Recent from talks
Nothing was collected or created yet.
4-Hydroxyamphetamine
View on Wikipedia
 | |
| Clinical data | |
|---|---|
| Trade names | Paredrine, Paremyd, Pedrolon, Mycadrine, Paredrinex, others |
| Other names | 4-Hydroxyamphetamine; 4-HA; Hydroxyamfetamine; Oxamphetamine; Norpholedrine; para-Hydroxyamphetamine; PHA; α-Methyltyramine; Methyltyramine, Hydroxyamphetamine (USAN US) |
| Routes of administration | Eye drops |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.866 |
| Chemical and physical data | |
| Formula | C9H13NO |
| Molar mass | 151.209 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydroxyamphetamine, also known as 4-hydroxyamphetamine or norpholedrine and sold under the brand names Paredrine and Paremyd among others, is a sympathomimetic medication used in eye drops to dilate the pupil for eye examinations.[1][2][3][4]
Hydroxyamfetamine acts as a norepinephrine releasing agent and hence is an indirectly acting sympathomimetic.[5][6] It is a substituted phenethylamine and amphetamine.[4]
Hydroxyamphetamine appeared to remain marketed only in the Czech Republic as of 2004.[3]
Medical uses
[edit]Hydroxyamphetamine is used in eye drops to dilate the pupil (a process called mydriasis) so that the back of the eye can be examined. This is a diagnostic test for Horner's syndrome. Patients with Horner's syndrome exhibit anisocoria brought about by lesions on the nerves that connect to the nasociliary branch of the ophthalmic nerve.[7] Application of hydroxyamphetamine to the eye can indicate whether the lesion is preganglionic or postganglionic based on the pupil's response. If the pupil dilates, the lesion is preganglionic. If the pupil does not dilate, the lesion is postganglionic.[7]
Hydroxyamphetamine has some limitations to its use as a diagnostic tool. If it is intended as an immediate follow up to another mydriatic drug (cocaine or apraclonidine), then the patient must wait anywhere from a day to a week before hydroxyamphetamine can be administered.[8][5] It also has the tendency to falsely localize lesions. False localization can arise in cases of acute onset; in cases where a postganglionic lesion is present, but the nerve still responds to residual norepinephrine; or in cases in which unrelated nerve damage masks the presence of a preganglionic lesion.[7][8]
Available forms
[edit]Hydroxyamphetamine is a component of two controlled (prescription only), name-brand ophthalmic mydriatics: Paredrine and Paremyd. Paredrine consists of a 1% solution of hydroxyamphetamine hydrobromide[9]: 543 while Paremyd consists of a combination of 1% hydroxyamphetamine hydrobromide and 0.25% tropicamide.[10]
Pharmacology
[edit]Pharmacodynamics
[edit]Hydroxyamphetamine acts as an indirect sympathomimetic and induces the release of norepinephrine which leads to mydriasis (pupil dilation).[5][6]
It has also been found to act as a serotonin releasing agent.[11] The drug produces the head-twitch response, a behavioral proxy of psychedelic effects, when it is given by intracerebroventricular injection in animals.[11] This effect is blocked by the serotonin receptor antagonists cyproheptadine and dimethothiazine, by the serotonin reuptake inhibitor fluoxetine, and by the serotonin synthesis inhibitor para-chlorophenylalanine (PCPA).[11] These findings suggest that hydroxyamphetamine-induced head twitches are due to activation of the serotonin 5-HT2A receptor and that they are mediated by induction of serotonin release as opposed to direct agonism of the serotonin 5-HT2A receptor.[11] Although hydroxyamphetamine produces the head-twitch response in animals, serotonin releasing agents are not necessarily hallucinogenic in humans, and hence their induction of head twitches in animals has been considered a false positive for psychedelic effects.[12][13][14]
It additionally decreases metabolism of serotonin and certain other monoamines by inhibiting the activity of monoamine oxidases (MAOs), particularly type A (MAO-A).[citation needed] The inhibition of MAO-A prevents metabolism of serotonin and catecholamines in the presynaptic terminal, and thus increases the amount of neurotransmitters available for release into the synaptic cleft.[11]
Like amphetamine, hydroxyamphetamine is an agonist of human TAAR1.[15]
Pharmacokinetics
[edit]Hydroxyamphetamine is a major metabolite of amphetamine and a minor metabolite of methamphetamine. In humans, amphetamine is metabolized to hydroxyamphetamine by CYP2D6, which is a member of the cytochrome P450 superfamily and is found in the liver.[16][17] 4-Hydroxyamphetamine is then metabolized by dopamine β-hydroxylase into 4-hydroxynorephedrine or eliminated in the urine.[6]
Metabolic pathways of amphetamine in humans[sources 1]
|
Chemistry
[edit]Hydroxyamphetamine, also known as 4-hydroxy-α-methylphenethylamine, 4-hydroxyamphetamine, or α-methyltyramine, is a substituted phenethylamine and amphetamine derivative. It is the 4-hydroxylated analogue of amphetamine, the N-demethylated analogue of pholedrine (4-hydroxy-N-methylamphetamine), and the α-methylated analogue of tyramine (4-hydroxyphenethylamine). Other analogues include α-methyldopamine, corbadrine (levonordefrin; α-methylnorepinephrine), and dioxifedrine (α-methylepinephrine).
