Recent from talks
Nothing was collected or created yet.
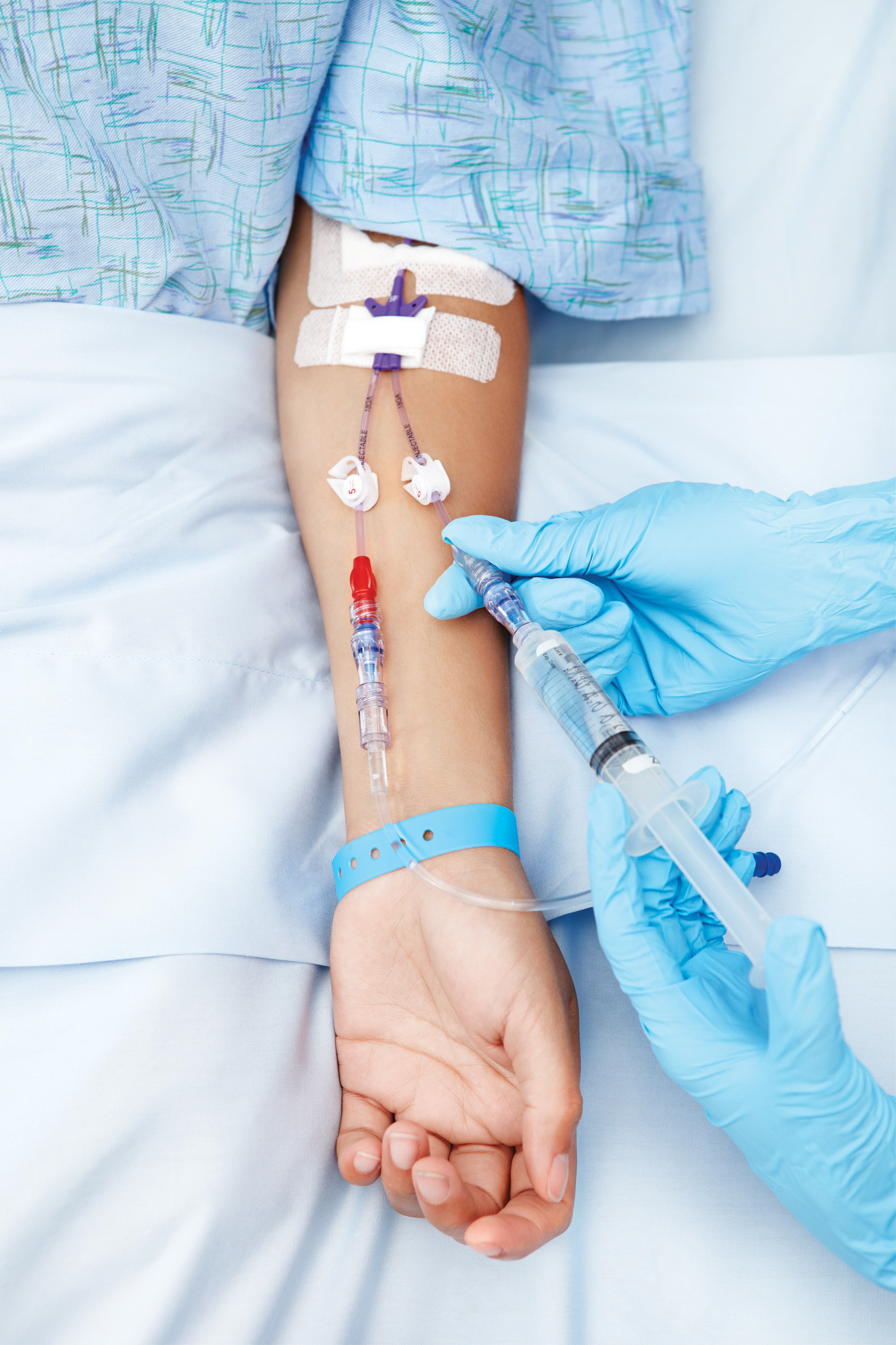
Intravenous therapy
View on Wikipedia
| Intravenous therapy | |
|---|---|
 A person receiving a medication through an intravenous line (cannula) | |
| Other names | IV therapy |
| ICD-9-CM | 38.93 |
| MeSH | D007262 |
Intravenous therapy (abbreviated as IV therapy) is a medical process that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use.
The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consumption of some recreational drugs. Many therapies are administered as a "bolus" or one-time dose, but they may also be administered as an extended infusion or drip. The act of administering a therapy intravenously, or placing an intravenous line ("IV line") for later use, is a procedure which should only be performed by a skilled professional. The most basic intravenous access consists of a needle piercing the skin and entering a vein which is connected to a syringe or to external tubing. This is used to administer the desired therapy. In cases where a patient is likely to receive many such interventions in a short period (with consequent risk of trauma to the vein), normal practice is to insert a cannula which leaves one end in the vein, and subsequent therapies can be administered easily through tubing at the other end. In some cases, multiple medications or therapies are administered through the same IV line.
IV lines are classified as "central lines" if they end in a large vein close to the heart, or as "peripheral lines" if their output is to a small vein in the periphery, such as the arm. An IV line can be threaded through a peripheral vein to end near the heart, which is termed a "peripherally inserted central catheter" or PICC line. If a person is likely to need long-term intravenous therapy, a medical port may be implanted to enable easier repeated access to the vein without having to pierce the vein repeatedly. A catheter can also be inserted into a central vein through the chest, which is known as a tunneled line. The specific type of catheter used and site of insertion are affected by the desired substance to be administered and the health of the veins in the desired site of insertion.
Placement of an IV line may cause pain, as it necessarily involves piercing the skin. Infections and inflammation (termed phlebitis) are also both common side effects of an IV line. Phlebitis may be more likely if the same vein is used repeatedly for intravenous access, and can eventually develop into a hard cord which is unsuitable for IV access. The unintentional administration of a therapy outside a vein, termed extravasation or infiltration, may cause other side effects.
Uses
[edit]Medical uses
[edit]Intravenous (IV) access is used to administer medications and fluid replacement which must be distributed throughout the body, especially when rapid distribution is desired. Another use of IV administration is the avoidance of first-pass metabolism in the liver. Substances that may be infused intravenously include volume expanders, blood-based products, blood substitutes, medications and nutrition.
Fluid solutions
[edit]Fluids may be administered as part of "volume expansion", or fluid replacement, through the intravenous route. Volume expansion consists of the administration of fluid-based solutions or suspensions designed to target specific areas of the body which need more water. There are two main types of volume expander: crystalloids and colloids. Crystalloids are aqueous solutions of mineral salts or other water-soluble molecules. Colloids contain larger insoluble molecules, such as gelatin. Blood itself is considered a colloid.[1]
The most commonly used crystalloid fluid is normal saline, a solution of sodium chloride at 0.9% concentration, which is isotonic with blood. Lactated Ringer's (also known as Ringer's lactate) and the closely related Ringer's acetate, are mildly hypotonic solutions often used in those who have significant burns. Colloids preserve a high colloid osmotic pressure in the blood, while, on the other hand, this parameter is decreased by crystalloids due to hemodilution.[2] Crystalloids generally are much cheaper than colloids.[2]
Buffer solutions which are used to correct acidosis or alkalosis are also administered through intravenous access. Lactated Ringer's solution used as a fluid expander or base solution to which medications are added also has some buffering effect. Another solution administered intravenously as a buffering solution is sodium bicarbonate.[3]
Medication and treatment
[edit]
Medications may be mixed into the fluids mentioned above, commonly normal saline, or dextrose solutions.[4] Compared with other routes of administration, such as oral medications, the IV route is the fastest way to deliver fluids and medications throughout the body.[5] For this reason, the IV route is commonly preferred in emergency situations or when a fast onset of action is desirable. In extremely high blood pressure (termed a hypertensive emergency), IV antihypertensives may be given to quickly decrease the blood pressure in a controlled manner to prevent organ damage.[6] In atrial fibrillation, IV amiodarone may be administered to attempt to restore normal heart rhythm.[7] IV medications can also be used for chronic health conditions such as cancer, for which chemotherapy drugs are commonly administered intravenously. In some cases, such as with vancomycin, a loading or bolus dose of medicine is given before beginning a dosing regimen to more quickly increase the concentration of medication in the blood.[8]
The bioavailability of an IV medication is by definition 100%, unlike oral administration where medication may not be fully absorbed, or may be metabolized prior to entering the bloodstream.[4] For some medications, there is virtually zero oral bioavailability. For this reason certain types of medications can only be given intravenously, as there is insufficient uptake by other routes of administration,[9] such is the case of severe dehydration where the patient is required to be treated via IV therapy for a quick recovery.[10] The unpredictability of oral bioavailability in different people is also a reason for a medication to be administered IV, as with furosemide.[11] Oral medications also may be less desirable if a person is nauseous or vomiting, or has severe diarrhea, as these may prevent the medicine from being fully absorbed from the gastrointestinal tract. In these cases, a medication may be given IV only until the patient can tolerate an oral form of the medication. The switch from IV to oral administration is usually performed as soon as viable, as there is generally cost and time savings over IV administration. Whether a medication can be potentially switched to an oral form is sometimes considered when choosing appropriate antibiotic therapy for use in a hospital setting, as a person is unlikely to be discharged if they still require IV therapy.[12]
Some medications, such as aprepitant, are chemically modified to be better suited for IV administration, forming a prodrug such as fosaprepitant. This can be for pharmacokinetic reasons or to delay the effect of the drug until it can be metabolized into the active form.[13]
Blood products
[edit]A blood product (or blood-based product) is any component of blood which is collected from a donor for use in a blood transfusion.[14] Blood transfusions can be used in massive blood loss due to trauma, or can be used to replace blood lost during surgery. Blood transfusions may also be used to treat a severe anaemia or thrombocytopenia caused by a blood disease. Early blood transfusions consisted of whole blood, but modern medical practice commonly uses only components of the blood, such as packed red blood cells, fresh frozen plasma or cryoprecipitate.[15]
Nutrition
[edit]
Parenteral nutrition is the act of providing required nutrients to a person through an intravenous line. This is used in people who are unable to get nutrients normally, by eating and digesting food. A person receiving parenteral nutrition will be given an intravenous solution which may contain salts, dextrose, amino acids, lipids and vitamins. The exact formulation of a parenteral nutrition used will depend on the specific nutritional needs of the person it is being given to. If a person is only receiving nutrition intravenously, it is called total parenteral nutrition (TPN), whereas if a person is only receiving some of their nutrition intravenously it is called partial parenteral nutrition (or supplemental parenteral nutrition).[16]
Imaging
[edit]Medical imaging relies on being able to clearly distinguish internal parts of the body from each other. One way this is accomplished is through the administration of a contrast agent into a vein.[17] The specific imaging technique being employed will determine the characteristics of an appropriate contrast agent to increase visibility of blood vessels or other features. Common contrast agents are administered into a peripheral vein from which they are distributed throughout the circulation to the imaging site.[18]
Other uses
[edit]Use in sports
[edit]IV rehydration was formerly a common technique for athletes.[19] The World Anti-Doping Agency prohibits intravenous injection of more than 100 mL per 12 hours, except under a medical exemption.[19] The United States Anti-Doping Agency notes that, as well as the dangers inherent in IV therapy, "IVs can be used to change blood test results (such as hematocrit where EPO or blood doping is being used), mask urine test results (by dilution) or by administering prohibited substances in a way that will more quickly be cleared from the body in order to beat an anti-doping test".[19] Players suspended after attending "boutique IV clinics" which offer this sort of treatment include footballer Samir Nasri in 2017[20] and swimmer Ryan Lochte in 2018.[21]
Use for hangover treatment
[edit]In the 1960s, John Myers developed the "Myers' cocktail", a non-prescription IV solution of vitamins and minerals marketed as a hangover cure and general wellness remedy.[22] The first "boutique IV" clinic, offering similar treatments, opened in Tokyo in 2008.[22] These clinics, whose target market was described by Elle as "health nuts who moonlight as heavy drinkers", have been publicized in the 2010s by glamorous celebrity customers.[22] Intravenous therapy is also used in people with acute ethanol toxicity to correct electrolyte and vitamin deficiencies which arise from alcohol consumption.[23]
Others
[edit]In some countries, non-prescription intravenous glucose is used to improve a person's energy, but is not a part of routine medical care in countries such as the United States where glucose solutions are prescription drugs.[24] Improperly administered intravenous glucose (called "ringer" [citation needed]), such as that which is administered clandestinely in store-front clinics, poses increased risks due to improper technique and oversight.[24] Intravenous access is also sometimes used outside of a medical setting for the self-administration of recreational drugs, such as heroin and fentanyl, cocaine, methamphetamine, DMT, and others.[25]
Intravenous therapy is also used for veterinary patient management.[26]
Types
[edit]Bolus
[edit]Some medications can be administered as a bolus dose, which is called an "IV push". A syringe containing the medication is connected to an access port in the primary tubing and the medication is administered through the port.[27] A bolus may be administered rapidly (with a fast depression of the syringe plunger) or may be administered slowly, over the course of a few minutes.[27] The exact administration technique depends on the medication and other factors.[27] In some cases, a bolus of plain IV solution (i.e. without medication added) is administered immediately after the bolus to further force the medicine into the bloodstream. This procedure is termed an "IV flush". Certain medications, such as potassium, are not able to be administered by IV push due to the extremely rapid onset of action and high level of effects.[27]
Infusion
[edit]An infusion of medication may be used when it is desirable to have a constant blood concentration of a medication over time, such as with some antibiotics including beta-lactams.[28] Continuous infusions, where the next infusion is begun immediately following the completion of the prior, may also be used to limit variation in drug concentration in the blood (i.e. between the peak drug levels and the trough drug levels).[28] They may also be used instead of intermittent bolus injections for the same reason, such as with furosemide.[29] Infusions can also be intermittent, in which case the medication is administered over a period of time, then stopped, and this is later repeated. Intermittent infusion may be used when there are concerns about the stability of medicine in solution for long periods of time (as is common with continuous infusions), or to enable the administration of medicines which would be incompatible if administered at the same time in the same IV line, for example vancomycin.[30]
Failure to properly calculate and administer an infusion can result in adverse effects, termed infusion reactions. For this reason, many medications have a maximum recommended infusion rate, such as vancomycin[30] and many monoclonal antibodies.[31] These infusion reactions can be severe, such as in the case of vancomycin, where the reaction is termed "red man syndrome".[30]
Secondary
[edit]Any additional medication to be administered intravenously at the same time as an infusion may be connected to the primary tubing; this is termed a secondary IV, or IV piggyback.[27] This prevents the need for multiple IV access lines on the same person. When administering a secondary IV medication, the primary bag is held lower than the secondary bag so that the secondary medication can flow into the primary tubing, rather than fluid from the primary bag flowing into the secondary tubing. The fluid from the primary bag is needed to help flush any remaining medication from the secondary IV from the tubing.[27] If a bolus or secondary infusion is intended for administration in the same line as a primary infusion, the molecular compatibility of the solutions must be considered.[27] Secondary compatibility is generally referred to as "y-site compatibility", named after the shape of the tubing which has a port for bolus administration.[27] Incompatibility of two fluids or medications can arise due to issues of molecular stability, changes in solubility, or degradation of one of the medications.[27]
Methods and equipment
[edit]Access
[edit]


