Recent from talks
Nothing was collected or created yet.
Scabies
View on Wikipedia
| Scabies | |
|---|---|
| Other names | Seven-year itch[1] |
 | |
| Magnified view of a burrowing trail of the scabies mite. The scaly patch on the left was caused by scratching and marks the mite's entry point into the skin. The mite has burrowed to the top-right, where it can be seen as a dark spot at the end. | |
| Specialty | Infectious disease, dermatology |
| Symptoms | itchiness, pimple-like rash[2] |
| Usual onset | 2–6 weeks (first infection), ~1 day (subsequent infections)[2] |
| Causes | Sarcoptes scabiei mite spread by close contact[3] |
| Risk factors | Crowded living conditions (child care facilities, group homes, prisons), lack of access to water, wearing second hand clothing[3][4][5] |
| Diagnostic method | Based on symptoms[6] |
| Differential diagnosis | seborrheic dermatitis, dermatitis herpetiformis, pediculosis, atopic dermatitis[7] |
| Medication | permethrin, crotamiton, lindane, ivermectin[8] |
| Frequency | 204 million / 2.8% (2015)[9] |
Scabies (/ˈskeɪb(i)iːz/, SKAY-b(ee-)eez;[10] also sometimes known as the seven-year itch)[1] is a contagious human skin infestation by the tiny (0.2–0.45 mm) mite Sarcoptes scabiei,[1][3] variety hominis. The word is from Latin: scabere, lit. 'to scratch'.[11] The most common symptoms are severe itchiness and a pimple-like rash.[2] Occasionally, tiny burrows may appear on the skin from eggs that are about to hatch.[2] In a first-ever infection, the infected person usually develops symptoms within two to six weeks.[2] During a second infection, symptoms may begin within 24 hours.[2] These symptoms can be present across most of the body or just in certain areas such as the wrists, between fingers, or along the waistline.[2] The head may be affected, but this is typically only in young children.[2] The itch is often worse at night.[2] Scratching may cause skin breakdown and an additional bacterial infection in the skin.[2]
Various names have been given to this condition and the name 'seven year itch' has been recorded in many documents from the 1800s.[12] Although the 1952 play The Seven Year Itch and modern treatment methods have generally changed this name to refer to human relationships, the condition was historically very difficult to treat.
Scabies is caused by infection with the female mite Sarcoptes scabiei var. hominis, an ectoparasite.[3] The mites burrow into the skin to live and deposit eggs.[3] The symptoms of scabies are due to an allergic reaction to the mites.[2] Often, only between 10 and 15 mites are involved in an infection.[2] Scabies most often spreads during a relatively long period of direct skin contact with an infected person (at least 10 minutes) such as that which may occur during sexual activity or living together.[3][13] Spread of the disease may occur even if the person has not developed symptoms yet.[14] Crowded living conditions, such as those found in child-care facilities, group homes, and prisons, increase the risk of spread.[3] Areas with a lack of access to water also have higher disease rates.[4] Crusted scabies is a more severe form of the disease, not essentially different but an infestation by huge numbers of mites[3] that typically only affects those with a poor immune system; the number of mites also makes them much more contagious.[3] In these cases, the spread of infection may occur during brief contact or by contaminated objects.[3] The mite is tiny and at the limit of detection with the human eye. It is not readily obvious; factors that aid in detection are good lighting, magnification, and knowing what to look for. Diagnosis is based either on detecting the mite (confirmed scabies), detecting typical lesions in a typical distribution with typical histological features (clinical scabies), or detecting atypical lesions or atypical distribution of lesions with only some histological features present (suspected scabies).[15]
Several medications are available to treat those infected, including oral and topical ivermectin, permethrin, crotamiton, and lindane creams.[8] Sexual contacts within the last month and people who live in the same house should also be treated at the same time.[14] Bedding and clothing used in the last three days should be washed in hot water and dried in a hot dryer.[14] As the mite does not live for more than three days away from human skin, more washing is not needed.[14] Symptoms may continue for two to four weeks following treatment.[14] If after this time symptoms continue, retreatment may be needed.[14]
Scabies is one of the three most common skin disorders in children, along with ringworm and bacterial skin infections.[16] As of 2015, it affects about 204 million people (2.8% of the world population).[9] It is equally common in both sexes.[17] The young and the old are more commonly affected.[6] It also occurs more commonly in the developing world and tropical climates.[6] Other animals do not spread human scabies;[3] similar infection in other animals is known as sarcoptic mange, and is typically caused by slightly different but related mites.[18]
Signs and symptoms
[edit]
The characteristic symptoms of a scabies infection include intense itching and superficial burrows.[20] Because the host develops the symptoms as a reaction to the mites' presence over time, typically a delay of four to six weeks occurs between the onset of infestation and the onset of itching. Similarly, symptoms often persist for one to several weeks after successful eradication of the mites. As noted, those re-exposed to scabies after successful treatment may exhibit symptoms of the new infestation in a much shorter period—as little as one to four days.[21]
Itching