It has a predicted log P of 0.58 to 1.4.[29][4][30]
Hydroxyamphetamine is used pharmaceutically as the hydrobromide salt.[1]
History
[edit]Hydroxyamphetamine was first synthesized by 1910.[1]
In the 1990s, the trade name rights, patents, and new drug applications (NDAs) for Paredrine and Paremyd were exchanged among a few different manufacturers after a shortage of the raw material required for their production, which caused both drugs to be indefinitely removed from the market.[31] Around 1997, Akorn, Inc., obtained the rights to both Paredrine and Paremyd,[32] and in 2002, the company reintroduced Paremyd to the market as a fast acting ophthalmic mydriatic agent.[10][33][34]
In 2004, hydroxyamphetamine appeared to remain marketed only in the Czech Republic.[3]
Society and culture
[edit]Names
[edit]Hydroxyamphetamine is the generic name of the drug and its BAN and DCF, while hydroxyamfetamine is its INN.[1][2][3] In the case of the hydrobromide salt, its generic name is hydroxyamphetamine hydrobromide and this is its USAN.[1][2][3] It is also known by synonyms including methyltyramine, norpholedrine, and oxamphetamine.[1][2][3][29] The drug is sold under brand names including Paredrine, Paredrinex, Paremyd, Pedrolon, and Mycadrine.[1][3]
Other drugs
[edit]4-Hydroxyamphetamine is also a metabolite of amphetamine and certain other amphetamines.[2]
Notes
[edit]- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[19][24] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[24][26] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[27][28]
Reference notes
[edit]References
[edit]- ^ a b c d e f g Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 74. ISBN 978-1-4757-2085-3. Retrieved August 30, 2024.
- ^ a b c d e Morton IK, Hall JM (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 90. ISBN 978-94-011-4439-1. Retrieved August 30, 2024.
- ^ a b c d e f g Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory. Medpharm Scientific Publishers. p. 609. ISBN 978-3-88763-101-7. Retrieved August 30, 2024.
- ^ a b c "Hydroxyamphetamine: Uses, Interactions, Mechanism of Action". DrugBank Online. January 30, 1992. Retrieved August 30, 2024.
- ^ a b c Lepore FE (1985). "Diagnostic pharmacology of the pupil". Clinical Neuropharmacology. 8 (1): 27–37. doi:10.1097/00002826-198503000-00003. PMID 3884149.
- ^ a b c Cho AK, Wright J (February 1978). "Pathways of metabolism of amphetamine and related compounds". Life Sciences. 22 (5): 363–372. doi:10.1016/0024-3205(78)90282-5. PMID 347211.
- ^ a b c Walton KA, Buono LM (December 2003). "Horner syndrome". Current Opinion in Ophthalmology. 14 (6): 357–363. doi:10.1097/00055735-200312000-00007. PMID 14615640. S2CID 11262166.
- ^ a b Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT (March 2013). "Adult Horner's syndrome: a combined clinical, pharmacological, and imaging algorithm". Eye. 27 (3): 291–298. doi:10.1038/eye.2012.281. PMC 3597883. PMID 23370415.