The simplest form of intravenous access is by passing a hollow needle through the skin directly into a vein. A syringe can be connected directly to this needle, which allows for a "bolus" dose to be administered. Alternatively, the needle may be placed and then connected to a length of tubing, allowing for an infusion to be administered.[32]: 344–348 The type and location of venous access (i.e. a central line versus peripheral line, and in which vein the line is placed) can be affected by the potential for some medications to cause peripheral vasoconstriction, which limits circulation to peripheral veins.[33]
A peripheral cannula is the most common intravenous access method utilized in hospitals, pre-hospital care, and outpatient medicine. This may be placed in the arm, commonly either the wrist or the median cubital vein at the elbow. A tourniquet may be used to restrict the venous drainage of the limb and make the vein bulge, making it easier to locate and place a line in a vein. When used, a tourniquet should be removed before injecting medication to prevent extravasation. The part of the catheter that remains outside the skin is called the connecting hub; it can be connected to a syringe or an intravenous infusion line, or capped with a heplock or saline lock, a needleless connection filled with a small amount of heparin or saline solution to prevent clotting, between uses of the catheter. Ported cannulae have an injection port on the top that is often used to administer medicine.[32]: 349–354
The thickness and size of needles and catheters can be given in Birmingham gauge or French gauge. A Birmingham gauge of 14 is a very large cannula (used in resuscitation settings) and 24-26 is the smallest. The most common sizes are 16-gauge (midsize line used for blood donation and transfusion), 18- and 20-gauge (all-purpose line for infusions and blood draws), and 22-gauge (all-purpose pediatric line). 12- and 14-gauge peripheral lines are capable of delivering large volumes of fluid very fast, accounting for their popularity in emergency medicine. These lines are frequently called "large bores" or "trauma lines".[32]: 188–191, 349
Peripheral lines
[edit]
A peripheral intravenous line is inserted in peripheral veins, such as the veins in the arms, hands, legs and feet. Medication administered in this way travels through the veins to the heart, from where it is distributed to the rest of the body through the circulatory system. The size of the peripheral vein limits the amount and rate of medication which can be administered safely.[35] A peripheral line consists of a short catheter inserted through the skin into a peripheral vein. This is usually in the form of a cannula-over-needle device, in which a flexible plastic cannula comes mounted over a metal trocar. Once the tip of the needle and cannula are placed, the cannula is advanced inside the vein over the trocar to the appropriate position and secured. The trocar is then withdrawn and discarded. Blood samples may also be drawn from the line directly after the initial IV cannula insertion.[32]: 344–348


Central lines
[edit]A central line is an access method in which a catheter empties into a larger, more central vein (a vein within the torso), usually the superior vena cava, inferior vena cava or the right atrium of the heart. There are several types of central IV access, categorized based on the route the catheter takes from the outside of the body to the central vein output.[36]: 17–22
Peripherally inserted central catheter
[edit]A peripherally inserted central catheter (also called a PICC line) is a type of central IV access which consists of a cannula inserted through a sheath into a peripheral vein and then carefully fed towards the heart, terminating at the superior vena cava or the right atrium. These lines are usually placed in peripheral veins in the arm, and may be placed using the Seldinger technique under ultrasound guidance. An X-ray is used to verify that the end of the cannula is in the right place if fluoroscopy was not used during the insertion. An EKG can also be used in some cases to determine if the end of the cannula is in the correct location.[37]: Ch.1, 5, 6
Tunneled lines
[edit]
A tunneled line is a type of central access which is inserted under the skin, and then travels a significant distance through surrounding tissue before reaching and penetrating the central vein. Using a tunneled line reduces the risk of infection as compared to other forms of access, as bacteria from the skin surface are not able to travel directly into the vein.[38] These catheters are often made of materials that resist infection and clotting. Types of tunneled central lines include the Hickman line or Broviac catheter. A tunnelled line is an option for long term venous access necessary for hemodialysis in people with poor kidney function.[39]
Implantable ports
[edit]An implanted port is a central line that does not have an external connector protruding from the skin for administration of medication. Instead, a port consists of a small reservoir covered with silicone rubber which is implanted under the skin, which then covers the reservoir. Medication is administered by injecting medication through the skin and the silicone port cover into the reservoir. When the needle is withdrawn, the reservoir cover reseals itself. A port cover is designed to function for hundreds of needle sticks during its lifetime. Ports may be placed in an arm or in the chest area.[40]
Infusions
[edit]Equipment used to place and administer an IV line for infusion consists of a bag, usually hanging above the height of the person, and sterile tubing through which the medicine is administered. In a basic "gravity" IV, a bag is simply hung above the height of the person and the solution is pulled via gravity through a tube attached to a needle inserted into a vein. Without extra equipment, it is not possible to precisely control the rate of administration. For this reason, a setup may also incorporate a clamp to regulate flow. Some IV lines may be placed with "Y-sites", devices which enable a secondary solution to be administered through the same line (known as piggybacking). Some systems employ a drip chamber, which prevents air from entering the bloodstream (causing an air embolism), and allows visual estimation of flow rate of the solution.[32]: 316–321, 344–348

Alternatively, an infusion pump allows precise control over the flow rate and total amount delivered. A pump is programmed based on the number and size of infusions being administered to ensure all medicine is fully administered without allowing the access line to run dry. Pumps are primarily utilized when a constant flow rate is important, or where changes in rate of administration would have consequences.[32]: 316–321, 344–348
Techniques
[edit]To reduce pain associated with the procedure, medical staff may apply a topical local anaesthetic (such as EMLA or Ametop) to the skin of the chosen venipuncture area about 45 minutes beforehand.[32]: 344–348
If the cannula is not inserted correctly, or the vein is particularly fragile and ruptures, blood may extravasate into the surrounding tissues; this situation is known as a blown vein or "tissuing". Using this cannula to administer medications causes extravasation of the drug, which can lead to edema, causing pain and tissue damage, and even necrosis depending on the medication. The person attempting to obtain the access must find a new access site proximal to the "blown" area to prevent extravasation of medications through the damaged vein. For this reason it is advisable to site the first cannula at the most distal appropriate vein.[32]: 355–359
Adverse effects
[edit]Pain
[edit]Placement of an intravenous line inherently causes pain when the skin is broken and is considered medically invasive. For this reason, when other forms of administration may suffice, intravenous therapy is usually not preferred. This includes the treatment of mild or moderate dehydration with oral rehydration therapy which is an option, as opposed to parenteral rehydration through an IV line.[41][42] Children in emergency departments being treated for dehydration have better outcomes with oral treatment than intravenous therapy due to the pain and complications of an intravenous line.[41] Cold spray may decrease the pain of putting in an IV.[43]
Certain medications also have specific sensations of pain associated with their administration IV. This includes potassium, which when administered IV can cause a burning or painful sensation.[44] The incidence of side effects specific to a medication can be affected by the type of access (peripheral versus central), the rate of administration, or the quantity of drug administered. When medications are administered too rapidly through an IV line, a set of vague symptoms such as redness or rash, fever, and others may occur; this is termed an "infusion reaction" and is prevented by decreasing the rate of administration of the medication. When vancomycin is involved, this is commonly termed "Red Man syndrome" after the rapid flushing which occurs after rapid administration.[45]
Infection and inflammation
[edit]As placement of an intravenous line requires breaking the skin, there is a risk of infection. Skin-dwelling organisms such as coagulase-negative staphylococcus or Candida albicans may enter through the insertion site around the catheter, or bacteria may be accidentally introduced inside the catheter from contaminated equipment. Infection of an IV access site is usually local, causing easily visible swelling, redness, and fever. However, pathogens may also enter the bloodstream, causing sepsis, which can be sudden and life-threatening. A central IV line poses a higher risk of sepsis, as it can deliver bacteria directly into the central circulation. A line which has been in place for a longer period of time also increases the risk of infection.[32]: 358, 373
Inflammation of the vein may also occur, called thrombophlebitis or simply phlebitis. This may be caused by infection, the catheter itself, or the specific fluids or medication being given. Repeated instances of phlebitis can cause scar tissue to build up along a vein. A peripheral IV line cannot be left in the vein indefinitely out of concern for the risk of infection and phlebitis, among other potential complications. However, recent studies have found that there is no increased risk of complications in those whose IVs were replaced only when clinically indicated versus those whose IVs were replaced routinely.[46] If placed with proper aseptic technique, it is not recommended to change a peripheral IV line more frequently than every 72–96 hours.[47]
Phlebitis is particularly common in intravenous drug users,[48] and those undergoing chemotherapy,[49] whose veins can become sclerotic and difficult to access over time, sometimes forming a hard, painful "venous cord". The presence of a cord is a cause of discomfort and pain associated with IV therapy, and makes it more difficult for an IV line to be placed as a line cannot be placed in an area with a cord.[50]
Infiltration and extravasation
[edit]Infiltration occurs when a non-vesicant IV fluid or medication enters the surrounding tissue as opposed to the desired vein. It may occur when the vein itself ruptures, when the vein is damaged during insertion of the intravascular access device, or from increased vein porosity. Infiltration may also occur if the puncture of the vein by the needle becomes the path of least resistance—such as a cannula which has been left inserted, causing the vein to scar. It can also occur upon insertion of an IV line if a tourniquet is not promptly removed. Infiltration is characterized by coolness and pallor to the skin as well as localized swelling or edema. It is treated by removing the intravenous line and elevating the affected limb so the collected fluids drain away. Injections of hyaluronidase around the area can be used to speed the dispersal of the fluid/drug.[51] Infiltration is one of the most common adverse effects of IV therapy[52] and is usually not serious unless the infiltrated fluid is a medication damaging to the surrounding tissue, most commonly a vesicant or chemotherapeutic agent. In such cases, the infiltration is termed extravasation, and may cause necrosis.[53]
Others
[edit]If the solutions administered are colder than the temperature of the body, induced hypothermia can occur. If the temperature change to the heart is rapid, ventricular fibrillation may result.[54] Furthermore, if a solution which is not balanced in concentration is administered, a person's electrolytes may become imbalanced. In hospitals, regular blood tests may be used to proactively monitor electrolyte levels.[55]
History
[edit]Discovery and development
[edit]The first recorded attempt at administering a therapeutic substance via IV injection was in 1492, when Pope Innocent VIII fell ill and was administered blood from healthy individuals.[56] If this occurred, the treatment did not work and resulted in the death of the donors while not healing the pope.[56] This story is disputed by some, who claim that the idea of blood transfusions could not have been considered by the medical professionals at the time, or that a complete description of blood circulation was not published until over 100 years later. The story is attributed to potential errors in translation of documents from the time, as well as potentially an intentional fabrication, whereas others still consider it to be accurate.[57] One of the leading medical history textbooks for medical and nursing students has claimed that the entire story was an anti-semitic fabrication.[58]
In 1656 Sir Christopher Wren and Robert Boyle worked on the subject. As stated by Wren, "I Have Injected Wine and Ale in a liveing Dog into the Mass of Blood by a Veine, in good Quantities, till I have made him extremely drunk, but soon after he Pisseth it out." The dog survived, grew fat, and was later stolen from his owner. Boyle attributed authorship to Wren.[59]
Richard Lower showed it was possible for blood to be transfused from animal to animal and from animal to man intravenously, a xenotransfusion. He worked with Edmund King to transfuse sheep's blood into a man who was mentally ill. Lower was interested in advancing science but also believed the man could be helped, either by the infusion of fresh blood or by the removal of old blood. It was difficult to find people who would agree to be transfused, but an eccentric scholar, Arthur Coga, consented and the procedure was carried out by Lower and King before the Royal Society on 23 November 1667.[60] Transfusion gathered some popularity in France and Italy, but medical and theological debates arose, resulting in transfusion being prohibited in France.
