Recent from talks
Contribute something
Nothing was collected or created yet.
Animal testing
View on Wikipedia
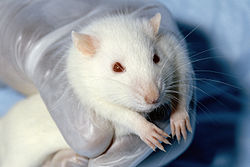 | |
| Description | Around 50–100 million vertebrate animals are used in experiments annually. |
|---|---|
| Subjects | Animal testing, science, medicine, animal welfare, animal rights, ethics |
Animal testing, also known as animal experimentation, animal research, and in vivo testing, is the use of animals, as model organisms, in experiments that seek answers to scientific and medical questions. This approach can be contrasted with field studies in which animals are observed in their natural environments or habitats. Experimental research with animals is usually conducted in universities, medical schools, pharmaceutical companies, defense establishments, and commercial facilities that provide animal-testing services to the industry.[1] The focus of animal testing varies on a continuum from pure research, focusing on developing fundamental knowledge of an organism, to applied research, which may focus on answering some questions of great practical importance, such as finding a cure for a disease.[2] Examples of applied research include testing disease treatments, breeding, defense research, and toxicology, including cosmetics testing. In education, animal testing is sometimes a component of biology or psychology courses.[3]
Research using animal models has been central to most of the achievements of modern medicine.[4][5][6] It has contributed to most of the basic knowledge in fields such as human physiology and biochemistry, and has played significant roles in fields such as neuroscience and infectious disease.[7][8] The results have included the near-eradication of polio and the development of organ transplantation, and have benefited both humans and animals.[4][9] From 1910 to 1927, Thomas Hunt Morgan's work with the fruit fly Drosophila melanogaster identified chromosomes as the vector of inheritance for genes,[10][11] and Eric Kandel wrote that Morgan's discoveries "helped transform biology into an experimental science".[12] Research in model organisms led to further medical advances, such as the production of the diphtheria antitoxin[13][14] and the 1922 discovery of insulin[15] and its use in treating diabetes, which was previously fatal.[16] Modern general anaesthetics such as halothane were also developed through studies on model organisms, and are necessary for modern, complex surgical operations.[17] Other 20th-century medical advances and treatments that relied on research performed in animals include organ transplant techniques,[18][19][20][21] the heart-lung machine,[22] antibiotics,[23][24] and the whooping cough vaccine.[25]
Animal testing is widely used to aid in research of human disease when human experimentation would be unfeasible or unethical.[26] This strategy is made possible by the common descent of all living organisms, and the conservation of metabolic and developmental pathways and genetic material over the course of evolution.[27] Performing experiments in model organisms allows for better understanding of the disease process without the added risk of harming an actual human. The species of the model organism is usually chosen so that it reacts to disease or its treatment in a way that resembles human physiology as needed. Biological activity in a model organism does not ensure an effect in humans, and care must be taken when generalizing from one organism to another.[28][page needed] However, many drugs, treatments and cures for human diseases are developed in part with the guidance of animal models.[29][30] Treatments for animal diseases have also been developed, including for rabies,[31] anthrax,[31] glanders,[31] feline immunodeficiency virus (FIV),[32] tuberculosis,[31] Texas cattle fever,[31] classical swine fever (hog cholera),[31] heartworm, and other parasitic infections.[33] Animal experimentation continues to be required for biomedical research,[34] and is used with the aim of solving medical problems such as Alzheimer's disease,[35] AIDS,[36] multiple sclerosis,[37] spinal cord injury,[38] and other conditions in which there is no useful in vitro model system available.
The annual use of vertebrate animals—from zebrafish to non-human primates—was estimated at 192 million as of 2015.[39] In the European Union, vertebrate species represent 93% of animals used in research,[39] and 11.5 million animals were used there in 2011.[40] The mouse (Mus musculus) is associated with many important biological discoveries of the 20th and 21st centuries,[41] and by one estimate, the number of mice and rats used in the United States alone in 2001 was 80 million.[42] In 2013, it was reported that mammals (mice and rats), fish, amphibians, and reptiles together accounted for over 85% of research animals.[43] In 2022, a law was passed in the United States that eliminated the FDA requirement that all drugs be tested on animals.[44]
Animal testing is regulated to varying degrees in different countries.[45] In some cases it is strictly controlled while others have more relaxed regulations. There are ongoing debates about the ethics and necessity of animal testing. Proponents argue that it has led to significant advancements in medicine and other fields while opponents raise concerns about cruelty towards animals and question its effectiveness and reliability.[46][47] There are efforts underway to find alternatives to animal testing such as computer simulation models, organs-on-chips technology that mimics human organs for lab tests,[48] microdosing techniques which involve administering small doses of test compounds to human volunteers instead of non-human animals for safety tests or drug screenings; positron emission tomography (PET) scans which allow scanning of the human brain without harming humans; comparative epidemiological studies among human populations; simulators and computer programs for teaching purposes; among others.[49][50][51]
Definitions
[edit]The terms animal testing, animal experimentation, animal research, in vivo testing, and vivisection have similar denotations but different connotations. Literally, "vivisection" means "live sectioning" of an animal, and historically referred only to experiments that involved the dissection of live animals. The term is occasionally used to refer pejoratively to any experiment using living animals; for example, the Encyclopædia Britannica defines "vivisection" as: "Operation on a living animal for experimental rather than healing purposes; more broadly, all experimentation on live animals",[52][53][54] although dictionaries point out that the broader definition is "used only by people who are opposed to such work".[55][56] The word has a negative connotation, implying torture, suffering, and death.[57] The word "vivisection" is preferred by those opposed to this research, whereas scientists typically use the term "animal experimentation".[58][59]
The following text excludes as much as possible practices related to in vivo veterinary surgery, which is left to the discussion of vivisection.
History
[edit]

The earliest references to animal testing are found in the writings of the Greeks in the 2nd and 4th centuries BCE. Aristotle and Erasistratus were among the first to perform experiments on living animals.[60] Galen, a 2nd-century Roman physician, performed post-mortem dissections of pigs and goats.[61] Avenzoar, a 12th-century Arabic physician in Moorish Spain introduced an experimental method of testing surgical procedures before applying them to human patients.[62][63] Discoveries in the 18th and 19th centuries included Antoine Lavoisier's use of a guinea pig in a calorimeter to prove that respiration was a form of combustion, and Louis Pasteur's demonstration of the germ theory of disease in the 1880s using anthrax in sheep.[64] Robert Koch used animal testing of mice and guinea pigs to discover the bacteria that cause anthrax and tuberculosis. In the 1890s, Ivan Pavlov famously used dogs to describe classical conditioning.[65]
Research using animal models has been central to most of the achievements of modern medicine.[4][5][6] It has contributed most of the basic knowledge in fields such as human physiology and biochemistry, and has played significant roles in fields such as neuroscience and infectious disease.[7][8] For example, the results have included the near-eradication of polio and the development of organ transplantation, and have benefited both humans and animals.[4][9] From 1910 to 1927, Thomas Hunt Morgan's work with the fruit fly Drosophila melanogaster identified chromosomes as the vector of inheritance for genes.[10][11] Drosophila became one of the first, and for some time the most widely used, model organisms,[66] and Eric Kandel wrote that Morgan's discoveries "helped transform biology into an experimental science".[12] D. melanogaster remains one of the most widely used eukaryotic model organisms. During the same time period, studies on mouse genetics in the laboratory of William Ernest Castle in collaboration with Abbie Lathrop led to generation of the DBA ("dilute, brown and non-agouti") inbred mouse strain and the systematic generation of other inbred strains.[67][68] The mouse has since been used extensively as a model organism and is associated with many important biological discoveries of the 20th and 21st centuries.[41]
In the late 19th century, Emil von Behring isolated the diphtheria toxin and demonstrated its effects in guinea pigs. He went on to develop an antitoxin against diphtheria in animals and then in humans, which resulted in the modern methods of immunization and largely ended diphtheria as a threatening disease.[13] The diphtheria antitoxin is famously commemorated in the Iditarod race, which is modeled after the delivery of antitoxin in the 1925 serum run to Nome. The success of animal studies in producing the diphtheria antitoxin has also been attributed as a cause for the decline of the early 20th-century opposition to animal research in the United States.[14]
Subsequent research in model organisms led to further medical advances, such as Frederick Banting's research in dogs, which determined that the isolates of pancreatic secretion could be used to treat dogs with diabetes. This led to the 1922 discovery of insulin (with John Macleod)[15] and its use in treating diabetes, which had previously meant death.[16][69] John Cade's research in guinea pigs discovered the anticonvulsant properties of lithium salts,[70] which revolutionized the treatment of bipolar disorder, replacing the previous treatments of lobotomy or electroconvulsive therapy. Modern general anaesthetics, such as halothane and related compounds, were also developed through studies on model organisms, and are necessary for modern, complex surgical operations.[17][71]
In the 1940s, Jonas Salk used rhesus monkey studies to isolate the most virulent forms of the polio virus,[72] which led to his invention of the polio vaccine. The vaccine, which was made publicly available in 1955, reduced the incidence of polio 15-fold in the United States over the following five years.[73] Albert Sabin improved the vaccine by passing the polio virus through animal hosts, including monkeys; the Sabin vaccine was produced for mass consumption in 1963, and had virtually eradicated polio in the United States by 1965.[74] It has been estimated that developing and producing the vaccines required the use of 100,000 rhesus monkeys, with 65 doses of vaccine produced from each monkey. Sabin wrote in 1992, "Without the use of animals and human beings, it would have been impossible to acquire the important knowledge needed to prevent much suffering and premature death not only among humans, but also among animals."[75]
On 3 November 1957, a Soviet dog, Laika, became the first of many animals to orbit the Earth. In the 1970s, antibiotic treatments and vaccines for leprosy were developed using armadillos,[76] then given to humans.[77] The ability of humans to change the genetics of animals took an enormous step forward in 1974 when Rudolf Jaenisch could produce the first transgenic mammal, by integrating DNA from simians into the genome of mice.[78] This genetic research progressed rapidly and, in 1996, Dolly the sheep was born, the first mammal to be cloned from an adult cell.[79][80]
Other 20th-century medical advances and treatments that relied on research performed in animals include organ transplant techniques,[18][19][20][21] the heart-lung machine,[22] antibiotics,[23][24] and the whooping cough vaccine.[25] Treatments for animal diseases have also been developed, including for rabies,[31] anthrax,[31] glanders,[31] feline immunodeficiency virus (FIV),[32] tuberculosis,[31] Texas cattle fever,[31] classical swine fever (hog cholera),[31] heartworm, and other parasitic infections.[33] Animal experimentation continues to be required for biomedical research,[34] and is used with the aim of solving medical problems such as Alzheimer's disease,[35] AIDS,[36][81][82] multiple sclerosis,[37] spinal cord injury, many headaches,[38] and other conditions in which there is no useful in vitro model system available.