- ^ Slamovits TL, Glaser JS (1999). "The Pupils and Accommodation.". In Glaser JS (ed.). Neuro-ophthalmology. Philadelphia, PA: Lippincott, Williams, & Wilkins. ISBN 978-0781717298.
- ^ a b "Hydroxyamphetamine Hydrobromide; Tropicamide". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Archived from the original on March 4, 2016.
- ^ a b c d e Nakagawasai O, Arai Y, Satoh SE, Satoh N, Neda M, Hozumi M, et al. (January 2004). "Monoamine oxidase and head-twitch response in mice. Mechanisms of alpha-methylated substrate derivatives". Neurotoxicology. 25 (1–2): 223–232. Bibcode:2004NeuTx..25..223N. doi:10.1016/S0161-813X(03)00101-3. PMID 14697897.
- ^ Halberstadt AL, Geyer MA (2018). "Effect of Hallucinogens on Unconditioned Behavior". In Halberstadt AL, Vollenweider FX, Nichols DE (eds.). Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. Vol. 36. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 159–199. doi:10.1007/7854_2016_466. ISBN 978-3-662-55878-2. PMC 5787039. PMID 28224459.
Amphetamine and methamphetamine, which act primarily by increasing carrier-mediated release of dopamine and norepinephrine, do not provoke head twitches (Corne and Pickering 1967; Silva and Calil 1975; Yamamoto and Ueki 1975; Jacobs et al. 1976; Bedard and Pycock 1977; Halberstadt and Geyer 2013). By contrast, the 5-HT releasing drugs fenfluramine and p-chloroamphetamine (PCA) do produce a robust HTR (Singleton and Marsden 1981; Darmani 1998a). Fenfluramine and PCA are thought to act indirectly, by increasing carrier-mediated release of 5-HT, because the response can be blocked by inhibition of the 5-HT transporter (Balsara et al. 1986; Darmani 1998a) or by depletion of 5-HT (Singleton and Marsden 1981; Balsara et al. 1986). [...] Because indirect 5-HT agonists such as fenfluramine, PCA, and 5-HTP are not hallucinogenic (Van Praag et al. 1971; Brauer et al. 1996; Turner et al. 2006), their effects on HTR can potentially be classified as false-positive responses.
- ^ Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD (May 2020). "Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species". Neuropharmacology. 167 107933. doi:10.1016/j.neuropharm.2019.107933. PMC 9191653. PMID 31917152.
Indirect 5-HT2A agonists such as fenfluramine, p-chloroamphetamine (PCA), and 5-hydroxytryptophan (5-HTP) induce head twitches in rodents (Corne et al. 1963; Singleton and Marsden 1981; Darmani 1998) but do not act as hallucinogens in humans (van Praag et al. 1971; Brauer et al. 1996; Turner et al. 2006), However, overdoses of compounds that increase serotonin (5-HT) release can result in 5-HT syndrome, which sometimes includes hallucinations (Birmes et al. 2003; Evans and Sebastian 2007).
- ^ Wojtas A, Gołembiowska K (December 2023). "Molecular and Medical Aspects of Psychedelics". Int J Mol Sci. 25 (1): 241. doi:10.3390/ijms25010241. PMC 10778977. PMID 38203411.
While some false positives have been identified, such as fenfluramine, p-chloroamphetamine, and 5-hydroxytryptophan, the test predominantly exhibits specificity for 5-HT2A receptor agonists [15].
- ^ Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorganic & Medicinal Chemistry. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ^ Markowitz JS, Patrick KS (2001). "Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder". Clinical Pharmacokinetics. 40 (10): 753–772. doi:10.2165/00003088-200140100-00004. PMID 11707061. S2CID 20884365.
- ^ Haefely W, Bartholini G, Pletscher A (1976). "Monoaminergic drugs: general pharmacology". Pharmacology & Therapeutics B. 2 (1): 185–218. doi:10.1016/0306-039x(76)90030-1. PMID 817330.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved December 30, 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved November 6, 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ a b "4-(2-Aminopropyl)phenol". PubChem. Retrieved August 30, 2024.
- ^ "C9H13NO". Hydroxyamphetamine. August 30, 2024. Retrieved August 30, 2024.
