Recent from talks
Nothing was collected or created yet.
Conjunctivitis
View on Wikipedia
This article needs additional citations for verification. (May 2024) |
| Conjunctivitis | |
|---|---|
| Other names | Pink eye, Madras eye |
 | |
| An eye with viral conjunctivitis | |
| Specialty | Ophthalmology, optometry |
| Symptoms | Reddish eye, scratchiness[1] |
| Duration | Viral conjunctivitis: up to two weeks[2] |
| Causes | Viral, bacterial, allergies[3] |
| Diagnostic method | Based on symptoms, microbial culture[1] |
| Prevention | Handwashing[1] |
| Treatment | Based on underlying cause[3] |
| Frequency | 3–6 million per year (US)[1][3] |
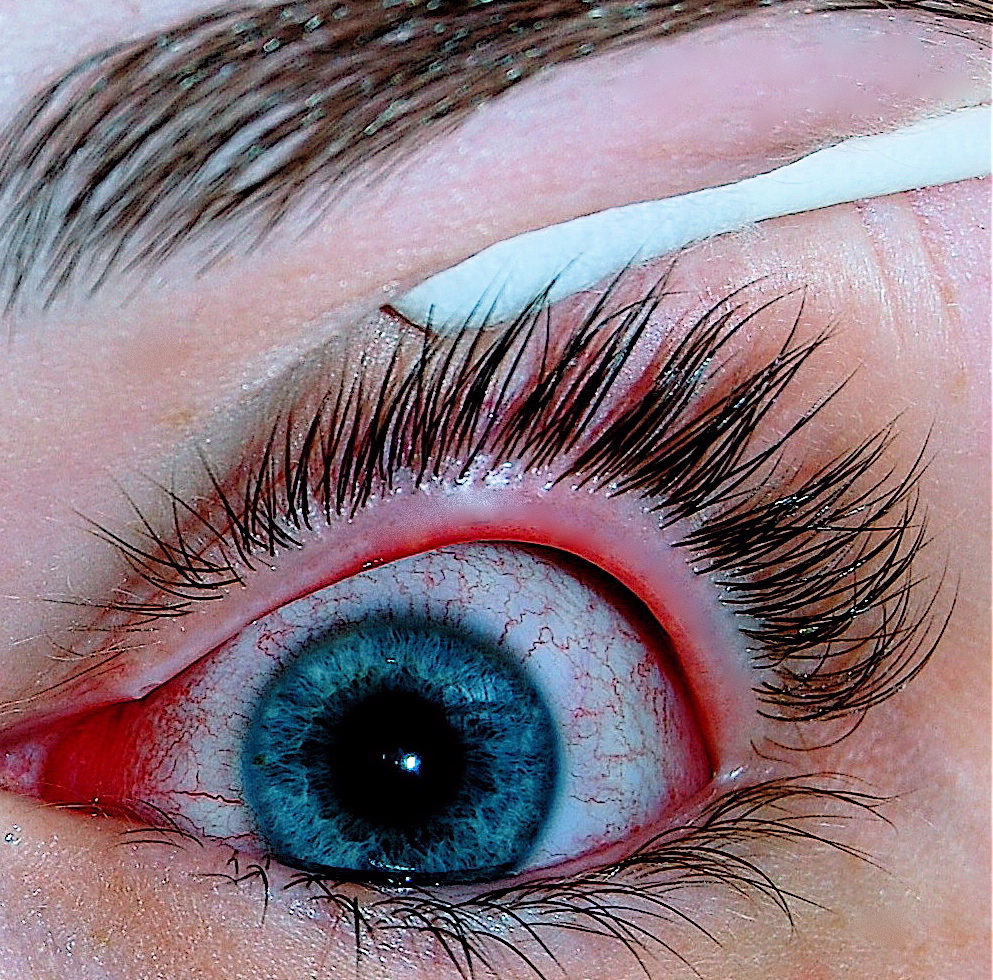
Conjunctivitis, also known as pink eye,[4][5] is inflammation of the conjunctiva, the thin, clear layer that covers the white surface of the eye and the inner eyelid.[6] It makes the eye appear pink or reddish.[1] Pain, burning, scratchiness, or itchiness may occur.[1] The affected eye may have increased tears or be stuck shut in the morning.[1] Swelling of the sclera may also occur.[1] Itching is more common in cases that are due to allergies.[3] Conjunctivitis can affect one or both eyes.[1]
The most common infectious causes in adults are viral, whereas in children bacterial causes predominate.[7][3] The viral infection may occur along with other symptoms of a common cold.[1] Both viral and bacterial cases are easily spread among people.[1] Allergies to pollen or animal hair are also a common cause.[3] Diagnosis is often based on signs and symptoms.[1] Occasionally a sample of the discharge is sent for culture.[1]
Prevention is partly by handwashing.[1] Treatment depends on the underlying cause.[1] In the majority of viral cases there is no specific treatment.[3] Most cases that are due to a bacterial infection also resolve without treatment; however antibiotics can shorten the illness.[1][3] People who wear contact lenses and those whose infection is caused by gonorrhea or chlamydia should be treated.[3] Allergic cases can be treated with antihistamines or mast cell inhibitor drops.[3]
Between three and six million people get acute conjunctivitis each year in the United States.[1][3] Typically they get better in one or two weeks.[1][3] If visual loss, significant pain, sensitivity to light or signs of herpes occur, or if symptoms do not improve after a week, further diagnosis and treatment may be required.[3] Conjunctivitis in a newborn, known as neonatal conjunctivitis, may also require specific treatment.[1]
Signs and symptoms
[edit]
Red eye, swelling of the conjunctiva, and watering of the eyes are symptoms common to all forms of conjunctivitis. However, the pupils should be normally reactive, and the visual acuity should be normal.[8]
Conjunctivitis is identified by inflammation of the conjunctiva, manifested by irritation and redness. Examination using a slit lamp (biomicroscope) may improve diagnostic accuracy. Examination of the palpebral conjunctiva, which overlies the inner aspects of the eyelids, is usually more diagnostic than examination of the bulbal conjunctiva, which overlies the sclera.[9]
Viral
[edit]
Approximately 80% of cases of conjunctivitis in adults and less than 20% in children are due to viruses, with 65% to 90% of these cases being attributed to adenoviruses.[3][7] Viral conjunctivitis is often associated with an infection of the upper respiratory tract, a common cold, or a sore throat. Other associated signs may include pre-auricular lymph node swelling and contact with another person with a red eye.[7] Eye pain may be present if the cornea is also involved.[7] Its symptoms include excessive watering and itching. The discharge in viral conjunctivitis is usually (but not always) watery.[7] The infection usually begins in one eye but may spread easily to the other eye.[citation needed]
Viral conjunctivitis manifests as a fine, diffuse pinkness of the conjunctiva which may be mistaken for iritis, but corroborative signs on microscopy, particularly numerous lymphoid follicles on the tarsal conjunctiva, and sometimes a punctate keratitis are seen.[10]
Allergic
[edit]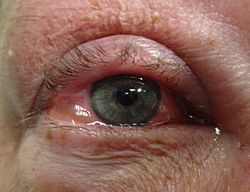
Allergic conjunctivitis is inflammation of the conjunctiva due to allergy.[11] The specific allergens may differ among patients. Symptoms result from the release of histamine and other active substances by mast cells, and consist of redness (mainly due to vasodilation of the peripheral small blood vessels), swelling of the conjunctiva, itching, and increased production of tears.[citation needed]
Bacterial
[edit]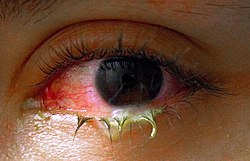
Bacteria are responsible for approximately 70% of conjunctivitis in children and less than 20% of cases in adults.[7] Common bacteria responsible for bacterial conjunctivitis are Staphylococcus including Staph aureus, Streptococcus such as strep pneumoniae,[12] Haemophilus species and Moraxella catarrhalis.[7] Less commonly, Chlamydia spp. and Niesseria species (Neisseria gonorrhoeae and Neisseria meningitidis) may be the cause.[7][13] Infection with Escherichia coli may also cause conjunctivitis, particularly in the neonatal subtype ophthalmia neonatorum.[14] Bacterial conjunctivitis usually causes a rapid onset of conjunctival redness, swelling of the eyelid, and a sticky discharge. Typically, symptoms develop first in one eye, but may spread to the other eye within 2–5 days. Conjunctivitis due to common pus-producing bacteria causes marked grittiness or irritation and a stringy, opaque, greyish or yellowish discharge that may cause the lids to stick together, especially after sleep. Severe crusting of the infected eye and the surrounding skin may also occur. The gritty or scratchy feeling is sometimes localized enough that patients may insist that they have a foreign body in the eye.[citation needed]

Bacteria such as Chlamydia trachomatis or Moraxella spp. can cause a nonexudative but persistent conjunctivitis without much redness. Bacterial conjunctivitis may cause the production of membranes or pseudomembranes that cover the conjunctiva. Pseudomembranes consist of a combination of inflammatory cells and exudates and adhere loosely to the conjunctiva, while true membranes are more tightly adherent and cannot be easily peeled away. Cases of bacterial conjunctivitis that involve the production of membranes or pseudomembranes are associated with Neisseria gonorrhoeae, β-hemolytic streptococci, and Corynebacterium diphtheriae. C. diphtheriae causes membrane formation in the conjunctiva of unimmunized children.[15]
Chemical
[edit]Chemical eye injury may result when an acidic or alkaline substance gets in the eye.[16] Alkali burns are typically worse than acidic burns.[17] Mild burns produce conjunctivitis, while more severe burns may cause the cornea to turn white.[17] Litmus paper may be used to test for chemical causes.[16] When a chemical cause has been confirmed, the eye or eyes should be flushed until the pH is in the range 6–8.[17] Anaesthetic eye drops can be used to decrease the pain.[17]
Irritant or toxic conjunctivitis is primarily marked by redness. If due to a chemical splash, it is often present in only the lower conjunctival sac. With some chemicals, above all with caustic alkalis such as sodium hydroxide, necrosis of the conjunctiva marked by a deceptively white eye due to vascular closure may occur, followed by sloughing off of the dead epithelium. A slit lamp examination is likely to show evidence of anterior uveitis.[18]
Biomarkers
[edit]Omics technologies have been used to identify biomarkers that inform on the emergence and progression of conjunctivitis. For example, in chronic inflammatory cicatrizing conjunctivitis, active oxylipins, lysophospholipids, fatty acids, and endocannabinoids alterations, from which potential biomarkers linked to inflammatory processes were identified.[19]
Other
[edit]
Inclusion conjunctivitis of the newborn is a conjunctivitis that may be caused by the bacterium Chlamydia trachomatis, and may lead to acute, purulent conjunctivitis.[20] However, it is usually self-healing.[20]
Causes
[edit]Viruses are the most common cause of infectious conjunctivitis.[3] Bacterial infections, allergies, other irritants, and dryness are also common causes. Both bacterial and viral infections are contagious, passing from person to person or spread through contaminated objects or water. Contact with contaminated fingers is a common cause of conjunctivitis. Bacteria may also reach the conjunctiva from the edges of the eyelids and surrounding skin, from the nasopharynx, from infected eye drops or contact lenses, from the genitals, or from the bloodstream.[21] Infection by human adenovirus accounts for 65% to 90% of cases of viral conjunctivitis.[22]