Toxicology testing became important in the 20th century. In the 19th century, laws regulating drugs were more relaxed. For example, in the US, the government could only ban a drug after they had prosecuted a company for selling products that harmed customers. However, in response to the Elixir Sulfanilamide disaster of 1937 in which the eponymous drug killed over 100 users, the US Congress passed laws that required safety testing of drugs on animals before they could be marketed. Other countries enacted similar legislation.[83] In the 1960s, in reaction to the Thalidomide tragedy, further laws were passed requiring safety testing on pregnant animals before a drug can be sold.[84]
Model organisms
[edit]Invertebrates
[edit]
Although many more invertebrates than vertebrates are used in animal testing, these studies are largely unregulated by law. The most frequently used invertebrate species are Drosophila melanogaster, a fruit fly, and Caenorhabditis elegans, a nematode worm. In the case of C. elegans, the worm's body is completely transparent and the precise lineage of all the organism's cells is known,[85] while studies in the fly D. melanogaster can use an amazing array of genetic tools.[86] These invertebrates offer some advantages over vertebrates in animal testing, including their short life cycle and the ease with which large numbers may be housed and studied. However, the lack of an adaptive immune system and their simple organs prevent worms from being used in several aspects of medical research such as vaccine development.[87] Similarly, the fruit fly immune system differs greatly from that of humans,[88] and diseases in insects can be different from diseases in vertebrates;[89] however, fruit flies and waxworms can be useful in studies to identify novel virulence factors or pharmacologically active compounds.[90][91][92]
Several invertebrate systems are considered acceptable alternatives to vertebrates in early-stage discovery screens.[93] Because of similarities between the innate immune system of insects and mammals, insects can replace mammals in some types of studies. Drosophila melanogaster and the Galleria mellonella waxworm have been particularly important for analysis of virulent traits of mammalian pathogens.[90][91] Waxworms and other insects have also proven valuable for the identification of pharmaceutical compounds with favorable bioavailability.[92] The decision to adopt such models generally involves accepting a lower degree of biological similarity with mammals for significant gains in experimental throughput.
Rodents
[edit]
In the U.S., the numbers of rats and mice used is estimated to be from 11 million[94] to between 20 and 100 million a year.[95] Other rodents commonly used are guinea pigs, hamsters, and gerbils. Mice are the most commonly used vertebrate species because of their size, low cost, ease of handling, and fast reproduction rate.[96][97] Mice are widely considered to be the best model of inherited human disease and share 95% of their genes with humans.[96] With the advent of genetic engineering technology, genetically modified mice can be generated to order and can provide models for a range of human diseases.[96] Rats are also widely used for physiology, toxicology and cancer research, but genetic manipulation is much harder in rats than in mice, which limits the use of these rodents in basic science.[98]
Dogs
[edit]

Dogs are widely used in biomedical research, testing, and education—particularly beagles, because they are gentle and easy to handle, and to allow for comparisons with historical data from beagles (a Reduction technique).[99] They are used as models for human and veterinary diseases in cardiology, endocrinology, and bone and joint studies, research that tends to be highly invasive, according to the Humane Society of the United States.[100] The most common use of dogs is in the safety assessment of new medicines[101] for human or veterinary use as a second species following testing in rodents, in accordance with the regulations set out in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. One of the most significant advancements in medical science involves the use of dogs in developing the answers to insulin production in the body for diabetics and the role of the pancreas in this process. They found that the pancreas was responsible for producing insulin in the body and that removal of the pancreas, resulted in the development of diabetes in the dog. After re-injecting the pancreatic extract (insulin), the blood glucose levels were significantly lowered.[102]
The U.S. Department of Agriculture's Animal Welfare Report shows that 60,979 dogs were used in USDA-registered facilities in 2016.[94] In the UK, according to the UK Home Office, there were 3,847 procedures on dogs in 2017.[103] Of the other large EU users of dogs, Germany conducted 3,976 procedures on dogs in 2016[104] and France conducted 4,204 procedures in 2016.[105] In both cases this represents under 0.2% of the total number of procedures conducted on animals in the respective countries.
Zebrafish
[edit]Zebrafish are commonly used for the basic study and development of various cancers. Used to explore the immune system and genetic strains. They are low in cost, small in size, have a fast reproduction rate, and able to observe cancer cells in real time. Humans and zebrafish share neoplasm similarities which is why they are used for research. The National Library of Medicine shows many examples of the types of cancer zebrafish are used in. The use of zebrafish have allowed them to find differences between MYC-driven pre-B vs T-ALL and be exploited to discover novel pre-B ALL therapies on acute lymphocytic leukemia.[106][107]
The National Library of Medicine also explains how a neoplasm is difficult to diagnose at an early stage. Understanding the molecular mechanism of digestive tract tumorigenesis and searching for new treatments is the current research. Zebrafish and humans share similar gastric cancer cells in the gastric cancer xenotransplantation model. This allowed researchers to find that Triphala could inhibit the growth and metastasis of gastric cancer cells. Since zebrafish liver cancer genes are related with humans they have become widely used in liver cancer search, as well as many other cancers.[108]

Non-human primates
[edit]

Non-human primates (NHPs) are used in toxicology tests, studies of AIDS and hepatitis, studies of neurology, behavior and cognition, reproduction, genetics, and xenotransplantation. They are caught in the wild or purpose-bred. In the United States and China, most primates are domestically purpose-bred, whereas in Europe the majority are imported purpose-bred.[109] The European Commission reported that in 2011, 6,012 monkeys were experimented on in European laboratories.[40] According to the U.S. Department of Agriculture, there were 71,188 monkeys in U.S. laboratories in 2016.[94] 23,465 monkeys were imported into the U.S. in 2014 including 929 who were caught in the wild.[110] Most of the NHPs used in experiments are macaques;[111] but marmosets, spider monkeys, and squirrel monkeys are also used, and baboons and chimpanzees are used in the US. As of 2015[update], there are approximately 730 chimpanzees in U.S. laboratories.[112]
In a survey in 2003, it was found that 89% of singly-housed primates exhibited self-injurious or abnormal stereotypyical behaviors including pacing, rocking, hair pulling, and biting among others.[113]
The first transgenic primate was produced in 2001, with the development of a method that could introduce new genes into a rhesus macaque.[114] This transgenic technology is now being applied in the search for a treatment for the genetic disorder Huntington's disease.[115] Notable studies on non-human primates have been part of the polio vaccine development, and development of Deep Brain Stimulation, and their current heaviest non-toxicological use occurs in the monkey AIDS model, SIV.[116][111][117] In 2008, a proposal to ban all primates experiments in the EU has sparked a vigorous debate.[118]
Other species
[edit]Over 500,000 fish and 9,000 amphibians were used in the UK in 2016.[103] The main species used is the zebrafish, Danio rerio, which are translucent during their embryonic stage, and the African clawed frog, Xenopus laevis. Over 20,000 rabbits were used for animal testing in the UK in 2004.[119] Albino rabbits are used in eye irritancy tests (Draize test) because rabbits have less tear flow than other animals, and the lack of eye pigment in albinos make the effects easier to visualize. The numbers of rabbits used for this purpose has fallen substantially over the past two decades. In 1996, there were 3,693 procedures on rabbits for eye irritation in the UK,[120] and in 2017 this number was just 63.[103] Rabbits are also frequently used for the production of polyclonal antibodies.
