Recent from talks
Nothing was collected or created yet.
Cluster headache
View on Wikipedia
| Cluster headache | |
|---|---|
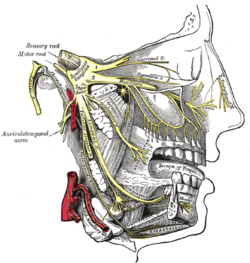 | |
| Trigeminal nerve | |
| Specialty | Neurology |
| Symptoms | Recurrent, severe headaches on one side of the head, eye watering, stuffy nose[1] |
| Usual onset | 20 to 40 years old[2] |
| Duration | 15 minutes to 3 hours[2] |
| Types | Episodic, chronic[2] |
| Causes | Unknown[2] |
| Risk factors | Tobacco smoke, family history[2] |
| Diagnostic method | Based on symptoms[2] |
| Differential diagnosis | Migraine, trigeminal neuralgia,[2] other trigeminal autonomic cephalgias[3] |
| Prevention | Verapamil, galcanezumab, oral glucocorticoids, steroid injections, civamide[4] |
| Treatment | Oxygen therapy, triptans[2][4] |
| Frequency | ~0.1% at some point in time[5] |
Cluster headache is a neurological disorder characterized by episodes of severe headaches on one side of the head, typically around the eye and temple, lasting between 15 minutes to three hours.[1] Episodes are often accompanied by eye watering, nasal congestion, drooping eyelids, or swelling around the eye on the affected side.[1] Cluster headaches are unique in their periodicity and regularity: the headaches occur at around the same hour every day during a cluster period, which typically lasts 8–10 weeks a year.[6] Between cluster periods are pain-free intervals without headaches, which last a little less than one year,[6] but some patients can have chronic cluster headaches without remission periods.[2] The disease is considered among the most painful conditions known to medical science.[7][8]
Triggers of cluster headaches may include alcohol, nitroglycerin, and histamine; a history of exposure to tobacco smoke (whether personal or secondhand smoke) is a significant risk factor.[2] The underlying cause is unknown, but may include a genetic component, as a family history of migraines increases risk. Structurally, the disease is likely related to dysfunction of the posterior hypothalamus.[6]
The diagnosis is based on the unique pattern of headaches and associated symptoms. There are no specific laboratory tests, physical exam maneuvers, or neuroimaging findings associated with the disease. However, neuroimaging may be required in the case of patients with red flag symptoms, such as a sudden change in the characteristics of the headache.[2]
Recommended management includes lifestyle adaptations, including smoking cessation and avoiding potential triggers.[2] Medical treatments for acute attacks include oxygen or a fast-acting triptan.[2][4] Preventative medications recommended to decrease the frequency of attacks include steroid injections, galcanezumab, civamide, verapamil, or oral glucocorticoids such as prednisone.[6][4][9] Nerve stimulation or surgery may occasionally be used if other measures are not effective.[2][6]
The condition affects about 0.1% of the general population at some point in their life and 0.05% in any given year.[5] The condition usually first occurs between 20 and 40 years of age.[2] Men are affected about four times more often than women.[5] These debilitating headaches significantly impact daily activities, and due to the severity of the pain, they have also been referred to as "suicide headaches".[2]
Signs and symptoms
[edit]Cluster headaches are recurring bouts of severe unilateral headache attacks.[10][11] The duration of a typical cluster headache ranges from about 15 to 180 minutes.[2] About 75% of untreated attacks last less than 60 minutes.[12] However, women may have longer and more severe cluster headaches.[13]
The onset of an attack is rapid and typically without an aura. Preliminary sensations of pain in the general area of attack, referred to as "shadows", may signal an imminent cluster headache, or these symptoms may linger after an attack has passed, or between attacks.[14] Though cluster headaches are strictly unilateral, there are some documented cases of "side-shift" between cluster periods,[15] or, rarely, simultaneous (within the same cluster period) bilateral cluster headaches.[16]
Pain
[edit]The pain occurs only on one side of the head, around the eye, particularly behind or above the eye, in the temple. The pain is typically greater than in other headache conditions, including migraines, and is usually described as burning, stabbing, drilling or squeezing.[17] While suicide is rare, those with cluster headaches may experience suicidal thoughts (giving the alternative name "suicide headache" or "suicidal headache").[18][19]
Dr. Peter Goadsby, Professor of Clinical Neurology at University College London, and Chair and Patron of OUCH(UK)[20], a leading researcher on the condition has commented:
"Cluster headache is probably the worst pain that humans experience. I know that's quite a strong remark to make, but if you ask a cluster headache patient if they've had a worse experience, they'll universally say they haven't. Women with cluster headache will tell you that an attack is worse than giving birth. So you can imagine that these people give birth without anesthetic once or twice a day, for six, eight, or ten weeks at a time, and then have a break. It's just awful."[21]
Other symptoms
[edit]The typical symptoms of cluster headache include grouped occurrence and recurrence (cluster) of headache attack, severe unilateral orbital, supraorbital and/or temporal pain. If left untreated, attack frequency may range from one attack every two days to eight attacks per day.[2][22] Cluster headache attack is accompanied by at least one of the following autonomic symptoms: drooping eyelid, pupil constriction, redness of the conjunctiva, tearing, runny nose and less commonly, facial blushing, swelling, or sweating, typically appearing on the same side of the head as the pain.[22] Similar to a migraine, sensitivity to light (photophobia) or noise (hyperacusis) may occur during a cluster headache. Nausea is a rare symptom although it has been reported.[10]
Restlessness (for example, pacing or rocking back and forth) may occur. Secondary effects may include the inability to organize thoughts and plans, physical exhaustion, confusion, agitation, aggressiveness, depression, and anxiety.[18]
People with cluster headaches may dread facing another headache and adjust their physical or social activities around a possible future occurrence. Likewise they may seek assistance to accomplish what would otherwise be normal tasks. They may hesitate to make plans because of the regularity, or conversely, the unpredictability of the pain schedule. These factors can lead to generalized anxiety disorders, panic disorder,[18] serious depressive disorders,[23] social withdrawal and isolation.[24]
Cluster headaches have been recently associated with obstructive sleep apnea comorbidity.[25]
Recurrence