There was virtually no recorded success with any attempts at injection therapy until the 1800s, when in 1831 Thomas Latta studied the use of IV fluid replacements for cholera treatment.[56][61] The first solutions which saw widespread use for IV injections were simple "saline-like solutions", which were followed by experiments with various other liquids, including milk, sugar, honey, and egg yolk.[56] In the 1830s, James Blundell, an English obstetrician, used intravenous administration of blood to treat women bleeding profusely during or after delivery.[56] This predated the understanding of blood type, leading to unpredictable results.
Modern usage
[edit]Intravenous therapy was expanded by Italian physician Guido Baccelli in the late 1890s[62] and further developed in the 1930s by Samuel Hirschfeld, Harold T. Hyman and Justine Johnstone Wanger[63][64] but was not widely available until the 1950s.[65] There was a time, roughly the 1910s–1920s, when fluid replacement that today would be done intravenously was likelier to be done with a Murphy drip, a rectal infusion; and IV therapy took years to increasingly displace that route. In the 1960s, the concept of providing a person's complete nutritional needs through an IV solution began to be seriously considered. The first parenteral nutrition supplementation consisted of hydrolyzed proteins and dextrose.[56] This was followed in 1975 with the introduction of intravenous fat emulsions and vitamins which were added to form "total parenteral nutrition", or that which includes protein, fat, and carbohydrates.[56]
See also
[edit]References
[edit]- ^ Noonpradej S, Akaraborworn O (3 August 2020). "Intravenous Fluid of Choice in Major Abdominal Surgery: A Systematic Review". Critical Care Research and Practice. 2020: 1–19. doi:10.1155/2020/2170828. PMC 7421038. PMID 32832150.
- ^ a b Martin GS. "An Update on Intravenous Fluids". Medscape. WebMD. Retrieved 25 August 2020.
- ^ Fujii T, Udy A, Licari E, Romero L, Bellomo R (June 2019). "Sodium bicarbonate therapy for critically ill patients with metabolic acidosis: A scoping and a systematic review". Journal of Critical Care. 51: 184–191. doi:10.1016/j.jcrc.2019.02.027. PMID 30852347. S2CID 73725286.
- ^ a b Flynn E (2007). "Pharmacokinetic Parameters". xPharm: The Comprehensive Pharmacology Reference. Elsevier. pp. 1–3. doi:10.1016/b978-008055232-3.60034-0. ISBN 978-0-08-055232-3.
- ^ "What is an IV Vitamin Therapy? A Complete Guide by Nepenthe". Retrieved 2022-09-02.
- ^ Peacock WF, Hilleman DE, Levy PD, Rhoney DH, Varon J (July 2012). "A systematic review of nicardipine vs labetalol for the management of hypertensive crises". The American Journal of Emergency Medicine. 30 (6): 981–993. doi:10.1016/j.ajem.2011.06.040. PMID 21908132.
- ^ Vardas PE, Kochiadakis GE (September 2003). "Amiodarone for the Restoration of Sinus Rhythm in Patients with Atrial Fibrillation". Cardiac Electrophysiology Review. 7 (3): 297–299. doi:10.1023/B:CEPR.0000012400.34597.00. PMID 14739732.
- ^ Álvarez R, López Cortés LE, Molina J, Cisneros JM, Pachón J (May 2016). "Optimizing the Clinical Use of Vancomycin". Antimicrobial Agents and Chemotherapy. 60 (5): 2601–2609. doi:10.1128/AAC.03147-14. PMC 4862470. PMID 26856841. S2CID 9560849.
- ^ Doyle GR, McCutcheon JA (13 November 2015). "7.5". Clinical Procedures for Safer Patient Care. Victoria, BC: BCcampus.
- ^ "IV Fluids". Cleveland Clinic. Retrieved 2023-09-30.
- ^ Boles Ponto LL, Schoenwald RD (1 May 1990). "Furosemide (Frusemide) A Pharmacokinetic/Pharmacodynamic Review (Part I)". Clinical Pharmacokinetics. 18 (5): 381–408. doi:10.2165/00003088-199018050-00004. PMID 2185908. S2CID 32352501.
- ^ Wetzstein GA (March 2000). "Intravenous to oral (iv:po) anti-infective conversion therapy". Cancer Control. 7 (2): 170–6. doi:10.1177/107327480000700211. PMID 10783821.
- ^ Patel P, Leeder JS, Piquette-Miller M, Dupuis LL (October 2017). "Aprepitant and fosaprepitant drug interactions: a systematic review". British Journal of Clinical Pharmacology. 83 (10): 2148–2162. doi:10.1111/bcp.13322. PMC 5595939. PMID 28470980.
- ^ "Blood Transfusion | National Heart, Lung, and Blood Institute (NHLBI)". www.nhlbi.nih.gov. Retrieved 2019-06-16.
- ^ Avery P, Morton S, Tucker H, Green L, Weaver A, Davenport R (June 2020). "Whole blood transfusion versus component therapy in adult trauma patients with acute major haemorrhage". Emergency Medicine Journal. 37 (6): 370–378. doi:10.1136/emermed-2019-209040. PMID 32376677. S2CID 218532376.
- ^ Halter JB, Ouslander JG, Studenski S, High KP, Asthana S, Supiano MA, Ritchie C (23 December 2016). "Chapter 35". In Edmonson KG, Davis KJ (eds.). Hazzard's geriatric medicine and gerontology (Seventh ed.). New York: McGraw Hill. ISBN 978-0-07-183345-5.
- ^ Runge VM, Ai T, Hao D, Hu X (December 2011). "The developmental history of the gadolinium chelates as intravenous contrast media for magnetic resonance". Investigative Radiology. 46 (12): 807–16. doi:10.1097/RLI.0b013e318237913b. PMID 22094366. S2CID 8425664.
- ^ Rawson JV, Pelletier AL (1 September 2013). "When to Order a Contrast-Enhanced CT". American Family Physician. 88 (5): 312–6. PMID 24010394.
- ^ a b c "IV Infusion: Explanatory Note". U.S. Anti-Doping Agency (USADA). 5 January 2018. Retrieved 24 July 2018.
- ^ Press Association (1 August 2018). "Samir Nasri's doping ban extended from six to 18 months after appeal by Uefa". The Guardian. Retrieved 2 August 2018.
- ^ Caron E (23 July 2018). "Ryan Lochte suspended 14 months for anti-doping violation". Sports Illustrated. Retrieved 24 July 2018.
- ^ a b c Hess A (23 April 2014). "The Party Girl Drip". Elle. Retrieved 24 July 2018.
- ^ Flannery AH, Adkins DA, Cook AM (August 2016). "Unpeeling the Evidence for the Banana Bag: Evidence-Based Recommendations for the Management of Alcohol-Associated Vitamin and Electrolyte Deficiencies in the ICU". Critical Care Medicine. 44 (8): 1545–1552. doi:10.1097/CCM.0000000000001659. PMID 27002274. S2CID 22431890.
- ^ a b Jiha Ham (March 20, 2015). "A Life Upended After an IV Glucose Treatment Popular Among Asian Immigrants". The New York Times. Retrieved March 21, 2015.
Although many doctors warn Asian immigrants in New York that the effects of injecting glucose differ little from drinking sugary water, many Asians, especially of older generations, still use the intravenous solution. In their homelands, it is commonly prescribed by doctors as a method to cure colds, fevers and sometimes an upset stomach.
- ^ Han Y, Yan W, Zheng Y, Khan MZ, Yuan K, Lu L (11 November 2019). "The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies". Translational Psychiatry. 9 (1): 282. doi:10.1038/s41398-019-0625-0. PMC 6848196. PMID 31712552.
- ^ Cooper E, Guillaumin J, Yaxley P, Her J, Young A (2022). Small Animal Fluid Therapy. CABI (Centre for Agriculture and Bioscience International). doi:10.1079/9781789243406.0000. ISBN 978-1-78924-338-3. S2CID 251612116. ISBN 978-1-78924-339-0. ISBN 978-1-78924-340-6.
- ^ a b c d e f g h i Kanji S, Lam J, Johanson C, Singh A, Goddard R, Fairbairn J, Lloyd T, Monsour D, Kakal J (September 2010). "Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units". Critical Care Medicine. 38 (9): 1890–1898. doi:10.1097/CCM.0b013e3181e8adcc. PMID 20562698. S2CID 205539703.
- ^ a b Dhaese S, Heffernan A, Liu D, Abdul-Aziz MH, Stove V, Tam VH, Lipman J, Roberts JA, De Waele JJ (25 July 2020). "Prolonged Versus Intermittent Infusion of β-Lactam Antibiotics: A Systematic Review and Meta-Regression of Bacterial Killing in Preclinical Infection Models". Clinical Pharmacokinetics. 59 (10): 1237–1250. doi:10.1007/s40262-020-00919-6. PMID 32710435. S2CID 220732187.
- ^ Chan JS, Kot TK, Ng M, Harky A (November 2019). "Continuous Infusion Versus Intermittent Boluses of Furosemide in Acute Heart Failure: A Systematic Review and Meta-Analysis". Journal of Cardiac Failure. 26 (9): 786–793. doi:10.1016/j.cardfail.2019.11.013. PMID 31730917. S2CID 208063606.
- ^ a b c Elbarbry F (June 2018). "Vancomycin Dosing and Monitoring: Critical Evaluation of the Current Practice". European Journal of Drug Metabolism and Pharmacokinetics. 43 (3): 259–268. doi:10.1007/s13318-017-0456-4. PMID 29260505. S2CID 13071392.
- ^ Bylsma LC, Dean R, Lowe K, Sangaré L, Alexander DD, Fryzek JP (September 2019). "The incidence of infusion reactions associated with monoclonal antibody drugs targeting the epidermal growth factor receptor in metastatic colorectal cancer patients: A systematic literature review and meta-analysis of patient and study characteristics". Cancer Medicine. 8 (12): 5800–5809. doi:10.1002/cam4.2413. PMC 6745824. PMID 31376243.
- ^ a b c d e f g h i Lippincott's nursing procedures (5th ed.). Philadelphia: Lippincott Williams & Wilkins. 2009. ISBN 978-0-7817-8689-8.
- ^ Raehl CL (July 1986). "Endotracheal drug therapy in cardiopulmonary resuscitation". Clinical Pharmacy. 5 (7): 572–9. PMID 3527527.
- ^ Roberts JR, Hedges JR (2013). Roberts and Hedges' Clinical Procedures in Emergency Medicine E-Book (6th ed.). Elsevier Health Sciences. p. 349. ISBN 978-1-4557-4859-4.
- ^ Rivera AM, Strauss KW, van Zundert A, Mortier E (2005). "The history of peripheral intravenous catheters: how little plastic tubes revolutionized medicine". Acta Anaesthesiologica Belgica. 56 (3): 271–82. PMID 16265830.
- ^ Marino PL (2014). "2. Central Venous Access". Marino's the ICU book (Fourth ed.). Philadelphia: LWW. ISBN 978-1-4511-2118-6.
- ^ Sandrucci S, Mussa B, eds. (5 July 2014). Peripherally inserted central venous catheters. Milan: Springer. ISBN 978-88-470-5665-7.
- ^ Agarwal AK, Haddad N, Boubes K (November 2019). "Avoiding problems in tunneled dialysis catheter placement". Seminars in Dialysis. 32 (6): 535–540. doi:10.1111/sdi.12845. PMID 31710156. S2CID 207955194.
- ^ Roca-Tey R (March 2016). "Permanent Arteriovenous Fistula or Catheter Dialysis for Heart Failure Patients". The Journal of Vascular Access. 17 (1_suppl): S23 – S29. doi:10.5301/jva.5000511. PMID 26951899. S2CID 44524962.