[edit]In the classic scenario, the itch is made worse by warmth and is usually experienced as being worse at night, possibly because distractions are fewer.[20] As a symptom, it is less common in the elderly.[20]
Rash
[edit]The superficial burrows of scabies usually occur in the area of the finger webs, feet, ventral wrists, elbows, back, buttocks, and external genitals.[20] Except in infants and the immunosuppressed, infection generally does not occur in the skin of the face or scalp. The burrows are created by the excavation of the adult mite in the epidermis.[20] Acropustulosis, or blisters and pustules on the palms and soles of the feet, are characteristic symptoms of scabies in infants.[22]
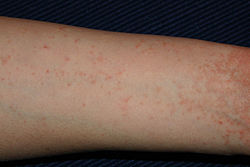
-
Scabies of the foot
-
Scabies of the arm
-
Scabies of the hand
In most people, the trails of the burrowing mites are linear or S-shaped tracks in the skin, often accompanied by rows of small, pimple-like mosquito or insect bites. Lesions are symmetrical and mainly affect the hands, wrists, axillae, thighs, buttocks, waist, soles of the feet, areola, and vulva in females, and penis and scrotum in males. The neck and above are usually not affected, except in cases of crusted scabies and infestations of infants, the elderly, and the immunocompromised.[22] Symptoms typically appear two to six weeks after infestation for individuals never before exposed to scabies. For those having been previously exposed, the symptoms can appear within several days after infestation. However, symptoms may appear after several months or years.[23]
Crusted scabies
[edit]
The elderly, disabled, and people with impaired immune systems, such as those with HIV/AIDS, cancer, or those on immunosuppressive medications, are susceptible to crusted scabies (also called Norwegian scabies).[20][23][24] On those with weaker immune systems, the host becomes a more fertile breeding ground for the mites, which spread over the host's body, except the face. The mites in crusted scabies are not more virulent than in noncrusted scabies but are much more numerous, sometimes up to two million. People with crusted scabies exhibit scaly rashes, slight itching, and thick crusts of skin that contain large numbers of scabies mites. For this reason, persons with crusted scabies are more contagious to others than those with typical scabies.[3][25] Such areas make eradication of mites particularly difficult, as the crusts protect the mites from topical miticides/scabicides, necessitating prolonged treatment of these areas.[citation needed]
Cause
[edit]Scabies mite
[edit]
In the 18th century, Italian biologists Giovanni Cosimo Bonomo and Diacinto Cestoni (1637–1718) described the mite now called Sarcoptes scabiei, variety hominis, as the cause of scabies. Sarcoptes is a genus of skin parasites and part of the larger family of mites collectively known as scab mites. These organisms have eight legs as adults and are placed in the same phylogenetic class (Arachnida) as spiders and ticks.[26]
S. scabiei mites are under 0.5 mm in size; they are sometimes visible as pinpoints of white. Gravid females tunnel into the dead, outermost layer (stratum corneum) of a host's skin and deposit eggs in the shallow burrows. The eggs hatch into larvae in three to ten days. These young mites move about on the skin and molt into a "nymphal" stage, before maturing as adults, which live three to four weeks in the host's skin. Males roam on top of the skin, occasionally burrowing into the skin. In general, the total number of adult mites infesting a healthy hygienic person with non-crusted scabies is small, about 11 females in burrows, on average.[27]
The movement of mites within and on the skin produces an intense itch, which has the characteristics of a delayed cell-mediated inflammatory response to allergens. IgE antibodies are present in the serum and the site of infection, which react to multiple protein allergens in the body of the mite. Some of these cross-react to allergens from house dust mites. Immediate antibody-mediated allergic reactions (wheals) have been elicited in infected persons, but not in those not infected; immediate hypersensitivity of this type is thought to explain the observed far more rapid allergic skin response to reinfection seen in persons who have been infected previously, especially within the previous year or two.[27]
Transmission
[edit]Scabies is contagious and can be contracted through prolonged physical contact with an infested person.[28] This includes sexual intercourse, although a majority of cases are acquired through other forms of skin-to-skin contact. Less commonly, scabies infestation can happen through the sharing of clothes, towels, and bedding, but this is not a major mode of transmission; individual mites can survive for only two to three days, at most, away from human skin at room temperature.[29][30] As with lice, a latex condom is ineffective against scabies transmission during intercourse, because mites typically migrate from one individual to the next at sites other than the sex organs.[31]
Healthcare workers are at risk of contracting scabies from patients, because they may be in extended contact with them.[32]
Pathophysiology
[edit]The symptoms are caused by an allergic reaction of the host's body to mite proteins, though exactly which proteins remains a topic of study. The mite proteins are also present in the gut, and in mite feces, which are deposited under the skin. The allergic reaction is both of the delayed (cell-mediated) and immediate (antibody-mediated) type, and involves IgE (antibodies are presumed to mediate the very rapid symptoms on reinfection).[27] The allergy-type symptoms (itching) continue for some days, and even several weeks, after all mites are killed. New lesions may appear for a few days after mites are eradicated. Nodular lesions from scabies may continue to be symptomatic for weeks after the mites have been killed.[27]