[edit]Cluster headaches may occasionally be referred to as "alarm clock headache" because of the regularity of their recurrence. Cluster headaches often awaken individuals from sleep. Both individual attacks and the cluster grouping can have a metronomic regularity; attacks typically strike at a precise time of day each morning or night. The recurrence of headache cluster grouping may occur more often around solstices, or seasonal changes, sometimes showing circannual periodicity. Conversely, attack frequency may be highly unpredictable, showing no periodicity at all. These observations have prompted researchers to speculate an involvement or dysfunction of the hypothalamus. The hypothalamus controls the body's "biological clock" and circadian rhythm.[26][27] In episodic cluster headache, attacks occur once or more daily, often at the same time each day for a period of several weeks, followed by a headache-free period lasting weeks, months, or years. Approximately 10–15% of cluster headaches are chronic, with multiple headaches occurring every day for years, sometimes without any remission.[28]
In accordance with the International Headache Society (IHS) diagnostic criteria, cluster headaches occurring in two or more cluster periods, lasting from 7 to 365 days with a pain-free remission of one month or longer between the headache attacks may be classified as episodic. If headache attacks occur for more than a year without pain-free remission of at least three months, the condition is classified as chronic.[22] Chronic cluster headaches both occur and recur without any remission periods between cycles; there may be variation in cycles, meaning the frequency and severity of attacks may change without predictability for a period of time. The frequency, severity, and duration of headache attacks experienced by people during these cycles varies between individuals and does not demonstrate complete remission of the episodic form. The condition may change unpredictably from chronic to episodic and from episodic to chronic.[29]
Causes
[edit] |
 |
 |
| Positron emission tomography (PET) shows brain areas being activated during pain. | ||
 |
 |
 |
| Voxel-based morphometry shows brain area structural differences. | ||
The specific causes and pathogenesis of cluster headaches are not fully understood.[6] The Third Edition of the International Classification of Headache disorders classifies cluster headaches as belonging to the trigeminal autonomic cephalalgias.[30]
Some experts consider the posterior hypothalamus to be important in the pathogenesis of cluster headaches. This is supported by a relatively high success ratio of deep-brain stimulation therapy on the posterior hypothalamic grey matter.[6]
Nerves
[edit]Therapies acting on the vagus nerve (cranial nerve X) and the greater occipital nerve have both shown efficacy in managing cluster headache, but the specific roles of these nerves are not well-understood.[6] Two nerves thought to play an important role in cluster headaches include the trigeminal nerve and the facial nerve.[31]
Genetics
[edit]Cluster headache may run in some families in an autosomal dominant inheritance pattern.[32][33] People with a first degree relative with the condition are about 14–48 times more likely to develop it themselves,[1] and around 8 to 10% of persons with cluster headaches have a family history.[32][34] Several studies have found a higher number of relatives affected among females.[34] Others have suggested these observations may be due to lower numbers of females in these studies.[34] Possible genetic factors warrant further research, current evidence for genetic inheritance is limited.[33]
Genes that are thought to play a role in the disease are the hypocretin/orexin receptor type 2 (HCRTR2), alcohol dehydrogenase 4 (ADH4), β3 subunit of G proteins (GNB3), pituitary adenylate cyclase-activating polypeptide type I receptor (ADCYAP1R1), and membrane metallo-endopeptidase (MME) genes.[32]
Tobacco smoking
[edit]About 65% of persons with cluster headache are, or have been, tobacco smokers.[1] Stopping smoking does not lead to improvement of the condition, and cluster headaches also occur in those who have never smoked (e.g., children);[1] it is thought unlikely that smoking is a cause.[1] People with cluster headaches may be predisposed to certain traits, including smoking or other lifestyle habits.[35]
Hypothalamus
[edit]A review suggests that the suprachiasmatic nucleus of the hypothalamus, which is the major biological clock in the human body, may be involved in cluster headaches, because cluster headaches occur with diurnal and seasonal rhythmicity.[36]
Positron emission tomography (PET) scans indicate the brain areas which are activated during attack only, compared to pain free periods. These pictures show brain areas that are active during pain in yellow/orange color (called "pain matrix"). The area in the center (in all three views) is activated only during cluster headaches. The bottom row voxel-based morphometry shows structural brain differences between individuals with and without CH; only a portion of the hypothalamus is different.[37]
Diagnosis
[edit]Cluster-like head pain may be diagnosed as secondary headache rather than cluster headache.[22]
A detailed oral history aids practitioners in correct differential diagnosis, as there are no confirmatory tests for cluster headache. A headache diary can be useful in tracking when and where pain occurs, how severe it is, and how long the pain lasts. A record of coping strategies used may help distinguish between headache type; data on frequency, severity and duration of headache attacks are a necessary tool for initial and correct differential diagnosis in headache conditions.[38]
Correct diagnosis presents a challenge as the first cluster headache attack may present where staff are not trained in the diagnosis of rare or complex chronic disease.[12] Experienced ER staff are sometimes trained to detect headache types.[39] While cluster headache attacks themselves are not directly life-threatening, suicide ideation has been observed.[18]
Individuals with cluster headaches typically experience diagnostic delay before correct diagnosis.[40] People are often misdiagnosed due to reported neck, tooth, jaw, and sinus symptoms and may unnecessarily endure many years of referral to ear, nose and throat (ENT) specialists for investigation of sinuses; dentists for tooth assessment; chiropractors and manipulative therapists for treatment; or psychiatrists, psychologists, and other medical disciplines before their headaches are correctly diagnosed.[41] Under-recognition of cluster headaches by health care professionals is reflected in consistent findings in Europe and the United States that the average time to diagnosis is around seven years.[42]
Differential
[edit]Cluster headache may be misdiagnosed as migraine or sinusitis.[42] Other types of headache are sometimes mistaken for, or may mimic closely, cluster headaches. Incorrect terms like "cluster migraine" confuse headache types, confound differential diagnosis and are often the cause of unnecessary diagnostic delay,[43] ultimately delaying appropriate specialist treatment.