- ^ "Akorn Acquires Paredrine - Specialty Ophthalmic Diagnostic Product From Pharmics, Inc". Akorn press release. March 24, 1999. Archived from the original on September 16, 2018. Retrieved December 9, 2014.
- ^ "Akorn press release". Archived from the original on December 9, 2014.
- ^ "Akorn timeline". Archived from the original on June 26, 2019. Retrieved December 9, 2014.
- ^ Lurcott R (December 1, 2002). "Unique Mydriatic Returns: The combination formula fosters patient flow efficiencies". Ophthalmology Management.
External links
[edit]- p-Hydroxyamphetamine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
4-Hydroxyamphetamine
View on GrokipediaMedical uses
Diagnostic applications
4-Hydroxyamphetamine serves as a mydriatic agent in topical ophthalmic formulations, primarily administered as 1% eye drops to induce pupil dilation (mydriasis) for facilitating fundus examinations and other diagnostic ocular assessments.[2] This application leverages its sympathomimetic properties to safely dilate the pupil in patients, including those with shallow anterior chambers, due to its relatively slow onset and minimal side effects compared to other mydriatics.[2] A key diagnostic role involves its use in the hydroxyamphetamine test, often following cocaine instillation, to localize lesions in the oculosympathetic pathway for Horner’s syndrome.[7] In this protocol, one drop of 1% hydroxyamphetamine is instilled into both eyes 48 hours after cocaine testing, with pupil responses evaluated after 45-60 minutes.[7] Dilation of the affected pupil indicates an intact postganglionic (third-order) neuron, suggesting a preganglionic or central lesion, whereas failure to dilate points to a postganglionic lesion.[7][8] Diagnostic limitations include the need for a 48-72 hour delay after cocaine or apraclonidine use to avoid interference from norepinephrine depletion, which can lead to false positives mimicking postganglionic lesions.[7] In acute Horner’s syndrome within the first week post-injury, false-negative results may occur due to incomplete degeneration of presynaptic terminals.[7] Additionally, normal variability in pupil response can limit localization accuracy, as a normal test does not reliably distinguish between central and preganglionic lesions, and transsynaptic degeneration may cause false positives in recent preganglionic cases.[8][9] Drug availability further restricts widespread clinical use, particularly following the discontinuation of commercial formulations in recent years.[7][10]Available forms and administration
4-Hydroxyamphetamine, also known as hydroxyamphetamine, was previously available as a 1% hydroxyamphetamine hydrobromide ophthalmic solution under the brand name Paredrine for topical ocular administration to induce mydriasis. However, Paredrine has been discontinued and is no longer commercially available. The combination product Paremyd, consisting of 1% hydroxyamphetamine hydrobromide and 0.25% tropicamide in a sterile ophthalmic solution packaged in 15 mL dropper bottles (NDC 17478-704-12), was introduced to enhance the speed and efficacy of mydriasis for diagnostic purposes but was discontinued by the manufacturer Akorn in 2023 and is no longer available as of November 2025.[11][12][13] Due to these discontinuations, hydroxyamphetamine is no longer routinely used in clinical practice, with alternatives such as phenylephrine employed for mydriasis and Horner syndrome evaluation. For the discontinued Paredrine, the standard dosing regimen involved instilling 1 drop of the 1% solution into the conjunctival sac of each eye, with an onset of mydriasis typically occurring in 45-60 minutes and a duration extending up to 24 hours.[10][7] For Paremyd, the recommended dose was 1-2 drops instilled into the conjunctival sac of the eye(s) requiring dilation, with onset within 15 minutes, maximum effect at 60 minutes, and recovery generally within 6-8 hours, though it may last up to 24 hours in some individuals.[11][14] Administration of either formulation followed standard ophthalmic instillation procedures: the patient should tilt their head backward, pull down the lower eyelid to create a conjunctival pouch, and apply the drops without allowing the dropper tip to touch the eye or any surface to prevent contamination. After instillation, the patient should close their eyes gently for 1-2 minutes, avoid blinking or rubbing the eye, and apply light finger pressure to the inner corner of the eye (lacrimal sac) for at least 1 minute to reduce systemic absorption and nasolacrimal drainage.