Viral
[edit]Adenoviruses are the most common cause of viral conjunctivitis (adenoviral keratoconjunctivitis).[23] Herpetic keratoconjunctivitis, caused by herpes simplex viruses, can be serious and requires treatment with aciclovir. Acute hemorrhagic conjunctivitis is a highly contagious disease caused by one of two enteroviruses, enterovirus 70 and coxsackievirus A24. These were first identified in an outbreak in Ghana in 1969 and have spread worldwide since then, causing several epidemics.[24]
Bacterial
[edit]The most common causes of acute bacterial conjunctivitis are Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae.[23][25] Though very rare, hyperacute cases are usually caused by Neisseria gonorrhoeae or Neisseria meningitidis. Chronic cases of bacterial conjunctivitis are those lasting longer than 3 weeks and are typically caused by S. aureus, Moraxella lacunata, or Gram-negative enteric flora.[citation needed]
Allergic
[edit]Conjunctivitis may also be caused by allergens such as pollen, perfumes, cosmetics, smoke,[26][unreliable medical source?] dust mites, Balsam of Peru,[27] or eye drops.[28] The most frequent cause of conjunctivitis is allergic conjunctivitis, and it affects 15% to 40% of the population.[29] Allergic conjunctivitis accounts for 15% of eye related primary care consultations; most including seasonal exposures in the spring and summer or perpetual conditions.[30]
Other
[edit]- Computer vision syndrome
- Dry eye syndrome
- Reactive arthritis: Conjunctivitis is part of the triad of reactive arthritis, which is thought to be caused by autoimmune cross-reactivity following certain bacterial infections. Reactive arthritis is highly associated with HLA-B27. Conjunctivitis is associated with the autoimmune disease relapsing polychondritis.[31][32]
Diagnosis
[edit]
Cultures are not often taken or needed, as most cases resolve either with time or typical antibiotics. If bacterial conjunctivitis is suspected, but no response to topical antibiotics is seen, swabs for bacterial culture should be taken and tested. Viral culture may be appropriate in epidemic case clusters.[33]
A patch test is used to identify the causative allergen in allergic conjunctivitis.[34]
Although conjunctival scrapes for cytology can be useful in detecting chlamydial and fungal infections, allergies, and dysplasia, they are rarely done because of the cost and the general dearth of laboratory staff experienced in handling ocular specimens. Conjunctival incisional biopsy is occasionally done when granulomatous diseases (e.g., sarcoidosis)[35] or dysplasia are suspected.[36]
Classification
[edit]Conjunctivitis may be classified either by cause or by extent of the inflamed area.[citation needed]
Causes
[edit]- Allergy
- Bacteria
- Viruses
- Chemicals
- Autoimmune
Neonatal conjunctivitis is often grouped separately from bacterial conjunctivitis because it is caused by different bacteria than the more common cases of bacterial conjunctivitis.[37]
By extent of involvement
[edit]Blepharoconjunctivitis is the dual combination of conjunctivitis with blepharitis (inflammation of the eyelids).[38]
Keratoconjunctivitis is the combination of conjunctivitis and keratitis (corneal inflammation).[39]
Blepharokeratoconjunctivitis is the combination of conjunctivitis with blepharitis and keratitis. It is clinically defined by changes of the lid margin, meibomian gland dysfunction, redness of the eye, conjunctival chemosis, and corneal inflammation.[40]
Differential diagnosis
[edit]Some more serious conditions can present with a red eye, such as infectious keratitis, angle-closure glaucoma, or iritis. These conditions require the urgent attention of an ophthalmologist. Signs of such conditions include decreased vision, significantly increased sensitivity to light, inability to keep the eye open, a pupil that does not respond to light, or a severe headache with nausea.[41] Fluctuating blurring is common, due to tearing and mucoid discharge. Mild photophobia is common. However, if any of these symptoms is prominent, considering other diseases such as glaucoma, uveitis, keratitis, and even meningitis or carotico-cavernous fistula is important.[citation needed]
A more comprehensive differential diagnosis for the red or painful eye includes:[41]
- Corneal abrasion
- Subconjunctival hemorrhage
- Pinguecula
- Blepharitis
- Dacryocystitis
- Keratoconjunctivitis sicca (dry eye)
- Keratitis
- Herpes simplex
- Herpes zoster
- Episcleritis – an inflammatory condition that produces a similar appearance to conjunctivitis, but without discharge or tearing
- Uveitis
- Acute angle-closure glaucoma
- Endophthalmitis
- Orbital cellulitis
Prevention
[edit]The most effective prevention is good hygiene, especially avoiding rubbing the eyes with infected hands. Vaccination against some of the causative pathogens, such as Haemophilus influenzae, pneumococcus, and Neisseria meningitidis is also effective.[42]
Povidone-iodine eye solution has been found to prevent neonatal conjunctivitis.[43] It is becoming more commonly used globally because of its low cost.[43]
Management
[edit]Conjunctivitis resolves in 65% of cases without treatment, within 2–5 days. The prescription of antibiotics is not necessary in most cases.[44]
Viral
[edit]Viral conjunctivitis usually resolves on its own and does not require any specific treatment.[3] Antihistamines (e.g., diphenhydramine) or mast cell stabilizers (e.g., cromolyn) may be used to help with the symptoms.[3] Povidone-iodine has been suggested as a treatment, but as of 2008, evidence to support it was poor.[45]
Allergic
[edit]For allergic conjunctivitis, cool water poured over the face with the head inclined downward constricts capillaries, and artificial tears sometimes relieve discomfort in mild cases. In more severe cases, nonsteroidal anti-inflammatory medications and antihistamines may be prescribed. Persistent allergic conjunctivitis may also require topical steroid drops.[46]
Bacterial
[edit]Bacterial conjunctivitis usually resolves without treatment.[3] Topical antibiotics may be needed only if no improvement is observed after 3 days.[47] No serious effects were noted either with or without treatment.[48] Because antibiotics speed healing in bacterial conjunctivitis, their use may be considered.[48] Antibiotics are also recommended for those who wear contact lenses, are immunocompromised, have disease which is thought to be due to chlamydia or gonorrhea, have a fair bit of pain, or have copious discharge.[3] Gonorrheal or chlamydial infections require both oral and topical antibiotics.[3]
The choice of antibiotic varies based on the strain or suspected strain of bacteria causing the infection. Fluoroquinolones, sodium sulfacetamide, or trimethoprim/polymyxin may be used, typically for 7–10 days.[23] Cases of meningococcal conjunctivitis can also be treated with systemic penicillin, as long as the strain is sensitive to penicillin.[citation needed]
When investigated as a treatment, povidone-iodine ophthalmic solution has also been observed to have some effectiveness against bacterial and chlamydial conjunctivitis, with a possible role suggested in locations where topical antibiotics are unavailable or costly.[49]
Chemical
[edit]Conjunctivitis due to chemicals is treated via irrigation with Ringer's lactate or saline solution. Chemical injuries, particularly alkali burns, are medical emergencies, as they can lead to severe scarring and intraocular damage. People with chemically induced conjunctivitis should not touch their eyes to avoid spreading the chemical.[50]
Epidemiology
[edit]Conjunctivitis is the most common eye disease.[51] Rates of disease are related to the underlying cause, which varies by age as well as the time of year. Acute conjunctivitis is most frequently found in infants, school-age children, and the elderly.[21] The most common cause of infectious conjunctivitis is viral conjunctivitis.[29]
It is estimated that acute conjunctivitis affects 6 million people annually in the United States.[3]
Some seasonal trends have been observed for the occurrence of different forms of conjunctivitis. In the Northern Hemisphere, the occurrence of bacterial conjunctivitis peaks from December to April, viral conjunctivitis peaks in the summer months, and allergic conjunctivitis is more prevalent throughout the spring and summer.[21]
History
[edit]An adenovirus was first isolated by Rowe et al. in 1953. Two years later, Jawetz et al. published on epidemic keratoconjunctivitis.[52]: 437 "Madras eye" is a colloquial term that has been used in India for the disease.
