Recent from talks
Nothing was collected or created yet.
Pentene
View on Wikipedia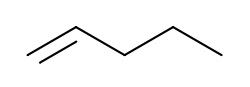 1-Pentene
| |
 cis-2-Pentene
| |
 trans-2-Pentene
| |
| Names | |
|---|---|
| IUPAC names
Pent-1-ene
cis-Pent-2-ene trans-Pent-2-ene | |
| Other names
amylene, n-amylene, n-pentene, beta-n-amylene, sym-methylethylethylene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.042.636 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10 | |
| Molar mass | 70.135 g·mol−1 |
| Density | 0.64 g/cm3 (1-pentene)[1] |
| Melting point | −165.2 °C (−265.4 °F; 108.0 K) (1-pentene)[1] |
| Boiling point | 30 °C (86 °F; 303 K) (1-pentene)[1] |
| −53.7·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pentenes are alkenes with the chemical formula C
5H
10. Each molecule contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure and whether the double bond has a cis or trans form.
Straight-chain isomers
[edit]1-Pentene is an alpha-olefin. Most often, 1-pentene is made as a byproduct of catalytic or thermal cracking of petroleum or during the production of ethylene and propylene via thermal cracking of hydrocarbon fractions.
As of 2010s, the only commercial manufacturer of 1-pentene was Sasol Ltd., where it is separated from crude by the Fischer-Tropsch process.[2]
2-Pentene has two geometric isomers: cis-2-pentene and trans-2-pentene. Cis-2-Pentene is used in olefin metathesis.
Branched-chain isomers
[edit]The branched isomers are 2-methylbut-1-ene, 3-methylbut-1-ene (isopentene), and 2-methylbut-2-ene (isoamylene).
Isoamylene is one of the three main byproducts of deep catalytic cracking (DCC), which is very similar to the operation of fluid catalytic cracking (FCC). The DCC uses vacuum gas oil (VGO) as a feedstock to produce primarily propylene, isobutylene, and isoamylene. The rise in demand for polypropylene has encouraged the growth of the DCC, which is operated very much like the FCC. Isobutylene and isoamylene feedstocks are necessary for the production of the much debated gasoline blending components methyl tert-butyl ether and tert-amyl methyl ether.
Production of fuels
[edit]Propylene, isobutene, and amylenes are feedstocks in the alkylation units of refineries. Using isobutane, blendstocks are generated with high branching for good combustion characteristics. Amylenes are valued as precursors to fuels, especially aviation fuels of relatively low volatility, as required by various regulations.[3]
References
[edit]- ^ a b c Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "RSA Olefins | cChange". www.cchange.ac.za. Retrieved 2017-10-19.
- ^ Bipin V. Vora; Joseph A. Kocal; Paul T. Barger; Robert J. Schmidt; James A. Johnson (2003). "Alkylation". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0112112508011313.a01.pub2. ISBN 0-471-23896-1.
Pentene
View on GrokipediaOverview
Definition and nomenclature
Pentenes are unsaturated hydrocarbons belonging to the class of alkenes, characterized by the presence of a single carbon-carbon double bond and having the molecular formula C₅H₁₀. These compounds represent the five-carbon analogs of ethene and propene, exhibiting reactivity typical of alkenes due to the sp²-hybridized carbon atoms in the double bond.[8] The term "pentene" derives from "pentane," the saturated hydrocarbon with formula C₅H₁₂, with the suffix "-ene" denoting unsaturation at the double bond.[9] According to IUPAC rules for alkene nomenclature, the parent chain is the longest continuous carbon chain that includes the double bond, numbered from the end that assigns the lowest possible locant to the double bond carbons. Substituents, if present, receive the lowest possible numbers, and the chain is named by replacing the "-ane" ending of the corresponding alkane with "-ene." For pentenes, this results in names like pent-1-ene for the terminal alkene where the double bond is between carbons 1 and 2.[8] In cases of geometric isomerism, such as in 2-pentene (CH₃CH=CHCH₂CH₃), the configurations are designated as cis-2-pentene or (2Z)-pent-2-ene when the methyl and ethyl groups are on the same side of the double bond, and trans-2-pentene or (2E)-pent-2-ene when they are on opposite sides, following the Cahn-Ingold-Prelog priority rules for stereodescriptors.[10]Molecular formula and general structure