[14] Soft contact lenses should be removed prior to administration due to potential preservative irritation and may be reinserted approximately 15 minutes after the drops are applied.[11] Paremyd should be stored at controlled room temperature (15°C to 25°C or 59°F to 77°F) and protected from light to maintain stability.[11] Historically, both Paredrine and Paremyd faced manufacturing and availability challenges in the late 1990s, leading to shortages; Akorn, Inc. acquired the rights from Allergan in 1997 and reintroduced Paremyd to the U.S. market in March 2002 following FDA approval.[15][16] However, following Akorn's bankruptcy in 2023, Paremyd was discontinued, resulting in its permanent withdrawal from the market.[13][12]Pharmacology
Pharmacodynamics
4-Hydroxyamphetamine functions primarily as a norepinephrine releasing agent (NERA) by promoting the reversal of the vesicular monoamine transporter 2 (VMAT2), which displaces norepinephrine from synaptic vesicles into the cytoplasm, and by inhibiting the norepinephrine transporter (NET), thereby preventing reuptake and increasing synaptic norepinephrine levels.[17][2] This indirect sympathomimetic action leads to enhanced stimulation of alpha- and beta-adrenergic receptors without direct agonism at these sites.[1] It also acts as a partial agonist at trace amine-associated receptor 1 (TAAR1), with an EC50 of approximately 0.05–0.2 μM in rat TAAR1-expressing cells, contributing to its overall sympathomimetic and stimulant effects by modulating monoamine signaling.[18][19] Additionally, 4-hydroxyamphetamine exhibits serotonin releasing agent (SRA) properties, facilitating serotonin release, and weakly inhibits monoamine oxidase A (MAO-A), reducing serotonin metabolism, though these effects are less pronounced compared to amphetamine.[20][21] The resulting physiological effects include elevated heart rate (tachycardia) and blood pressure due to beta-adrenergic stimulation and alpha-adrenergic vasoconstriction, respectively, as well as mydriasis (pupil dilation) mediated by alpha-adrenergic activation in the iris.[22][21] Relative to amphetamine, 4-hydroxyamphetamine demonstrates reduced central nervous system stimulation owing to its greater polarity and limited blood-brain barrier penetration, while retaining comparable peripheral sympathomimetic potency.Pharmacokinetics
4-Hydroxyamphetamine is administered topically to the eye as a 1% solution (Paredrine), producing primarily local mydriatic effects with minimal systemic absorption due to its ophthalmic route of delivery. The onset of mydriasis occurs within 15-30 minutes, with peak dilation achieved at approximately 45-60 minutes and mydriasis lasting 3-6 hours; pupil size returns to normal within 24 hours.[23][22] In cases of systemic absorption, 4-hydroxyamphetamine undergoes rapid distribution to peripheral tissues, including sympathetic nerve terminals. It is extensively metabolized in the liver, primarily via conjugation to form water-soluble metabolites, with further involvement of cytochrome P450 2D enzymes in its hydroxylation and elimination pathways; inhibitors of CYP2D reduce urinary elimination of the compound.[24] Excretion occurs mainly through the kidneys, with up to 3% of an administered dose (or equivalent from parent compounds) appearing as free 4-hydroxyamphetamine in urine and the majority as the conjugated form, totaling around 60-65% recovery in humans over 3-4 days; renal clearance is pH-dependent, accelerating in acidic urine.[4] The elimination half-life, inferred from studies of related amphetamines and its metabolic profile, ranges from 7 to 13 hours in adults, though specific data for standalone administration are limited.[25] Pharmacokinetic variability is notable in certain populations. In the elderly, reduced hepatic metabolism and CYP2D6 activity may prolong exposure, while hepatic impairment inhibits elimination, leading to higher plasma levels and extended effects. Renal dysfunction similarly delays clearance due to the urinary excretion pathway. Drug interactions can alter kinetics: monoamine oxidase inhibitors potentiate sympathomimetic effects by preventing norepinephrine breakdown, risking enhanced toxicity, and CYP2D6 inhibitors or substrates (e.g., certain antidepressants) may decrease 4-hydroxyamphetamine metabolism.[26][27]Metabolism and role as an amphetamine metabolite