Outbreak in Pakistan
[edit]In September 2023, a significant outbreak of conjunctivitis occurred in Pakistan. The outbreak began in Karachi and quickly spread to Lahore, Rawalpindi, and Islamabad. By the end of the month, over 86,133 cases had been reported in Punjab alone. The rapid spread of the disease led to the temporary closure of schools in the region. This event marked one of the largest outbreaks of Pink Eye in the country's recent history.[53][54][55][56]
Society and culture
[edit]Conjunctivitis imposes economic and social burdens. The cost of treating bacterial conjunctivitis in the United States was estimated to be $377 million to $857 million per year.[3] Approximately 1% of all primary care office visits in the United States are related to conjunctivitis. Approximately 70% of all people with acute conjunctivitis present to primary care and urgent care.[3]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s "Facts About Pink Eye". National Eye Institute. November 2015. Archived from the original on 9 March 2016. Retrieved 8 March 2016.
- ^ Long SS, Prober CG, Fischer M (2017). Principles and Practice of Pediatric Infectious Diseases E-Book. Elsevier Health Sciences. p. 502. ISBN 978-0-323-46132-0.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Azari AA, Barney NP (October 2013). "Conjunctivitis: a systematic review of diagnosis and treatment". JAMA. 310 (16): 1721–1729. doi:10.1001/jama.2013.280318. PMC 4049531. PMID 24150468.
- ^ "What is Viral Conjunctivitis a.k.a. Sore Eyes? | National Institutes of Health". Archived from the original on 9 November 2023. Retrieved 14 September 2020.
- ^ Mohanasundaram AS, Gurnani B, Kaur K, Manikkam R (May 2023). "Madras eye outbreak in India: Why should we foster a better understanding of acute conjunctivitis?". Indian Journal of Ophthalmology. 71 (5): 2298–2299. doi:10.4103/IJO.IJO_3317_22. PMC 10391441. PMID 37202982.
- ^ Richards A, Guzman-Cottrill JA (May 2010). "Conjunctivitis". Pediatrics in Review. 31 (5): 196–208. doi:10.1542/pir.31-5-196. PMID 20435711. S2CID 245084568.
- ^ a b c d e f g h Durand ML, Barshak MB, Sobrin L (December 2023). "Eye Infections". The New England Journal of Medicine. 389 (25): 2363–2375. doi:10.1056/NEJMra2216081. PMID 38118024. S2CID 266433325.
- ^ Hashmi MF, Gurnani B, Benson S (2023). "Conjunctivitis". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31082078. Retrieved 23 May 2023.
- ^ "Conjunctivitis". nhs.uk. 19 October 2017. Retrieved 13 February 2025.
- ^ "Conjunctivitis and variants". www.pathologyoutlines.com. Retrieved 29 August 2025.
- ^ Bielory L, Friedlaender MH (February 2008). "Allergic conjunctivitis". Immunology and Allergy Clinics of North America. 28 (1): 43–58, vi. doi:10.1016/j.iac.2007.12.005. PMID 18282545. S2CID 34371872.
- ^ "Pink Eye (Conjunctivitis)". MedicineNet. Archived from the original on 22 June 2013.
- ^ "Acute Bacterial Conjunctivitis – Eye Disorders". Merck Manuals Professional Edition. Archived from the original on 28 December 2016. Retrieved 31 December 2016.
- ^ Saadeh-Jackson S, Rodriguez L, Leffler CT, Freymiller C, Wolf E, Wijesooriya N, et al. (August 2022). "Ophthalmia neonatorum due to Escherichia coli: A rare cause or an emerging bacterial etiology of neonatal conjunctivitis?". Clinical Case Reports. 10 (8) e6201. doi:10.1002/ccr3.6201. PMC 9354094. PMID 35949413.
- ^ Hamborsky J, Kroger A, Wolfe C, eds. (2015). Epidemiology and Prevention of Vaccine-Preventable Diseases. U.S. Dept. of Health & Human Services, Centers for Disease Control and Prevention. p. 112. ISBN 978-0-9904491-1-9.
- ^ a b Zentani A, Burslem J (December 2009). "Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 4: use of litmus paper in chemical eye injury". Emergency Medicine Journal. 26 (12): 887. doi:10.1136/emj.2009.086124. PMID 19934140. S2CID 38124735.
- ^ a b c d Hodge C, Lawless M (July 2008). "Ocular emergencies". Australian Family Physician. 37 (7): 506–509. PMID 18592066.
- ^ "Diagnosing Conjunctivitis". nyulangone.org. Retrieved 13 February 2025.
- ^ Di Zazzo A, Yang W, Coassin M, Micera A, Antonini M, Piccinni F, et al. (May 2020). "Signaling lipids as diagnostic biomarkers for ocular surface cicatrizing conjunctivitis". Journal of Molecular Medicine. 98 (5): 751–760. doi:10.1007/s00109-020-01907-w. PMC 7220886. PMID 32313985.
- ^ a b Fisher B, Harvey RP, Champe PC (2007). Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstown MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-8215-9.
- ^ a b c Høvding G (February 2008). "Acute bacterial conjunctivitis". Acta Ophthalmologica. 86 (1): 5–17. doi:10.1111/j.1600-0420.2007.01006.x. PMID 17970823. S2CID 20629824.
- ^ Singh MP, Ram J, Kumar A, Rungta T, Gupta A, Khurana J, et al. (2018). "Molecular epidemiology of circulating human adenovirus types in acute conjunctivitis cases in Chandigarh, North India". Indian Journal of Medical Microbiology. 36 (1): 113–115. doi:10.4103/ijmm.ijmm_17_258. PMID 29735838.
- ^ a b c Yanoff M, Duker JS (2008). Ophthalmology (3rd ed.). Edinburgh: Mosby. pp. 227–236. ISBN 978-0-323-05751-6.
- ^ Lévêque N, Huguet P, Norder H, Chomel JJ (April 2010). "[Enteroviruses responsible for acute hemorrhagic conjunctivitis]". Médecine et Maladies Infectieuses (in French). 40 (4): 212–218. doi:10.1016/j.medmal.2009.09.006. PMID 19836177.