- ^ Li G, Zhang Y, Ma H, Zheng J (3 July 2019). "Arm port vs chest port: a systematic review and meta-analysis". Cancer Management and Research. 11: 6099–6112. doi:10.2147/CMAR.S205988. PMC 6613605. PMID 31308748. S2CID 196610436.
- ^ a b American College of Emergency Physicians, "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Emergency Physicians, archived from the original on March 7, 2014, retrieved January 24, 2014
- ^ Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W (July 2006). "Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children". The Cochrane Database of Systematic Reviews. 2006 (3) CD004390. doi:10.1002/14651858.CD004390.pub2. PMC 6532593. PMID 16856044.
- ^ Griffith RJ, Jordan V, Herd D, Reed PW, Dalziel SR (April 2016). "Vapocoolants (cold spray) for pain treatment during intravenous cannulation" (PDF). The Cochrane Database of Systematic Reviews. 2016 (4) CD009484. doi:10.1002/14651858.CD009484.pub2. PMC 8666144. PMID 27113639.
- ^ Heng SY, Yap RT, Tie J, McGrouther DA (April 2020). "Peripheral Vein Thrombophlebitis in the Upper Extremity: A Systematic Review of a Frequent and Important Problem". The American Journal of Medicine. 133 (4): 473–484.e3. doi:10.1016/j.amjmed.2019.08.054. PMID 31606488. S2CID 204545798.
- ^ Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, da Luz Gonçalves Pedreira M, Sorgini Peterlini MA, Azzalis LA, Campos Junqueira VB, Fonseca FL (February 2015). "The use of vancomycin with its therapeutic and adverse effects: a review". European Review for Medical and Pharmacological Sciences. 19 (4): 694–700. PMID 25753888.
- ^ Webster J, Osborne S, Rickard CM, Marsh N (23 January 2019). "Clinically-indicated replacement versus routine replacement of peripheral venous catheters". The Cochrane Database of Systematic Reviews. 1 (1) CD007798. doi:10.1002/14651858.CD007798.pub5. ISSN 1469-493X. PMC 6353131. PMID 30671926.
- ^ O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S (May 2011). "Guidelines for the prevention of intravascular catheter-related infections". Clinical Infectious Diseases. 52 (9): e162-93. doi:10.1093/cid/cir257. PMC 3106269. PMID 21460264.
- ^ Jaffe RB (July 1983). "Cardiac and vascular involvement in drug abuse". Seminars in Roentgenology. 18 (3): 207–212. doi:10.1016/0037-198x(83)90024-x. PMID 6137064.
- ^ Lv L, Zhang J (May 2020). "The incidence and risk of infusion phlebitis with peripheral intravenous catheters: A meta-analysis". The Journal of Vascular Access. 21 (3): 342–349. doi:10.1177/1129729819877323. PMID 31547791. S2CID 202745746.
- ^ Mihala G, Ray-Barruel G, Chopra V, Webster J, Wallis M, Marsh N, McGrail M, Rickard CM (2018). "Phlebitis Signs and Symptoms With Peripheral Intravenous Catheters: Incidence and Correlation Study". Journal of Infusion Nursing. 41 (4): 260–263. doi:10.1097/NAN.0000000000000288. PMID 29958263. S2CID 49613143.
- ^ Reynolds PM, MacLaren R, Mueller SW, Fish DN, Kiser TH (June 2014). "Management of extravasation injuries: a focused evaluation of noncytotoxic medications". Pharmacotherapy. 34 (6): 617–32. doi:10.1002/phar.1396. PMID 24420913. S2CID 25278254.
- ^ Schwamburger NT, Hancock RH, Chong CH, Hartup GR, Vandewalle KS (2012). "The rate of adverse events during IV conscious sedation". General Dentistry. 60 (5): e341-4. PMID 23032244.
- ^ Hadaway L (August 2007). "Infiltration and extravasation". The American Journal of Nursing. 107 (8): 64–72. doi:10.1097/01.NAJ.0000282299.03441.c7. PMID 17667395.
- ^ Campbell G, Alderson P, Smith AF, Warttig S (13 April 2015). "Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia". Cochrane Database of Systematic Reviews. 2015 (4) CD009891. doi:10.1002/14651858.CD009891.pub2. PMC 6769178. PMID 25866139.
- ^ Wang W (25 July 2015). "Tolerability of hypertonic injectables". International Journal of Pharmaceutics. 490 (1–2): 308–15. doi:10.1016/j.ijpharm.2015.05.069. PMID 26027488.
- ^ a b c d e f g Millam D (January 1996). "The history of intravenous therapy". Journal of Intravenous Nursing. 19 (1): 5–14. PMID 8708844.
- ^ Lindeboom GA (1954). "The Story of a Blood Transfusion to a Pope". Journal of the History of Medicine and Allied Sciences. IX (4): 455–459. doi:10.1093/jhmas/IX.4.455. PMID 13212030.
- ^ Duffin J (2010). History of medicine: a scandalously short introduction (2nd ed.). Toronto [Ont.]: University of Toronto Press. pp. 198–199. ISBN 978-0-8020-9825-2.
- ^ Jorge Dagnino; Wren, Boyle, and the Origins of Intravenous Injections and the Royal Society of London. Anesthesiology 2009; 111:923–924 https://doi.org/10.1097/ALN.0b013e3181b56163
- ^ Felts, J. H. (2000). Richard Lower: anatomist and physiologist. Annals of internal medicine, 133(6), 485.
- ^ MacGillivray N (2009). "Dr Thomas Latta: the father of intravenous infusion therapy". Journal of Infection Prevention. 10 (Suppl. 1): 3–6. doi:10.1177/1757177409342141.
- ^ See, for example, the Nobel Prize Nomination Database: https://www.nobelprize.org/nomination/redirector/?redir=archive/
- ^ Stanley A (1995). Mothers and daughters of invention: notes for a revised history of technology. Rutgers University Press. pp. 141–142. ISBN 978-0-8135-2197-8. Retrieved 2011-06-05.
Wanger and colleagues had in effect invented the modern I.V.-drip method of drug delivery [...]
- ^ Hirschfeld S, Hyman HT, Wanger JJ (February 1931). "Influence of velocity on the response to intravenous injections". Archives of Internal Medicine. 47 (2): 259–287. doi:10.1001/archinte.1931.00140200095007.
- ^ Geggel L (3 December 2012). "A Royal Spotlight on a Rare Condition". The New York Times.
Further reading
[edit]- Royal College of Nursing, Standards for Infusion Therapy (Archive of the 4th edition (December 2016) via the Internet Wayback Machine)
External links
[edit] Media related to Intravenous therapy at Wikimedia Commons
Media related to Intravenous therapy at Wikimedia Commons
Intravenous therapy
View on GrokipediaOverview
Definition and Principles
Intravenous therapy, commonly abbreviated as IV therapy, is a medical procedure that delivers fluids, medications, nutrients, or blood products directly into a patient's vein using a needle or catheter, enabling rapid systemic distribution while bypassing gastrointestinal absorption. This method provides immediate access to the bloodstream, achieving 100% bioavailability of the administered substance, unlike oral routes where absorption can be incomplete or delayed due to first-pass metabolism.[7] The physiological basis relies on the vein's direct connection to the circulatory system, allowing for onset of action within seconds to minutes, which is critical for urgent interventions requiring swift therapeutic effects.[8] Key to IV therapy is the anatomy of superficial veins, particularly in the upper extremities, such as the dorsal metacarpal veins of the hand, cephalic and basilic veins of the forearm, and those in the antecubital fossa, which are preferred for their accessibility and lower risk of complications.[9] These veins facilitate precise dosage control through adjustable infusion rates, ensuring the substance enters the bloodstream at a controlled pace to maintain hemodynamic stability and avoid overload.[1] Basic components include sterile solutions, such as crystalloids like 0.9% sodium chloride (normal saline), which serve as the primary vehicle for delivery, along with equipment comprising IV bags or bottles to hold the solution, flexible tubing for conduction, and needles or catheters for venous insertion.[10] Flow dynamics can be managed via gravity-dependent systems, where hydrostatic pressure drives the infusion, or electronic pumps that provide more accurate regulation for precise volume control.[1] Infusion rates are typically measured in milliliters per hour (mL/hour) for volumetric delivery or drops per minute (gtt/min) using drip chambers, with standard macro-drip sets calibrated at 20 drops per milliliter to facilitate manual adjustments.[11] Solutions are formulated to match physiological osmolarity, with isotonic fluids having an osmolarity of approximately 280-300 mOsm/L to prevent cellular damage from osmotic shifts, such as hemolysis or edema.[10]Indications and Contraindications
Intravenous therapy is indicated in clinical scenarios where rapid restoration of fluid volume, electrolyte balance, or medication delivery is essential, such as in cases of dehydration, where isotonic solutions like 0.9% saline are used to correct extracellular isotonic dehydration and hypovolemia.[12] It is also primary for managing electrolyte imbalances, including hyponatremia, through targeted fluid administration to restore sodium levels and prevent neurological complications.[12] Hemodynamic instability, particularly in shock states like sepsis or trauma, necessitates IV therapy for resuscitation with boluses of at least 30 mL/kg of crystalloid within the first 3 hours (for sepsis) or 20 mL/kg initial bolus (for hypovolemic shock), administered rapidly and reassessed for response.[13] Additionally, IV access is required when patients cannot tolerate oral intake, such as during perioperative periods, severe vomiting, or gastrointestinal obstruction, to provide maintenance fluids meeting daily water and electrolyte needs.[1] For rapid drug action, it is indicated in emergencies like anaphylaxis, where immediate intravenous administration ensures quick systemic effects.[14] Patient selection criteria for IV therapy incorporate age- and condition-specific factors to optimize safety and efficacy. In pediatrics, indications mirror adult scenarios but require dose adjustments and careful vein selection due to smaller vessel sizes, with heightened monitoring to prevent fluid overload in infants and children under 16 years.[15] Geriatric patients often need IV therapy for dehydration or medication delivery, but fragile veins and reduced physiological reserve demand slower infusion rates and frequent assessments to avoid complications like phlebitis.[1] For those with chronic conditions, such as renal failure, IV fluids must be closely monitored to prevent exacerbation of impairment, using smaller volumes like bags under 1,000 mL to mitigate overload risks.[1] Contraindications to IV therapy are categorized as absolute or relative to guide safe application. Absolute contraindications include the absence of suitable peripheral veins for access or explicit patient refusal, precluding insertion altogether.[14] Relative contraindications encompass local infection at the potential insertion site, which increases sepsis risk, and coagulopathy, which elevates bleeding potential during cannulation.[14] Systemic conditions like heart failure or fluid overload states are also relative, as excessive volume can worsen pulmonary edema or cardiac strain, necessitating alternative routes or cautious titration.[12] Decision-making for initiating IV therapy follows structured assessment protocols, such as the ABCDE approach (Airway, Breathing, Circulation, Disability, Exposure), to prioritize interventions in acutely ill patients by first evaluating circulatory status for hypovolemia and determining the need for fluid resuscitation.[16] This framework ensures IV use is reserved for scenarios where oral or enteral routes are inadequate, integrating vital signs, laboratory results, and clinical history to tailor therapy.[17]Clinical Uses
Fluid and Electrolyte Replacement
Intravenous therapy plays a critical role in fluid and electrolyte replacement by restoring intravascular volume and correcting imbalances caused by dehydration, hemorrhage, or metabolic disturbances, thereby maintaining homeostasis and preventing organ dysfunction. This approach is particularly essential in conditions like hypovolemic shock or severe electrolyte derangements, where oral intake is insufficient or contraindicated.[18] Crystalloid solutions are the primary fluids used for volume expansion and electrolyte replenishment due to their ability to distribute across extracellular spaces. Isotonic crystalloids, such as 0.9% saline, provide rapid intravascular expansion without causing significant shifts in cellular fluid; it contains 154 mEq/L of sodium and 154 mEq/L of chloride.[19] Another common isotonic option is lactated Ringer's solution, a balanced crystalloid that approximates plasma electrolyte composition, including 130 mEq/L sodium, 4 mEq/L potassium, 109 mEq/L chloride, and 28 mEq/L lactate, which is metabolized to bicarbonate to help buffer acidosis.[20] Hypotonic crystalloids like 5% dextrose in water are employed when free water replacement is needed, such as in hypernatremia, as they provide calories and dilute serum solutes after the dextrose is metabolized.[21] Colloids, such as 5% albumin, are reserved for cases requiring maintenance of oncotic pressure to prevent edema, particularly in hypoalbuminemic patients, as their larger molecules remain in the vascular space longer than crystalloids.[22] Electrolyte correction via IV therapy targets specific deficiencies to avert complications like cardiac arrhythmias or neuromuscular weakness. For hypokalemia, intravenous potassium chloride is administered at 20-40 mEq per liter of fluid in monitored settings, with infusion rates not exceeding 10 mEq per hour to avoid hyperkalemia or cardiac toxicity.[23] Magnesium sulfate is used for hypomagnesemia associated with arrhythmias, typically as one-time IV doses of 4 g in 100 mL premixed solutions infused over 1-2 hours, often preceding or accompanying potassium replacement to enhance efficacy.[24] Basic calculations for electrolyte deficits guide dosing; for instance, the potassium or sodium deficit can be estimated as total body water (TBW) multiplied by the difference between desired and current serum concentration, where TBW is approximately 0.6 times body weight in kilograms for adults, allowing tailored replacement to normalize levels gradually.[25] Administration guidelines emphasize controlled delivery to match physiological needs and avoid overload. Maintenance fluid rates follow the 4-2-1 rule: 4 mL/kg/hour for the first 10 kg of body weight, 2 mL/kg/hour for the next 10 kg, and 1 mL/kg/hour for each additional kilogram, ensuring daily requirements without excess. For rehydration in dehydration or shock, protocols recommend an initial 20 mL/kg bolus of isotonic crystalloid over 10-20 minutes, which may be repeated if hemodynamic improvement is inadequate, as seen in pediatric or adult hypovolemic states. Bolus techniques for rapid replacement are detailed in the types of administration section. Monitoring is vital to assess response and prevent complications during IV fluid and electrolyte replacement. Key parameters include urine output, targeted at greater than 0.5 mL/kg/hour to confirm adequate renal perfusion and fluid balance.[26] Vital signs such as blood pressure, heart rate, and central venous pressure should be tracked frequently, alongside laboratory trends like serum sodium checked every 4-6 hours to guide adjustments and detect hyponatremia or hypernatremia early. Overload risks, such as pulmonary edema, are addressed in the complications section.| Fluid Type | Example | Key Composition (per liter) | Primary Use |