Cats are most commonly used in neurological research. In 2016, 18,898 cats were used in the United States alone,[94] around a third of which were used in experiments which have the potential to cause "pain and/or distress"[121] though only 0.1% of cat experiments involved potential pain which was not relieved by anesthetics/analgesics. In the UK, just 198 procedures were carried out on cats in 2017. The number has been around 200 for most of the last decade.[103]
Care and use of animals
[edit]Regulations and laws
[edit]
| | Nationwide ban on all cosmetic testing on animals | | Partial ban on cosmetic testing on animals1 |
| | Ban on the sale of cosmetics tested on animals | | No ban on any cosmetic testing on animals |
| | Unknown |
The regulations that apply to animals in laboratories vary across species. In the U.S., under the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (the Guide), published by the National Academy of Sciences, any procedure can be performed on an animal if it can be successfully argued that it is scientifically justified. Researchers are required to consult with the institution's veterinarian and its Institutional Animal Care and Use Committee (IACUC), which every research facility is obliged to maintain.[122] The IACUC must ensure that alternatives, including non-animal alternatives, have been considered, that the experiments are not unnecessarily duplicative, and that pain relief is given unless it would interfere with the study. The IACUCs regulate all vertebrates in testing at institutions receiving federal funds in the USA. Although the Animal Welfare Act does not include purpose-bred rodents and birds, these species are equally regulated under Public Health Service policies that govern the IACUCs.[123][124] The Public Health Service policy oversees the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). The CDC conducts infectious disease research on nonhuman primates, rabbits, mice, and other animals, while FDA requirements cover use of animals in pharmaceutical research.[125] Animal Welfare Act (AWA) regulations are enforced by the USDA, whereas Public Health Service regulations are enforced by OLAW and in many cases by AAALAC.
According to the 2014 U.S. Department of Agriculture Office of the Inspector General (OIG) report—which looked at the oversight of animal use during a three-year period—"some Institutional Animal Care and Use Committees ...did not adequately approve, monitor, or report on experimental procedures on animals". The OIG found that "as a result, animals are not always receiving basic humane care and treatment and, in some cases, pain and distress are not minimized during and after experimental procedures". According to the report, within a three-year period, nearly half of all American laboratories with regulated species were cited for AWA violations relating to improper IACUC oversight.[126] The USDA OIG made similar findings in a 2005 report.[127] With only a broad number of 120 inspectors, the United States Department of Agriculture (USDA) oversees more than 12,000 facilities involved in research, exhibition, breeding, or dealing of animals.[125] Others have criticized the composition of IACUCs, asserting that the committees are predominantly made up of animal researchers and university representatives who may be biased against animal welfare concerns.[128]
Larry Carbone, a laboratory animal veterinarian, writes that, in his experience, IACUCs take their work very seriously regardless of the species involved, though the use of non-human primates always raises what he calls a "red flag of special concern".[129] A study published in Science magazine in July 2001 confirmed the low reliability of IACUC reviews of animal experiments. Funded by the National Science Foundation, the three-year study found that animal-use committees that do not know the specifics of the university and personnel do not make the same approval decisions as those made by animal-use committees that do know the university and personnel. Specifically, blinded committees more often ask for more information rather than approving studies.[130]
Scientists in India are protesting a recent guideline issued by the University Grants Commission to ban the use of live animals in universities and laboratories.[131]
On April 10, 2025, the FDA announced[132] a Roadmap to Reducing Animal Testing in Preclinical Safety Studies[133] to reduce and phase out animal testing and promote alternatives such as advanced computer simulations and lab grown human "organoids" and organ-on-a-chip systems. Over 90% of drugs that appear safe and effective in animals do not go on to receive FDA approval in humans predominantly due to safety and/or efficacy issues.[134]
On April 29, 2025, the US NIH announced[135] a new initiative to prioritize human-based research technologies and reduce use of animals in NIH-funded research. On July 8, 2025 at a collaborative FDA & NIH Workshop on Reducing Animal Testing, the NIH announced[136] that NIH will no longer seek proposals exclusively for animal models and that all new NIH funding opportunities moving forward should incorporate language on consideration of NAMs.
Numbers
[edit]Accurate global figures for animal testing are difficult to obtain; it has been estimated that 100 million vertebrates are experimented on around the world every year,[137] 10–11 million of them in the EU.[138] The Nuffield Council on Bioethics reports that global annual estimates range from 50 to 100 million animals. None of the figures include invertebrates such as shrimp and fruit flies.[139]

The USDA/APHIS has published the 2016 animal research statistics. Overall, the number of animals (covered by the Animal Welfare Act) used in research in the US rose 6.9% from 767,622 (2015) to 820,812 (2016).[140] This includes both public and private institutions. By comparing with EU data, where all vertebrate species are counted, Speaking of Research estimated that around 12 million vertebrates were used in research in the US in 2016.[94] A 2015 article published in the Journal of Medical Ethics, argued that the use of animals in the US has dramatically increased in recent years. Researchers found this increase is largely the result of an increased reliance on genetically modified mice in animal studies.[141]
In 1995, researchers at Tufts University Center for Animals and Public Policy estimated that 14–21 million animals were used in American laboratories in 1992, a reduction from a high of 50 million used in 1970.[142] In 1986, the U.S. Congress Office of Technology Assessment reported that estimates of the animals used in the U.S. range from 10 million to upwards of 100 million each year, and that their own best estimate was at least 17 million to 22 million.[143] In 2016, the Department of Agriculture listed 60,979 dogs, 18,898 cats, 71,188 non-human primates, 183,237 guinea pigs, 102,633 hamsters, 139,391 rabbits, 83,059 farm animals, and 161,467 other mammals, a total of 820,812, a figure that includes all mammals except purpose-bred mice and rats. The use of dogs and cats in research in the U.S. decreased from 1973 to 2016 from 195,157 to 60,979, and from 66,165 to 18,898, respectively.[94]
In the UK, Home Office figures show that 3.79 million procedures were carried out in 2017.[144] 2,960 procedures used non-human primates, down over 50% since 1988. A "procedure" refers here to an experiment that might last minutes, several months, or years. Most animals are used in only one procedure: animals are frequently euthanized after the experiment; however death is the endpoint of some procedures.[139] The procedures conducted on animals in the UK in 2017 were categorised as: 43% (1.61 million) sub-threshold, 4% (0.14 million) non-recovery, 36% (1.35 million) mild, 15% (0.55 million) moderate, and 4% (0.14 million) severe.[145] A 'severe' procedure would be, for instance, any test where death is the end-point or fatalities are expected, whereas a 'mild' procedure would be something like a blood test or an MRI scan.[144]
The Three Rs
[edit]The Three Rs (3Rs) are guiding principles for more ethical use of animals in testing. These were first described by W.M.S. Russell and R.L. Burch in 1959.[146] The 3Rs state:
- Replacement which refers to the preferred use of methods that do not use animals over methods that use animals whenever possible to achieve the same scientific aims. These methods include computer modeling.
- Reduction which means using the minimum number of animals possible and to obtain as much information as possible from the same number of animals.
