Recent from talks
Nothing was collected or created yet.
Tachykinin receptor 1
View on Wikipedia
The tachykinin receptor 1 (TACR1) also known as neurokinin 1 receptor (NK1R) or substance P receptor (SPR) is a G protein coupled receptor found in the central nervous system and peripheral nervous system. The endogenous ligand for this receptor is Substance P, although it has some affinity for other tachykinins. The protein is the product of the TACR1 gene.[5]
Structure
[edit]Tachykinins are a family of neuropeptides that share the same hydrophobic C-terminal region with the amino acid sequence Phe-X-Gly-Leu-Met-NH2, where X represents a hydrophobic residue that is either an aromatic or a beta-branched aliphatic. The N-terminal region varies between different tachykinins.[6][7][8] The term tachykinin originates in the rapid onset of action caused by the peptides in smooth muscles.[8]
Substance P (SP) is the most researched and potent member of the tachykinin family. It is an undecapeptide with the amino acid sequence Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2.[6] SP binds to all three of the tachykinin receptors, but it binds most strongly to the NK1 receptor.[7]
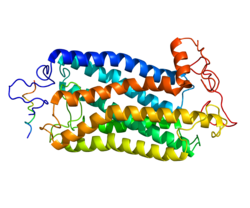
The tachykinin NK1 receptor consists of 407 amino acid residues, and it has a molecular weight of 58,000.[6][9] NK1 receptor, as well as the other tachykinin receptors, is made of seven hydrophobic transmembrane (TM) domains with three extracellular and three intracellular loops, an amino-terminus and a cytoplasmic carboxy-terminus. The loops have functional sites, including two cysteines for a disulfide bridge, Asp-Arg-Tyr, responsible for association with arrestin, and Lys/Arg-Lys/Arg-X-X-Lys/Arg, which interacts with G-proteins.[10][9] The binding site for substance P and other agonists and antagonists is found between the second and third transmembrane domains. The NK-1 receptor is found on human chromosome 2 and is located on the cell's surface as a cytoplasmic receptor.[11]
Function
[edit]The binding of SP to the NK1 receptor has been associated with the transmission of stress signals and pain, the contraction of smooth muscles, and inflammation.[12] NK1 receptor antagonists have also been studied in migraine, emesis, and psychiatric disorders. In fact, aprepitant has been proved effective in a number of pathophysiological models of anxiety and depression.[13] Other diseases in which the NK1 receptor system is involved include asthma, rheumatoid arthritis, and gastrointestinal disorders.[14]
Tissue distribution
[edit]The NK1 receptor can be found in both the central and peripheral nervous system. It is present in neurons, brainstem, vascular endothelial cells, muscle, gastrointestinal tracts, genitourinary tract, pulmonary tissue, thyroid gland, and different types of immune cells.[10][15][8][9]
Mechanisms of action
[edit]SP is synthesized by neurons and transported to synaptic vesicles; the release of SP is accomplished through the depolarizing action of calcium-dependent mechanisms.[6] When NK1 receptors are stimulated, they can generate various second messengers, which can trigger a wide range of effector mechanisms that regulate cellular excitability and function.
There are three well-defined, independent second messenger systems:
- Stimulation via phospholipase C, leading to phosphatidyl inositol turnover and Ca mobilization from both intra- and extracellular sources.
- Arachidonic acid mobilization via phospholipase A2.
- cAMP accumulation via stimulation of adenylate cyclase.[16]
It has also been reported that SP elicits interleukin-1 (IL-1) production in macrophages, sensitizes neutrophils, and enhances dopamine release in the substantia nigra region in cat brain. From spinal neurons, SP is known to evoke release of neurotransmitters like acetylcholine, histamine, and GABA. It also secretes catecholamines and plays a role in the regulation of blood pressure and hypertension. Likewise, SP is known to bind to N-methyl-D-aspartate (NMDA) receptors, eliciting excitation with calcium ion influx, which further releases nitric oxide. Studies in frogs have shown that SP elicits the release of prostaglandin E2 and prostacyclin by the arachidonic acid pathway, which leads to an increase in corticosteroid output.[8]
Clinical significance
[edit]In combination therapy, NK1 receptor antagonists appear to offer better control of delayed emesis and post-operative emesis than drug therapy without NK1 receptor antagonists. NK1 receptor antagonists block responses to a broader range of emetic stimuli than the established 5-HT3 antagonist treatments.[14] It has been reported that centrally-acting NK1 receptor antagonists, such as CP-99994, inhibit emesis induced by apomorphine and loperimidine, which are two compounds that act through central mechanisms.[15]
This receptor is considered an attractive drug target, particularly with regards to potential analgesics and anti-depressants.[17][18] It is also a potential treatment for alcoholism and opioid addiction.[19] In addition, it has been identified as a candidate in the etiology of bipolar disorder.[20] Finally NK1R antagonists may also have a role as novel antiemetics[21] and hypnotics.[22][23]
Neurokinin receptor 1 (NK-1R) also plays a significant role in cancer progression. NK-1R is overexpressed in various cancer types and is activated by substance P (SP).[24][25] This activation promotes tumor cell proliferation, migration, and invasion while inhibiting apoptosis.[25][26] The SP/NK-1R system is involved in angiogenesis, chronic inflammation, and the Warburg effect, all of which contribute to tumor growth.[24][25] NK-1R antagonists, such as aprepitant, have shown promise as potential anticancer treatments by inhibiting tumor growth, inducing apoptosis, and blocking metastasis.[25][27] The overexpression of NK-1R in tumors may also serve as a prognostic biomarker.[25]
Ligands
[edit]Many selective ligands for NK1 are now available, several of which have gone into clinical use as antiemetics.