Other types of headaches that may be confused with cluster headache include:
- Chronic paroxysmal hemicrania is a unilateral headache condition, without the male predominance usually seen in cluster headaches. Paroxysmal hemicrania may also be episodic but the episodes of pain seen in chronic paroxysmal hemicrania are usually shorter than those seen with cluster headaches. Chronic paroxysmal hemicrania typically responds "absolutely" to treatment with the anti-inflammatory drug indomethacin[22] where in most cases cluster headaches typically show no indomethacin response, making "indomethacin response" an important diagnostic tool for specialist practitioners seeking correct differential diagnosis between the conditions.[44][45]
- Hemicrania continua[46]
- Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) is a headache syndrome belonging to the group of TACs.[22][47]
- Trigeminal neuralgia is a unilateral headache syndrome,[41] or "cluster-like" headache.[48]
Prevention
[edit]Management for cluster headache is divided into three primary categories: abortive, transitional, and preventive.[49] Preventive treatments are used to reduce or eliminate cluster headache attacks; they are generally used in combination with abortive and transitional techniques.[10]
Verapamil
[edit]The recommended first-line preventive therapy is verapamil, a calcium channel blocker.[2][50] Verapamil was previously underused in people with cluster headache.[10] Improvement can be seen in an average of 1.7 weeks for episodic cluster headache and 5 weeks for chronic cluster headache when using a dosage of ranged between 160 and 720 mg (mean 240 mg/day).[51] Preventive therapy with verapamil is believed to work because it has an effect on the circadian rhythm and on CGRPs as CGRP-release is controlled by voltage-gated calcium channels.[51]
Glucocorticoids
[edit]Since these compounds are steroids, there is little evidence to support long-term benefits from glucocorticoids,[2] but they may be used until other medications take effect as they appear to be effective at three days.[2] They are generally discontinued after 8–10 days of treatment.[10] Prednisone is given at a starting dose of 60–80 milligrams daily; then it is reduced by 5 milligrams every day. Corticosteroids are also used to break cycles, especially in chronic patients.[52]
Surgery
[edit]Nerve stimulators may be an option in the small number of people who do not improve with medications.[53][54] Two procedures, deep brain stimulation or occipital nerve stimulation, may be useful;[2] early experience shows a benefit in about 60% of cases.[55] It typically takes weeks or months for this benefit to appear.[54] A non-invasive method using transcutaneous electrical nerve stimulation (TENS) is being studied.[54]
A number of surgical procedures, such as a rhizotomy or microvascular decompression, may also be considered,[54] but evidence to support them is limited and there are cases of people whose symptoms worsen after these procedures.[54]
Other
[edit]Lithium, methysergide, and topiramate are recommended alternative treatments,[50][56] although there is little evidence supporting the use of topiramate or methysergide.[2][57] This is also true for tianeptine, melatonin, and ergotamine.[2] Valproate, sumatriptan, and oxygen are not recommended as preventive measures.[2] Botulinum toxin injections have shown limited success.[58] Evidence for baclofen, botulinum toxin, and capsaicin is unclear.[57]
Management
[edit]There are two primary treatments for acute CH: oxygen and triptans,[2] but they are underused due to misdiagnosis of the syndrome.[10] During bouts of headaches, triggers such as alcohol, nitroglycerine, and naps during the day should be avoided.[12]
Oxygen
[edit]Oxygen therapy may help to abort attacks, though it does not prevent future episodes.[2] Typically it is given via a non-rebreather mask at 12–15 liters per minute for 15–20 minutes.[2] One review found about 70% of patients improve within 15 minutes.[12] The evidence for effectiveness of 100% oxygen, however, is weak.[12][59] Hyperbaric oxygen at pressures of ~2 times greater than atmospheric pressure may relieve cluster headaches.[59]
Triptans
[edit]The other primarily recommended treatment of acute attacks is subcutaneous or intranasal sumatriptan.[50][60] Sumatriptan and zolmitriptan have both been shown to improve symptoms during an attack with sumatriptan being superior.[61] Because of the vasoconstrictive side-effect of triptans, they may be contraindicated in people with ischemic heart disease.[2] The vasoconstrictor ergot compounds may be useful,[12] but have not been well studied in acute attacks.[61]
Opioids
[edit]The use of opioid medication in management of cluster headache is not recommended[62] and may make headache syndromes worse.[63][64] Long-term opioid use is associated with well known dependency, addiction, and withdrawal syndromes.[65] Prescription of opioid medication may additionally lead to further delay in differential diagnosis, undertreatment, and mismanagement.[62]
Other
[edit]Intranasal lidocaine (sprayed in the ipsilateral nostril) may be an effective treatment with patient resistant to more conventional treatment.[13]