|---|---|---|---|
| Isotonic Crystalloid | 0.9% Saline | 154 mEq Na⁺, 154 mEq Cl⁻ | Isotonic expansion, hypovolemia |
| Balanced Crystalloid | Lactated Ringer's | 130 mEq Na⁺, 4 mEq K⁺, 109 mEq Cl⁻, 28 mEq lactate | Electrolyte-balanced resuscitation, acidosis buffer |
| Hypotonic Crystalloid | 5% Dextrose | 50 g dextrose (hypotonic after metabolism) | Free water replacement, hypernatremia |
| Colloid | 5% Albumin | 25-50 g albumin | Oncotic pressure maintenance, hypoalbuminemia |
Medication Delivery
Intravenous therapy offers significant pharmacokinetic advantages for medication delivery by providing direct access to the systemic circulation, thereby achieving 100% bioavailability and bypassing gastrointestinal absorption and first-pass hepatic metabolism.[27] This route ensures rapid onset of action and predictable plasma concentrations, which is particularly beneficial for time-sensitive treatments.[28] For instance, continuous IV infusions enable zero-order kinetics, where the drug input rate is constant, allowing steady-state levels to be maintained without dependence on absorption variability, as seen with antibiotics.[29] Common classes of medications administered via IV therapy include antimicrobials, analgesics, and vasopressors. Antimicrobials such as IV penicillin G are frequently used for severe infections like sepsis to achieve immediate therapeutic levels.[30] Analgesics like morphine are given as IV boluses, typically 2 to 5 mg for acute pain management in opioid-naive adults, providing faster analgesia compared to oral routes.[31] Vasopressors, such as norepinephrine, are infused continuously at rates of 0.01 to 0.3 mcg/kg/min to support blood pressure in septic shock, titrated based on hemodynamic response.[32] Compatibility is a critical consideration in IV medication delivery to prevent adverse reactions. Medications may be administered via Y-site injection into an existing line or through dedicated lines to avoid interactions; for example, incompatible drugs like ceftriaxone and calcium-containing solutions can form precipitates, risking embolism or reduced efficacy, and should never be mixed.[33] Dilution requirements further mitigate risks, with many IV antibiotics, such as vancomycin, needing reconstitution in at least 200 to 250 mL of normal saline to ensure stability and prevent phlebitis.[34] Dosing regimens for IV medications often involve loading doses followed by maintenance infusions, adjusted via therapeutic drug monitoring to optimize efficacy and safety. For vancomycin, a loading dose of 15 to 20 mg/kg actual body weight is recommended to rapidly achieve therapeutic levels in serious infections, with subsequent maintenance doses every 8 to 12 hours.[35] Trough levels of 10 to 20 mcg/mL are targeted for monitoring to ensure adequate exposure while minimizing nephrotoxicity, with levels drawn just before the next dose.[36]Blood and Blood Product Administration
Intravenous administration of blood and blood products is a critical application of IV therapy used to restore oxygen-carrying capacity, correct coagulopathies, and address platelet deficiencies in patients with hematologic compromise. This process involves transfusing components such as whole blood, packed red blood cells (PRBCs), platelets, and fresh frozen plasma (FFP), each selected based on specific clinical needs to support hemostasis and tissue oxygenation. Unlike synthetic medications, these biologic products require rigorous immunologic matching to prevent adverse reactions, making compatibility testing a cornerstone of safe administration.[37] Whole blood is indicated primarily for life-threatening hemorrhage, such as in severe trauma, where simultaneous replacement of oxygen-carrying red cells, clotting factors, and volume is essential; it contains all blood elements and is preferred in scenarios requiring rapid resuscitation with balanced components. PRBCs are used for anemia, particularly when hemoglobin levels fall below 7 g/dL in stable adults or in cases of symptomatic anemia from chronic conditions like chemotherapy-induced bone marrow suppression, with one unit typically raising hemoglobin by approximately 1 g/dL. Platelets are transfused prophylactically or therapeutically for thrombocytopenia below 10,000/μL in non-bleeding patients or to manage active bleeding in platelet dysfunction, aiming to increase platelet count by 30,000-60,000/μL per unit in adults. FFP addresses coagulopathies, such as elevated INR greater than 1.6 with active bleeding or prior to invasive procedures in anticoagulated patients, providing clotting factors to reverse deficiencies from massive transfusion or liver disease.[38][39][39][39][39] Transfusion protocols emphasize ABO and Rh compatibility to minimize hemolytic risks, with type and screen testing valid for up to 72 hours; full cross-matching, which detects additional incompatibilities, typically requires 30-60 minutes before issuance. For PRBCs, infusion rates are generally 1-2 mL/kg/hour to avoid transfusion-associated circulatory overload (TACO), with a standard unit (approximately 350 mL) completed within 4 hours, often over 90-180 minutes; slower rates of 1 mL/kg/hour are used in at-risk patients, such as those with heart failure. Platelets are infused over 30-60 minutes at rates up to 250-350 mL/hour, while FFP is given at 10-20 mL/kg over 30-120 minutes, not exceeding 4 hours total to prevent bacterial growth. In massive transfusions involving large volumes, central venous access may be preferred for efficient delivery, though peripheral IVs suffice for routine units. Unit documentation includes donor identification, transfusion start/stop times, volume administered, and patient response.[37][40][37][41][37][37] Monitoring begins with baseline vital signs (temperature, pulse, blood pressure, respirations) immediately before transfusion and every 15 minutes for the first hour, then hourly until completion, continuing for at least 20-30 minutes post-transfusion to detect delayed reactions. Clinicians watch for signs of acute hemolytic reactions, including fever, chills, flank or back pain, hypotension, or hemoglobinuria, which necessitate immediate cessation of the transfusion, disconnection of the blood tubing, and maintenance of IV access with normal saline while notifying the provider. All units must be double-checked by two qualified personnel at the bedside against patient identification and documentation to ensure traceability. Infectious risks, such as bacterial contamination, are minimized through donor screening but remain a focus in post-transfusion surveillance as detailed in complication management guidelines.[37][39][37]Nutritional Support
Intravenous nutritional support through total parenteral nutrition (TPN) delivers essential macronutrients, micronutrients, electrolytes, and fluids directly into the bloodstream, bypassing the gastrointestinal tract to sustain patients with impaired enteral intake.[42] This method ensures complete nutritional provision, preventing malnutrition in scenarios where oral or enteral feeding is infeasible or insufficient.[43] TPN is indicated for conditions such as bowel obstruction, severe malabsorption disorders like short gut syndrome, and post-operative recovery periods involving prolonged gastrointestinal rest.[42][44] These situations often arise in critically ill patients or those with hypercatabolic states, where enteral nutrition cannot meet metabolic demands.[43] TPN formulations are customized admixtures comprising carbohydrates, proteins, lipids, electrolytes, vitamins, and trace elements to mimic balanced dietary intake.[42] Carbohydrates are primarily provided as 10-20% dextrose solutions, supplying the majority of non-protein calories while supporting glucose-dependent energy needs.[43] Proteins consist of crystalline amino acids at 3-5% concentrations, including essential and non-essential types to promote nitrogen balance and tissue repair.[42] Lipids are administered as 10-30% intravenous fat emulsions, often soybean- or multi-oil based, contributing 25-30% of total caloric intake and preventing essential fatty acid deficiencies.[44] Electrolytes such as sodium, potassium, magnesium, calcium, and phosphate are adjusted based on serum levels to maintain homeostasis.[43] Vitamins are added via standardized multivitamin preparations providing daily requirements of fat- and water-soluble forms, while trace elements include zinc at 2.5-5 mg/day for adults, along with copper, selenium, manganese, and chromium to support enzymatic functions and prevent deficiencies.[42][45] Nutritional requirements in TPN are calculated to align with individual metabolic needs, typically aiming for 25-30 kcal/kg/day of total energy to cover basal expenditures and stress factors.[44] Protein provision targets 1-1.5 g/kg/day to achieve positive nitrogen balance, with adjustments for critically ill patients requiring higher amounts.[42] The glucose infusion rate is limited to less than 5-7 mg/kg/min to minimize risks of hyperglycemia and related complications like hepatic steatosis.[43] These calculations often incorporate patient weight, indirect calorimetry results if available, and serial monitoring of serum glucose, electrolytes, and prealbumin levels.[44] Administration of TPN requires central venous access due to the hyperosmolar nature of solutions exceeding 900 mOsm/L, which could cause phlebitis if infused peripherally.[42] Continuous infusion over 24 hours is standard initially to stabilize metabolism, but cyclic regimens—typically 12-18 hours daily—may be employed for long-term therapy to simulate natural eating patterns, improve quality of life, and reduce hepatic complications.[43][44] Infusion rates are advanced gradually, starting at 50% of goal to prevent refeeding syndrome.[42]Diagnostic and Imaging Applications
Intravenous therapy plays a crucial role in diagnostic and imaging applications by delivering contrast agents that enhance visualization of anatomical structures, particularly in computed tomography (CT) and magnetic resonance imaging (MRI). These agents are administered via intravenous routes to improve the differentiation between normal and pathological tissues, aiding in the detection of abnormalities such as tumors and vascular anomalies.[46] The primary agents used include iodinated contrasts for CT scans and gadolinium-based agents for MRI, often followed by saline flushes to optimize delivery.[47] Iodinated contrast media, such as iohexol, are commonly employed for CT imaging due to their high radiodensity and ability to opacify vascular and parenchymal structures. Iohexol is available in concentrations of 300-370 mgI/mL and is typically dosed at 1-2 mL/kg body weight, depending on the protocol and patient size, to achieve adequate enhancement without excessive risk.[48] For MRI, gadolinium-based contrast agents are administered at a standard dose of 0.1 mmol/kg to shorten T1 relaxation times, thereby increasing signal intensity in targeted tissues.[49] Saline flushes, usually 20-50 mL of 0.9% normal saline, are injected immediately after the contrast bolus to displace residual agent from the tubing and veins, reducing the required contrast volume by up to 20-40% and minimizing artifacts.[47] Key procedures utilizing these agents include intravenous pyelography (IVP) and angiography. In IVP, iodinated contrast is injected intravenously to evaluate the urinary tract, with serial X-rays capturing the agent's excretion through the kidneys, ureters, and bladder for assessment of obstructions or anomalies.[50] Angiography, often performed as CT angiography (CTA), involves rapid intravenous injection of iodinated contrast at power injection rates of 3-5 mL/sec to map vascular anatomy, such as coronary or peripheral arteries, enabling real-time visualization of blood flow and stenoses.[51] These injections are typically delivered via peripheral intravenous catheters compatible with power injectors, ensuring high-flow delivery while monitoring for extravasation.[52] Indications for intravenous contrast in these applications center on enhancing diagnostic accuracy for tumor detection and vascular mapping. In oncology, iodinated or gadolinium contrasts highlight tumor vascularity and margins, facilitating early identification of lesions in organs like the liver or brain by increasing contrast-to-noise ratios.[53] For vascular mapping, CTA with iodinated agents delineates arterial occlusions, aneurysms, or malformations, guiding interventions such as stent placement.[46] Prior to administration, allergy risk assessment is essential, as iodinated contrasts carry a risk of hypersensitivity reactions in patients with prior exposure. Premedication protocols for those with a history of moderate or severe reactions typically include oral prednisone 50 mg at 13, 7, and 1 hours before the procedure, combined with diphenhydramine 50 mg orally 1 hour prior, to mitigate anaphylactoid responses.[54] Post-procedure care focuses on preventing contrast-induced nephropathy (CIN), particularly in at-risk patients with renal impairment. Hydration protocols recommend intravenous 0.9% normal saline at 1 mL/kg/hour for 3-12 hours before and 6-24 hours after contrast administration to maintain renal perfusion and reduce CIN incidence.[55] Monitoring serum creatinine levels 48-72 hours post-procedure is advised to detect any acute kidney injury.[55]Non-Clinical Uses
Non-clinical uses of intravenous therapy are often conducted in unregulated or non-medical environments, including wellness clinics, spas, mobile services, and at home, frequently without the supervision of qualified medical professionals. This absence of professional oversight significantly heightens the risks of serious complications. These risks include infection at the injection site, thrombophlebitis (vein inflammation or clotting), fluid infiltration into surrounding tissues causing damage or swelling, air embolism, electrolyte imbalances, overhydration, overdose (such as from potassium leading to arrhythmias), allergic reactions, and the lack of immediate monitoring for life-threatening events. Professional medical supervision is essential for safe IV therapy. For detailed information on complications and their management, see the Complications and Management section.[5][56]Athletic and Performance Enhancement