Agonists
[edit]- GR-73632 - potent and selective agonist, EC50 2nM, 5-amino acid polypeptide chain. CAS# 133156-06-6
Antagonists
[edit]- Aprepitant
- Casopitant
- Elinzanetant
- Ezlopitant
- Fosaprepitant
- Lanepitant
- Maropitant
- Rolapitant
- Vestipitant
- L-733,060
- L-741,671
- L-742,694
- RP-67580 - potent and selective antagonist, Ki 2.9nM, (3aR,7aR)-Octahydro-2-[1-imino-2-(2-methoxyphenyl)ethyl ]-7,7-diphenyl-4H-isoindol, CAS# 135911-02-3
- RPR-100,893
- CP-96345
- CP-99994
- GR-205,171/Vofopitant
- TAK-637
- T-2328
See also
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000115353 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000030043 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Takeda Y, Chou KB, Takeda J, Sachais BS, Krause JE (September 1991). "Molecular cloning, structural characterization and functional expression of the human substance P receptor". Biochemical and Biophysical Research Communications. 179 (3): 1232–1240. doi:10.1016/0006-291X(91)91704-G. PMID 1718267.
- ^ a b c d Ho WZ, Douglas SD (December 2004). "Substance P and neurokinin-1 receptor modulation of HIV". Journal of Neuroimmunology. 157 (1–2): 48–55. doi:10.1016/j.jneuroim.2004.08.022. PMID 15579279. S2CID 14975995.
- ^ a b Page NM (August 2005). "New challenges in the study of the mammalian tachykinins". Peptides. 26 (8): 1356–1368. doi:10.1016/j.peptides.2005.03.030. PMID 16042976. S2CID 23094292.
- ^ a b c d Datar P, Srivastava S, Coutinho E, Govil G (2004). "Substance P: structure, function, and therapeutics". Current Topics in Medicinal Chemistry. 4 (1): 75–103. doi:10.2174/1568026043451636. PMID 14754378.
- ^ a b c Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, et al. (August 2004). "Tachykinins and tachykinin receptors: structure and activity relationships". Current Medicinal Chemistry. 11 (15): 2045–2081. doi:10.2174/0929867043364748 (inactive 1 July 2025). PMID 15279567.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ a b Satake H, Kawada T (August 2006). "Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors". Current Drug Targets. 7 (8): 963–974. doi:10.2174/138945006778019273. PMID 16918325.
- ^ Graefe SB, Mohiuddin SS (April 2022). Biochemistry, Substance P. Treasure Island, FL: StatPearls Publishing. PMID 32119470. Retrieved 28 January 2023.
- ^ Seto S, Tanioka A, Ikeda M, Izawa S (March 2005). "Design and synthesis of novel 9-substituted-7-aryl-3,4,5,6-tetrahydro-2H-pyrido[4,3-b]- and [2,3-b]-1,5-oxazocin-6-ones as NK(1) antagonists". Bioorganic & Medicinal Chemistry Letters. 15 (5): 1479–1484. doi:10.1016/j.bmcl.2004.12.091. PMID 15713411.
- ^ Quartara L, Altamura M (August 2006). "Tachykinin receptors antagonists: from research to clinic". Current Drug Targets. 7 (8): 975–992. doi:10.2174/138945006778019381. PMID 16918326.
- ^ a b Humphrey JM (2003). "Medicinal chemistry of selective neurokinin-1 antagonists". Current Topics in Medicinal Chemistry. 3 (12): 1423–1435. doi:10.2174/1568026033451925. PMID 12871173.