Octreotide administered subcutaneously has been demonstrated to be more effective than placebo for the treatment of acute attacks.[66]
Sub-occipital steroid injections have shown benefit and are recommended for use as a transitional therapy to provide temporary headache relief as more long term prophylactic therapies are instituted.[67]
Epidemiology
[edit]Cluster headache affects about 0.1% of the general population at some point in their life.[5] Males are affected about four times more often than females.[5] The condition usually starts between the ages of 20 and 50 years, although it can occur at any age.[1] About one in five affected adults report the onset of cluster headache between 10 and 19 years of age.[68]
History
[edit]The first complete description of cluster headache was given by the London neurologist Wilfred Harris in 1926, who named the disease migrainous neuralgia.[69][70][71] Descriptions of cluster headache date to 1745 and probably earlier.[72]
The condition was originally named Horton's cephalalgia after Bayard Taylor Horton, a US neurologist who postulated the first theory as to their pathogenesis. His original paper describes the severity of the headaches as being able to take normal men and force them to attempt or die by suicide; his 1939 paper said:
"Our patients were disabled by the disorder and suffered from bouts of pain from two to twenty times a week. They had found no relief from the usual methods of treatment. Their pain was so severe that several of them had to be constantly watched for fear of suicide. Most of them were willing to submit to any operation which might bring relief."[73]
CH has alternately been called erythroprosopalgia of Bing, ciliary neuralgia, erythromelalgia of the head, Horton's headache, histaminic cephalalgia, petrosal neuralgia, sphenopalatine neuralgia, vidian neuralgia, Sluder's neuralgia, Sluder's syndrome, and hemicrania angioparalyticia.[74]
Society and culture
[edit]Robert Shapiro, a professor of neurology, says that while cluster headaches are about as common as multiple sclerosis with a similar disability level, as of 2013, the US National Institutes of Health had spent $1.872 billion on research into multiple sclerosis in one decade, but less than $2 million on cluster headache research in 25 years.[75]
Research directions
[edit]Some case reports suggest that ingesting lysergamides such as LSD, tryptamines such as psilocybin (as found in hallucinogenic mushrooms), or DMT can abort attacks and interrupt cluster headache cycles.[76][77] The hallucinogen DMT has a chemical structure that is similar to the triptan sumatriptan, indicating a possible shared mechanism in preventing or stopping migraine and TACs.[52] In a 2006 survey of 53 individuals, 18 of 19 psilocybin users reported extended remission periods. The survey was not a blinded or a controlled study, and was "limited by recall and selection bias".[76] The safety and efficacy of psilocybin is currently being studied in cluster headache, with the extension phase of one randomized controlled trial demonstrating reduced cluster attack burden after a 3-dose pulse of psilocybin.[78][79][80] In Canada, a first cluster headache patient was granted approval to receive treatment with psilocybin under the country's Special Access Program.[81]
Fremanezumab, a humanized monoclonal antibody directed against calcitonin gene-related peptides alpha and beta, was in phase 3 clinical trials for cluster headaches, but the studies were stopped early due to a futility analysis demonstrating that a successful outcome was unlikely.[82][83]
References
[edit]- ^ a b c d e f g h Nesbitt, A. D.; Goadsby, P. J. (2012). "Cluster headache". BMJ. 344 e2407. doi:10.1136/bmj.e2407. PMID 22496300. S2CID 5479248.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Weaver-Agostoni, J (2013). "Cluster headache". American Family Physician. 88 (2): 122–8. PMID 23939643. Archived from the original on 30 December 2019. Retrieved 24 July 2017.
- ^ Rizzoli, P; Mullally, WJ (20 September 2017). "Headache". The American Journal of Medicine. 131 (1): 17–24. doi:10.1016/j.amjmed.2017.09.005. PMID 28939471.
- ^ a b c d Robbins, Matthew S.; Starling, Amaal J.; Pringsheim, Tamara M.; Becker, Werner J.; Schwedt, Todd J. (2016). "Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines". Headache. 56 (7): 1093–106. doi:10.1111/head.12866. PMID 27432623.
- ^ a b c d e Fischera, M; Marziniak, M; Gralow, I; Evers, S (2008). "The Incidence and Prevalence of Cluster Headache: A Meta-Analysis of Population-Based Studies". Cephalalgia. 28 (6): 614–8. doi:10.1111/j.1468-2982.2008.01592.x. PMID 18422717. S2CID 2471915.
- ^ a b c d e f g h Goadsby, Peter J. (2022). "Chapter 430". Harrison's Principles of Internal Medicine (21st ed.). McGraw Hill. ISBN 978-1-264-26850-4.
- ^ Matharu M, Goadsby P (2001). "Cluster Headache". Practical Neurology. 1: 42. doi:10.1046/j.1474-7766.2001.00505.x. S2CID 19601387.
- ^ Matharu, Manjit S; Goadsby, Peter J (2014). "Cluster headache: Focus on emerging therapies". Expert Review of Neurotherapeutics. 4 (5): 895–907. doi:10.1586/14737175.4.5.895. PMID 15853515. S2CID 43918900.