Intravenous therapy has been employed by athletes for rapid rehydration following intense exercise, typically involving the administration of 1 to 2 liters of normal saline solution to restore fluid balance more quickly than oral methods.[57] Vitamin infusions, such as those containing B-complex vitamins, are also used to purportedly boost energy levels and aid recovery by delivering nutrients directly into the bloodstream, bypassing gastrointestinal absorption limitations. However, IV vitamin therapy lacks clear benefits for routine health enhancement in healthy individuals, with risks potentially outweighing unproven advantages; it is useful only in specific medical cases like severe deficiencies or malabsorption disorders.[58][59][60] These practices are promoted in athletic contexts for optimizing performance in endurance sports like marathon running or cycling, where dehydration from sweat loss can impair endurance.[57] The World Anti-Doping Agency (WADA) strictly regulates intravenous infusions to prevent misuse, prohibiting any IV administration exceeding 100 mL per 12-hour period at all times, both in- and out-of-competition, unless it occurs in a hospital setting, during surgical procedures, or as part of a clinical diagnostic investigation with a Therapeutic Use Exemption (TUE).[61] This ban targets routine use in healthy athletes to avoid masking underlying conditions or facilitating prohibited substance delivery. In cycling, for instance, Team Sky faced accusations in 2017 of violating the Union Cycliste Internationale's (UCI) no-needles policy through unauthorized IV recovery sessions during events like the Tour de France, highlighting enforcement challenges in high-profile competitions.[62] As of 2025, sports organizations such as USA Swimming have cautioned against the normalization of non-essential IV infusions, noting the lack of robust medical research supporting their routine use for performance or recovery.[63] Scientific evidence on the efficacy of IV rehydration compared to oral methods in endurance athletes is limited and shows only marginal, transient benefits, such as slightly faster restoration of plasma volume, but no significant improvements in subsequent exercise performance or prolonged recovery.[64] Studies indicate that for mild to moderate dehydration, oral rehydration is equally effective and safer, with IV approaches offering no clear ergogenic advantage in most scenarios.[65] In the athletic context, IV therapy carries risks including the masking of dehydration symptoms, which may encourage overexertion and increase injury susceptibility, as well as potential for abuse in delivering prohibited substances like erythropoietin (EPO) intravenously—though such practices have become rarer with shifts to subcutaneous administration.[57][66] Ethical concerns arise from the potential to undermine fair competition, prompting WADA's prohibitions to preserve the integrity of sports.[61]Hangover and Detoxification Treatments
Intravenous therapy for hangover and detoxification treatments typically involves elective infusions administered in non-clinical settings, such as wellness clinics, to alleviate symptoms following alcohol consumption. These treatments aim to address acute effects like dehydration, electrolyte depletion, and oxidative stress without medical necessity. Common formulations include the Myers' Cocktail, which consists of intravenous fluids combined with high doses of vitamin C (typically 2500 mg ascorbic acid), B-complex vitamins (such as thiamine, riboflavin, and pyridoxine), magnesium chloride, calcium gluconate, and sometimes hydroxocobalamin (vitamin B12). However, IV vitamin therapy lacks clear benefits for routine health enhancement in healthy individuals, with risks potentially outweighing unproven advantages; it is useful only in specific medical cases like severe deficiencies or malabsorption disorders.[58][59][60][67][68] Another frequent approach is hydration therapy with added antiemetics, such as ondansetron at a dose of 4 mg, to combat nausea alongside saline fluids and electrolytes like sodium and potassium.[69][70] The purported mechanisms center on rapid rehydration to counteract alcohol-induced diuresis, which increases urinary output and leads to fluid and electrolyte loss, contributing to symptoms like headache, fatigue, and weakness.[71] Vitamin C in these infusions provides antioxidant effects by neutralizing free radicals generated during alcohol metabolism, potentially reducing inflammation and supporting liver detoxification processes.[72] Magnesium and B vitamins are included to replenish those depleted by alcohol, aiding energy metabolism and neuromuscular function, though bioavailability via IV exceeds oral routes for faster symptom mitigation.[73] These treatments have gained popularity through clinic-based services, often costing $100-300 per session, with mobile options available for convenience.[74] The non-clinical IV hydration therapy market, driven by such elective uses, is valued at $2.93 billion as of 2025 and projected to grow significantly, amid calls for greater regulatory oversight of spas and clinics to address safety risks.[75][76] Celebrity endorsements, including from figures in entertainment promoting quick recovery, have boosted demand, particularly in urban areas hosting nightlife events.[77] However, the U.S. Food and Drug Administration (FDA) has not approved IV drips for hangover relief claims, issuing warnings against unverified wellness infusions due to risks like contamination in unregulated facilities.[78] Brief mention of infection risks in such settings underscores the need for sterile practices, as detailed in broader complication guidelines.[79] Evidence for efficacy remains largely anecdotal, with users reporting quicker relief from symptoms like nausea and headache compared to oral rehydration alone.[80] Limited clinical studies on IV hydration indicate faster restoration of fluid balance versus oral methods, potentially shortening recovery time in dehydration scenarios, though no large-scale trials specifically validate hangover-specific benefits.[81] Small observational reports suggest symptom resolution may occur sooner with IV approaches, but rigorous randomized controlled trials are lacking, and oral fluids with rest remain the evidence-based standard.[82]Other Non-Medical Applications
Intravenous chelation therapy, often using ethylenediaminetetraacetic acid (EDTA), is promoted in alternative medicine for "detoxification" by purportedly removing heavy metals such as lead and mercury from the body.[83] This approach is established for treating confirmed heavy metal poisoning, where EDTA binds to metals and facilitates their excretion via urine.[84] However, its extension to unverified detox claims lacks robust evidence, and it remains controversial for non-medical uses like preventing or treating heart disease, where clinical trials show minimal or no benefit.[85][86] Another example is intravenous glutathione infusions, commonly marketed as "beauty drips" for skin lightening and anti-aging effects. These typically involve doses of 600-1200 mg administered once or twice weekly, with proponents claiming reduced melanin production for brighter skin. However, IV vitamin therapy lacks clear benefits for routine health enhancement in healthy individuals, with risks potentially outweighing unproven advantages; it is useful only in specific medical cases like severe deficiencies or malabsorption disorders.[58][59][60] Such treatments are popular in wellness spas and cosmetic clinics, particularly in regions emphasizing aesthetic enhancements.[87][88] These non-medical applications occur primarily in alternative medicine settings, such as wellness spas and holistic centers, where IV therapy is offered for purported vitality boosts without underlying medical conditions.[79] In the United States, such uses are not approved by the Food and Drug Administration (FDA) for non-medical claims, and many IV products for these purposes are unapproved or compounded under potentially insanitary conditions.[89][90] Federal oversight is limited, with regulations varying by state and no standardized procedures for med spas, raising concerns about practitioner qualifications and product safety.[76][56] Significant risks accompany these therapies, often rooted in pseudoscientific assertions like unproven anti-aging or detoxification benefits that exceed available evidence.[58] For instance, high-dose IV vitamin therapies have been linked to acute kidney injury, including cases of renal failure from oxalate crystal formation, as seen in reports of patients receiving megadoses of vitamin C.[91][92] Similarly, IV glutathione carries risks of liver and kidney toxicity, severe allergic reactions, and nervous system effects, particularly with repeated or high doses.[87][93] Globally, variations include the popularity of IV vitamin C as an alternative cancer therapy in Asia, where high-dose IV vitamin C is investigated in supervised clinical trials for potential adjunctive benefits, though evidence for tumor reduction or survival improvements remains limited and emerging.[94] In countries like Thailand, such alternative therapies are commonly sought by cancer patients, often alongside conventional treatments, though systematic reviews highlight gaps in rigorous data supporting efficacy.[95][96]Types of Administration
Bolus Delivery
Bolus delivery in intravenous therapy involves the rapid administration of a discrete volume of medication or fluid, typically ranging from 1 to 100 mL, directly into the bloodstream over a short duration of 1 to 5 minutes, often via syringe injection or high-speed infusion to achieve immediate therapeutic effects.[97] This method bypasses absorption barriers, providing nearly 100% bioavailability and enabling precise control over dosing in acute scenarios.[97] Unlike slower infusions, bolus delivery is designed for single, non-repeated events to elicit a swift pharmacological response.[98] Applications of bolus delivery are prominent in emergency medicine, where rapid onset is critical, such as administering epinephrine at doses of 0.1 to 0.5 mg intravenously for anaphylaxis to counteract hypotension and bronchospasm within seconds.[99] Another key use is in diagnostic imaging, where contrast agents are delivered as boluses at rates of 5 to 10 mL per second—often 50 to 100 mL total—to optimize vascular enhancement during computed tomography scans, ensuring clear visualization of arterial structures.[100] These applications leverage the method's ability to produce high peak concentrations quickly for time-sensitive interventions.[98] Techniques for bolus delivery emphasize controlled rates to minimize risks, typically performed through peripheral venous access such as the antecubital vein using a syringe or automated injector.[97] For opioids like morphine, administration occurs at rates not exceeding 2 mg per minute (e.g., 2 to 4 mg diluted in 10 mL over 2 to 5 minutes) to prevent respiratory depression and chest wall rigidity.[101][102] Irritant drugs are often diluted in compatible fluids prior to push to reduce vein irritation, with a saline flush immediately following to clear the line and confirm patency.[97] For sequential administration of multiple bolus medications, such as diphenhydramine followed by hydromorphone, the first drug is administered slowly (e.g., diphenhydramine undiluted at a rate not exceeding 25 mg/min; hydromorphone over 2–3 minutes), the line is flushed with 5–10 mL normal saline, a brief wait is observed if needed, and then the second drug is administered slowly.[103][104][105] In arrhythmias, drugs like adenosine are given as a 6 mg rapid push over 1 to 2 seconds, followed by a 20 mL saline flush to expedite delivery to the heart.[106] From a pharmacodynamic perspective, IV bolus administration results in peak plasma concentrations within less than 1 minute, facilitating immediate distribution to highly perfused organs like the brain and heart before slower equilibration with peripheral tissues.[98] This rapid profile suits drugs with short half-lives, such as adenosine (approximately 10 seconds) for transient AV nodal blockade in supraventricular tachycardia or epinephrine (2 to 3 minutes) for acute hemodynamic stabilization.[98][99] The ensuing decline follows a bi-exponential curve in multi-compartment models, with initial fast elimination reflecting central compartment clearance and subsequent slower phases tied to tissue redistribution.[98]Continuous Infusion
Continuous intravenous infusion involves the steady administration of fluids, medications, or nutrients directly into the bloodstream at a controlled rate, typically ranging from 1 to 500 mL per hour, over extended periods such as hours or days, to maintain therapeutic levels without fluctuations.[2] This method relies on gravity-based systems or electronic infusion pumps to ensure precise delivery, distinguishing it from intermittent or bolus techniques by providing uninterrupted flow for sustained effects.[107] Common applications include the delivery of vasopressors to support hemodynamic stability in critically ill patients, such as dopamine administered at 5 to 20 mcg/kg/min to enhance cardiac output and renal perfusion.[108] Total parenteral nutrition (TPN) is another key use, often infused continuously over 24-hour cycles via central venous access to provide complete nutritional support when enteral feeding is not feasible, as in cases of gastrointestinal obstruction or severe malabsorption.[42] Maintenance hydration represents a foundational application, where electrolyte-balanced solutions are delivered at rates like 100 mL/hour to prevent dehydration in postoperative or immobile patients.[2] Stability in continuous infusions depends on aligning the delivery rate with the drug's pharmacokinetic profile, particularly its elimination half-life, to achieve and sustain steady-state plasma concentrations; for instance, insulin, with a short half-life of about 5-15 minutes, is infused at 0.1 units/kg body weight per hour to maintain glycemic control in diabetic ketoacidosis.[109] Ensuring line patency is critical, with protocols recommending saline flushes every 1 to 2 hours in high-risk scenarios or when flow interruptions are suspected, to prevent occlusion and verify catheter function.[110] Rate calculations for continuous infusions are based on the formula of total volume divided by infusion duration, yielding mL per hour; for example, 1000 mL of fluid over 8 hours equates to 125 mL per hour, which can be fine-tuned using microdrip tubing (60 drops per mL) for lower volumes in pediatrics or precise adjustments.[2] These computations prioritize patient-specific factors like weight and clinical response, often supported by infusion pumps for accuracy, though detailed device operations are outlined in equipment guidelines.[107] Prolonged infusions require monitoring to mitigate risks like electrolyte imbalances, addressed in complication management protocols.[42]Intermittent or Secondary Infusion