- ^ "Protect Yourself From Pink Eye". Centers for Disease Control and Prevention (CDC). 2 October 2017. Retrieved 7 December 2018.
- ^ "Allergic Conjunctivitis". familydoctor.org. Archived from the original on 6 September 2015. Retrieved 18 September 2015.
- ^ Brooks P (25 October 2012). The Daily Telegraph: Complete Guide to Allergies. Little, Brown Book. ISBN 978-1-4721-0394-9. Retrieved 15 April 2014.[permanent dead link]
- ^ "What Is Allergic Conjunctivitis? What Causes Allergic Conjunctivitis?". medicalnewstoday.com. Archived from the original on 16 March 2010. Retrieved 6 April 2010.
- ^ a b Mourad MS, Rihan RA (April 2018). "Prevalence of Different Eye Diseases excluding Refractive Errors Presented at the Outpatient Clinic in Beheira Eye Hospital". The Egyptian Journal of Hospital Medicine. 71 (2): 2484–2489. doi:10.12816/0045645. S2CID 80882721.
- ^ Perkin MR, Bader T, Rudnicka AR, Strachan DP, Owen CG (24 November 2015). "Inter-Relationship between Rhinitis and Conjunctivitis in Allergic Rhinoconjunctivitis and Associated Risk Factors in Rural UK Children". PLOS ONE. 10 (11) e0143651. Bibcode:2015PLoSO..1043651P. doi:10.1371/journal.pone.0143651. PMC 4658044. PMID 26600465.
- ^ Puéchal X, Terrier B, Mouthon L, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C (March 2014). "Relapsing polychondritis". Joint Bone Spine. 81 (2): 118–124. doi:10.1016/j.jbspin.2014.01.001. PMID 24556284. S2CID 205754989.
- ^ Cantarini L, Vitale A, Brizi MG, Caso F, Frediani B, Punzi L, et al. (2014). "Diagnosis and classification of relapsing polychondritis". Journal of Autoimmunity. 48–49: 53–59. doi:10.1016/j.jaut.2014.01.026. PMID 24461536.
- ^ Sheikh A, Hurwitz B (2008). "BACTERIAL CONJUNCTIVITIS 372.05 (Infective Conjunctivitis, Mucopurulent Conjunctivitis, Purulent Conjunctivitis)". Roy and Fraunfelder's Current Ocular Therapy. Elsevier. pp. 332–334. doi:10.1016/b978-1-4160-2447-7.50182-1. ISBN 978-1-4160-2447-7.
- ^ Mannis MJ, Macsai MS, Huntley AC (1996). Eye and skin disease. Lippincott-Raven. ISBN 978-0-7817-0269-0. Archived from the original on 5 July 2014. Retrieved 23 April 2014.
- ^ Korkmaz Ekren P, Mogulkoc N, Toreyin ZN, Egrilmez S, Veral A, Akalın T, et al. (October 2016). "Conjunctival Biopsy as a First Choice to Confirm a Diagnosis of Sarcoidosis". Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 33 (3): 196–200. PMID 27758983.
- ^ Roberts F, Thum CK (2021). "The Conjunctival Biopsy". In Roberts F (ed.). Lee's Ophthalmic Histopathology. Cham: Springer International Publishing. pp. 343–388. doi:10.1007/978-3-030-76525-5_11. ISBN 978-3-030-76525-5.
- ^ Makker K, Nassar GN, Kaufman EJ (2025), "Neonatal Conjunctivitis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28722870, retrieved 4 July 2025
- ^ Fazal MI, Patel BC (2025), "Blepharoconjunctivitis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32644328, retrieved 18 June 2025
- ^ Burrow MK, Gurnani B, Patel BC (2025), "Keratoconjunctivitis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31194419, retrieved 7 July 2025
- ^ O'Gallagher M, Banteka M, Bunce C, Larkin F, Tuft S, Dahlmann-Noor A (May 2016). "Systemic treatment for blepharokeratoconjunctivitis in children". The Cochrane Database of Systematic Reviews. 2016 (5) CD011750. doi:10.1002/14651858.CD011750.pub2. PMC 9257284. PMID 27236587.
- ^ a b Longo DL (2012). "Disorders of the Eye(Horton JC)". Harrison's Principles of Internal Medicine. McGra-Hill.
- ^ "Protect Yourself From Pink Eye". Centers for Disease Control and Prevention. 2 October 2017. Retrieved 18 October 2017.
- ^ a b Isenberg SJ (2003). "The ocular application of povidone-iodine". Community Eye Health. 16 (46): 30–31. PMC 1705857. PMID 17491857.
- ^ Rose P (August 2007). "Management strategies for acute infective conjunctivitis in primary care: a systematic review". Expert Opinion on Pharmacotherapy. 8 (12): 1903–1921. doi:10.1517/14656566.8.12.1903. PMID 17696792. S2CID 45899988.
- ^ Bartlett JD, Jaanus SD (2008). Clinical Ocular Pharmacology. Elsevier Health Sciences. pp. 454–. ISBN 978-0-7506-7576-5. Archived from the original on 3 December 2016.
- ^ Patel DS, Arunakirinathan M, Stuart A, Angunawela R (November 2017). "Allergic eye disease". BMJ. 359 j4706. doi:10.1136/bmj.j4706. PMID 29097360. S2CID 5316455.
- ^ Visscher KL, Hutnik CM, Thomas M (November 2009). "Evidence-based treatment of acute infective conjunctivitis: Breaking the cycle of antibiotic prescribing". Canadian Family Physician. 55 (11): 1071–1075. PMC 2776793. PMID 19910590.
- ^ a b Chen YY, Liu SH, Nurmatov U, van Schayck OC, Kuo IC (March 2023). "Antibiotics versus placebo for acute bacterial conjunctivitis". The Cochrane Database of Systematic Reviews. 3 (3) CD001211. doi:10.1002/14651858.CD001211.pub4. PMC 10014114. PMID 36912752.
- ^ Isenberg SJ, Apt L, Valenton M, Del Signore M, Cubillan L, Labrador MA, et al. (November 2002). "A controlled trial of povidone-iodine to treat infectious conjunctivitis in children". American Journal of Ophthalmology. 134 (5): 681–688. doi:10.1016/S0002-9394(02)01701-4. PMID 12429243.
- ^ "Conjunctivitis". American Optometric Association. Retrieved 15 March 2024.
- ^ Smeltzer SC (2010). Brunner & Suddarth's textbook of medical-surgical nursing (12th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1787. ISBN 978-0-7817-8589-1. Archived from the original on 15 August 2016.
- ^ Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB (September–October 2015). "Adenoviral keratoconjunctivitis". Survey of Ophthalmology. 60 (5): 435–443. doi:10.1016/j.survophthal.2015.04.001. PMID 26077630.
- ^ "From pink eye to blindness". 28 September 2023.
- ^ "86,133 pink eye cases in Punjab in September and counting". 28 September 2023.
- ^ Ahmed S (27 September 2023). "Punjab Announces Holiday for Schools Amid Pink Eye Outbreak". Propakistani.
- ^ "85 new cases of Pink Eye infection reported in Pakistan's Lahore". ETHealthworld.com. Asian News International.
External links
[edit]- "Pink Eye". MedlinePlus. U.S. National Library of Medicine.
- "Metabolomics for Ocular Surface Disease". MSBB Group, Leiden University. 28 February 2021.