- ^ Gaul, C; Diener, H; Müller, OM (2011). "Cluster Headache Clinical Features and Therapeutic Options". Deutsches Ärzteblatt International. 108 (33): 543–549. doi:10.3238/arztebl.2011.0543. PMC 3167933. PMID 21912573.
- ^ a b c d e f Beck E, Sieber WJ, Trejo R (February 2005). "Management of cluster headache". American Family Physician (Review). 71 (4): 717–24. PMID 15742909. Archived from the original on 13 November 2015.
- ^ Capobianco, David; Dodick, David (2006). "Diagnosis and Treatment of Cluster Headache". Seminars in Neurology. 26 (2): 242–59. doi:10.1055/s-2006-939925. PMID 16628535. S2CID 260319925.
- ^ a b c d e f Friedman, Benjamin Wolkin; Grosberg, Brian Mitchell (2009). "Diagnosis and Management of the Primary Headache Disorders in the Emergency Department Setting". Emergency Medicine Clinics of North America. 27 (1): 71–87, viii. doi:10.1016/j.emc.2008.09.005. PMC 2676687. PMID 19218020.
- ^ a b Vollesen AL, Benemei S, Cortese F, Labastida-Ramírez A, Marchese F, Pellesi L, Romoli M, Ashina M, Lampl C, School of Advanced Studies of the European Headache Federation (EHF-SAS) (2018). "Migraine and cluster headache - the common link". The Journal of Headache and Pain. 19 (1): 89. doi:10.1186/s10194-018-0909-4. PMC 6755613. PMID 30242519.
- ^ Marmura, Michael J; Pello, Scott J; Young, William B (2010). "Interictal pain in cluster headache". Cephalalgia. 30 (12): 1531–4. doi:10.1177/0333102410372423. PMID 20974600. S2CID 153838.
- ^ Meyer, Eva Laudon; Laurell, Katarina; Artto, Ville; Bendtsen, Lars; Linde, Mattias; Kallela, Mikko; Tronvik, Erling; Zwart, John-Anker; Jensen, Rikke M.; Hagen, Knut (2009). "Lateralization in cluster headache: A Nordic multicenter study". The Journal of Headache and Pain. 10 (4): 259–63. doi:10.1007/s10194-009-0129-z. PMC 3451747. PMID 19495933.
- ^ Bahra, A; May, A; Goadsby, PJ (2002). "Cluster headache: A prospective clinical study with diagnostic implications". Neurology. 58 (3): 354–61. doi:10.1212/wnl.58.3.354. PMID 11839832. S2CID 46463344.
- ^ Noshir Mehta; George E. Maloney; Dhirendra S. Bana; Steven J. Scrivani (20 September 2011). Head, Face, and Neck Pain Science, Evaluation, and Management: An Interdisciplinary Approach. John Wiley & Sons. p. 199. ISBN 978-1-118-20995-0. Archived from the original on 14 February 2017.
- ^ a b c d Robbins, Matthew S. (2013). "The Psychiatric Comorbidities of Cluster Headache". Current Pain and Headache Reports. 17 (2): 313. doi:10.1007/s11916-012-0313-8. PMID 23296640. S2CID 35296409.
- ^ The 5-Minute Sports Medicine Consult (2 ed.). Lippincott Williams & Wilkins. 2012. p. 87. ISBN 978-1-4511-4812-1. Archived from the original on 10 September 2017.
- ^ "Trustees & Officers - OUCH(UK)".
- ^ Goadsby P, Mitchell N (1999). "Cluster Headaches". Australian Broadcasting Corporation. Archived from the original on 22 September 2011.
- ^ a b c d e f "IHS Classification ICHD-3 3.1.2 Cluster headache". The International Headache Society. Archived from the original on 8 February 2024. Retrieved 8 February 2024.
- ^ Liang, Jen-Feng; Chen, Yung-Tai; Fuh, Jong-Ling; Li, Szu-Yuan; Liu, Chia-Jen; Chen, Tzeng-Ji; Tang, Chao-Hsiun; Wang, Shuu-Jiun (2012). "Cluster headache is associated with an increased risk of depression: A nationwide population-based cohort study". Cephalalgia. 33 (3): 182–9. doi:10.1177/0333102412469738. PMID 23212294. S2CID 23184973.
- ^ Jensen, RM; Lyngberg, A; Jensen, RH (2016). "Burden of Cluster Headache". Cephalalgia. 27 (6): 535–41. doi:10.1111/j.1468-2982.2007.01330.x. PMID 17459083. S2CID 38485245.
- ^ Tabaee D., Payam; Rizzoli, P; Pecis, M (2020). "Right-to-left shunt and obstructive sleep apnea in cluster headache". Neurology & Neurosc. 1 (1): 1–3. Archived from the original on 24 October 2020. Retrieved 22 January 2021.
- ^ Pringsheim, Tamara (2014). "Cluster Headache: Evidence for a Disorder of Circadian Rhythm and Hypothalamic Function". The Canadian Journal of Neurological Sciences. 29 (1): 33–40. doi:10.1017/S0317167100001694. PMID 11858532.
- ^ Dodick, David W.; Eross, Eric J.; Parish, James M. (2003). "Clinical, Anatomical, and Physiologic Relationship Between Sleep and Headache". Headache: The Journal of Head and Face Pain. 43 (3): 282–92. doi:10.1046/j.1526-4610.2003.03055.x. PMID 12603650. S2CID 6029272.