- Refinement which refers to methods that alleviate or minimize potential pain, suffering or distress, enhance animal welfare for the animals used, and use non-invasive techniques.[147]
The 3Rs have a broader scope than simply encouraging alternatives to animal testing, but aim to improve animal welfare and scientific quality where the use of animals can not be avoided. These 3Rs are now implemented in many testing establishments worldwide and have been adopted by various pieces of legislation and regulations.[2]
Despite the widespread acceptance of the 3Rs, many countries—including Canada, Australia, Israel, South Korea, and Germany—have reported rising experimental use of animals in recent years with increased use of mice and, in some cases, fish while reporting declines in the use of cats, dogs, primates, rabbits, guinea pigs, and hamsters. Along with other countries, China has also escalated its use of GM animals, resulting in an increase in overall animal use.[148][149][150][151][152][153][excessive citations]
Sources
[edit]Animals used by laboratories are largely supplied by specialist dealers. Sources differ for vertebrate and invertebrate animals. Most laboratories breed and raise flies and worms themselves, using strains and mutants supplied from a few main stock centers.[154] For vertebrates, sources include breeders and dealers including Fortrea and Charles River Laboratories, which supply purpose-bred and wild-caught animals; businesses that trade in wild animals such as Nafovanny; and dealers who supply animals sourced from pounds, auctions, and newspaper ads. Animal shelters also supply the laboratories directly.[155] Large centers also exist to distribute strains of genetically modified animals; the International Knockout Mouse Consortium, for example, aims to provide knockout mice for every gene in the mouse genome.[156]

In the U.S., Class A breeders are licensed by the U.S. Department of Agriculture (USDA) to sell animals for research purposes, while Class B dealers are licensed to buy animals from "random sources" such as auctions, pound seizure, and newspaper ads. Some Class B dealers have been accused of kidnapping pets and illegally trapping strays, a practice known as bunching.[157][158][159][160][161][162] It was in part out of public concern over the sale of pets to research facilities that the 1966 Laboratory Animal Welfare Act was ushered in—the Senate Committee on Commerce reported in 1966 that stolen pets had been retrieved from Veterans Administration facilities, the Mayo Institute, the University of Pennsylvania, Stanford University, and Harvard and Yale Medical Schools.[163] The USDA recovered at least a dozen stolen pets during a raid on a Class B dealer in Arkansas in 2003.[164]
Four states in the U.S.—Minnesota, Utah, Oklahoma, and Iowa—require their shelters to provide animals to research facilities. Fourteen states explicitly prohibit the practice, while the remainder either allow it or have no relevant legislation.[165]
In the European Union, animal sources are governed by Council Directive 86/609/EEC, which requires lab animals to be specially bred, unless the animal has been lawfully imported and is not a wild animal or a stray. The latter requirement may also be exempted by special arrangement.[166] In 2010 the Directive was revised with EU Directive 2010/63/EU.[167] In the UK, most animals used in experiments are bred for the purpose under the 1988 Animal Protection Act, but wild-caught primates may be used if exceptional and specific justification can be established.[168][169] The United States also allows the use of wild-caught primates; between 1995 and 1999, 1,580 wild baboons were imported into the U.S. Most of the primates imported are handled by Charles River Laboratories or by Fortrea, which are very active in the international primate trade.[170]
Pain and suffering
[edit]
It is generally accepted that animals can feel pain.[171] The extent to which animal testing causes pain and suffering, and the capacity of animals to experience and comprehend them, has been debated.[172][173]
According to the USDA, in 2016 501,560 animals (61%) (not including rats, mice, birds, or invertebrates) were used in procedures that did not include more than momentary pain or distress. 247,882 (31%) animals were used in procedures in which pain or distress was relieved by anesthesia, while 71,370 (9%) were used in studies that would cause pain or distress that would not be relieved.[94]
The idea that animals might not feel pain as human beings feel it traces back to the 17th-century French philosopher, René Descartes, who argued that animals do not experience pain and suffering because they lack consciousness.[139][174] Bernard Rollin of Colorado State University, the principal author of two U.S. federal laws regulating pain relief for animals,[175] writes that researchers remained unsure into the 1980s as to whether animals experience pain, and that veterinarians trained in the U.S. before 1989 were simply taught to ignore animal pain.[176] In his interactions with scientists and other veterinarians, he was regularly asked to "prove" that animals are conscious, and to provide "scientifically acceptable" grounds for claiming that they feel pain.[176] Carbone writes that the view that animals feel pain differently is now a minority view. Academic reviews of the topic are more equivocal, noting that although the argument that animals have at least simple conscious thoughts and feelings has strong support,[177] some critics continue to question how reliably animal mental states can be determined.[139][178] However, some canine experts are stating that, while intelligence does differ animal to animal, dogs have the intelligence of a two to two-and-a-half-year old. This does support the idea that dogs, at the very least, have some form of consciousness.[179] The ability of invertebrates to experience pain and suffering is less clear, however, legislation in several countries (e.g. U.K., New Zealand,[180] Norway[181]) protects some invertebrate species if they are being used in animal testing.
In the U.S., the defining text on animal welfare regulation in animal testing is the Guide for the Care and Use of Laboratory Animals.[182] This defines the parameters that govern animal testing in the U.S. It states "The ability to experience and respond to pain is widespread in the animal kingdom...Pain is a stressor and, if not relieved, can lead to unacceptable levels of stress and distress in animals." The Guide states that the ability to recognize the symptoms of pain in different species is vital in efficiently applying pain relief and that it is essential for the people caring for and using animals to be entirely familiar with these symptoms. On the subject of analgesics used to relieve pain, the Guide states "The selection of the most appropriate analgesic or anesthetic should reflect professional judgment as to which best meets clinical and humane requirements without compromising the scientific aspects of the research protocol". Accordingly, all issues of animal pain and distress, and their potential treatment with analgesia and anesthesia, are required regulatory issues in receiving animal protocol approval.[183] Currently, traumatic methods of marking laboratory animals are being replaced with non-invasive alternatives.[184][185]
In 2019, Katrien Devolder and Matthias Eggel proposed gene editing research animals to remove the ability to feel pain. This would be an intermediate step towards eventually stopping all experimentation on animals and adopting alternatives.[186] Additionally, this would not stop research animals from experiencing psychological harm.