4-Hydroxyamphetamine is formed as a primary metabolite of amphetamine through aromatic hydroxylation primarily catalyzed by the cytochrome P450 enzyme CYP2D6 in the liver.[26] This pathway accounts for approximately 2% of amphetamine's overall metabolism in humans, though the exact fraction can vary based on individual enzyme activity.[2] The process involves the addition of a hydroxyl group at the para position of the amphetamine phenyl ring, yielding the pharmacologically active 4-hydroxyamphetamine.[26] Following formation, 4-hydroxyamphetamine undergoes further metabolism, including oxidation by dopamine β-hydroxylase to produce 4-hydroxynorephedrine, a norepinephrine analog that retains sympathomimetic activity. It is also subject to phase II conjugation reactions, forming glucuronide and sulfate conjugates that facilitate renal excretion.[28] These conjugated forms are primarily eliminated in urine, contributing to the detection of amphetamine use in drug screens where 4-hydroxyamphetamine serves as a biomarker.[29] Genetic polymorphisms in CYP2D6 significantly influence 4-hydroxyamphetamine production; poor metabolizers exhibit reduced levels of this metabolite due to diminished enzyme function, potentially altering amphetamine's overall disposition.[30] In terms of pharmacological implications, 4-hydroxyamphetamine contributes to the peripheral sympathomimetic effects of amphetamine, such as vasoconstriction and increased heart rate.[2] Toxicologically, 4-hydroxyamphetamine and its downstream metabolite 4-hydroxynorephedrine play roles in amphetamine-induced neurotoxicity, with 4-hydroxynorephedrine demonstrating greater cytotoxicity in dopaminergic cells and longer persistence in the brain compared to the parent compound.[31] This persistence underscores their potential contribution to long-term neuronal damage observed in amphetamine abuse.[5]Chemistry
Chemical structure and properties
4-Hydroxyamphetamine has the molecular formula C₉H₁₃NO and the systematic IUPAC name 4-(2-aminopropyl)phenol.[32][33] The free base has a molecular weight of 151.21 g/mol, while the hydrobromide salt, which is the form used clinically, has the formula C₉H₁₄BrNO and a molecular weight of 232.12 g/mol.[32] It appears as a white to off-white crystalline solid.[34] Key physical properties include a predicted logP value ranging from 0.58 to 1.0, indicating moderate lipophilicity; a pKa of approximately 9.8 for the amine group and 10.5 for the phenolic hydroxyl; and solubility in water (predicted ~3 mg/mL) and ethanol (up to 30 mg/mL for the hydrochloride salt).[35][3] These properties reflect its increased polarity due to the para-hydroxyl substitution on the phenyl ring compared to amphetamine, which reduces its ability to penetrate the blood-brain barrier.[36] For analytical identification, 4-hydroxyamphetamine exhibits characteristic spectroscopic features. In mass spectrometry, the molecular ion appears at m/z 151, with prominent fragments at m/z 136 (loss of CH₃) and m/z 108.[33] Infrared spectroscopy shows absorption bands at approximately 3300 cm⁻¹ (O-H and N-H stretch) and 1600-1500 cm⁻¹ (aromatic C=C stretch), while ¹H NMR reveals signals for the phenolic proton around 5-6 ppm, aromatic protons at 6.7-7.2 ppm, and the benzylic CH₂ at ~2.7 ppm.[33][32]Synthesis and preparation
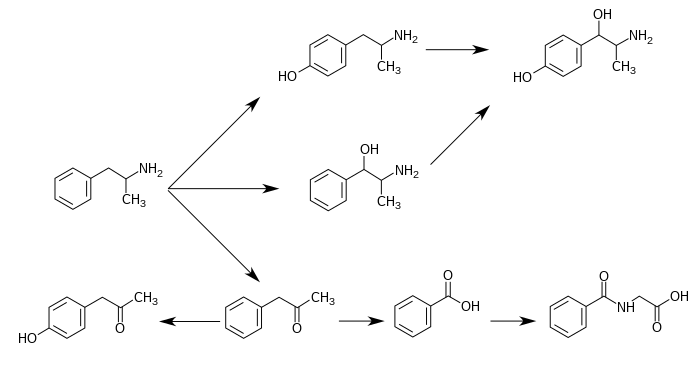
4-Hydroxyamphetamine is typically synthesized in the laboratory through reductive amination of 4-hydroxyphenylacetone (p-hydroxyphenyl-2-propanone) with ammonia, using a reducing agent such as sodium cyanoborohydride or catalytic hydrogenation.[37] This method, which involves the formation of an imine intermediate followed by reduction to the primary amine, has been a standard approach since the early 20th century, with early descriptions appearing around 1910 via reduction of the ketone precursor.[38] Yields for this reaction can reach 70-90% under optimized conditions, such as in supercritical carbon dioxide at 35°C and 1500 psi, which facilitates the process while minimizing side products.