Rates of scabies are negatively related to temperature and positively related to humidity.[33]
Diagnosis
[edit]
Scabies may be diagnosed clinically in geographical areas where it is common when diffuse itching presents along with either a lesion in two typical spots or itchiness is present in another household member.[16] The classical sign of scabies is the burrow made by a mite within the skin.[16] To detect the burrow, the suspected area is rubbed with ink from a fountain pen or a topical tetracycline solution, which glows under a special light. The skin is then wiped with an alcohol pad. If the person is infected with scabies, the characteristic zigzag or S pattern of the burrow will appear across the skin; however, interpreting this test may be difficult, as the burrows are scarce and may be obscured by scratch marks.[16] A definitive diagnosis is made by finding either the scabies mites or their eggs and fecal pellets.[16] Searches for these signs involve either scraping a suspected area, mounting the sample in potassium hydroxide and examining it under a microscope, or using dermoscopy to examine the skin directly.[20]
Differential diagnosis
[edit]Symptoms of early scabies infestation mirror other skin diseases, including dermatitis, syphilis, erythema multiforme, various urticaria-related syndromes, allergic reactions, ringworm-related diseases, and other ectoparasites such as lice and fleas.[34]
Prevention of passing on scabies to other people
[edit]Mass-treatment programs that use topical permethrin or oral ivermectin have been effective in reducing the prevalence of scabies in several populations.[16] No vaccine is available for scabies. The simultaneous treatment of all close contacts is recommended, even if they show no symptoms of infection (asymptomatic), to reduce rates of recurrence.[16] Since mites can survive for only two to three days without a host, other objects in the environment pose little risk of transmission except in the case of crusted scabies. Therefore, cleaning is of little importance.[16] Rooms used by those with crusted scabies require thorough cleaning.[35]
Management
[edit]Treatment
[edit]Several medications are effective in treating scabies. Treatment should involve the entire household and any others who have had recent, prolonged contact with the infested individual.[16] In addition to treating the infestation, options to control itchiness include antihistamines and prescription anti-inflammatory agents.[36] Bedding, clothing and towels used during the previous three days should be washed in hot water and dried in a hot dryer.[37]
Treatment protocols for crusted scabies are significantly more intense than for common scabies.[8][38][39]
Permethrin
[edit]Permethrin, a pyrethroid insecticide, is the most effective treatment for scabies,[40] and remains the treatment of choice.[16][41] It is applied from the neck down, usually before sleep, and left on for about 8 to 14 hours, then washed off in the morning.[16] Care should be taken to coat the entire skin surface, not just symptomatic areas; any patch of skin left untreated can provide a "safe haven" for one or more mites to survive. One application is normally sufficient, as permethrin kills eggs, hatchlings, and adult mites, though many physicians recommend a second application three to seven days later as a precaution. Crusted scabies may require multiple applications or supplemental treatment with oral ivermectin (below).[16][41][42] Permethrin may cause slight irritation of the skin that is usually tolerable.[20] In recent years, concern is growing about permethrin-resistant scabies,[43][44] although some researchers refer to this as pseudo-resistance.[45]
Ivermectin
[edit]Oral ivermectin is effective in eradicating scabies, often in a single dose.[4][16] It is the treatment of choice for crusted scabies, and is sometimes prescribed in combination with a topical agent.[16][20] It has not been tested on infants, and is not recommended for children under six years of age.[20]
Topical ivermectin preparations are effective for scabies in adults.[46] It has also been useful for sarcoptic mange, the veterinary analog of human scabies.[47][48]
One review found that the efficacy of permethrin is similar to that of systemic or topical ivermectin.[49] A separate review found that although oral ivermectin is usually effective for the treatment of scabies, it does have a higher treatment failure rate than topical permethrin.[50] Another review found that oral ivermectin provided a reasonable balance between efficacy and safety.[51] A study has demonstrated that scabies is markedly reduced in populations taking ivermectin regularly;[52] the drug is widely used for treating scabies and other parasitic diseases, particularly among the poor and disadvantaged in the tropics, beginning with the developer Merck providing the drug at no cost to treat onchocerciasis from 1987.[53]
Others
[edit]Other treatments include lindane, benzyl benzoate, crotamiton, malathion, and sulfur preparations.[16][20] Lindane is effective, but concerns over potential neurotoxicity have limited its availability in many countries.[20] It is banned in California,[54] but may be used in other states as a second-line treatment.[55] Sulfur ointments or benzyl benzoate are often used in the developing world due to their low cost;[20] some 10% sulfur solutions have been shown to be effective,[56] and sulfur ointments are typically used for at least a week, though many people find the odor of sulfur products unpleasant.[20] Crotamiton has been found to be less effective than permethrin in limited studies.[20] Crotamiton or sulfur preparations are sometimes recommended instead of permethrin for children, due to concerns over dermal absorption of permethrin.[16]