- ^ "Cluster headaches:Pattern of attacks". NHS. Gov.UK. 22 May 2017. Archived from the original on 20 June 2019. Retrieved 13 December 2018.
- ^ Torelli, Paola; Manzoni, Gian Camillo (2002). "What predicts evolution from episodic to chronic cluster headache?". Current Pain and Headache Reports. 6 (1): 65–70. doi:10.1007/s11916-002-0026-5. PMID 11749880. S2CID 37173661.
- ^ Headache Classification Committee of the International Headache Society (IHS) (2013). "The International Classification of Headache Disorders, 3rd edition (beta version)" (PDF). Cephalalgia. 33 (9): 629–808. doi:10.1177/0333102413485658. PMID 23771276. S2CID 78846027. Archived (PDF) from the original on 9 February 2020. Retrieved 16 August 2019.
- ^ Ferraro S, Nigri A, Bruzzone MG, Demichelis G, Pinardi C, Brivio L, Giani L, Proietti A, Leone M, Chiapparini L (2019). "Cluster headache: insights from resting-state functional magnetic resonance imaging". Neurological Sciences. 40 (Suppl 1): 45–47. doi:10.1007/s10072-019-03874-8. PMID 30941629. S2CID 91190597.
- ^ a b c Waung MW, Taylor A, Qualmann KJ, Burish MJ (2020). "Family History of Cluster HeadacheA Systematic Review". JAMA Neurology. 77 (7): 887–896. doi:10.1001/jamaneurol.2020.0682. PMC 7644512. PMID 32310255.
- ^ a b Pinessi, L.; Rainero, I.; Rivoiro, C.; Rubino, E.; Gallone, S. (2005). "Genetics of cluster headache: An update". The Journal of Headache and Pain. 6 (4): 234–6. doi:10.1007/s10194-005-0194-x. PMC 3452030. PMID 16362673.
- ^ a b c O'Connor, Emer; Simpson, Benjamin S.; Houlden, Henry; Vandrovcova, Jana; Matharu, Manjit (25 April 2020). "Prevalence of familial cluster headache: a systematic review and meta-analysis". The Journal of Headache and Pain. 21 (1): 37. doi:10.1186/s10194-020-01101-w. ISSN 1129-2377. PMC 7183702. PMID 32334514.
- ^ Schürks, Markus; Diener, Hans-Christoph (2008). "Cluster headache and lifestyle habits". Current Pain and Headache Reports. 12 (2): 115–21. doi:10.1007/s11916-008-0022-5. PMID 18474191. S2CID 29434840.
- ^ Pringsheim, Tamara (February 2002). "Cluster headache: evidence for a disorder of circadian rhythm and hypothalamic function". Canadian Journal of Neurological Sciences. 29 (1): 33–40. doi:10.1017/S0317167100001694. PMID 11858532.
- ^ Dasilva, Alexandre F. M.; Goadsby, Peter J.; Borsook, David (2007). "Cluster headache: A review of neuroimaging findings". Current Pain and Headache Reports. 11 (2): 131–6. doi:10.1007/s11916-007-0010-1. PMID 17367592. S2CID 35178080.
- ^ "Headache diary: helping you manage your headache" (PDF). NPS.org.au. Archived from the original (PDF) on 21 September 2013. Retrieved 2 January 2014.
- ^ Clarke, C E (2005). "Ability of a nurse specialist to diagnose simple headache disorders compared with consultant neurologists". Journal of Neurology, Neurosurgery & Psychiatry. 76 (8): 1170–2. doi:10.1136/jnnp.2004.057968. PMC 1739753. PMID 16024902.
- ^ Bahra, A.; Goadsby, P. J. (2004). "Diagnostic delays and mis-management in cluster headache". Acta Neurologica Scandinavica. 109 (3): 175–9. doi:10.1046/j.1600-0404.2003.00237.x. PMID 14763953. S2CID 22500766.
- ^ a b Van Alboom, E; Louis, P; Van Zandijcke, M; Crevits, L; Vakaet, A; Paemeleire, K (2009). "Diagnostic and therapeutic trajectory of cluster headache patients in Flanders". Acta Neurologica Belgica. 109 (1): 10–7. PMID 19402567.
- ^ a b Tfelt-Hansen, Peer C.; Jensen, Rigmor H. (2012). "Management of Cluster Headache". CNS Drugs. 26 (7): 571–80. doi:10.2165/11632850-000000000-00000. PMID 22650381. S2CID 22522914.
- ^ Klapper, Jack A.; Klapper, Amy; Voss, Tracy (2000). "The Misdiagnosis of Cluster Headache: A Nonclinic, Population-Based, Internet Survey". Headache. 40 (9): 730–5. doi:10.1046/j.1526-4610.2000.00127.x. PMID 11091291. S2CID 40116437.
- ^ Prakash, Sanjay; Shah, Nilima D; Chavda, Bhavna V (2010). "Cluster headache responsive to indomethacin: Case reports and a critical review of the literature". Cephalalgia. 30 (8): 975–82. doi:10.1177/0333102409357642. PMID 20656709. S2CID 5938778.
- ^ Sjaastad, O; Vincent, M (2010). "Indomethacin responsive headache syndromes: Chronic paroxysmal hemicrania and Hemicrania continua. How they were discovered and what we have learned since". Functional Neurology. 25 (1): 49–55. PMID 20626997.