Conjunctivitis
View on GrokipediaOverview
Definition
Conjunctivitis, commonly known as pink eye, is an inflammation of the conjunctiva, the thin, transparent mucous membrane that lines the inner surface of the eyelids and covers the anterior portion of the sclera, or white part of the eye.[1][3] This condition arises from various triggers leading to vascular congestion and tissue swelling in the affected area.[3] The term "pink eye" stems from the characteristic redness of the ocular surface, caused by dilation and engorgement of the conjunctival blood vessels.[4] Conjunctivitis affects individuals across all age groups and is generally self-limiting, often resolving spontaneously within one to two weeks, though its potential for contagion depends on the specific etiology.[5][6] In the United States, the condition accounts for an estimated 6 million cases annually.[7]Classification
Conjunctivitis is primarily classified by its etiology into infectious and non-infectious categories, with infectious forms further subdivided into viral and bacterial subtypes, while non-infectious includes allergic, chemical or irritant-induced, and other causes such as autoimmune or neoplastic conditions.[3][8] Viral conjunctivitis, often caused by adenoviruses, represents the most common infectious type, followed by bacterial infections from pathogens like Staphylococcus aureus or Haemophilus influenzae.[9] Non-infectious types encompass allergic reactions mediated by IgE and histamine release, as well as irritant exposures from chemicals or foreign bodies.[1] Other forms, such as those linked to systemic autoimmune diseases like ocular mucous membrane pemphigoid, fall into miscellaneous categories due to their distinct underlying mechanisms.[8] A secondary classification considers the extent of involvement, distinguishing between unilateral (affecting one eye) and bilateral (affecting both eyes) presentations, as well as localized versus diffuse conjunctival inflammation, which aids in differentiating etiologies during clinical assessment.[9] For instance, bacterial conjunctivitis may initially present unilaterally before becoming bilateral, whereas allergic forms typically involve both eyes symmetrically with diffuse involvement.[8] This spatial categorization is crucial for guiding diagnostic approaches, as unilateral cases often prompt evaluation for foreign bodies or focal infections.[1] Conjunctivitis is also categorized by duration and pattern, with acute forms lasting less than 4 weeks, chronic persisting beyond 4 weeks, and recurrent or hyperacute variants indicating severe or repeated episodes.[9][6] Allergic conjunctivitis specifically includes seasonal (triggered by pollen or environmental allergens) and perennial (year-round, often due to indoor irritants) subtypes, reflecting exposure patterns.[10] These temporal distinctions help predict prognosis and management strategies, such as monitoring chronic cases for underlying systemic issues.[8] Neonatal conjunctivitis, also known as ophthalmia neonatorum, forms a distinct subgroup occurring within the first month of life, primarily due to perinatal exposure to bacteria like Chlamydia trachomatis or Neisseria gonorrhoeae during passage through the birth canal, necessitating prompt evaluation to prevent complications like corneal scarring.[11] This category is separated owing to its unique risks and higher potential for severe outcomes compared to adult-onset forms.[1]Pathophysiology
General Mechanisms
Conjunctivitis is fundamentally an inflammatory condition of the conjunctiva, where the initial response to injury, infection, or irritation involves vasodilation of the conjunctival blood vessels. This dilation increases blood flow to the area, resulting in the characteristic redness or hyperemia observed clinically. Concurrently, increased vascular permeability allows the leakage of plasma proteins and fluid into the surrounding tissues, leading to conjunctival edema (chemosis) and the production of exudate, which manifests as tearing, discharge, or crusting depending on the severity. These vascular changes are mediated by local release of vasoactive mediators and represent a universal hallmark across all forms of conjunctivitis.[3][6] The inflammatory cascade in conjunctivitis is driven by a complex interplay of signaling molecules, including pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These cytokines are secreted by activated epithelial cells, resident immune cells, and infiltrating leukocytes, promoting further amplification of inflammation through recruitment of neutrophils, eosinophils, and macrophages to the conjunctival tissues. Histamine, primarily released from degranulated mast cells upon stimulation, exacerbates this process by binding to H1 receptors on endothelial cells, enhancing vasodilation and permeability while contributing to symptoms like itching and irritation. This cascade establishes a self-perpetuating cycle of tissue damage and repair unless resolved.[12][13][14] Conjunctival goblet cells, specialized epithelial cells within the conjunctival mucosa, are integral to the ocular surface defense by secreting gel-forming mucins, primarily MUC5AC, which form a protective layer in the tear film. In the context of inflammation, these cells respond to stimuli by increasing mucin production, which can lead to excessive mucus secretion and contribute to the viscous exudate seen in many cases; conversely, chronic inflammation induced by cytokines like TNF-α can reduce goblet cell density and function, disrupting tear film stability and exacerbating dryness and epithelial damage. This dual role underscores goblet cells' contribution to both protective mucus barriers and pathological responses in conjunctival inflammation.[15][16] The immune response underlying conjunctivitis exhibits variations between innate and adaptive arms, tailored to the underlying trigger. Innate immunity provides rapid, non-specific protection through antimicrobial components in the tear film, such as lysozyme and immunoglobulins, which limit pathogen spread and initiate the inflammatory cascade via pattern recognition receptors on epithelial cells. In contrast, adaptive immunity, predominant in allergic forms, involves antigen-specific T-cell activation and antibody production, leading to sustained lymphocytic infiltration and chronic inflammation. These distinctions highlight how general mechanisms adapt to specific contexts without altering the core vascular and cellular responses.[3][9]Etiology-Specific Pathways
Infectious pathways in conjunctivitis involve pathogen invasion of the conjunctival epithelium, leading to cellular damage and inflammation. For viral causes, such as adenoviruses, which cause up to 90% of viral conjunctivitis cases, the pathogen adheres to epithelial cell receptors via projecting fibers like pentons, facilitating entry and intracellular replication. This replication culminates in epithelial cell lysis, releasing viral particles and inducing superficial punctate keratopathy along with subepithelial infiltrates, particularly in epidemic keratoconjunctivitis caused by serotypes 8, 19, or 37.[17] Bacterial pathogens, including Haemophilus influenzae, Streptococcus pneumoniae, and Staphylococcus aureus, colonize the ocular surface through direct contact or compromised epithelial barriers, particularly in children, contact lens wearers, and immunocompromised individuals. These bacteria produce toxins and enzymes that exacerbate epithelial disruption, promoting purulent discharge and potential keratitis.[4] Secondary bacterial superinfection frequently complicates viral conjunctivitis, as epithelial lysis weakens local defenses, allowing opportunistic bacterial overgrowth and intensified inflammatory responses.[17] Allergic pathways are characterized by an IgE-mediated type I hypersensitivity reaction, where allergen exposure triggers rapid mast cell degranulation in the conjunctiva. Upon re-exposure, allergens cross-link IgE antibodies bound to high-affinity FcεRI receptors on sensitized mast cells, prompting the release of preformed mediators such as histamine, tryptase, and leukotrienes, alongside newly synthesized cytokines and prostaglandins. This degranulation induces immediate vascular permeability, itching, and chemotaxis of eosinophils and basophils, amplifying ocular surface inflammation.[10] Dominance of Th2 immune responses further sustains the process, with cytokines like IL-4 promoting B-cell class switching to IgE production and IL-13 enhancing goblet cell mucus secretion and epithelial barrier dysfunction. These Th2-skewed signals, including IL-5 for eosinophil recruitment, create a chronic inflammatory milieu in forms like vernal or atopic keratoconjunctivitis.[10][18] Chemical pathways initiate direct toxic injury to the conjunctival epithelium upon exposure to irritants, acids, or alkalis, bypassing immune mediation. Alkalis, such as ammonia or sodium hydroxide, penetrate deeply by saponifying cell membrane lipids, causing liquefactive necrosis and widespread epithelial denudation that extends to stromal layers. Acids, like sulfuric acid, provoke protein coagulation on the surface, limiting deeper invasion but still resulting in ischemic necrosis and sloughing of the epithelium.[19] Irritants, including alcohols or environmental pollutants, induce oxidative stress through reactive oxygen species generation, leading to lipid peroxidation, protein modification, and apoptosis in conjunctival cells. This oxidative damage disrupts tear film stability and triggers secondary inflammatory cascades via cytokine release, such as TNF-α, exacerbating surface toxicity.[19][20] Chronic forms of conjunctivitis arise from persistent antigen exposure, driving progressive fibrosis and tissue remodeling in the conjunctiva. In conditions like vernal or atopic keratoconjunctivitis, ongoing allergen or autoantigen stimulation activates conjunctival fibroblasts and myofibroblasts, marked by α-smooth muscle actin expression, leading to excessive extracellular matrix deposition and subepithelial collagen accumulation. This results in forniceal shortening, symblepharon, and ankyloblepharon, impairing ocular motility and lubrication.[21] Th2-derived cytokines, particularly IL-13 and TGF-β from CD11b+ dendritic cells and T-helper cells, form a feedback loop that sustains fibroblast proliferation and inhibits matrix degradation, culminating in irreversible scarring. In autoimmune variants like ocular mucous membrane pemphigoid, chronic inflammation progresses through phases of injury, proliferation, and fibrosis, with persistent autoantigen-driven responses amplifying remodeling.[21][22]Types of Conjunctivitis