-
Day 4
-
Day 8 (treatment begins)
-
Day 12 (under treatment)
-
Healed
Communities
[edit]Scabies is endemic in many developing countries,[16] where it tends to be particularly problematic in rural and remote areas. In such settings, community-wide control strategies are required to reduce the rate of disease, as treatment of only individuals is ineffective due to the high rate of reinfection. Large-scale mass drug administration strategies may be required where coordinated interventions aim to treat whole communities in one concerted effort.[57] Although such strategies have shown to be able to reduce the burden of scabies in these kinds of communities, debate remains about the best strategy to adopt, including the choice of drug.[57][58]
The resources required to implement such large-scale interventions in a cost-effective and sustainable way are significant. Furthermore, since endemic scabies is largely restricted to poor and remote areas, it is a public health issue that has not attracted much attention from policymakers and international donors.[57][58]
Epidemiology
[edit]Scabies is one of the three most common skin disorders in children, along with tinea and pyoderma.[16] As of 2010, it affects about 100 million people (1.5% of the population) and its frequency is not related to gender.[17] The mites are distributed around the world and equally infect all ages, races, and socioeconomic classes in different climates.[25] Scabies is more often seen in crowded areas with unhygienic living conditions.[59] Globally as of 2009, an estimated 300 million cases of scabies occur each year, although various parties claim the figure is either over- or underestimated.[23][60] About 1–10% of the global population is estimated to be infected with scabies, but in certain populations, the infection rate may be as high as 50–80%.[16]
History
[edit]
Scabies has been observed in humans since ancient times. Archeological evidence from Egypt and the Middle East suggests scabies was present as early as 494 BC.[21][61] In the fourth century BC, Aristotle reported on "lice" that "escape from little pimples if they are pricked" – a description consistent with scabies.[62] Arab physician Ibn Zuhr is believed to have been the first to provide a clinical description of the scabies mites.[63]
Roman encyclopedist and medical writer Aulus Cornelius Celsus (circa 25 BC – 50 AD) is credited with naming the disease "scabies" and describing its characteristic features.[62] The parasitic etiology of scabies was documented by Italian physician Giovanni Cosimo Bonomo (1663–1696) in his 1687 letter, "Observations concerning the fleshworms of the human body".[62] Bonomo's description established scabies as one of the first human diseases with a well-understood cause.[21][61]
In Europe in the late 19th through mid-20th centuries, a sulfur-bearing ointment called by the medical eponym of Wilkinson's ointment was widely used for topical treatment of scabies. The contents and origins of several versions of the ointment were detailed in correspondence published in the British Medical Journal in 1945.[64]
In the 1995 documentary Anne Frank Remembered, Bloeme Evers-Emden told of how she was selected from Auschwitz and sent to a work camp where conditions were sufficiently improved that she was able to survive until the liberation. She said Anne Frank was rejected for this transfer because she had contracted scabies.[65]
Scabies in animals
[edit]
Scabies may occur in some domestic and wild animals; the mites that cause these infestations are of different subspecies from the one typically causing the human form.[20] These subspecies can infest animals that are not their usual hosts, but such infections do not last long.[20] Scabies-infected animals experience severe itching and secondary skin infections. They often lose weight and become frail.[27]
The most frequently diagnosed form of scabies in domestic animals is sarcoptic mange, caused by the subspecies Sarcoptes scabiei canis, most commonly in dogs and cats. Sarcoptic mange is transmissible to humans who come into prolonged contact with infested animals,[66] and is distinguished from human scabies by its distribution on skin surfaces covered by clothing. Scabies-infected domestic fowl develop what is known as "scaly leg". Domestic animals that have gone feral and have no veterinary care are frequently affected by scabies.[67] Nondomestic animals have also been observed to develop scabies. Gorillas, for instance, are known to be susceptible to infection by contact with items used by humans,[68] and it is a fatal disease of wombats.[69]
Society and culture
[edit]
The International Alliance for the Control of Scabies was started in 2012,[6][58][74] and brings together over 150 researchers, clinicians, and public-health experts from more than 15 countries. It has managed to bring the global health implications of scabies to the attention of the World Health Organization (WHO).[58] Consequently, the WHO has included scabies on its official list of neglected tropical diseases and other neglected conditions.[75]
Research
[edit]Moxidectin is being evaluated as a treatment for scabies.[76] It is established in veterinary medicine to treat a range of parasites, including sarcoptic mange. Its advantage over ivermectin is its longer half-life in humans, thus the potential duration of action.[77]
Tea tree oil (TTO) exhibits scabicidal action in a laboratory setting.[78]
References
[edit]- ^ a b c Gates RH (2003). Infectious disease secrets (2nd ed.). Philadelphia: Elsevier, Hanley Belfus. p. 355. ISBN 978-1-56053-543-0.