Euthanasia
[edit]Regulations require that scientists use as few animals as possible, especially for terminal experiments.[187] However, while policy makers consider suffering to be the central issue and see animal euthanasia as a way to reduce suffering, others, such as the RSPCA, argue that the lives of laboratory animals have intrinsic value.[188] Regulations focus on whether particular methods cause pain and suffering, not whether their death is undesirable in itself.[189] The animals are euthanized at the end of studies for sample collection or post-mortem examination; during studies if their pain or suffering falls into certain categories regarded as unacceptable, such as depression, infection that is unresponsive to treatment, or the failure of large animals to eat for five days;[190] or when they are unsuitable for breeding or unwanted for some other reason.[191]
Methods of euthanizing laboratory animals are chosen to induce rapid unconsciousness and death without pain or distress.[192] The methods that are preferred are those published by councils of veterinarians. The animal can be made to inhale a gas, such as carbon monoxide and carbon dioxide, by being placed in a chamber, or by use of a face mask, with or without prior sedation or anesthesia. Sedatives or anesthetics such as barbiturates can be given intravenously, or inhalant anesthetics may be used. Amphibians and fish may be immersed in water containing an anesthetic such as tricaine. Physical methods are also used, with or without sedation or anesthesia depending on the method. Recommended methods include decapitation (beheading) for small rodents or rabbits. Cervical dislocation (breaking the neck or spine) may be used for birds, mice, rats, and rabbits depending on the size and weight of the animal.[193] High-intensity microwave irradiation of the brain can preserve brain tissue and induce death in less than 1 second, but this is currently only used on rodents. Captive bolts may be used, typically on dogs, ruminants, horses, pigs and rabbits. It causes death by a concussion to the brain. Gunshot may be used, but only in cases where a penetrating captive bolt may not be used. Some physical methods are only acceptable after the animal is unconscious. Electrocution may be used for cattle, sheep, swine, foxes, and mink after the animals are unconscious, often by a prior electrical stun. Pithing (inserting a tool into the base of the brain) is usable on animals already unconscious. Slow or rapid freezing, or inducing air embolism are acceptable only with prior anesthesia to induce unconsciousness.[194]
Research classification
[edit]Pure research
[edit]Basic or pure research investigates how organisms behave, develop, and function. Those opposed to animal testing object that pure research may have little or no practical purpose, but researchers argue that it forms the necessary basis for the development of applied research, rendering the distinction between pure and applied research—research that has a specific practical aim—unclear.[195] Pure research uses larger numbers and a greater variety of animals than applied research. Fruit flies, nematode worms, mice and rats together account for the vast majority, though small numbers of other species are used, ranging from sea slugs through to armadillos.[196] Examples of the types of animals and experiments used in basic research include:
- Studies on embryogenesis and developmental biology. Mutants are created by adding transposons into their genomes, or specific genes are deleted by gene targeting.[197][198] By studying the changes in development these changes produce, scientists aim to understand both how organisms normally develop, and what can go wrong in this process. These studies are particularly powerful since the basic controls of development, such as the homeobox genes, have similar functions in organisms as diverse as fruit flies and man.[199][200]
- Experiments into behavior, to understand how organisms detect and interact with each other and their environment, in which fruit flies, worms, mice, and rats are all widely used.[201][202] Studies of brain function, such as memory and social behavior, often use rats and birds.[203][204] For some species, behavioral research is combined with enrichment strategies for animals in captivity because it allows them to engage in a wider range of activities.[205]
- Breeding experiments to study evolution and genetics. Laboratory mice, flies, fish, and worms are inbred through many generations to create strains with defined characteristics.[206] These provide animals of a known genetic background, an important tool for genetic analyses. Larger mammals are rarely bred specifically for such studies due to their slow rate of reproduction, though some scientists take advantage of inbred domesticated animals, such as dog or cattle breeds, for comparative purposes. Scientists studying how animals evolve use many animal species to see how variations in where and how an organism lives (their niche) produce adaptations in their physiology and morphology. As an example, sticklebacks are now being used to study how many and which types of mutations are selected to produce adaptations in animals' morphology during the evolution of new species.[207][208]
Applied research
[edit]Applied research aims to solve specific and practical problems. These may involve the use of animal models of diseases or conditions, which are often discovered or generated by pure research programmes. In turn, such applied studies may be an early stage in the drug discovery process. Examples include:
- Genetic modification of animals to study disease. Transgenic animals have specific genes inserted, modified or removed, to mimic specific conditions such as single gene disorders, such as Huntington's disease.[209] Other models mimic complex, multifactorial diseases with genetic components, such as diabetes,[210] or even transgenic mice that carry the same mutations that occur during the development of cancer.[211] These models allow investigations on how and why the disease develops, as well as providing ways to develop and test new treatments.[212] The vast majority of these transgenic models of human disease are lines of mice, the mammalian species in which genetic modification is most efficient.[96] Smaller numbers of other animals are also used, including rats, pigs, sheep, fish, birds, and amphibians.[169]
- Studies on models of naturally occurring disease and condition. Certain domestic and wild animals have a natural propensity or predisposition for certain conditions that are also found in humans. Cats are used as a model to develop immunodeficiency virus vaccines and to study leukemia because their natural predisposition to FIV and Feline leukemia virus.[213][214] Certain breeds of dog experience narcolepsy making them the major model used to study the human condition. Armadillos and humans are among only a few animal species that naturally have leprosy; as the bacteria responsible for this disease cannot yet be grown in culture, armadillos are the primary source of bacilli used in leprosy vaccines.[196]
- Studies on induced animal models of human diseases. Here, an animal is treated so that it develops pathology and symptoms that resemble a human disease. Examples include restricting blood flow to the brain to induce stroke, or giving neurotoxins that cause damage similar to that seen in Parkinson's disease.[215] Much animal research into potential treatments for humans is wasted because it is poorly conducted and not evaluated through systematic reviews.[216] For example, although such models are now widely used to study Parkinson's disease, the British anti-vivisection interest group BUAV argues that these models only superficially resemble the disease symptoms, without the same time course or cellular pathology.[217] In contrast, scientists assessing the usefulness of animal models of Parkinson's disease, as well as the medical research charity The Parkinson's Appeal, state that these models were invaluable and that they led to improved surgical treatments such as pallidotomy, new drug treatments such as levodopa, and later deep brain stimulation.[117][215][218]
- Animal testing has also included the use of placebo testing. In these cases animals are treated with a substance that produces no pharmacological effect, but is administered in order to determine any biological alterations due to the experience of a substance being administered, and the results are compared with those obtained with an active compound.
Xenotransplantation
[edit]Xenotransplantation research involves transplanting tissues or organs from one species to another, as a way to overcome the shortage of human organs for use in organ transplants.[219] Current research involves using primates as the recipients of organs from pigs that have been genetically modified to reduce the primates' immune response against the pig tissue.[220] Although transplant rejection remains a problem,[220] recent clinical trials that involved implanting pig insulin-secreting cells into diabetics did reduce these people's need for insulin.[221][222]
Documents released to the news media by the animal rights organization Uncaged Campaigns showed that, between 1994 and 2000, wild baboons imported to the UK from Africa by Imutran Ltd, a subsidiary of Novartis Pharma AG, in conjunction with Cambridge University and Huntingdon Life Sciences, to be used in experiments that involved grafting pig tissues, had serious and sometimes fatal injuries. A scandal occurred when it was revealed that the company had communicated with the British government in an attempt to avoid regulation.[223][224]
Toxicology testing
[edit]Toxicology testing, also known as safety testing, is conducted by pharmaceutical companies testing drugs, or by contract animal testing facilities, such as Huntingdon Life Sciences, on behalf of a wide variety of customers.[225] According to 2005 EU figures, around one million animals are used every year in Europe in toxicology tests; which are about 10% of all procedures.[226] According to Nature, 5,000 animals are used for each chemical being tested, with 12,000 needed to test pesticides.[227] The tests are conducted without anesthesia, because interactions between drugs can affect how animals detoxify chemicals, and may interfere with the results.[228][229]
Toxicology tests are used to examine finished products such as pesticides, medications, food additives, packing materials, and air freshener, or their chemical ingredients. Most tests involve testing ingredients rather than finished products, but according to BUAV, manufacturers believe these tests overestimate the toxic effects of substances; they therefore repeat the tests using their finished products to obtain a less toxic label.[225]
The substances are applied to the skin or dripped into the eyes; injected intravenously, intramuscularly, or subcutaneously; inhaled either by placing a mask over the animals and restraining them, or by placing them in an inhalation chamber; or administered orally, through a tube into the stomach, or simply in the animal's food. Doses may be given once, repeated regularly for many months, or for the lifespan of the animal.[230]
There are several different types of acute toxicity tests. The LD50 ("Lethal Dose 50%") test is used to evaluate the toxicity of a substance by determining the dose required to kill 50% of the test animal population. This test was removed from OECD international guidelines in 2002, replaced by methods such as the fixed dose procedure, which use fewer animals and cause less suffering.[231][232] Abbott writes that, as of 2005, "the LD50 acute toxicity test ... still accounts for one-third of all animal [toxicity] tests worldwide".[227]
Irritancy can be measured using the Draize test, where a test substance is applied to an animal's eyes or skin, usually an albino rabbit. For Draize eye testing, the test involves observing the effects of the substance at intervals and grading any damage or irritation, but the test should be halted and the animal killed if it shows "continuing signs of severe pain or distress".[233] The Humane Society of the United States writes that the procedure can cause redness, ulceration, hemorrhaging, cloudiness, or even blindness.[234] This test has also been criticized by scientists for being cruel and inaccurate, subjective, over-sensitive, and failing to reflect human exposures in the real world.[235] Although no accepted in vitro alternatives exist, a modified form of the Draize test called the low volume eye test may reduce suffering and provide more realistic results and this was adopted as the new standard in September 2009.[236][237] However, the Draize test will still be used for substances that are not severe irritants.[237]
The most stringent tests are reserved for drugs and foodstuffs. For these, a number of tests are performed, lasting less than a month (acute), one to three months (subchronic), and more than three months (chronic) to test general toxicity (damage to organs), eye and skin irritancy, mutagenicity, carcinogenicity, teratogenicity, and reproductive problems. The cost of the full complement of tests is several million dollars per substance and it may take three or four years to complete.