Viral Conjunctivitis
Viral conjunctivitis, the most prevalent form of infectious conjunctivitis in adults, is primarily caused by adenoviruses, which account for approximately 80% of cases, followed by herpes simplex virus and enteroviruses.[23] Adenoviral serotypes such as 3, 4, 7, 8, 19, and 37 are most commonly implicated, often leading to epidemic keratoconjunctivitis (EKC) in outbreaks.[24] Emerging strains, including coxsackievirus A24 variant (CVA24v), have been associated with acute hemorrhagic conjunctivitis outbreaks since 2023, notably in regions like Pakistan, India, Bhutan, and the Indian Ocean islands, with continued outbreaks reported in 2024-2025 in East Africa (e.g., Kenya, Tanzania) and the Indian Ocean (e.g., Madagascar, Mayotte).[25][26][27][28] The pathophysiology involves viral entry and replication within conjunctival and corneal epithelial cells, resulting in cytopathic effects such as cell swelling, lysis, and punctate epithelial erosions.[29] This replication triggers an inflammatory response, including recruitment of immune cells and release of cytokines, which contributes to conjunctival hyperemia and edema.[30] In adenoviral cases, late-phase subepithelial infiltrates in the cornea arise from a hypersensitivity reaction to viral antigens, potentially persisting beyond the acute infection.[23] Characteristic signs and symptoms include unilateral or bilateral ocular redness, watery discharge, foreign body sensation, burning, itching, and photophobia, often with preauricular lymphadenopathy indicating viral etiology.[17] Symptoms typically emerge after a 5- to 12-day incubation period and peak within 3 to 5 days, resolving over 1 to 2 weeks in most cases, though discomfort may linger.[31] Severe adenoviral infections may present with follicular conjunctivitis or, in EKC, corneal involvement manifesting as subepithelial opacities.[32] Diagnosis relies on clinical pattern recognition, including the presence of watery discharge and lymphadenopathy, to differentiate from other forms.[3] In outbreak settings or atypical cases, confirmatory polymerase chain reaction (PCR) testing of conjunctival swabs identifies the specific virus, serving as the gold standard for etiologic confirmation.[33] Rapid antigen tests may aid initial assessment but have lower sensitivity compared to PCR.[34] Treatment is predominantly supportive, emphasizing cold compresses, artificial tears, and vaseline ointment to alleviate symptoms and prevent secondary bacterial infection.[35] For herpetic cases, topical or oral antivirals such as acyclovir are indicated to reduce duration and complications.[36] No specific antiviral therapy is approved for adenoviral conjunctivitis, though Phase 2 trials evaluating IVIEW-1201 were completed in 2024, with Phase 3 studies planned or initiated as of 2025 to explore targeted interventions to shorten viral shedding and symptom resolution.[37][38] Strict hygiene measures, including handwashing and isolation, are essential to curb transmission.[17] Complications are uncommon but may include pseudomembrane formation on the tarsal conjunctiva in severe adenoviral infections, potentially leading to scarring if not managed promptly.[39] Keratitis, manifesting as punctate or geographic epithelial defects, occurs rarely and can impair vision temporarily due to corneal inflammation.[32] Persistent subepithelial infiltrates may require topical corticosteroids in refractory cases, under specialist supervision to avoid exacerbating infection.[17]Bacterial Conjunctivitis
Bacterial conjunctivitis, a common form of infectious conjunctivitis, is particularly prevalent in children and results from bacterial colonization of the conjunctival surface. The primary causative agents include Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae, which account for most cases in both children and adults.[40] In neonates, Neisseria gonorrhoeae is a significant pathogen, often acquired through vertical transmission during birth.[41] This condition is more frequent in pediatric populations due to higher exposure in school settings and immature immune responses.[42] The pathophysiology involves bacterial adherence to the compromised conjunctival epithelium, where normal flora such as staphylococci and streptococci overgrow following disruptions in host defenses, such as minor trauma or viral coinfection.[4] Certain bacteria produce toxins that exacerbate inflammation and tissue damage, while biofilm formation by pathogens like S. aureus promotes persistent infection and resistance to clearance, ultimately leading to the accumulation of purulent exudate.[43][44] This inflammatory response causes conjunctival hyperemia and edema, distinguishing it from noninfectious forms. Characteristic signs and symptoms include sticky yellow-green purulent or mucopurulent discharge that often mats the eyelids upon waking, conjunctival injection, and mild ocular discomfort or foreign body sensation.[1][5] Unlike viral or allergic variants, itching is typically absent, and the condition is unilateral at onset but may become bilateral.[4] Most uncomplicated cases resolve spontaneously within 7 to 10 days without intervention, though symptoms can persist longer in the absence of treatment.[6] Diagnosis is primarily clinical, relying on the history of purulent discharge and eyelid crusting in the absence of severe pain or vision changes that might suggest deeper involvement.[45] In mild cases, no further testing is needed, but for severe presentations, neonates, or treatment failures, conjunctival swabs for Gram staining or bacterial culture are recommended to identify the pathogen and guide therapy.[4] Treatment focuses on topical antibiotics to accelerate resolution and reduce contagiousness, with options including erythromycin ointment or fluoroquinolone drops applied four times daily for 5 to 7 days.[46][47] For gonococcal cases, systemic antibiotics such as ceftriaxone are essential alongside topical agents. Emerging therapies, such as the antibiotic-NSAID combination NTC014 (a quinolone with bromfenac), completed Phase II trials in late 2024 (MIRAKLE study), demonstrating non-inferiority to antibiotic monotherapy with faster symptom relief and microbiological eradication.[48] Complications are uncommon in routine cases but can be severe in untreated gonococcal infections, including corneal ulceration, perforation, and potential blindness due to rapid progression.[49][50]Allergic Conjunctivitis
Allergic conjunctivitis is an immune-mediated inflammatory condition of the conjunctiva triggered by environmental allergens, distinguishing it as a non-infectious form of ocular allergy. It affects approximately 15-40% of the global population and is characterized by a type I hypersensitivity reaction. Primary causes include exposure to airborne allergens such as pollen, dust mites, animal dander, and mold spores, often exacerbated by genetic predisposition, atopy, and environmental factors like air pollution.[10][51][52] The condition manifests in several types, with seasonal allergic conjunctivitis (SAC) being the most common, occurring during high-pollen seasons like spring and summer due to tree and grass pollens. Perennial allergic conjunctivitis (PAC) presents year-round with milder symptoms, primarily triggered by indoor allergens such as house dust mites and pet dander, often worsening in autumn. A more severe variant, vernal keratoconjunctivitis (VKC), typically affects young males aged 5-20 in warm, dry climates and involves recurrent episodes linked to allergens and climatic factors.[10][51][52] Pathophysiologically, allergic conjunctivitis involves type I hypersensitivity where allergens cross-link IgE antibodies on sensitized mast cells and basophils, leading to rapid degranulation and release of mediators like histamine, tryptase, and leukotrienes. This initiates an immediate phase of inflammation, characterized by vasodilation and increased vascular permeability. A late-phase response follows, involving recruitment of eosinophils, T cells, and additional inflammatory cells, which perpetuate conjunctival edema and hyperemia through cytokine release.[10][51][52] Clinically, patients experience intense typically bilateral ocular itching as the hallmark symptom, though rarely unilateral if allergen exposure is asymmetric, often accompanied by excessive tearing, conjunctival chemosis (swelling), and injection (redness) from dilated blood vessels, which can be localized in such cases. Symptoms typically persist or recur with ongoing allergen exposure and may include watery or mucoid discharge; in VKC, additional features like photophobia, thick mucus, and giant papillae on the tarsal conjunctiva are common. These manifestations are usually self-limiting but can significantly impair quality of life during peak exposure periods.[10][51][52][53] Diagnosis relies primarily on a detailed patient history of allergen exposure and atopic conditions, supplemented by clinical examination revealing characteristic signs like conjunctival hyperemia and chemosis. Conjunctival scrapings or impression cytology can confirm the presence of eosinophils, supporting the allergic etiology, while allergy testing such as skin prick or serum IgE levels may identify specific triggers. Differential diagnosis excludes infectious causes through absence of purulent discharge or lymphadenopathy.[10][51][52] Treatment focuses on symptom relief and inflammation control, beginning with allergen avoidance strategies. Topical antihistamines (e.g., emedastine) and dual-action antihistamine-mast cell stabilizers (e.g., olopatadine) are first-line therapies, providing rapid relief from itching and redness by blocking histamine receptors and preventing mediator release. For severe or refractory cases, particularly VKC, short-term topical corticosteroids (e.g., loteprednol) are used to suppress inflammation, though with caution due to risks like glaucoma. Emerging therapies include reproxalap, a reactive aldehyde species modulator under FDA review as of 2025 for potential use in allergic conjunctivitis to reduce oxidative stress and symptoms. Immunomodulators like cyclosporine may be employed in chronic forms.[10][51][52][54] Complications are rare in mild SAC and PAC but more frequent in VKC, where chronic inflammation can lead to corneal involvement, including superficial punctate keratitis or shield ulcers—raised epithelial defects that occur in 3-20% of cases and risk permanent scarring or vision loss if untreated. Prompt management is essential to prevent these sequelae.[10][51][52]Chemical and Irritant Conjunctivitis