- ^ a b c d e f g h i j k l "Parasites – Scabies Disease". Center for Disease Control and Prevention. 2 November 2010. Archived from the original on 2 May 2015. Retrieved 18 May 2015.
- ^ a b c d e f g h i j k l "Parasites - Scabies: Epidemiology & Risk Factors". Centers for Disease Control and Prevention. 2 November 2010. Archived from the original on 29 April 2015. Retrieved 1 January 2024.
- ^ a b c "WHO -Water-related Disease". World Health Organization. Archived from the original on 22 October 2010. Retrieved 10 October 2010.
- ^ Muthiani YM (21 June 2017). "Potential skin pathogens on second hand clothes and the effectiveness of disinfection methods". JKUAT Annual Scientific Conference.
- ^ a b c d "Scabies". World Health Organization. Archived from the original on 18 May 2015. Retrieved 18 May 2015.
- ^ Ferri FF (2010). "Chapter S". Ferri's differential diagnosis: a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. ISBN 978-0-323-07699-9.
- ^ a b c "Parasites – Scabies Medications". Center for Disease Control and Prevention. 2 October 2019. Archived from the original on 30 April 2015.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ Wells JC (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- ^ Mosby's Medical, Nursing & Allied Health Dictionary (4 ed.). Mosby-Year Book Inc. 1994. p. 1395. ISBN 978-0-8016-7225-5.
- ^ "Seven-year itch".
- ^ Dressler C, Rosumeck S, Sunderkötter C, Werner RN, Nast A (November 2016). "The Treatment of Scabies". Deutsches Ärzteblatt International. 113 (45): 757–762. doi:10.3238/arztebl.2016.0757. PMC 5165060. PMID 27974144.
- ^ a b c d e f "Parasites - Scabies Treatment". Center for Disease Control and Prevention. 2 November 2010. Archived from the original on 28 April 2015. Retrieved 18 May 2015.
- ^ Engelman D, Yoshizumi J, Hay RJ, Osti M, Micali G, Norton S, et al. (November 2020). "The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies". The British Journal of Dermatology. 183 (5): 808–820. doi:10.1111/bjd.18943. PMC 7687112. PMID 32034956.
- ^ a b c d e f g h i j k l m n o p q r s Andrews RM, McCarthy J, Carapetis JR, Currie BJ (December 2009). "Skin disorders, including pyoderma, scabies, and tinea infections". Pediatric Clinics of North America. 56 (6): 1421–1440. doi:10.1016/j.pcl.2009.09.002. PMID 19962029.
- ^ a b Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. (December 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2163–2196. doi:10.1016/S0140-6736(12)61729-2. PMC 6350784. PMID 23245607.
- ^ Georgis' Parasitology for Veterinarians (10 ed.). Elsevier Health Sciences. 2014. p. 68. ISBN 978-1-4557-3988-2.
- ^ a b "Scabies". CDC Parasitology Diagnostic Web Site. Archived from the original on 20 February 2009. Retrieved 9 February 2009.
- ^ a b c d e f g h i j k l m n o p q Hay RJ (2009). "Scabies and pyodermas--diagnosis and treatment". Dermatologic Therapy. 22 (6): 466–474. doi:10.1111/j.1529-8019.2009.01270.x. PMID 19889132. S2CID 41376428.