- ^ Sanjay Prakash; Nilima D Shah; Bhavna V Chavda (2010). "Cluster headache responsive to indomethacin: Case reports and a critical review of the literature". Cephalalgia. 30 (8): 975–982. doi:10.1177/0333102409357642. PMID 20656709. S2CID 5938778.
- ^ Rizzoli, P; Mullally, WJ (September 2017). "Headache". American Journal of Medicine (Review). S0002-9343 (17): 30932–4. doi:10.1016/j.amjmed.2017.09.005. PMID 28939471.
- ^ Benoliel, Rafael (2012). "Trigeminal autonomic cephalgias". British Journal of Pain. 6 (3): 106–23. doi:10.1177/2049463712456355. PMC 4590147. PMID 26516482.
- ^ Nalini Vadivelu; Alan David Kaye; Jack M. Berger (28 November 2012). Essentials of palliative care. New York, NY: Springer. p. 335. ISBN 978-1-4614-5164-8. Archived from the original on 10 September 2017.
- ^ a b c May, A.; Leone, M.; Áfra, J.; Linde, M.; Sándor, P. S.; Evers, S.; Goadsby, P. J. (2006). "EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias". European Journal of Neurology. 13 (10): 1066–77. doi:10.1111/j.1468-1331.2006.01566.x. PMID 16987158.
- ^ a b Petersen AS, Barloese MC, Snoer A, Soerensen AM, Jensen RH (2019). "Verapamil and Cluster Headache: Still a Mystery. A Narrative Review of Efficacy, Mechanisms and Perspectives". Headache. 59 (8): 1198–1211. doi:10.1111/head.13603. PMID 31339562. S2CID 198193843.
- ^ a b Butticè, Claudio (2022). What you need to know about headaches. Santa Barbara, California: Greenwood. ISBN 978-1-4408-7531-1. OCLC 1259297708. Archived from the original on 28 November 2022. Retrieved 19 September 2022.
- ^ Magis, Delphine; Schoenen, Jean (2011). "Peripheral Nerve Stimulation in Chronic Cluster Headache". Peripheral Nerve Stimulation. Progress in Neurological Surgery. Vol. 24. pp. 126–32. doi:10.1159/000323045. ISBN 978-3-8055-9489-9. PMID 21422783.
- ^ a b c d e Martelletti, Paolo; Jensen, Rigmor H; Antal, Andrea; Arcioni, Roberto; Brighina, Filippo; De Tommaso, Marina; Franzini, Angelo; Fontaine, Denys; Heiland, Max; Jürgens, Tim P; Leone, Massimo; Magis, Delphine; Paemeleire, Koen; Palmisani, Stefano; Paulus, Walter; May, Arne (2013). "Neuromodulation of chronic headaches: Position statement from the European Headache Federation". The Journal of Headache and Pain. 14 (1): 86. doi:10.1186/1129-2377-14-86. PMC 4231359. PMID 24144382.
- ^ Bartsch, Thorsten; Paemeleire, Koen; Goadsby, Peter J (2009). "Neurostimulation approaches to primary headache disorders". Current Opinion in Neurology. 22 (3): 262–8. doi:10.1097/wco.0b013e32832ae61e. PMID 19434793. S2CID 2063863.
- ^ Evers, Stefan (2010). "Pharmacotherapy of cluster headache". Expert Opinion on Pharmacotherapy. 11 (13): 2121–7. doi:10.1517/14656566.2010.496454. PMID 20569084. S2CID 40081324.
- ^ a b Matharu M (9 February 2010). "Cluster headache". Clinical Evidence (Review). 2010. PMC 2907610. PMID 21718584.
- ^ Ailani, Jessica; Young, William B. (2009). "The role of nerve blocks and botulinum toxin injections in the management of cluster headaches". Current Pain and Headache Reports. 13 (2): 164–7. doi:10.1007/s11916-009-0028-7. PMID 19272284. S2CID 10284630.
- ^ a b Bennett, Michael H; French, Christopher; Schnabel, Alexander; Wasiak, Jason; Kranke, Peter; Weibel, Stephanie (2015). "Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache". Cochrane Database of Systematic Reviews. Vol. 2016. doi:10.1002/14651858.CD005219.pub3. PMC 8720466. PMID 26709672.
- ^ "Cluster headache". MedlinePlus Medical Encyclopedia. 2 November 2012. Archived from the original on 5 April 2014. Retrieved 5 April 2014.
- ^ a b Law, Simon; Derry, Sheena; Moore, R Andrew (2013). "Triptans for acute cluster headache". Cochrane Database of Systematic Reviews. Vol. 2018. doi:10.1002/14651858.cd008042.pub3. PMC 4170909. PMID 20393964.
- ^ a b Paemeleire, Koen; Evers, Stefan; Goadsby, Peter J. (2008). "Medication-overuse headache in patients with cluster headache". Current Pain and Headache Reports. 12 (2): 122–7. doi:10.1007/s11916-008-0023-4. PMID 18474192. S2CID 28752169.
- ^ Johnson, Jacinta L; Hutchinson, Mark R; Williams, Desmond B; Rolan, Paul (2012). "Medication-overuse headache and opioid-induced hyperalgesia: A review of mechanisms, a neuroimmune hypothesis and a novel approach to treatment". Cephalalgia. 33 (1): 52–64. doi:10.1177/0333102412467512. hdl:2440/78280. PMID 23144180. S2CID 5697283.