- ^ a b Saria A (June 1999). "The tachykinin NK1 receptor in the brain: pharmacology and putative functions". European Journal of Pharmacology. 375 (1–3): 51–60. doi:10.1016/S0014-2999(99)00259-9. PMID 10443564.
- ^ Quartara L, Maggi CA (December 1997). "The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation". Neuropeptides. 31 (6): 537–563. doi:10.1016/S0143-4179(97)90001-9. PMID 9574822. S2CID 13735836.
- ^ Humphrey JM (2003). "Medicinal chemistry of selective neurokinin-1 antagonists". Current Topics in Medicinal Chemistry. 3 (12): 1423–1435. doi:10.2174/1568026033451925. PMID 12871173.
- ^ Duffy RA (May 2004). "Potential therapeutic targets for neurokinin-1 receptor antagonists". Expert Opinion on Emerging Drugs. 9 (1): 9–21. doi:10.1517/eoed.9.1.9.32956. PMID 15155133.
- ^ Schank JR (October 2014). "The neurokinin-1 receptor in addictive processes". The Journal of Pharmacology and Experimental Therapeutics. 351 (1): 2–8. doi:10.1124/jpet.113.210799. PMID 25038175. S2CID 16533561.
- ^ Perlis RH, Purcell S, Fagerness J, Kirby A, Petryshen TL, Fan J, et al. (January 2008). "Family-based association study of lithium-related and other candidate genes in bipolar disorder". Archives of General Psychiatry. 65 (1): 53–61. doi:10.1001/archgenpsychiatry.2007.15. PMID 18180429.
- ^ Munoz M, Covenas R, Esteban F, Redondo M (June 2015). "The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs". Journal of Biosciences. 40 (2): 441–463. doi:10.1007/s12038-015-9530-8. PMID 25963269. S2CID 3048287.
- ^ Brasure M, MacDonald R, Fuchs E, Olson CM, Carlyle M, Diem S, et al. (2015). "Management of Insomnia Disorder". Comparative Effectiveness Reviews. 159. PMID 26844312.
- ^ Jordan K (February 2006). "Neurokinin-1-receptor antagonists: a new approach in antiemetic therapy". Onkologie. 29 (1–2): 39–43. doi:10.1159/000089800. PMID 16514255. S2CID 34016787.
- ^ a b Esteban F, Ramos-García P, Muñoz M, González-Moles MÁ (December 2021). "Substance P and Neurokinin 1 Receptor in Chronic Inflammation and Cancer of the Head and Neck: A Review of the Literature". International Journal of Environmental Research and Public Health. 19 (1): 375. doi:10.3390/ijerph19010375. PMC 8751191. PMID 35010633.
- ^ a b c d e Coveñas R, Muñoz M (July 2022). "Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer". Cancers. 14 (14): 3539. doi:10.3390/cancers14143539. PMC 9317685. PMID 35884599.
- ^ Muñoz M, Coveñas R (2016). "Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: A new approach". Saudi Journal of Gastroenterology. 22 (4): 260–268. doi:10.4103/1319-3767.187601. PMC 4991196. PMID 27488320.
- ^ Muñoz M, González-Ortega A, Salinas-Martín MV, Carranza A, Garcia-Recio S, Almendro V, et al. (October 2014). "The neurokinin-1 receptor antagonist aprepitant is a promising candidate for the treatment of breast cancer". International Journal of Oncology. 45 (4): 1658–1672. doi:10.3892/ijo.2014.2565. PMID 25175857.
Further reading
[edit]- Burcher E (June 1989). "The study of tachykinin receptors". Clinical and Experimental Pharmacology & Physiology. 16 (6): 539–543. doi:10.1111/j.1440-1681.1989.tb01602.x. PMID 2548782. S2CID 30578296.
- Kowall NW, Quigley BJ, Krause JE, Lu F, Kosofsky BE, Ferrante RJ (July 1993). "Substance P and substance P receptor histochemistry in human neurodegenerative diseases". Regulatory Peptides. 46 (1–2): 174–185. doi:10.1016/0167-0115(93)90028-7. PMID 7692486. S2CID 54379670.
- Patacchini R, Maggi CA (October 2001). "Peripheral tachykinin receptors as targets for new drugs". European Journal of Pharmacology. 429 (1–3): 13–21. doi:10.1016/S0014-2999(01)01301-2. PMID 11698023.
- Saito R, Takano Y, Kamiya HO (February 2003). "Roles of substance P and NK(1) receptor in the brainstem in the development of emesis". Journal of Pharmacological Sciences. 91 (2): 87–94. doi:10.1254/jphs.91.87. PMID 12686752.
