among young adults, reflect causal environmental dominance, as genetic evolution cannot account for decadal surges; this view is bolstered by animal models demonstrating environmentally induced axial elongation absent in controls.[55][56] Resolution remains elusive, with calls for larger-scale interaction studies using polygenic scores and environmental tracking to disentangle effects, though methodological challenges like unmeasured confounders persist.[37] Emerging evidence from multi-ancestry GWAS hints at ancestry-specific interactions, where East Asian genomes may confer heightened vulnerability to modern visual demands.[51]Epidemiology
Global Prevalence and Projections
The global prevalence of myopia across all age groups was estimated at 22.9% (1.4 billion people) in 2000, rising to approximately 30% (around 2.6 billion people) by the early 2020s, reflecting a sustained upward trend driven primarily by increases among younger populations.[57][6] Among children and adolescents specifically, meta-analyses of studies spanning 1990 to 2023 report a pooled prevalence escalating from 24.3% to 35.8%, with current estimates around 30.5% globally.[4][58] These figures derive from systematic reviews aggregating cycloplegic refraction data across diverse populations, though variations exist due to differences in diagnostic criteria and underreporting in low-resource regions.[4] Projections to 2050, modeled on age-, gender-, and ethnicity-stratified trends from 1990 onward, forecast that myopia will affect nearly 50% of the world's population (approximately 4.8 to 5 billion individuals), representing a roughly twofold increase from early 21st-century levels.[57][59] High myopia (typically defined as ≤ -6 diopters) is anticipated to reach 10% prevalence globally, heightening risks of associated complications like retinal detachment.[57] For children and adolescents, prevalence could exceed 39-40% by mid-century, potentially impacting over 740 million individuals in that demographic alone, assuming continuation of current urbanization and lifestyle patterns without widespread interventions.[60][61] These estimates, from meta-regression analyses, carry uncertainties related to demographic shifts and potential mitigation efforts, such as increased outdoor activity, but underscore the trajectory toward a public health challenge of unprecedented scale.[57][4]Regional and Demographic Variations
Myopia prevalence varies markedly by region, with East and Southeast Asia exhibiting the highest rates globally. In urban East Asian populations, myopia affects over 80% of young adults, driven by high incidence in school-aged children.[62] In contrast, rates in Europe are substantially lower, with a meta-analysis reporting childhood and adolescent prevalence ranging from 11.9% in Finland to 49.7% in Sweden, influenced by age and national differences.[63] In the United States and Europe, adult myopia prevalence hovers around 30-33%, far below East Asian figures.[64] Regions like sub-Saharan Africa and the Eastern Mediterranean show even lower childhood rates, with pooled prevalence of 5.23% in the latter from 2000-2022 studies.[65] Demographic factors further delineate variations. Ethnically, East Asians consistently demonstrate the highest myopia rates, exceeding those of white Europeans by more than twofold in comparable age groups; South Asian children face a ninefold risk relative to white Europeans, while black African Caribbeans experience a threefold increase.[66][67] Gender disparities appear in several contexts, with females showing higher prevalence than males, such as 4.90% versus 3.94% in Eastern Mediterranean schoolchildren.[65] Age-related patterns reveal escalating prevalence through childhood and adolescence, from under 3% in ages 0-4 to over 67% in late teens in aggregated global data, stabilizing in adulthood.[68]| Ethnicity (Children) | Myopia Risk Relative to White Europeans | Source |
|---|---|---|
| East Asian | >2-fold | [67] |
| South Asian | 9-fold | [66] |
| Black African Caribbean | 3-fold | [66] |