These toxicity tests provide, in the words of a 2006 United States National Academy of Sciences report, "critical information for assessing hazard and risk potential".[238] Animal tests may overestimate risk, with false positive results being a particular problem,[227][239] but false positives appear not to be prohibitively common.[240] Variability in results arises from using the effects of high doses of chemicals in small numbers of laboratory animals to try to predict the effects of low doses in large numbers of humans.[241] Although relationships do exist, opinion is divided on how to use data on one species to predict the exact level of risk in another.[242]
Scientists face growing pressure to move away from using traditional animal toxicity tests to determine whether manufactured chemicals are safe.[243] Among variety of approaches to toxicity evaluation the ones which have attracted increasing interests are in vitro cell-based sensing methods applying fluorescence.[244]
Cosmetics testing
[edit]
Cosmetics testing on animals is particularly controversial. Such tests, which are still conducted in the U.S., involve general toxicity, eye and skin irritancy, phototoxicity (toxicity triggered by ultraviolet light) and mutagenicity.[245]
Cosmetics testing on animals is banned in India, the United Kingdom, the European Union,[246] Israel and Norway[247][248] while legislation in the U.S. and Brazil is currently considering similar bans.[249] In 2002, after 13 years of discussion, the European Union agreed to phase in a near-total ban on the sale of animal-tested cosmetics by 2009, and to ban all cosmetics-related animal testing. France, which is home to the world's largest cosmetics company, L'Oreal, has protested the proposed ban by lodging a case at the European Court of Justice in Luxembourg, asking that the ban be quashed.[250] The ban is also opposed by the European Federation for Cosmetics Ingredients, which represents 70 companies in Switzerland, Belgium, France, Germany, and Italy.[250] In October 2014, India passed stricter laws that also ban the importation of any cosmetic products that are tested on animals.[251]
Drug testing
[edit]Before the early 20th century, laws regulating drugs were lax. Currently, all new pharmaceuticals undergo rigorous animal testing before being licensed for human use. Tests on pharmaceutical products involve:
- metabolic tests, investigating pharmacokinetics—how drugs are absorbed, metabolized and excreted by the body when introduced orally, intravenously, intraperitoneally, intramuscularly, or transdermally.
- toxicology tests, which gauge acute, sub-acute, and chronic toxicity. Acute toxicity is studied by using a rising dose until signs of toxicity become apparent. Current European legislation demands that "acute toxicity tests must be carried out in two or more mammalian species" covering "at least two different routes of administration".[252]: 1. Single dose toxicity Sub-acute toxicity is where the drug is given to the animals for four to six weeks in doses below the level at which it causes rapid poisoning, in order to discover if any toxic drug metabolites build up over time. Testing for chronic toxicity can last up to two years and, in the European Union, is required to involve two species of mammals, one of which must be non-rodent.[252]: 2. Repeated dose toxicity (sub-acute or chronic toxicity)
- efficacy studies, which test whether experimental drugs work by inducing the appropriate illness in animals. The drug is then administered in a double-blind controlled trial, which allows researchers to determine the effect of the drug and the dose-response curve.
- Specific tests on reproductive function, embryonic toxicity, or carcinogenic potential can all be required by law, depending on the result of other studies and the type of drug being tested.
Education
[edit]It is estimated that 20 million animals are used annually for educational purposes in the United States including, classroom observational exercises, dissections and live-animal surgeries.[253][254] Frogs, fetal pigs, perch, cats, earthworms, grasshoppers, crayfish and starfish are commonly used in classroom dissections.[255] Alternatives to the use of animals in classroom dissections are widely used, with many U.S. States and school districts mandating students be offered the choice to not dissect.[256] Citing the wide availability of alternatives and the decimation of local frog species, India banned dissections in 2014.[257][258]
The Sonoran Arthropod Institute hosts an annual Invertebrates in Education and Conservation Conference to discuss the use of invertebrates in education.[259] There also are efforts in many countries to find alternatives to using animals in education.[260] The NORINA database, maintained by Norecopa, lists products that may be used as alternatives or supplements to animal use in education, and in the training of personnel who work with animals.[261] These include alternatives to dissection in schools. InterNICHE has a similar database and a loans system.[262]
In November 2013, the U.S.-based company Backyard Brains released for sale to the public what they call the "Roboroach", an "electronic backpack" that can be attached to cockroaches. The operator is required to amputate a cockroach's antennae, use sandpaper to wear down the shell, insert a wire into the thorax, and then glue the electrodes and circuit board onto the insect's back. A mobile phone app can then be used to control it via Bluetooth.[263] It has been suggested that the use of such a device may be a teaching aid that can promote interest in science. The makers of the "Roboroach" have been funded by the National Institute of Mental Health and state that the device is intended to encourage children to become interested in neuroscience.[263][264]
Defense
[edit]Animals are used by the military to develop weapons, vaccines, battlefield surgical techniques, and defensive clothing.[195] For example, in 2008 the United States Defense Advanced Research Projects Agency used live pigs to study the effects of improvised explosive device explosions on internal organs, especially the brain.[265]
In the US military, goats are commonly used to train combat medics. (Goats have become the main animal species used for this purpose after the Pentagon phased out using dogs for medical training in the 1980s.[266]) While modern mannequins used in medical training are quite efficient in simulating the behavior of a human body, some trainees feel that "the goat exercise provide[s] a sense of urgency that only real life trauma can provide".[267] Nevertheless, in 2014, the U.S. Coast Guard announced that it would reduce the number of animals it uses in its training exercises by half after PETA released video showing Guard members cutting off the limbs of unconscious goats with tree trimmers and inflicting other injuries with a shotgun, pistol, ax and a scalpel.[268] That same year, citing the availability of human simulators and other alternatives, the Department of Defense announced it would begin reducing the number of animals it uses in various training programs.[269] In 2013, several Navy medical centers stopped using ferrets in intubation exercises after complaints from PETA.[270]
Besides the United States, six out of 28 NATO countries, including Poland and Denmark, use live animals for combat medic training.[266]
Ethics
[edit]Most animals are euthanized after being used in an experiment.[57] Sources of laboratory animals vary between countries and species; most animals are purpose-bred, while a minority are caught in the wild or supplied by dealers who obtain them from auctions and pounds.[271][272][157] Supporters of the use of animals in experiments, such as the British Royal Society, argue that virtually every medical achievement in the 20th century relied on the use of animals in some way.[116] The Institute for Laboratory Animal Research of the United States National Academy of Sciences has argued that animal testing cannot be replaced by even sophisticated computer models, which are unable to deal with the extremely complex interactions between molecules, cells, tissues, organs, organisms and the environment.[273] Animal rights organizations—such as PETA and BUAV—question the need for and legitimacy of animal testing, arguing that it is cruel and poorly regulated, that medical progress is actually held back by misleading animal models that cannot reliably predict effects in humans, that some of the tests are outdated, that the costs outweigh the benefits, or that animals have the intrinsic right not to be used or harmed in experimentation.[52][274][275][276][277][278]
Viewpoints
[edit]
| Part of a series on |
| Animal rights |
|---|
The moral and ethical questions raised by performing experiments on animals are subject to debate, and viewpoints have shifted significantly over the 20th century.[279] There remain disagreements about which procedures are useful for which purposes, as well as disagreements over which ethical principles apply to which species.
A 2015 Gallup poll found that 67% of Americans were "very concerned" or "somewhat concerned" about animals used in research.[280] A Pew poll taken the same year found 50% of American adults opposed the use of animals in research.[281]
Still, a wide range of viewpoints exist. The view that animals have moral rights (animal rights) is a philosophical position proposed by Tom Regan, among others, who argues that animals are beings with beliefs and desires, and as such are the "subjects of a life" with moral value and therefore moral rights.[282] Regan still sees ethical differences between killing human and non-human animals, and argues that to save the former it is permissible to kill the latter. Likewise, a "moral dilemma" view suggests that avoiding potential benefit to humans is unacceptable on similar grounds, and holds the issue to be a dilemma in balancing such harm to humans to the harm done to animals in research.[283] In contrast, an abolitionist view in animal rights holds that there is no moral justification for any harmful research on animals that is not to the benefit of the individual animal.[283] Bernard Rollin argues that benefits to human beings cannot outweigh animal suffering, and that human beings have no moral right to use an animal in ways that do not benefit that individual. Donald Watson has stated that vivisection and animal experimentation "is probably the cruelest of all Man's attack on the rest of Creation."[284] Another prominent position is that of philosopher Peter Singer, who argues that there are no grounds to include a being's species in considerations of whether their suffering is important in utilitarian moral considerations.[285] Malcolm Macleod and collaborators argue that most controlled animal studies do not employ randomization, allocation concealment, and blinding outcome assessment, and that failure to employ these features exaggerates the apparent benefit of drugs tested in animals, leading to a failure to translate much animal research for human benefit.[286][287][288][289][290]
Governments such as the Netherlands and New Zealand have responded to the public's concerns by outlawing invasive experiments on certain classes of non-human primates, particularly the great apes.[291][292] In 2015, captive chimpanzees in the U.S. were added to the Endangered Species Act adding new road blocks to those wishing to experiment on them.[293] Similarly, citing ethical considerations and the availability of alternative research methods, the U.S. NIH announced in 2013 that it would dramatically reduce and eventually phase out experiments on chimpanzees.[294]
The British government has required that the cost to animals in an experiment be weighed against the gain in knowledge.[295] Some medical schools and agencies in China, Japan, and South Korea have built cenotaphs for killed animals.[296] In Japan there are also annual memorial services Ireisai (Japanese: 慰霊祭) for animals sacrificed at medical school.