[37] Alternative routes include the Henry reaction (nitroaldol condensation) of p-hydroxybenzaldehyde with nitroethane to form a nitroalkene, followed by reduction to the ketone and subsequent reductive amination.[38] A protected variant starts from 4-methoxybenzaldehyde, condensing with nitroethane in the presence of a primary amine catalyst to yield the nitropropene, which is reduced (e.g., with iron and HCl) to 4-methoxyphenylacetone, then aminated and demethylated.[38] These multi-step processes allow access from commercially available aromatic aldehydes and are adaptable for analog synthesis. The hydrobromide salt, used in pharmaceutical formulations like ophthalmic solutions, is prepared by treating the free base with hydrobromic acid in a suitable solvent, followed by precipitation and recrystallization to achieve purity greater than 99%.[38] This salt formation enhances stability and solubility for diagnostic applications. Synthesis challenges include achieving stereoselectivity for the (S)-enantiomer, which exhibits greater sympathomimetic activity; asymmetric reductive amination using chiral catalysts or enzymes can yield enantiomeric excesses over 95%.[37] Impurities such as 4-hydroxymethamphetamine must be avoided by using ammonia exclusively, as methylamine would lead to N-methylation; rigorous control of reagents and purification steps mitigates this.[38] For commercial scale-up, the process employs continuous flow reductive amination in large reactors, with purification via acid-base extraction and crystallization from ethanol or isopropyl alcohol to isolate the hydrobromide salt at kilogram scales, ensuring compliance with pharmaceutical standards.[37]History
Early development
4-Hydroxyamphetamine, also known as p-hydroxyamphetamine, emerged in the scientific literature during the 1940s as a sympathomimetic agent derived from amphetamine analogs. It was first synthesized around 1910 as part of early investigations into sympathomimetic amines. Early investigations focused on its potential as a nasal decongestant and pressor agent, with initial clinical testing reported in 1948 using hydroxyamphetamine hydrobromide (Paredrine) as a spray for upper respiratory conditions, demonstrating its vasoconstrictive effects comparable to those of other sympathomimetics.[39] These studies highlighted its ability to mimic epinephrine's actions on alpha-adrenergic receptors, though with a more indirect mechanism involving catecholamine release rather than direct agonism.[21] In the 1940s and 1950s, researchers explored its mydriatic potential through topical application, noting pupil dilation in animal models and human subjects as a result of norepinephrine release from postganglionic sympathetic neurons. Preclinical animal testing during this period demonstrated that 4-hydroxyamphetamine induced significant norepinephrine efflux from sympathetic nerve endings, leading to ocular effects such as mydriasis and elevated intraocular pressure, which were less pronounced than those of direct agonists like epinephrine but sustained due to its releasing properties. These findings positioned it as a useful tool for assessing sympathetic function in the eye, with comparisons to epinephrine underscoring its role in indirect sympathomimetic activity.[2] Naming conventions during this period standardized it as p-hydroxyamphetamine or hydroxyamfetamine, distinguishing it from other amphetamine derivatives in pharmacological literature.[40] By the 1960s, metabolic studies revealed 4-hydroxyamphetamine's significance as a key metabolite of amphetamine in humans, with research detecting its presence in urine following amphetamine administration, confirming aromatic hydroxylation as a primary pathway. These investigations, building on earlier animal data, quantified its excretion as conjugated 4-hydroxyamphetamine, accounting for a notable portion of amphetamine's biotransformation and contributing to its overall sympathomimetic profile.[41]Commercial introduction and availability