- ^ Watkins, Linda R.; Hutchinson, Mark R.; Rice, Kenner C.; Maier, Steven F. (2009). "The "Toll" of Opioid-Induced Glial Activation: Improving the Clinical Efficacy of Opioids by Targeting Glia". Trends in Pharmacological Sciences. 30 (11): 581–91. doi:10.1016/j.tips.2009.08.002. PMC 2783351. PMID 19762094.
- ^ Saper, Joel R.; Da Silva, Arnaldo Neves (2013). "Medication Overuse Headache: History, Features, Prevention and Management Strategies". CNS Drugs. 27 (11): 867–77. doi:10.1007/s40263-013-0081-y. PMID 23925669. S2CID 39617729.
- ^ Matharu, M (2010). "Cluster headache". BMJ Clinical Evidence. 2010. PMC 2907610. PMID 21718584.
- ^ Malu, Omojo Odihi; Bailey, Jonathan; Hawks, Matthew Kendall (January 2022). "Cluster Headache: Rapid Evidence Review". American Family Physician. 105 (1): 24–32. ISSN 1532-0650. PMID 35029932. Archived from the original on 30 October 2022. Retrieved 30 October 2022.
- ^ Ishaq Abu-Arafeh; Aynur Özge (2016). Headache in Children and Adolescents: A Case-Based Approach. Springer International Publishing Switzerland. p. 62. ISBN 978-3-319-28628-0. Archived from the original on 10 September 2017.
- ^ Harris W.: Neuritis and Neuralgia. p. 307-12. Oxford: Oxford University Press 1926.
- ^ Bickerstaff E (1959). "The periodic migrainous neuralgia of Wilfred Harris". The Lancet. 273 (7082): 1069–71. doi:10.1016/S0140-6736(59)90651-8. PMID 13655672.
- ^ Boes, CJ; Capobianco, DJ; Matharu, MS; Goadsby, PJ (2016). "Wilfred Harris' Early Description of Cluster Headache". Cephalalgia. 22 (4): 320–6. doi:10.1046/j.1468-2982.2002.00360.x. PMID 12100097. S2CID 25747361.
- ^ Pearce, J M S (2007). "Gerardi van Swieten: Descriptions of episodic cluster headache". Journal of Neurology, Neurosurgery & Psychiatry. 78 (11): 1248–9. doi:10.1136/jnnp.2007.123091. PMC 2117620. PMID 17940171.
- ^ Horton BT, MacLean AR, Craig WM (1939). "A new syndrome of vascular headache: results of treatment with histamine: preliminary report". Mayo Clinic Proceedings. 14: 257.
- ^ Silberstein SD, Lipton RB, Goadsby PJ (2002). Headache in Clinical Practice (Second ed.). Taylor & Francis.[page needed]
- ^ Johnson, Tim (16 May 2013). "Researcher works to unlock mysteries of migraines". USA Today. Archived from the original on 17 May 2013. Retrieved 4 January 2013.
- ^ a b Sun-Edelstein, Christina; Mauskop, Alexander (2011). "Alternative Headache Treatments: Nutraceuticals, Behavioral and Physical Treatments". Headache. 51 (3): 469–83. doi:10.1111/j.1526-4610.2011.01846.x. PMID 21352222.
- ^ Vollenweider, Franz X.; Kometer, Michael (2010). "The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders". Nature Reviews Neuroscience. 11 (9): 642–51. doi:10.1038/nrn2884. PMID 20717121. S2CID 16588263.
- ^ Brandt, Roemer B.; Doesborg, Patty G. G.; Haan, Joost; Ferrari, Michel D.; Fronczek, Rolf (1 February 2020). "Pharmacotherapy for Cluster Headache". CNS Drugs. 34 (2): 171–184. doi:10.1007/s40263-019-00696-2. ISSN 1179-1934. PMC 7018790. PMID 31997136.
- ^ "Psilocybin for the Treatment of Cluster Headache - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 27 May 2020. Retrieved 15 February 2020.
- ^ Schindler, Emmanuelle A.D.; Sewell, R. Andrew; Gottschalk, Christopher H.; Flynn, L. Taylor; Zhu, Yutong; Pittman, Brian P.; Cozzi, Nicholas V.; D'Souza, Deepak C. (May 2024). "Psilocybin pulse regimen reduces cluster headache attack frequency in the blinded extension phase of a randomized controlled trial". Journal of the Neurological Sciences. 460 122993. doi:10.1016/j.jns.2024.122993. ISSN 0022-510X. PMID 38581739.
- ^ Busby, Mattha (27 June 2024). "Cluster Headache Patient Wins Federal Court Case to Access Mushrooms". DoubleBlind Mag. Retrieved 12 March 2025.
- ^ "A Study Comparing the Efficacy and Safety of TEV-48125 (Fremanezumab) for the Prevention of Chronic Cluster Headache (CCH)". ClinicalTrials.gov. 28 January 2021. Archived from the original on 3 May 2020. Retrieved 30 November 2017.
- ^ "A Study to Evaluate the Efficacy and Safety of TEV-48125 (Fremanezumab) for the Prevention of Episodic Cluster Headache (ECH)". ClinicalTrials.gov. 2 July 2020. Archived from the original on 1 May 2020. Retrieved 30 November 2017.
External links
[edit]- OUCH(UK) (Organisation for the Understanding of Cluster Headache) - UK charity providing advice and support for sufferers
- Clusterbusters patient support and advocacy
- Organisation for the Prevention of Intense Suffering (OPIS) resource page on cluster headaches