Chemical and irritant conjunctivitis arises from direct exposure to external agents that damage the conjunctival surface without involving infectious or allergic pathways. Primary causes include environmental irritants such as chlorine in swimming pools, tobacco smoke, and airborne pollutants, as well as chemical exposures like acids (e.g., sulfuric acid from battery leaks) and alkalis (e.g., ammonia from cleaning products or lime from construction materials).[55][56] Overuse or improper care of contact lenses, including exposure to contaminated solutions, also commonly triggers this form by causing mechanical irritation and toxic buildup on the ocular surface.[57][58] The pathophysiology involves direct cytotoxicity to the conjunctival epithelium, where irritants disrupt the pH balance, leading to protein denaturation and tissue necrosis. Acids typically cause coagulative necrosis by precipitating proteins, forming a barrier that limits deeper penetration, whereas alkalis induce liquefactive necrosis through saponification of cell membranes and free radical generation, resulting in more severe and penetrating damage.[55] This acute injury provokes an inflammatory response with vasodilation, edema, and release of inflammatory mediators, exacerbating conjunctival hyperemia without the immune-mediated features seen in allergic cases; exposure to irritants like dust, smoke, chlorine, or chemicals can cause localized redness by affecting specific areas of the eye, leading to dilated blood vessels in those regions, and uneven dry eye can contribute to localized irritation.[3][58][59] Symptoms manifest immediately upon exposure, characterized by intense burning pain, conjunctival redness, and a foreign body sensation, often accompanied by tearing and mild mucous discharge. Severity varies by agent; for instance, alkali exposures produce more profound symptoms and tissue destruction than acids due to their deeper penetration.[55][1] In cases involving chlorine gas or smoke, patients may experience photophobia and blurred vision from epithelial sloughing, while contact lens-related irritation can lead to discomfort that worsens with prolonged wear.[60][56] Diagnosis relies primarily on a detailed history of recent exposure to confirm the irritant source, supplemented by clinical examination revealing conjunctival injection and edema. Testing the tear film pH with litmus paper is essential for chemical exposures, aiming to neutralize it to 7.0–7.2 to assess the need for further intervention; slit-lamp biomicroscopy may reveal epithelial defects or corneal involvement.[55][3] Unlike infectious forms, routine cultures are unnecessary unless secondary infection is suspected.[58] Treatment prioritizes immediate removal of the irritant through copious irrigation with normal saline or balanced salt solution, ideally for at least 30 minutes or until pH normalizes, to minimize ongoing damage.[55][1] Symptomatic relief includes cycloplegic agents like cyclopentolate to reduce ciliary spasm and pain, along with topical lubricants; antibiotics are reserved for cases with secondary bacterial infection and are not routinely indicated.[3] For milder irritants like smoke or chlorine, artificial tears and avoidance of the trigger often suffice for resolution within hours to days.[60][61] Complications are more likely with severe chemical burns, particularly from alkalis, and can include conjunctival scarring, symblepharon (adhesions between conjunctiva and eyelid), and ischemic damage leading to limbal stem cell deficiency.[55] Untreated exposures may progress to corneal opacification or perforation, necessitating surgical interventions like amniotic membrane transplantation in refractory cases.[58]Other Forms
Other forms of conjunctivitis encompass specialized variants such as neonatal infections, chronic infectious conditions like trachoma, drug-induced reactions, and autoimmune-associated cases, which differ from the primary viral, bacterial, allergic, or irritant categories due to their unique etiologies and presentations. Neonatal conjunctivitis, also known as ophthalmia neonatorum, arises from perinatal transmission of pathogens during delivery, with chlamydial and gonococcal infections being prominent causes. Chlamydial neonatal conjunctivitis is caused by Chlamydia trachomatis serovars D-K acquired from maternal genital tract infection, while gonococcal cases stem from Neisseria gonorrhoeae.[62][63] In pathophysiology, chlamydial infection involves intracellular replication of the bacterium in conjunctival epithelial cells, leading to an inflammatory response with inclusion bodies, whereas gonococcal infection triggers a hyperacute purulent reaction due to bacterial endotoxin release. Signs and symptoms of gonococcal neonatal conjunctivitis typically manifest within 24-48 hours of birth, featuring severe eyelid edema, copious purulent discharge, and chemosis, potentially progressing to corneal ulceration if untreated. Chlamydial cases present later, around 5-14 days, with milder mucopurulent discharge, conjunctival injection, and pseudomembrane formation. Diagnosis relies on Gram staining and culture for gonorrhea, revealing gram-negative diplococci, and Giemsa staining or nucleic acid amplification tests (NAATs) for chlamydia to detect intracytoplasmic inclusions. Treatment for gonococcal conjunctivitis requires systemic ceftriaxone (25-50 mg/kg IV single dose) or cefotaxime, alongside saline irrigation to remove discharge, while chlamydial infection is managed with oral erythromycin (50 mg/kg/day for 14 days) or azithromycin (20 mg/kg single dose), evaluating the mother and partner for concurrent infections.[64][65][62] Trachoma represents a chronic form of infectious conjunctivitis endemic in certain regions, caused by repeated ocular exposure to Chlamydia trachomatis serovars A-C, often through direct contact or fly vectors in areas with poor sanitation. The pathophysiology involves persistent follicular hyperplasia in the tarsal conjunctiva from repeated infections, progressing through inflammatory stages to cicatricial scarring, entropion, and trichiasis. Early signs include conjunctival follicles and lymphoid aggregates, evolving to chronic dryness, corneal opacity, and scarring; symptoms encompass irritation, tearing, and photophobia, with late-stage vision loss from corneal abrasion by inturned lashes. Diagnosis is primarily clinical using the WHO simplified grading system, supplemented by PCR detection of C. trachomatis DNA in conjunctival swabs, and Giemsa staining for elementary bodies in resource-limited settings. Treatment follows the SAFE strategy: systemic azithromycin (20 mg/kg single oral dose for children, 1 g for adults) for active infection, surgical correction of trichiasis, facial cleanliness, and environmental improvements to reduce transmission. Complications include irreversible blindness from corneal scarring, making trachoma the leading infectious cause of blindness worldwide, affecting an estimated 1.9 million people.[66][67][68] Drug-induced conjunctivitis can emerge as a hypersensitivity reaction to medications, including recent reports of ocular adverse events from immune checkpoint inhibitors (ICIs) used in cancer immunotherapy, such as pembrolizumab and nivolumab. These agents provoke immune-related adverse events through T-cell activation against self-antigens, leading to conjunctival inflammation in approximately 1% of cases, often manifesting as part of dry eye syndrome. Pathophysiology involves immune-mediated epithelial damage and vascular changes, with symptoms including red eyes, irritation, foreign body sensation, and mild discharge, typically appearing weeks to months after initiation. Diagnosis is clinical, supported by exclusion of other causes via slit-lamp examination and discontinuation trials, with the Naranjo algorithm aiding causality assessment. Management entails prompt discontinuation of the offending drug, artificial tears for mild cases, and topical corticosteroids for persistent inflammation, rarely requiring ICI cessation.[69] Autoimmune conjunctivitis, exemplified by keratoconjunctivitis sicca in Sjögren's syndrome, results from systemic autoimmune attack on lacrimal glands, primarily affecting women over 40. The underlying pathophysiology features lymphocytic infiltration and autoimmune epithelitis of exocrine glands, reducing tear production and causing evaporative dry eye with secondary conjunctival inflammation. Signs and symptoms include chronic ocular dryness, grittiness, burning, redness, and filamentary keratitis, often accompanied by xerostomia. Diagnosis involves Schirmer's test for tear quantity (<5 mm/5 min), fluorescein staining for ocular surface damage, and confirmatory salivary gland biopsy showing focal lymphocytic sialadenitis, alongside serologic tests for anti-SSA/Ro and anti-SSB/La antibodies. Treatment focuses on supportive measures like preservative-free artificial tears and punctal occlusion, with anti-inflammatory options such as topical cyclosporine or lifitegrast, and systemic immunosuppressants (e.g., hydroxychloroquine) for severe cases.[70][71]Diagnosis