- Fong TM, Yu H, Huang RR, Strader CD (December 1992). "The extracellular domain of the neurokinin-1 receptor is required for high-affinity binding of peptides". Biochemistry. 31 (47): 11806–11811. doi:10.1021/bi00162a019. PMID 1280161.
- Fong TM, Huang RR, Strader CD (December 1992). "Localization of agonist and antagonist binding domains of the human neurokinin-1 receptor". The Journal of Biological Chemistry. 267 (36): 25664–25667. doi:10.1016/S0021-9258(18)35657-6. PMID 1281469.
- Fong TM, Anderson SA, Yu H, Huang RR, Strader CD (January 1992). "Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor". Molecular Pharmacology. 41 (1): 24–30. doi:10.1016/S0026-895X(25)08831-5. PMID 1310144.
- Takahashi K, Tanaka A, Hara M, Nakanishi S (March 1992). "The primary structure and gene organization of human substance P and neuromedin K receptors". European Journal of Biochemistry. 204 (3): 1025–1033. doi:10.1111/j.1432-1033.1992.tb16724.x. PMID 1312928.
- Walsh DA, Mapp PI, Wharton J, Rutherford RA, Kidd BL, Revell PA, et al. (March 1992). "Localisation and characterisation of substance P binding to human synovial tissue in rheumatoid arthritis". Annals of the Rheumatic Diseases. 51 (3): 313–317. doi:10.1136/ard.51.3.313. PMC 1004650. PMID 1374227.
- Gerard NP, Garraway LA, Eddy RL, Shows TB, Iijima H, Paquet JL, et al. (November 1991). "Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones". Biochemistry. 30 (44): 10640–10646. doi:10.1021/bi00108a006. PMID 1657150.
- Hopkins B, Powell SJ, Danks P, Briggs I, Graham A (October 1991). "Isolation and characterisation of the human lung NK-1 receptor cDNA". Biochemical and Biophysical Research Communications. 180 (2): 1110–1117. doi:10.1016/S0006-291X(05)81181-7. PMID 1659396.
- Takeda Y, Chou KB, Takeda J, Sachais BS, Krause JE (September 1991). "Molecular cloning, structural characterization and functional expression of the human substance P receptor". Biochemical and Biophysical Research Communications. 179 (3): 1232–1240. doi:10.1016/0006-291X(91)91704-G. PMID 1718267.
- Giuliani S, Barbanti G, Turini D, Quartara L, Rovero P, Giachetti A, et al. (October 1991). "NK2 tachykinin receptors and contraction of circular muscle of the human colon: characterization of the NK2 receptor subtype". European Journal of Pharmacology. 203 (3): 365–370. doi:10.1016/0014-2999(91)90892-T. PMID 1723045.
- Ichinose H, Katoh S, Sueoka T, Titani K, Fujita K, Nagatsu T (August 1991). "Cloning and sequencing of cDNA encoding human sepiapterin reductase--an enzyme involved in tetrahydrobiopterin biosynthesis". Biochemical and Biophysical Research Communications. 179 (1): 183–189. doi:10.1016/0006-291X(91)91352-D. PMID 1883349.
- Thöny B, Heizmann CW, Mattei MG (March 1995). "Human GTP-cyclohydrolase I gene and sepiapterin reductase gene map to region 14q21-q22 and 2p14-p12, respectively, by in situ hybridization". Genomics. 26 (1): 168–170. doi:10.1016/0888-7543(95)80101-Q. PMID 7782081.
- Fong TM, Cascieri MA, Yu H, Bansal A, Swain C, Strader CD (March 1993). "Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345". Nature. 362 (6418): 350–353. Bibcode:1993Natur.362..350M. doi:10.1038/362350a0. PMID 8384323. S2CID 4339311.
- Derocq JM, Ségui M, Blazy C, Emonds-Alt X, Le Fur G, Brelire JC, et al. (December 1996). "Effect of substance P on cytokine production by human astrocytic cells and blood mononuclear cells: characterization of novel tachykinin receptor antagonists". FEBS Letters. 399 (3): 321–325. Bibcode:1996FEBSL.399..321D. doi:10.1016/S0014-5793(96)01346-4. PMID 8985172. S2CID 84912440.
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. (March 1998). "Altered nociception, analgesia and aggression in mice lacking the receptor for substance P". Nature. 392 (6674): 394–397. Bibcode:1998Natur.392..394D. doi:10.1038/32904. PMID 9537323. S2CID 4324247.
External links
[edit]- "Tachykinin Receptors: NK1". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. Archived from the original on 2011-05-16. Retrieved 2007-10-25.
- Receptors,+Neurokinin-1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P25103 (Substance-P receptor) at the PDBe-KB.