- ^ a b c Markell EK, John DC, Petri WH (2006). Markell and Voge's medical parasitology (9th ed.). St. Louis, Mo: Elsevier Saunders. ISBN 978-0-7216-4793-7.
- ^ a b Maguire JR (March 2022). "Scabies: Diagnosis and Treatment with Images". DermNet. With link to many images
- ^ a b c Bouvresse S, Chosidow O (April 2010). "Scabies in healthcare settings". Current Opinion in Infectious Diseases. 23 (2): 111–118. doi:10.1097/QCO.0b013e328336821b. PMID 20075729. S2CID 206001293.
- ^ Hicks MI, Elston DM (2009). "Scabies". Dermatologic Therapy. 22 (4): 279–292. doi:10.1111/j.1529-8019.2009.01243.x. PMID 19580575. S2CID 221647574.
- ^ a b "DPDx – Scabies". Laboratory Identification of Parasites of Public Health Concern. CDC. Archived from the original on 20 February 2009.
- ^ André HM (30 June 2019). "The true identity of Pascal's mite and the diachronic use of ciron". Acarologia. 59 (2) 4330: 261–278. doi:10.24349/acarologia/20194330. ISSN 0044-586X.
- ^ a b c d e Walton SF, Currie BJ (April 2007). "Problems in diagnosing scabies, a global disease in human and animal populations". Clinical Microbiology Reviews. 20 (2): 268–279. doi:10.1128/CMR.00042-06. PMC 1865595. PMID 17428886.
- ^ Turkington C, Dover JS (2006). The Encyclopedia of Skin and Skin Disorders. New York: Facts on File inc. ISBN 978-0-8160-6403-8.
- ^ "Scabies Causes". WebMD. October 2010. Archived from the original on 22 September 2010. Retrieved 9 October 2010.
- ^ Chosidow O (April 2006). "Clinical practices. Scabies". The New England Journal of Medicine. 354 (16): 1718–1727. doi:10.1056/NEJMcp052784. PMID 16625010.
- ^ "Scabies – Fast Facts". American Social Health Association. Archived from the original on 22 April 2011. Retrieved 9 October 2010.
- ^ FitzGerald D, Grainger RJ, Reid A (February 2014). "Interventions for preventing the spread of infestation in close contacts of people with scabies". The Cochrane Database of Systematic Reviews. 2014 (2) CD009943. doi:10.1002/14651858.CD009943.pub2. PMC 10819104. PMID 24566946.
- ^ Liu JM, Wang HW, Chang FW, Liu YP, Chiu FH, Lin YC, et al. (2016). "The effects of climate factors on scabies. A 14-year population-based study in Taiwan". Parasite. 23: 54. doi:10.1051/parasite/2016065. PMC 5134670. PMID 27905271.

- ^ Arlian LG (1989). "Biology, host relations, and epidemiology of Sarcoptes scabiei". Annual Review of Entomology. 34 (1): 139–161. doi:10.1146/annurev.en.34.010189.001035. PMID 2494934.
- ^ "Prevention and Control – Scabies". Center for Disease Control and Prevention. Archived from the original on 7 March 2010. Retrieved 9 October 2010.
- ^ Vañó-Galván S, Moreno-Martin P (July 2008). "Generalized pruritus after a beach vacation. Diagnosis: scabies". Cleveland Clinic Journal of Medicine. 75 (7): 474, 478. doi:10.3949/ccjm.75.7.474 (inactive 12 July 2025). PMID 18646583. S2CID 72142958.
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ "Parasites - Scabies". cdc.gov. 2 November 2010. Archived from the original on 11 December 2014. Retrieved 11 December 2014.
- ^ Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS (August 2017). "European guideline for the management of scabies". Journal of the European Academy of Dermatology and Venereology. 31 (8): 1248–1253. doi:10.1111/jdv.14351. PMID 28639722. S2CID 32956377.
- ^ Thomas L (October 2021). "Crusted scabies". DermNet.
- ^ Strong M, Johnstone P (July 2007). Strong M (ed.). "Interventions for treating scabies". The Cochrane Database of Systematic Reviews. 2007 (3) CD000320. doi:10.1002/14651858.CD000320.pub2. PMC 6532717. PMID 17636630.
- ^ a b "Scabies". Illinois Department of Public Health. January 2008. Archived from the original on 5 December 2010. Retrieved 7 October 2010.
- ^ The Pill Book. Bantam Books. 2010. pp. 867–69. ISBN 978-0-553-59340-2.
- ^ Mounsey KE, Pasay CJ, Arlian LG, Morgan MS, Holt DC, Currie BJ, et al. (May 2010). "Increased transcription of Glutathione S-transferases in acaricide exposed scabies mites". Parasites & Vectors. 3: 43. doi:10.1186/1756-3305-3-43. PMC 2890653. PMID 20482766.