4-Hydroxyamphetamine was first commercialized in the mid-20th century as Paredrine, a 1% hydroxyamphetamine hydrobromide ophthalmic solution developed by Smith Kline & French Laboratories for inducing mydriasis in diagnostic eye examinations.[42] In the 1990s, a combination formulation known as Paremyd—containing 1% hydroxyamphetamine hydrobromide and 0.25% tropicamide—was introduced by Allergan Inc. to provide faster-onset pupil dilation compared to hydroxyamphetamine alone, with a marketing start date of January 30, 1992.[43] A nationwide shortage of hydroxyamphetamine-based products, including Paredrine and Paremyd, occurred in the early 2000s due to raw material supply disruptions, leading to their temporary removal from the U.S. market. In 1998, Akorn Inc. acquired the rights to both brands from Allergan; the company resolved the shortage by reintroducing Paremyd in March 2002 as a faster-acting mydriatic option.[15][1] As of 2025, Paremyd continues to face availability challenges stemming from ongoing manufacturing delays at Akorn, which halted production following its 2023 bankruptcy and shutdown. Paredrine is no longer commercially manufactured and is typically prepared on-demand by compounding pharmacies for limited neuro-ophthalmic applications. Internationally, distribution remains restricted due to the drug's niche diagnostic role, with approvals noted in select markets such as the Czech Republic by 2004, though global supply is minimal. Current production is primarily handled by specialized pharmaceutical firms, with heightened discontinuation risks for legacy brands amid supply chain vulnerabilities.[44][2][45]Society and culture
Nomenclature
The generic name for 4-hydroxyamphetamine is hydroxyamphetamine, with the International Nonproprietary Name (INN) designated as hydroxyamfetamine.[1][46] The systematic IUPAC name is 4-(2-aminopropyl)phenol, equivalently expressed as 1-(4-hydroxyphenyl)propan-2-amine or 4-(1-methyl-2-aminoethyl)phenol in chemical nomenclature.[47][1] Other synonyms include 4-hydroxy-α-methylphenethylamine and p-hydroxyamphetamine.[47][48] Brand names include Paremyd for the combination product with tropicamide, manufactured by Akorn. The standalone formulation was formerly marketed as Paredrine but is no longer commercially available and is now prepared as a compounded 1% ophthalmic solution by some pharmacies.[49][50][2] In international contexts, the INN hydroxyamfetamine is used alongside abbreviations such as 4-HA or p-OHA in scientific literature.[46][3]Legal status
In the United States, 4-Hydroxyamphetamine is classified as a prescription-only (℞) medication under FDA regulations, primarily available as an ophthalmic solution in products like Paremyd for diagnostic mydriasis.[11] It is not federally scheduled as a controlled substance by the DEA, unlike amphetamine itself, which is Schedule II.[51] However, it is listed as a controlled substance in select state schedules, such as Alabama's state-controlled list (Schedule I, as of January 16, 2025), where it falls under provisions for amphetamine derivatives.[52] Under DEA considerations, 4-Hydroxyamphetamine may be treated as a controlled substance analog in non-medical abuse contexts due to its structural and pharmacological similarity to amphetamine, invoking the Federal Analogue Act for prosecution if intended for human consumption outside approved uses.[53] Prescription guidelines restrict its use primarily to ophthalmologists for eye examinations, with requirements for patient monitoring to prevent misuse given its sympathomimetic properties.[54] Internationally, 4-Hydroxyamphetamine is regulated as a prescription-only medicinal product in regions with controls on sympathomimetics, such as the European Union under pharmaceutical laws. As of November 2025, no major regulatory changes specific to this compound have been reported since 2020.Related compounds
4-Hydroxyamphetamine is structurally and pharmacologically related to several compounds in the amphetamine family.- Amphetamine: The parent compound, from which 4-hydroxyamphetamine is primarily metabolized via CYP2D6-mediated para-hydroxylation.[55]
- Methamphetamine: A precursor that also metabolizes to 4-hydroxyamphetamine, sharing similar sympathomimetic effects.[2]
- 4-Hydroxynorephedrine (p-Hydroxynorephedrine): A β-hydroxylated metabolite of amphetamine, exhibiting related noradrenergic activity.[2]
- Pholedrine (4-Hydroxymethamphetamine): An N-methylated analog used historically as a bronchodilator.[2]
- Norephedrine (Phenylpropanolamine): Another metabolite of amphetamine, with sympathomimetic properties.[2]
- 3-Hydroxyamphetamine: The meta-isomer metabolite, less potent in norepinephrine release.[2]