Clinical Evaluation
The clinical evaluation of suspected conjunctivitis begins with a detailed patient history to identify key features that guide diagnosis and management. Clinicians assess the onset of symptoms, distinguishing acute presentations (typically within days) from chronic ones (lasting weeks to months), as well as whether involvement is unilateral or bilateral, which may suggest infectious versus systemic etiologies.[3] The character of ocular discharge is critical: purulent or mucopurulent discharge often points to bacterial causes, while watery discharge is more common in viral or allergic forms.[6] Symptom differentiation includes evaluating for prominent itching, which strongly suggests allergic conjunctivitis, versus pain or foreign body sensation, which may indicate infectious or irritant processes; photophobia or discomfort in bright light further refines the assessment.[58] Exposures and contacts are probed, including recent upper respiratory infections, close contact with individuals having red eyes, environmental allergens like pollen or animal dander, contact lens use, or chemical irritants, as these can pinpoint transmission routes or triggers.[3] Physical examination follows, starting with measurement of visual acuity to detect any impairment, which is uncommon in uncomplicated conjunctivitis but warrants further investigation if present. External inspection reveals eyelid swelling, crusting, or matting, while slit-lamp biomicroscopy allows detailed evaluation of conjunctival injection—diffuse redness of the bulbar and tarsal conjunctiva—and assessment for corneal involvement such as infiltrates or ulcers.[58] Eversion of the eyelids exposes the superior tarsal conjunctiva to identify follicles (small, avascular elevations typical of viral processes) or papillae (vascularized projections seen in allergic or bacterial cases), aiding in etiological clues.[3] Preauricular or submandibular lymphadenopathy may also be palpated, particularly in viral infections. Type-specific signs, such as preauricular nodes in adenoviral cases, provide additional context but require correlation with history.[6] Red flags during evaluation necessitate urgent ophthalmologic referral to rule out sight-threatening conditions. These include significant vision loss, severe ocular pain disproportionate to findings, hypopyon (pus in the anterior chamber), or profound photophobia, which may signal corneal ulceration, scleritis, or acute angle-closure glaucoma rather than isolated conjunctivitis.[3] In resource-limited settings, basic tools like a penlight can suffice for initial injection assessment, but slit-lamp use is standard for precision.[58] For chronic or recurrent conjunctivitis, emerging evaluation includes analysis of tear biomarkers to differentiate inflammatory subtypes. Tear cytokines, such as interleukin-6 or tumor necrosis factor-alpha, are elevated in inflammatory dry eye-associated conjunctivitis and can help distinguish it from noninflammatory causes.[72] Similarly, oxylipins—lipid-derived inflammatory mediators like hydroxyeicosatetraenoic acids—serve as diagnostic biomarkers in ocular cicatrizing conjunctivitis, a chronic scarring form, with specific profiles aiding in targeted management.[73] These non-invasive tear sampling techniques enhance clinical differentiation when standard history and exam are inconclusive.[74]Laboratory and Imaging Tests
Laboratory tests for conjunctivitis are typically reserved for cases where clinical evaluation is inconclusive, such as severe, recurrent, or atypical presentations, to identify infectious agents or underlying immune responses. Bacterial conjunctivitis diagnosis may involve conjunctival swabs for Gram staining and culture to isolate pathogens like Staphylococcus aureus or Haemophilus influenzae, particularly in neonates or immunocompromised patients where empirical treatment fails.[3] Viral etiologies, especially adenovirus, are confirmed via polymerase chain reaction (PCR) on conjunctival swabs, which offers high sensitivity and specificity compared to traditional viral culture; for instance, real-time PCR detects adenoviral DNA in over 90% of epidemic keratoconjunctivitis cases.[17][75] Point-of-care immunochromatographic assays, such as the AdenoPlus or QuickVue test, provide rapid detection of adenovirus in ocular swabs within 10 minutes; these CLIA-waived tests have a sensitivity of 85-96% and specificity of 96-100% relative to PCR or culture, enabling quick identification of contagious cases in outpatient or emergency settings.[76] Allergy testing is indicated when allergic conjunctivitis is suspected based on history of atopy or bilateral symptoms. Skin prick testing (SPT) identifies IgE-mediated sensitization to common aeroallergens like pollen or dust mites by wheal-and-flare response within 15-20 minutes, correlating well with clinical symptoms in seasonal allergic conjunctivitis.[58] Alternatively, serum-specific IgE assays quantify allergen-specific antibodies, providing a non-invasive option for patients on antihistamines that could interfere with SPT, though tear IgE measurement is emerging for direct ocular assessment.[77] Imaging modalities are infrequently required but aid in evaluating corneal complications. Fluorescein staining, applied topically and viewed under cobalt blue light, reveals epithelial defects or ulcers associated with corneal involvement in infectious or chemical conjunctivitis, guiding management to prevent progression.[58] Optical coherence tomography (OCT) of the anterior segment visualizes subepithelial infiltrates in adenoviral cases, measuring corneal thickness and monitoring resolution non-invasively, which is particularly useful in persistent photophobia.[78] Conjunctival biopsy is performed in chronic or suspected autoimmune conjunctivitis to confirm diagnoses like ocular cicatricial pemphigoid. Histopathology and direct immunofluorescence on biopsy specimens detect linear IgG or C3 deposits at the basement membrane, essential for distinguishing cicatrizing conditions from infections. These tests' interpretations align with specific etiologies, such as PCR positivity supporting viral pathways detailed elsewhere.[3]Differential Diagnosis
Conjunctivitis, characterized by diffuse conjunctival injection and often accompanied by discharge, must be differentiated from other causes of red eye to avoid missing vision-threatening conditions. Key mimics include keratitis, which involves corneal inflammation and typically presents with severe pain, photophobia, decreased visual acuity, and fluorescein staining revealing epithelial defects or infiltrates, unlike the usually preserved vision and lack of corneal involvement in conjunctivitis.[79][80] Uveitis, an intraocular inflammation, is distinguished by ciliary flush (perilimbal injection), blurred vision, photophobia, and anterior chamber cells or flare on slit-lamp examination, contrasting with the diffuse redness and absence of pupillary changes or intraocular findings in uncomplicated conjunctivitis.[79][80] Acute angle-closure glaucoma manifests as a painful red eye with marked vision reduction, halos around lights, mid-dilated fixed pupils, and a firm globe due to elevated intraocular pressure, features not seen in conjunctivitis where pain is mild or absent and intraocular pressure remains normal.[79][80] Dry eye syndrome often mimics mild conjunctivitis with bilateral redness, foreign body sensation, and reflex tearing but lacks significant discharge and shows punctate epithelial erosions on staining rather than overt conjunctival inflammation.[79] Episcleritis presents with sectoral redness, mild tenderness, and dilated episcleral vessels that blanch with topical phenylephrine, differing from the diffuse injection and potential discharge of conjunctivitis, with normal vision preserved in both but episcleritis lacking systemic associations common in some conjunctivitis forms.[80][9] In neonates, conjunctivitis differentials include dacryocystitis, a congenital nasolacrimal duct obstruction causing unilateral epiphora, lid swelling, and purulent reflux from the punctum upon medial canthal pressure, unlike the more diffuse bilateral involvement and lack of a palpable mass in infectious neonatal conjunctivitis.[62] Gonococcal conjunctivitis, a severe form, is distinguished by its hyperacute onset with profuse purulent discharge, lid edema, and risk of corneal perforation, often bilateral and requiring urgent Gram staining to identify gram-negative diplococci, contrasting with milder bacterial causes.[62][81] Drug-related conjunctivitis, such as from topical medications or preservatives, typically arises shortly after exposure with watery discharge and unilaterality if unilateral instillation occurred, while infectious forms like viral (watery, preauricular lymphadenopathy) or bacterial (purulent) develop more gradually and may spread bilaterally, aiding distinction based on timing and exposure history.[9][79]| Condition | Key Distinguishing Features | Citation |
|---|---|---|
| Keratitis | Severe pain, photophobia, corneal defects on fluorescein staining, decreased vision | [80] |
| Uveitis | Ciliary flush, anterior chamber inflammation, blurred vision | [80] |
| Glaucoma | Halos, firm globe, elevated IOP, severe pain | [80] |
| Dry Eye Syndrome | Bilateral itching, no discharge, punctate erosions | [79] |
| Episcleritis | Sectoral redness, blanches with phenylephrine, mild discomfort | [80] |
| Neonatal Dacryocystitis | Unilateral mass, reflux on pressure, minimal redness | [62] |
| Gonococcal Conjunctivitis | Hyperacute purulent discharge, lid edema, gram-negative diplococci | [62] |