- ^ Meyersburg D, Hoellwerth M, Brandlmaier M, Handisurya A, Kaiser A, Prodinger C, et al. (2024). "Comparison of topical permethrin 5% vs. Benzyl benzoate 25% treatment in scabies: A double-blinded randomized controlled trial". British Journal of Dermatology. pp. 486–491. doi:10.1093/bjd/ljad501. PMID 38112640.
- ^ Yürekli A (March 2022). "Is there a really resistance to scabies treatment with permethrin? In vitro killing activity of permethrin on Sarcoptes scabiei from patients with resistant scabies". Dermatologic Therapy. 35 (3) e15260. doi:10.1111/dth.15260. PMID 34897912.
- ^ Victoria J, Trujillo R (2001). "Topical ivermectin: a new successful treatment for scabies". Pediatric Dermatology. 18 (1): 63–65. doi:10.1046/j.1525-1470.2001.018001063.x. PMID 11207977. S2CID 39384922.
- ^ Soll MD, d'Assonville JA, Smith CJ (1992). "Efficacy of topically applied ivermectin against sarcoptic mange (Sarcoptes scabiei var. bovis) of cattle". Parasitology Research. 78 (2): 120–122. doi:10.1007/BF00931652. PMID 1557323. S2CID 28579947.
- ^ Carr PC, Brodell RT (March 2016). "IMAGES IN CLINICAL MEDICINE. Scabies". The New England Journal of Medicine. 374 (11): e13. doi:10.1056/NEJMicm1500116. PMID 26981951.
- ^ Rosumeck S, Nast A, Dressler C (April 2018). "Ivermectin and permethrin for treating scabies". The Cochrane Database of Systematic Reviews. 2018 (4) CD012994. doi:10.1002/14651858.CD012994. PMC 6494415. PMID 29608022.
- ^ Dhana A, Yen H, Okhovat JP, Cho E, Keum N, Khumalo NP (January 2018). "Ivermectin versus permethrin in the treatment of scabies: A systematic review and meta-analysis of randomized controlled trials". Journal of the American Academy of Dermatology. 78 (1): 194–198. doi:10.1016/j.jaad.2017.09.006. PMID 29241784.
- ^ Thadanipon K, Anothaisintawee T, Rattanasiri S, Thakkinstian A, Attia J (May 2019). "Efficacy and safety of antiscabietic agents: A systematic review and network meta-analysis of randomized controlled trials". Journal of the American Academy of Dermatology. 80 (5): 1435–1444. doi:10.1016/j.jaad.2019.01.004. PMID 30654070.

- ^ Crump A, Ōmura S (10 February 2011). "Ivermectin, 'wonder drug' from Japan: the human use perspective". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 87 (2): 13–28. Bibcode:2011PJAB...87...13C. doi:10.2183/pjab.87.13. PMC 3043740. PMID 21321478.
- ^ Laing R, Gillan V, Devaney E (June 2017). "Ivermectin - Old Drug, New Tricks?". Trends in Parasitology. 33 (6): 463–472. doi:10.1016/j.pt.2017.02.004. PMC 5446326. PMID 28285851.

- ^ Humphreys EH, Janssen S, Heil A, Hiatt P, Solomon G, Miller MD (March 2008). "Outcomes of the California ban on pharmaceutical lindane: clinical and ecologic impacts". Environmental Health Perspectives. 116 (3): 297–302. Bibcode:2008EnvHP.116..297H. doi:10.1289/ehp.10668. PMC 2265033. PMID 18335094.
- ^ "FDA Public Health Advisory: Safety of Topical Lindane Products for the Treatment of Scabies and Lice". Fda.gov. 30 April 2009. Archived from the original on 26 November 2010. Retrieved 14 November 2010.
- ^ Jin-gang A, Sheng-xiang X, Sheng-bin X, Jun-min W, Song-mei G, Ying-ying D, et al. (October 2010). "Quality of life of patients with scabies". Journal of the European Academy of Dermatology and Venereology. 24 (10): 1187–1191. doi:10.1111/j.1468-3083.2010.03618.x. PMID 20236379. S2CID 21544520.
- ^ a b c Hay RJ, Steer AC, Chosidow O, Currie BJ (April 2013). "Scabies: a suitable case for a global control initiative". Current Opinion in Infectious Diseases. 26 (2): 107–109. doi:10.1097/QCO.0b013e32835e085b. PMID 23302759. S2CID 26416151.