Various specific cases of animal testing have drawn attention, including both instances of beneficial scientific research, and instances of alleged ethical violations by those performing the tests. The fundamental properties of muscle physiology were determined with work done using frog muscles (including the force generating mechanism of all muscle,[297] the length-tension relationship,[298] and the force-velocity curve[299]), and frogs are still the preferred model organism due to the long survival of muscles in vitro and the possibility of isolating intact single-fiber preparations (not possible in other organisms).[300] Modern physical therapy and the understanding and treatment of muscular disorders is based on this work and subsequent work in mice (often engineered to express disease states such as muscular dystrophy).[301] In February 1997 a team at the Roslin Institute in Scotland announced the birth of Dolly the sheep, the first mammal to be cloned from an adult somatic cell.[79]
Concerns have been raised over the mistreatment of primates undergoing testing. In 1985, the case of Britches, a macaque monkey at the University of California, Riverside, gained public attention. He had his eyelids sewn shut and a sonar sensor on his head as part of an experiment to test sensory substitution devices for blind people. The laboratory was raided by Animal Liberation Front in 1985, removing Britches and 466 other animals.[302] The National Institutes of Health conducted an eight-month investigation and concluded, however, that no corrective action was necessary.[303] During the 2000s other cases have made headlines, including experiments at the University of Cambridge[304] and Columbia University in 2002.[305] In 2004 and 2005, undercover footage of staff of in an animal testing facility in Virginia owned by Covance (now Fortrea) was shot by People for the Ethical Treatment of Animals (PETA). Following release of the footage, the U.S. Department of Agriculture fined the company $8,720 for 16 citations, three of which involved lab monkeys; the other citations involved administrative issues and equipment.[306][307]
Threats to researchers
[edit]Threats of violence to animal researchers are not uncommon.[vague][308]
In 2006, a primate researcher at the University of California, Los Angeles (UCLA) shut down the experiments in his lab after threats from animal rights activists. The researcher had received a grant to use 30 macaque monkeys for vision experiments; each monkey was anesthetized for a single physiological experiment lasting up to 120 hours, and then euthanized.[309] The researcher's name, phone number, and address were posted on the website of the Primate Freedom Project. Demonstrations were held in front of his home. A Molotov cocktail was placed on the porch of what was believed to be the home of another UCLA primate researcher; instead, it was accidentally left on the porch of an elderly woman unrelated to the university. The Animal Liberation Front claimed responsibility for the attack.[310] As a result of the campaign, the researcher sent an email to the Primate Freedom Project stating "you win", and "please don't bother my family anymore".[311] In another incident at UCLA in June 2007, the Animal Liberation Brigade placed a bomb under the car of a UCLA children's ophthalmologist who experiments on cats and rhesus monkeys; the bomb had a faulty fuse and did not detonate.[312]
In 1997, PETA filmed staff from Huntingdon Life Sciences, showing dogs being mistreated.[313][314] The employees responsible were dismissed,[315] with two given community service orders and ordered to pay £250 costs, the first lab technicians to have been prosecuted for animal cruelty in the UK.[316] The Stop Huntingdon Animal Cruelty campaign used tactics ranging from non-violent protest to the alleged firebombing of houses owned by executives associated with HLS's clients and investors. The Southern Poverty Law Center, which monitors US domestic extremism, has described SHAC's modus operandi as "frankly terroristic tactics similar to those of anti-abortion extremists", and in 2005 an official with the FBI's counter-terrorism division referred to SHAC's activities in the United States as domestic terrorist threats.[317][318] 13 members of SHAC were jailed for between 15 months and eleven years on charges of conspiracy to blackmail or harm HLS and its suppliers.[319][320]
These attacks—as well as similar incidents that caused the Southern Poverty Law Center to declare in 2002 that the animal rights movement had "clearly taken a turn toward the more extreme"—prompted the US government to pass the Animal Enterprise Terrorism Act and the UK government to add the offense of "Intimidation of persons connected with animal research organisation" to the Serious Organised Crime and Police Act 2005. Such legislation and the arrest and imprisonment of activists may have decreased the incidence of attacks.[321]
Scientific criticism
[edit]Systematic reviews have pointed out that animal testing often fails to accurately mirror outcomes in humans.[322][323] For instance, a 2013 review noted that some 100 vaccines have been shown to prevent HIV in animals, yet none of them have worked on humans.[323] Effects seen in animals may not be replicated in humans, and vice versa. Many corticosteroids cause birth defects in animals, but not in humans. Conversely, thalidomide causes serious birth defects in humans, but not in some animals such as mice (however, it does cause birth defects in rabbits).[324] A 2004 paper concluded that much animal research is wasted because systemic reviews are not used, and due to poor methodology.[325] A 2006 review found multiple studies where there were promising results for new drugs in animals, but human clinical studies did not show the same results. The researchers suggested that this might be due to researcher bias, or simply because animal models do not accurately reflect human biology.[326] Lack of meta-reviews may be partially to blame.[324] Poor methodology is an issue in many studies. A 2009 review noted that many animal experiments did not use blinded experiments, a key element of many scientific studies in which researchers are not told about the part of the study they are working on to reduce bias.[324][327] A 2021 paper found, in a sample of Open Access Alzheimer Disease studies, that if the authors omitted from the title that the experiment was performed in mice, the news headline followed suit and the Twitter repercussion was higher.[328]
Activism
[edit]There are various examples of activists utilizing Freedom of Information Act (FOIA) requests to obtain information about taxpayer funding of animal testing. For example, the White Coat Waste Project, a group of activists that hold that taxpayers should not have

to pay $20 billion every year for experiments on animals,[329] highlighted that the National Institute of Allergy and Infectious Diseases provided $400,000 in taxpayer money to fund experiments in which 28 beagles were infected by disease-causing parasites.[330] The White Coat Project found reports that said dogs taking part in the experiments were "vocalizing in pain" after being injected with foreign substances.[331] Following public outcry, People for the Ethical Treatment of Animals (PETA) made a call to action that all members of the National Institute of Health resign effective immediately[332] and that there is a "need to find a new NIH director to replace the outgoing Francis Collins who will shut down research that violates the dignity of nonhuman animals."[333]
Historical debate
[edit]
As the experimentation on animals increased, especially the practice of vivisection, so did criticism and controversy. In 1655, the advocate of Galenic physiology Edmund O'Meara said that "the miserable torture of vivisection places the body in an unnatural state".[336][337] O'Meara and others argued pain could affect animal physiology during vivisection, rendering results unreliable. There were also objections ethically, contending that the benefit to humans did not justify the harm to animals.[337] Early objections to animal testing also came from another angle—many people believed animals were inferior to humans and so different that results from animals could not be applied to humans.[2][337]
On the other side of the debate, those in favor of animal testing held that experiments on animals were necessary to advance medical and biological knowledge. Claude Bernard—who is sometimes known as the "prince of vivisectors"[334] and the father of physiology, and whose wife, Marie Françoise Martin, founded the first anti-vivisection society in France in 1883[338]—famously wrote in 1865 that "the science of life is a superb and dazzlingly lighted hall which may be reached only by passing through a long and ghastly kitchen".[339] Arguing that "experiments on animals [. . .] are entirely conclusive for the toxicology and hygiene of man [. . . T]he effects of these substances are the same on man as on animals, save for differences in degree",[335] Bernard established animal experimentation as part of the standard scientific method.[340]