Intermittent or secondary infusion refers to the scheduled delivery of medications or fluids intravenously over a short duration, typically 30 to 60 minutes, using volumes of 100 to 250 mL, repeated at intervals of every 4 to 8 hours, often through a secondary bag connected to a primary IV line.[111][112] This approach allows for precise control of drug delivery while maintaining venous access via the primary line.[1] Common applications include antibiotic therapy, such as cefazolin administered at 1 to 2 g diluted in 50 to 100 mL of compatible fluid over 30 minutes every 8 hours for moderate to severe infections.[113][114] In chemotherapy, agents like doxorubicin are given intermittently, for example, 50 to 75 mg/m² infused over 15 to 30 minutes every 3 to 4 weeks in cycles for cancers such as breast or ovarian malignancies.[115] These discrete episodes enable targeted dosing aligned with therapeutic needs while minimizing continuous exposure. Setup involves attaching the secondary bag to a piggyback port on the primary IV tubing, with the secondary bag positioned higher than the primary to facilitate gravity or pump-driven flow.[11] The line is flushed with 10 mL of 0.9% normal saline before and after the infusion to ensure complete drug delivery and prevent residual buildup or incompatibility issues.[116][117] For home or outpatient use, the access can be converted to a saline lock post-infusion, maintaining patency with periodic flushes.[118] This method offers advantages such as reduced line manipulation compared to repeated direct accesses, which lowers the risk of contamination and phlebitis.[1] Dosing intervals are optimized based on drug pharmacokinetics, for instance, allowing beta-lactam antibiotics like cefazolin to achieve desired peak concentrations and appropriate trough levels for efficacy against bacterial pathogens.[119][120]Methods and Equipment
Vascular Access Options
Vascular access for intravenous therapy involves selecting appropriate entry sites and device categories based on patient needs, therapy requirements, and risk minimization. Peripheral sites, typically in the upper extremities such as the hands and forearms while avoiding areas of high flexion like the antecubital fossa, are preferred for short-term access due to their accessibility and lower complication risks compared to lower extremity sites, which should be avoided in adults except in emergencies.[121][122][110] Central sites, including the subclavian, internal jugular, or femoral veins, are selected for long-term therapy or administration of irritant or hyperosmolar solutions, with the subclavian site favored over jugular or femoral in adults to reduce infection risk.[122][121][110] Site selection prioritizes veins with adequate size, patency, and distance from nerves or arteries, often assessed via ultrasound to ensure vessel health and avoid areas of compromised circulation, infection, or lymphedema.[121][2] Device categories for vascular access include short peripheral catheters (SPCs), midline catheters, central venous catheters (CVCs), and implanted ports, each suited to specific therapy durations and needs. SPCs are over-the-needle devices inserted into superficial peripheral veins for brief infusions, while midline catheters extend from the upper arm to the axilla for intermediate access without entering central circulation.[121][123] CVCs, such as peripherally inserted central catheters (PICCs) or nontunneled/tunneled lines, provide central access for prolonged or high-volume therapy, and implanted ports offer subcutaneous, long-term intermittent access via a reservoir connected to a central vein.[121][124] Selection factors encompass therapy duration, vein size, and flow requirements to optimize outcomes and preserve vascular integrity. For durations under 14 days, peripheral options like SPCs are appropriate, whereas midline or CVCs are indicated for 1-4 weeks or longer, respectively, to avoid repeated insertions.[121][123] Vein size influences gauge choice, with 18-22 gauge catheters commonly used for adults to match vessel diameter and maintain a catheter-to-vein ratio of 45% or less, reducing endothelial damage.[121][2] Flow needs dictate larger lumens or central access for rapid infusions, such as blood products requiring rates over 20 mL/min, while smaller gauges suffice for standard fluids.[121][125] Guidelines from the Infusion Nurses Society emphasize evidence-based practices, including the use of the lowest effective gauge and shortest dwell time to minimize complications, with devices removed upon therapy completion or site issues.[121][110] Ultrasound guidance is recommended for difficult intravenous access, enhancing first-attempt success in peripheral and central placements by visualizing vessel depth and patency.[121][122] These standards align with CDC recommendations for site and device choices to support safe, patient-centered intravenous therapy.[122]Peripheral Intravenous Catheters
Peripheral intravenous catheters (PIVCs) are short-term, non-central vascular access devices designed for insertion into superficial peripheral veins, typically in the upper extremities, to deliver fluids, medications, electrolytes, and blood products. They represent the most frequently used form of intravenous access in acute care settings due to their relative simplicity, cost-effectiveness, and minimal invasiveness compared to central venous options. PIVCs are intended for therapies requiring short durations, with insertion performed using a needle-over-catheter technique that allows the needle to be withdrawn after venipuncture, leaving the flexible catheter in place.[126] Common types of PIVCs include over-the-needle catheters made from materials such as Teflon (polytetrafluoroethylene) or Vialon (a polyurethane biomaterial). These catheters are sized by gauge, ranging from 14 to 24 gauge, where lower gauge numbers indicate larger diameters suitable for rapid infusions, such as in trauma or surgical patients, while higher gauges are used for slower infusions or in patients with smaller veins. Vialon catheters demonstrate superior performance over Teflon, with clinical trials showing a 36% lower incidence of phlebitis and extended dwell times due to reduced thrombogenicity and improved flexibility. For patients with fragile or difficult-to-access veins, such as the elderly or children, winged infusion sets—also known as butterfly needles—are employed, featuring stabilizing plastic wings for precise control and flexible tubing to minimize vein trauma during short-term access.[126][127][128] Insertion sites for PIVCs are selected based on vein accessibility, patient mobility, and risk of complications, with the upper extremities preferred over lower ones. Ideal veins include the cephalic and basilic veins in the forearm, as well as dorsal metacarpal veins on the hands, while avoiding areas of flexion such as the antecubital fossa or wrist to reduce risks of nerve injury and infection. Sites near the dominant hand should be avoided to preserve functionality and reduce dislodgement risks from daily activities. Sites are replaced when clinically indicated rather than on a fixed schedule, in line with current evidence to prevent phlebitis and infection while minimizing unnecessary interventions.[126][122][110][129] PIVC materials often incorporate features to enhance safety and longevity, including polyurethane bases that are less irritating to vessel walls than older polyvinyl chloride options. Some catheters feature antimicrobial coatings, such as those impregnated with chlorhexidine gluconate, to mitigate biofilm formation and infection risks, though routine use of topical antimicrobial ointments at the site is not recommended. Securement is critical to prevent movement and complications; transparent, semi-permeable polyurethane dressings are standard, allowing site visualization while providing a barrier to microbes, whereas sutures are discouraged due to increased infection potential, skin erosion, and needlestick hazards—sutureless devices are preferred per guidelines.[126][130][131][122] Limitations of PIVCs include a maximum dwell time of 3 to 5 days (72 to 120 hours) to minimize risks like infiltration and thrombosis, after which reassessment or replacement is necessary. They are unsuitable for hyperosmolar solutions exceeding 600 mOsm/L, as these can cause endothelial damage, phlebitis, and vein sclerosis; such therapies require central access to dilute the infusate adequately.[122][132]Central Venous Access Devices
Central venous access devices (CVADs) are specialized catheters designed for prolonged intravenous access into large central veins, such as the superior vena cava (SVC), to deliver therapies that peripheral lines cannot safely handle. These devices are essential for patients requiring extended treatment durations, multiple simultaneous infusions, or administration of irritating substances like chemotherapy agents, vasopressors, or total parenteral nutrition (TPN). Unlike short-term peripheral catheters, CVADs minimize vein irritation and enable higher flow rates, reducing the need for frequent venipunctures.[133][134] Common types of CVADs include non-tunneled central venous catheters (CVCs), peripherally inserted central catheters (PICCs), tunneled CVCs, and implanted ports. Non-tunneled CVCs, often triple-lumen designs with lumens sized 16-18 gauge, are used for short-term access in acute settings like intensive care units (ICUs), allowing rapid administration of fluids, medications, and blood products. PICCs are inserted through a peripheral arm vein (e.g., basilic or cephalic) and advanced to the SVC, providing mid- to long-term access suitable for home care. Tunneled CVCs, such as Hickman or Broviac catheters, feature a subcutaneous tunnel to secure the device and reduce infection risk, ideal for extended use over months. Implanted ports (e.g., Port-a-Cath) are fully subcutaneous reservoirs connected to a central vein catheter, accessed percutaneously for intermittent long-term therapy.[134][135][124] Indications for CVADs primarily involve therapies that demand central placement to avoid endothelial damage or ensure reliable delivery, including TPN for nutritional support, chemotherapy for cancer treatment, vasopressor infusions in hemodynamic instability, and hemodialysis or plasmapheresis in renal failure. Multi-lumen configurations support concurrent administration of incompatible drugs, such as antibiotics and sedatives in critically ill patients. These devices are particularly beneficial for patients with poor peripheral veins or those needing frequent blood sampling without repeated sticks.[133][134][124] Placement typically employs the Seldinger technique, involving ultrasound-guided venipuncture, guidewire insertion, and catheter advancement, with post-insertion X-ray confirmation to verify tip position in the SVC or right atrium. Common sites include the internal jugular (preferred for ultrasound ease), subclavian, or femoral veins, selected based on patient anatomy and infection risk. Dwell times vary: non-tunneled CVCs last days to weeks, PICCs endure 1-6 months or longer with proper care, tunneled catheters support months to years, and ports remain in place indefinitely until removal.[133][134][135] Advantages of CVADs include high infusion rates up to 300 mL/hour, which facilitate rapid resuscitation, and reduced risk of phlebitis or infiltration when infusing vesicants or hyperosmolar solutions. They also lower overall procedural discomfort by enabling long-term access with fewer interventions, improving patient quality of life during chronic therapies. Brief reference to insertion details aligns with standard protocols outlined in dedicated maintenance guidelines.[133][134][124]Infusion Pumps and Devices
Infusion pumps and devices are mechanical systems designed to deliver fluids, medications, or nutrients intravenously at controlled rates, enhancing precision and safety over manual methods.[136] These tools attach to vascular access sites and regulate flow to prevent under- or over-delivery, particularly for critical therapies like chemotherapy or analgesics.[1] Common types include gravity sets, volumetric pumps, syringe pumps, and patient-controlled analgesia (PCA) pumps, each suited to specific volume and rate needs. Gravity sets rely on manual roller clamps to adjust flow from an elevated fluid bag, allowing basic control without electricity but requiring frequent monitoring to maintain accuracy.[1] Volumetric pumps, also known as large-volume pumps, handle higher fluid volumes and operate via peristaltic mechanisms to deliver rates from 0.1 to 999 mL/hr with typical accuracy of ±5%.[137][138] Syringe pumps, ideal for low-volume infusions under 50 mL, use a motorized plunger for precise delivery at rates as low as 0.1 mL/hr and accuracy of ±1-3%, making them suitable for concentrated medications in critical care.[139] PCA pumps enable patient self-administration of analgesics, such as fentanyl at basal rates of 10-50 mcg/hr, combined with on-demand boluses within programmed limits.[140][141] Key features across these devices include occlusion alarms to detect blockages, free-flow protection to prevent unintended rapid delivery, and air-in-line detectors for safety.[136] Programming involves setting the infusion rate in mL/hr, volume to be infused (VTBI) for finite deliveries, and optional bolus overrides for intermittent dosing.[1] Smart pumps incorporate drug libraries with dose error reduction software (DERS), which cross-references programmed parameters against predefined limits to alert users of potential errors, reducing medication mistakes by up to 50% in some studies. Maintenance ensures reliable operation, with battery life typically lasting 8-12 hours under standard use to support portability during power interruptions.[142] Tubing priming, a critical step, involves flushing the line to eliminate air bubbles and confirm patency, often automated in modern pumps to prevent air embolism risks.[1] Regular calibration and software updates are recommended to uphold accuracy and compliance with safety standards.[136]Insertion and Maintenance Techniques