- ^ a b c d Engelman D, Kiang K, Chosidow O, McCarthy J, Fuller C, Lammie P, et al. (2013). "Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies". PLOS Neglected Tropical Diseases. 7 (8) e2167. doi:10.1371/journal.pntd.0002167. PMC 3738445. PMID 23951369.
- ^ Green MS (1989). "Epidemiology of scabies". Epidemiologic Reviews. 11 (1): 126–150. doi:10.1093/oxfordjournals.epirev.a036033. PMID 2509232.
- ^ Hicks MI, Elston DM (July–August 2009). "Scabies". Dermatologic Therapy. 22 (4): 279–292. doi:10.1111/j.1529-8019.2009.01243.x. PMID 19580575. S2CID 221647574.
- ^ a b "Scabies homepage". Stanford University. Archived from the original on 13 May 2010. Retrieved 9 October 2010.
- ^ a b c Roncalli RA (July 1987). "The history of scabies in veterinary and human medicine from biblical to modern times". Veterinary Parasitology. 25 (2): 193–198. doi:10.1016/0304-4017(87)90104-X. PMID 3307123.
- ^ "Ibn Zuhr | Encyclopedia.com". www.encyclopedia.com.
- ^ Goldsmith WN (1945). "Wilkinson's ointment". Br Med J. 1 (4392): 347–48. doi:10.1136/bmj.1.4392.347-c. PMC 2056959.
- ^ Blair J (1995). Anne Frank Remembered (motion picture).
- ^ Borgman W (June 30, 2006). Dog mange called scabies can transfer to humans. Orlando Sentinel archive Archived 2015-02-16 at the Wayback Machine. Retrieved February 16, 2015.
- ^ "Bali Animal Welfare Association". Archived from the original on 26 February 2010. Retrieved 28 July 2009.
- ^ "Uganda: Out of the Wild". Frontline. PBS. Archived from the original on 5 November 2013. Transcript | A Death In Tehran | FRONTLINE | PBS (section on rare diseases in Uganda). Retrieved 4 November 2013.
- ^ Old JM, Sengupta C, Narayan E, Wolfenden J (2018). Sarcoptic mange in wombats – A review and future research directions. Transboundary and Emerging Diseases. 65, 399-407. DOI: 10.1111/tbed.12770
- ^ Reichard MV (15 May 2015). "Mange in Cattle - Integumentary System". Merck Veterinary Manual. Retrieved 22 April 2022.
- ^ Patrick CD (2014). "Cattle Scabies" (PDF). Beef Cattle Handbook. Texas A & M University Extension Beef Cattle Resource Committee. pp. 1–3.
- ^ Roberts IH, Cobbett NG. "Cattle scabies". Yearbook of Agriculture. Washington, DC: US Department of Agriculture. p. 591.: 292–297
- ^ "Beef cattle-Scabies mite". Pacific Northwest Pest Management Handbooks. Pacific Northwest Extension (Oregon, Washington, Idaho). 22 October 2015. Retrieved 22 April 2022.
- ^ "International Alliance for the Control of Scabies". International Alliance for the Control of Scabies. Archived from the original on 2 February 2014. Retrieved 1 February 2014.
- ^ "The 17 neglected tropical diseases". Neglected tropical diseases. World Health Organization. Archived from the original on 22 February 2014. Retrieved 1 February 2014.
- ^ Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS (March 2016). "Prospects for Moxidectin as a New Oral Treatment for Human Scabies". PLOS Neglected Tropical Diseases. 10 (3) e0004389. doi:10.1371/journal.pntd.0004389. PMC 4795782. PMID 26985995.
- ^ Prichard R, Ménez C, Lespine A (December 2012). "Moxidectin and the avermectins: Consanguinity but not identity". International Journal for Parasitology. Drugs and Drug Resistance. 2: 134–153. doi:10.1016/j.ijpddr.2012.04.001. PMC 3862425. PMID 24533275.
- ^ Thomas J, Carson CF, Peterson GM, Walton SF, Hammer KA, Naunton M, et al. (February 2016). "Therapeutic Potential of Tea Tree Oil for Scabies". The American Journal of Tropical Medicine and Hygiene. 94 (2): 258–266. doi:10.4269/ajtmh.14-0515. PMC 4751955. PMID 26787146.
Further reading
[edit]- Lokuge B, Burke T (2014). A Doctor's Dream: A Story of Hope from the Top End. Sydney: Allen & Unwin. ISBN 978-1-76011-098-7. OCLC 1348975937.
- Craig E, ed. (2022). The Itch: Scabies. Oxford University Press. ISBN 978-0-19-284840-6. OCLC 1317800330.
- Friedman R (1947). The Story of Scabies. Froben Press. ASIN B0007FK79C. OCLC 9127739.