Pentene has the molecular formula , which represents the empirical and molecular formula for this class of alkenes.[11] This formula aligns with the general pattern for monoalkenes, where .[12] The degree of unsaturation for is calculated by comparing it to the saturated alkane pentane (), revealing two fewer hydrogen atoms and thus one degree of unsaturation.[12] This unsaturation is accounted for by a single carbon-carbon double bond in the structure. The general structure of pentenes consists of five carbon atoms connected by single bonds except for one carbon-carbon double bond, forming either linear or branched chains, with the remaining valences filled by hydrogen atoms.[11] The two carbon atoms in the double bond exhibit hybridization, forming three sigma bonds each in a trigonal planar arrangement.[13] In terms of bonding geometry, the double bond has a length of approximately 1.34 Å, which is shorter than the typical single bond of 1.54 Å due to the additional pi bond.[14] The bond angles around the -hybridized carbons are approximately 120°, reflecting the planar trigonal geometry.[14] Skeletal formulas and line diagrams are commonly used to represent the general structure of pentene, depicting the carbon chain as a zigzag line with the double bond shown as two parallel lines between the relevant carbons, while hydrogen atoms are omitted for simplicity.[11] This notation emphasizes the connectivity and the position of the double bond without specifying exact isomer configurations.[15]Physical properties
Boiling and melting points
Pentene isomers exhibit boiling points typically ranging from approximately 20°C to 38°C, reflecting their relatively low molecular weights and nonpolar nature, while melting points are generally very low, often below -130°C, due to weak intermolecular forces.[11][16][17] These phase transition temperatures vary among isomers primarily due to structural differences, such as the position of the double bond, geometric isomerism, and branching. The boiling point of 1-pentene is 29.9°C, lower than that of internal alkenes like cis-2-pentene at 36.9°C and trans-2-pentene at 36.3°C, as the terminal double bond results in a more linear shape with slightly stronger van der Waals interactions.[11][16][18] Branching tends to lower boiling points compared to linear analogs of similar mass because the more spherical shape reduces surface area and thus weakens London dispersion forces; for example, 3-methylbut-1-ene boils at 20.1°C compared to 1-pentene at 29.9°C.[4][11][19] For geometric isomers, cis-2-pentene has a higher boiling point than trans-2-pentene due to its bent structure, which creates a net dipole moment and enables stronger dipole-dipole interactions, whereas the more symmetric trans isomer relies solely on dispersion forces.[20][16][18] Melting points follow a similar trend influenced by packing efficiency; for example, 1-pentene melts at -165.2°C, cis-2-pentene at -151.4°C, and trans-2-pentene at -140.2°C, with trans isomers often showing higher values due to better crystal lattice formation.[11][16][18] The following table compares these properties for major pentene isomers:| Isomer | Boiling Point (°C) | Melting Point (°C) |
|---|---|---|
| 3-Methylbut-1-ene | 20.1 | -168.5 |
| 1-Pentene | 29.9 | -165.2 |
| 2-Methylbut-1-ene | 31.2 | -137.5 |
| cis-2-Pentene | 36.9 | -151.4 |
| trans-2-Pentene | 36.3 | -140.2 |
| 2-Methylbut-2-ene | 38.0 | -133.6 |
Solubility and density
Pentenes exhibit low solubility in water due to their hydrophobic, nonpolar character, primarily resulting from the predominance of C-H and C=C bonds, which limit interactions with polar water molecules.[22] For instance, 1-pentene has a water solubility of 148 mg/L (0.0148 g/100 mL) at 25 °C, rendering it effectively immiscible.[23] Similarly, cis-2-pentene and trans-2-pentene each display a solubility of 203 mg/L at 25 °C, while 2-methyl-1-butene is soluble to 130 mg/L at 20 °C.[24][25][26] In contrast, pentenes are highly soluble in nonpolar organic solvents, such as ethanol, ethyl ether, benzene, and hexane, often miscible in all proportions, which facilitates their use in organic synthesis and extraction processes.[23][24][26] The densities of pentenes are characteristically low, reflecting their hydrocarbon composition and reliance on weak van der Waals forces for intermolecular interactions, which contribute to their overall lightness compared to water.[27] Typical values range from 0.62 to 0.66 g/cm³ at 20 °C across isomers, with specific examples including 1-pentene at 0.6405 g/cm³, cis-2-pentene at 0.6556 g/cm³, trans-2-pentene at 0.6482 g/cm³, 3-methyl-1-butene at 0.627 g/cm³, 2-methyl-1-butene at 0.6504 g/cm³, and 2-methyl-2-butene at 0.66 g/cm³.[23][24][25][26][28]| Isomer | Density (g/cm³ at 20 °C) | Water Solubility (mg/L at 25 °C unless noted) |
|---|---|---|
| 1-Pentene | 0.6405 | 148 |
| cis-2-Pentene | 0.6556 | 203 |
| trans-2-Pentene | 0.6482 | 203 |
| 3-Methyl-1-butene | 0.627 | 130 |
| 2-Methyl-1-butene | 0.6504 | 130 (at 20 °C) |
| 2-Methyl-2-butene | 0.66 | 190 |