Insertion of intravenous (IV) catheters begins with rigorous hand hygiene, performed using either soap and water or alcohol-based hand rubs to reduce microbial contamination. [143] A tourniquet is then applied proximal to the selected vein, typically 10-15 cm above the insertion site, to distend the vein and facilitate visualization while avoiding excessive pressure that could cause trauma. [126] The skin is prepared with a 2% chlorhexidine gluconate solution in 70% isopropyl alcohol, applied in a circular motion from the insertion site outward for at least 30 seconds, allowing it to dry completely to maximize antimicrobial efficacy. [144] Venipuncture for peripheral IV catheters involves advancing the catheter-over-needle assembly with the bevel facing up at an angle of 15-30 degrees to the skin, entering the vein smoothly to minimize vessel wall damage. [145] A no-touch technique is employed throughout, ensuring that the catheter tip and insertion site remain uncontaminated after skin preparation. [146] For central venous catheters (CVCs), insertion adheres to maximal sterile barrier precautions, including the use of a sterile cap, mask, gown, gloves, and a full-body sterile drape to significantly lower infection risk compared to standard precautions. [122] The Seldinger technique is the standard method: a hollow needle is inserted into the vein under ultrasound guidance when feasible, followed by advancement of a guidewire through the needle, removal of the needle, dilation of the tract with a serial dilator, and finally threading the catheter over the guidewire to secure venous access. [133] Maintenance of IV sites prioritizes ongoing asepsis and monitoring to prevent complications. Sites are assessed at least every nursing shift using the Visual Infusion Phlebitis (VIP) scale, which evaluates for signs such as pain, erythema, swelling, warmth, induration, and palpable venous cord, with scores guiding decisions for site rotation or removal. [147] Transparent semipermeable dressings are applied post-insertion to allow continuous visual inspection while securing the device, and changed every 7 days or sooner if soiled, loose, or showing signs of infection. [143] Flushing maintains patency; for saline-locked peripheral catheters, 5-10 mL of 0.9% sodium chloride is instilled after each use or every 8-12 hours if unused, using a pulsatile technique to clear the lumen without force that could dislodge clots. [148] Heparin is no longer routinely recommended for locking peripheral devices due to equivalent efficacy of saline and risks of heparin-induced thrombocytopenia. [149] Removal of peripheral IV catheters involves first discontinuing any infusion, then using aseptic technique to remove the dressing and gently withdrawing the catheter parallel to the vein while applying direct pressure with sterile gauze to achieve hemostasis and prevent bleeding or hematoma formation. [118] For peripherally inserted central catheters (PICCs), removal follows similar principles of gentle traction and pressure application at the site, followed by immediate capping or dressing if partial removal is not indicated, though full removal typically requires physician oversight. [133]Complications and Management
Local Site Reactions
Local site reactions in intravenous (IV) therapy encompass a range of immediate, localized complications arising from catheter insertion or fluid/medication administration at the access point, primarily affecting the surrounding skin and subcutaneous tissues. These reactions, distinct from systemic issues, include inflammation, leakage, discomfort, and bleeding, often resulting from mechanical irritation, chemical properties of infusates, or vascular trauma. Early recognition through routine site assessment is essential to mitigate progression to more severe tissue damage.[150] Phlebitis, or inflammation of the vein wall, is a frequent local complication of peripheral IV catheters, classified into mechanical and chemical types. Mechanical phlebitis occurs due to friction from catheter movement or oversized devices relative to the vein, leading to endothelial damage.[151] Chemical phlebitis results from the irritant effects of infused substances, such as hypertonic solutions or certain medications, which erode the vein intima.[152] Severity is commonly assessed using the Visual Infusion Phlebitis (VIP) scale or the Infusion Nurses Society (INS) standards, both employing a 0-4 or 0-5 grading system based on symptoms like pain, erythema, swelling, and induration; for instance, grade 2 typically involves pain with erythema at the site, indicating early-stage phlebitis requiring catheter removal.[153] Incidence varies by patient factors and catheter dwell time but has been reported in up to 41% of peripheral IV catheters in clinical settings.[154] Infiltration and extravasation represent unintended leakage of IV fluids or medications into perivascular tissues, with infiltration involving non-vesicant solutions and extravasation concerning vesicant agents capable of severe injury. Symptoms of both include localized swelling, pallor, coolness, and pain at the insertion site, potentially progressing to blistering or necrosis in extravasation cases.[155] Management protocols emphasize immediate cessation of infusion, aspiration of residual fluid via the catheter if possible, limb elevation, and avoidance of heat or tourniquets to prevent further spread.[156] For vesicant extravasation, such as with anthracyclines like doxorubicin, specific antidotes like dexrazoxane are administered to neutralize tissue damage and reduce ulceration risk.[157] Infiltration is among the most common IV complications, occurring in a substantial proportion of peripheral lines due to dislodgement or vein perforation.[2] Pain at the IV site manifests during insertion as a sharp sting from needle puncture or during infusion as a burning sensation from irritant infusates. Insertion-related pain affects a notable fraction of patients, with studies indicating discomfort in 20-50% of cases without intervention, often mitigated by pretreatment with topical or intradermal lidocaine to numb the area and improve cannulation success.[158] Infusion pain, particularly burning, is associated with rapid administration of electrolytes like potassium chloride at rates exceeding 10 mEq/hour, which irritates the endothelium and prompts site rotation or dilution strategies.[159] Overall, local pain contributes to patient distress and may signal emerging complications like phlebitis or infiltration. Brief reference to optimal vascular access selection, as detailed in methods sections, can help minimize such reactions through reduced trauma. Hematoma formation involves localized bleeding into tissues following IV insertion, more prevalent in patients on anticoagulant therapy due to impaired clotting. It presents as ecchymosis, swelling, and tenderness at the site, resulting from vessel puncture or seepage.[160] Management focuses on direct compression to achieve hemostasis, typically for 5-10 minutes, combined with cold application to vasoconstrict and limit expansion, avoiding invasive interventions unless expansion threatens neurovascular structures.[161] In anticoagulated individuals, monitoring for progression is critical, as hematomas can delay therapy resumption. Techniques for secure catheter stabilization, covered in maintenance guidelines, aid in prevention.Infectious Risks
Infectious risks associated with intravenous therapy primarily arise from microbial colonization and invasion at the catheter insertion site or along the device, leading to local or systemic infections. The most serious complication is catheter-related bloodstream infection (CRBSI), defined as a laboratory-confirmed bloodstream infection (LCBI) attributable to an intravascular catheter, with incidence rates typically ranging from 1 to 5 episodes per 1,000 catheter-days in central venous catheters (CVCs).[162] Other types include exit-site infections, characterized by erythema, tenderness, or purulent drainage at the catheter exit point without systemic involvement, and tunnel infections, which involve inflammation extending more than 2 cm from the exit site along the subcutaneous tract of tunneled catheters.[163] These infections can progress to CRBSI if untreated, particularly in long-term devices.[164] Common pathogens in IV catheter infections include coagulase-negative staphylococci (the most frequent), Staphylococcus aureus, and Gram-negative bacilli such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli.[164] Fungal pathogens like Candida species are less common but more prevalent in immunocompromised patients or prolonged catheterization.[165] Risk factors for these infections encompass catheter dwell time exceeding 7 days, use of multi-lumen catheters, inadequate skin antisepsis or hand hygiene, and patient factors like immunosuppression or ICU admission.[146] Infections occur at higher rates with CVCs compared to peripheral intravenous catheters (PIVCs), with CVCs associated with approximately 2- to 5-fold greater risk per catheter-day due to their proximity to dense skin flora and longer indwelling times.[166] Overall incidence is lower for PIVCs at about 0.5 per 1,000 catheter-days (equivalent to approximately 0.1% of catheters), versus 1-3 per 1,000 for short-term CVCs, though cumulative rates in long-term CVCs without preventive bundles can reach up to 20%.[167][168] Diagnosis of CRBSI relies on clinical signs such as fever, chills, or hypotension in the presence of a catheter, confirmed by paired blood cultures showing differential time to positivity (DTP), where the culture from the catheter draws positive at least 2 hours earlier than peripheral blood.[163] The Centers for Disease Control and Prevention (CDC) defines central line-associated bloodstream infection (CLABSI) as a primary LCBI in a patient with a CVC in place for more than 2 calendar days, with the infection not attributable to another site, often requiring fever plus a positive blood culture excluding contaminants like coagulase-negative staphylococci unless two positive cultures are obtained.[169] For exit-site and tunnel infections, diagnosis involves clinical assessment of local inflammation, with cultures of exudate or tissue if available; imaging such as ultrasound may identify abscesses or thrombi in tunnel infections.[164] Early recognition is critical, as untreated CRBSI carries high morbidity, including sepsis and endocarditis.[170]Fluid and Medication Overload
Fluid and medication overload in intravenous (IV) therapy occurs when excessive volumes of fluids or drugs are administered, leading to systemic complications that can compromise cardiovascular, respiratory, and renal function. This iatrogenic issue is particularly prevalent in critically ill patients, where aggressive fluid resuscitation or continuous infusions may inadvertently exceed physiological tolerances. Overload manifests through circulatory strain, toxic accumulation, and electrolyte derangements, necessitating vigilant monitoring to mitigate risks. Fluid overload, defined as an increase in body weight exceeding 10%, arises from excessive IV fluid administration and can precipitate pulmonary edema and exacerbate heart failure. In pulmonary edema, fluid accumulates in the alveolar spaces, impairing gas exchange and causing severe dyspnea, hypoxemia, and potential respiratory failure. This condition is especially dangerous in patients with preexisting heart failure, where elevated filling pressures lead to acute decompensation and reduced cardiac output. Patients with renal impairment face heightened risks, as diminished excretory capacity delays fluid clearance, prolonging exposure and increasing mortality; for instance, acute kidney injury recovery is hindered in those with >10% weight gain from overload. Medication overload primarily stems from over-infusion of therapeutic agents or errors in drug compatibility, resulting in toxic serum levels and adverse effects. For example, excessive IV digoxin administration can elevate serum levels above 2 ng/mL, inducing life-threatening arrhythmias such as bidirectional ventricular tachycardia due to enhanced myocardial automaticity and conduction delays. Compatibility errors, such as mixing incompatible drugs in IV lines, may cause precipitation, leading to particulate emboli that obstruct pulmonary or renal vasculature and provoke infarction or inflammation. These incidents underscore the need for precise dosing and admixture verification to prevent such toxicities. Electrolyte shifts represent another critical consequence of IV overload, often triggered by rapid or unbalanced infusions. Hyperkalemia from swift IV potassium administration (>5.5 mmol/L serum levels) disrupts cardiac electrophysiology, manifesting as peaked T-waves on ECG and progressing to arrhythmias or asystole if untreated. Conversely, hyponatremia induced by hypotonic IV fluids (e.g., 0.18% NaCl in dextrose) dilutes serum sodium below 135 mmol/L, causing cerebral edema, seizures, and neurological impairment through osmotic fluid shifts into brain cells. These imbalances highlight the importance of tailored fluid composition in vulnerable populations. Effective monitoring thresholds help avert overload by providing early indicators of excessive administration. Central venous pressure (CVP) targets of 8-12 mmHg signal adequate preload without hypervolemia, guiding fluid cessation in responsive patients. Daily body weight assessments serve as the gold standard for detecting cumulative overload, with gains prompting immediate intervention. Infusion pumps incorporate alarms for volume to be infused (VTBI) completion, alerting clinicians to prevent unintended exceedance and reduce the risk of sustained overdelivery.Risks of Non-Professional or Home Administration
Administering intravenous (IV) drips at home without a qualified medical professional carries significant risks, as this bypasses essential sterile techniques, proper vascular access procedures, appropriate fluid and medication dosing, and continuous clinical monitoring. These risks overlap substantially with the complications discussed in other subsections but are markedly increased due to the absence of trained personnel and emergency response capabilities.[5] Key risks include:- Infection at the injection site, potentially progressing to systemic infection, amplified by non-sterile home environments (see Infectious Risks).
- Vein inflammation or clotting (thrombophlebitis) from improper catheter placement, movement, or irritant infusates (see Local Site Reactions).
- Fluid infiltration into surrounding tissue, leading to swelling, tissue damage, or necrosis (see Local Site Reactions).
- Air embolism from inadvertent air entry into the vein due to poor line priming or management, which can be fatal.[171]
- Electrolyte imbalances or overhydration from inappropriate volume or composition of fluids (see Fluid and Medication Overload).
- Overdose effects, such as rapid potassium administration causing cardiac arrhythmias (see Fluid and Medication Overload).
- Allergic reactions to infused substances without immediate treatment availability.
- Lack of immediate monitoring and intervention for life-threatening complications, increasing the likelihood of severe outcomes.