In 1896, the physiologist and physician Dr. Walter B. Cannon said "The antivivisectionists are the second of the two types Theodore Roosevelt described when he said, 'Common sense without conscience may lead to crime, but conscience without common sense may lead to folly, which is the handmaiden of crime.'"[341] These divisions between pro- and anti-animal testing groups first came to public attention during the Brown Dog affair in the early 1900s, when hundreds of medical students clashed with anti-vivisectionists and police over a memorial to a vivisected dog.[342]
In 1822, the first animal protection law was enacted in the British parliament, followed by the Cruelty to Animals Act (1876), the first law specifically aimed at regulating animal testing. The legislation was promoted by Charles Darwin, who wrote to Ray Lankester in March 1871: "You ask about my opinion on vivisection. I quite agree that it is justifiable for proper investigations on physiology; but not for mere damnable and detestable curiosity. It is a subject which makes me sick with horror, so I will not say another word about it, else I shall not sleep to-night."[343][344] In response to the lobbying by anti-vivisectionists, several organizations were set up in Britain to defend animal research: The Physiological Society was formed in 1876 to give physiologists "mutual benefit and protection",[345] the Association for the Advancement of Medicine by Research was formed in 1882 and focused on policy-making, and the Research Defence Society (now Understanding Animal Research) was formed in 1908 "to make known the facts as to experiments on animals in this country; the immense importance to the welfare of mankind of such experiments and the great saving of human life and health directly attributable to them".[346]
Opposition to the use of animals in medical research first arose in the United States during the 1860s, when Henry Bergh founded the American Society for the Prevention of Cruelty to Animals (ASPCA), with America's first specifically anti-vivisection organization being the American AntiVivisection Society (AAVS), founded in 1883. Antivivisectionists of the era generally believed the spread of mercy was the great cause of civilization, and vivisection was cruel. However, in the USA the antivivisectionists' efforts were defeated in every legislature, overwhelmed by the superior organization and influence of the medical community. Overall, this movement had little legislative success until the passing of the Laboratory Animal Welfare Act, in 1966.[347]
Real progress in thinking about animal rights build on the "theory of justice" (1971) by the philosopher John Rawls and work on ethics by philosopher Peter Singer.[2]
Alternatives
[edit]Most scientists and governments state that animal testing should cause as little suffering to animals as possible, and that animal tests should only be performed when there is no alternative.[citation needed] The "Three Rs" are guiding principles for the use of animals in research in most countries.[146][187] Whilst replacement of animals, i.e. alternatives to animal testing, is one of the principles, their scope is much broader.[348] Although such principles have been welcomed as a step forwards by some animal welfare groups,[349] they have also been criticized as both outdated by current research,[350] and of little practical effect in improving animal welfare.[351] The scientists and engineers at Harvard's Wyss Institute have created "organs-on-a-chip", including the "lung-on-a-chip" and "gut-on-a-chip". Researchers at cellasys in Germany developed a "skin-on-a-chip".[352] These tiny devices contain human cells in a 3-dimensional system that mimics human organs. The chips can be used instead of animals in in vitro disease research, drug testing, and toxicity testing.[353] Researchers have also begun using 3-D bioprinters to create human tissues for in vitro testing.[354]
Another non-animal research method is in silico or computer simulation and mathematical modeling which seeks to investigate and ultimately predict toxicity and drug effects on humans without using animals. This is done by investigating test compounds on a molecular level using recent advances in technological capabilities with the ultimate goal of creating treatments unique to each patient.[355][356] Microdosing is another alternative to the use of animals in experimentation. Microdosing is a process whereby volunteers are administered a small dose of a test compound allowing researchers to investigate its pharmacological affects without harming the volunteers. Microdosing can replace the use of animals in pre-clinical drug screening and can reduce the number of animals used in safety and toxicity testing.[357] Additional alternative methods include positron emission tomography (PET), which allows scanning of the human brain in vivo,[358] and comparative epidemiological studies of disease risk factors among human populations.[359] Simulators and computer programs have also replaced the use of animals in dissection, teaching and training exercises.[360][361]
Official bodies such as the European Centre for the Validation of Alternative Test Methods of the European Commission, the Interagency Coordinating Committee for the Validation of Alternative Methods in the US,[362] ZEBET in Germany,[363] and the Japanese Center for the Validation of Alternative Methods[364] (among others) also promote and disseminate the 3Rs. These bodies are mainly driven by responding to regulatory requirements, such as supporting the cosmetics testing ban in the EU by validating alternative methods. The European Partnership for Alternative Approaches to Animal Testing serves as a liaison between the European Commission and industries.[365] The European Consensus Platform for Alternatives coordinates efforts amongst EU member states.[366] Academic centers also investigate alternatives, including the Center for Alternatives to Animal Testing at the Johns Hopkins University[367] and the NC3Rs in the UK.[368]
See also
[edit]References
[edit]Citations
[edit]- ^ ""Introduction", Select Committee on Animals in Scientific Procedures Report". UK Parliament. Retrieved 13 July 2012.
- ^ a b c d Liguori, G., et al. (2017). "Ethical Issues in the Use of Animal Models for Tissue Engineering: Reflections on Legal Aspects, Moral Theory, 3Rs Strategies, and Harm-Benefit Analysis" (PDF). Tissue Engineering Part C: Methods. 23 (12): 850–62. doi:10.1089/ten.TEC.2017.0189. PMID 28756735. S2CID 206268293.
- ^ Hajar R (2011). "Animal Testing and Medicine". Heart Views. 12 (1): 42. doi:10.4103/1995-705X.81548. ISSN 1995-705X. PMC 3123518. PMID 21731811.
- ^ a b c d Royal Society of Medicine (13 May 2015). "Statement of the Royal Society's position on the use of animals in research".
From antibiotics and insulin to blood transfusions and treatments for cancer or HIV, virtually every medical achievement in the past century has depended directly or indirectly on research using animals, including veterinary medicine.
- ^ a b National Research Council and Institute of Medicine (1988). Use of Laboratory Animals in Biomedical and Behavioral Research. National Academies Press. p. 37. ISBN 978-0-309-03839-3. NAP:13195.
The...methods of scientific inquiry have greatly reduced the incidence of human disease and have substantially increased life expectancy. Those results have come largely through experimental methods based in part on the use of animals.
- ^ a b Lieschke GJ, Currie PD (May 2007). "Animal models of human disease: zebrafish swim into view". Nature Reviews Genetics. 8 (5): 353–367. doi:10.1038/nrg2091. PMID 17440532. S2CID 13857842.
Biomedical research depends on the use of animal models to understand the pathogenesis of human disease at a cellular and molecular level and to provide systems for developing and testing new therapies.
- ^ a b National Research Council and Institute of Medicine (1988). Use of Laboratory Animals in Biomedical and Behavioral Research. National Academies Press. p. 27. ISBN 978-0-309-03839-3. NAP:13195.
Animal studies have been an essential component of every field of medical research and have been crucial for the acquisition of basic knowledge in biology.
- ^ a b Hau and Shapiro 2011:
- Jann Hau, Steven J. Schapiro (2011). Handbook of Laboratory Animal Science, Volume I, Third Edition: Essential Principles and Practices. CRC Press. p. 2. ISBN 978-1-4200-8456-6.
Animal-based research has played a key role in understanding infectious diseases, neuroscience, physiology, and toxicology. Experimental results from animal studies have served as the basis for many key biomedical breakthroughs.
- Jann Hau, Steven J. Schapiro (2011). Handbook of Laboratory Animal Science, Volume II, Third Edition: Animal Models. CRC Press. p. 1. ISBN 978-1-4200-8458-0.
Most of our basic knowledge of human biochemistry, physiology, endocrinology, and pharmacology has been derived from initial studies of mechanisms in animal models.
- Jann Hau, Steven J. Schapiro (2011). Handbook of Laboratory Animal Science, Volume I, Third Edition: Essential Principles and Practices. CRC Press. p. 2. ISBN 978-1-4200-8456-6.
- ^ a b Institute of Medicine (1991). Science, Medicine, and Animals. National Academies Press. p. 3. ISBN 978-0-309-56994-1.
...without this fundamental knowledge, most of the clinical advances described in these pages would not have occurred.
- ^ a b "The Nobel Prize in Physiology or Medicine 1933". Nobel Web AB. Retrieved 20 June 2015.
- ^ a b "Thomas Hunt Morgan and his Legacy". Nobel Web AB. Retrieved 20 June 2015.
- ^ a b Kandel, Eric. 1999. "Genes, Chromosomes, and the Origins of Modern Biology", Columbia Magazine
- ^ a b Bering Nobel Biography
- ^ a b Walter B. Cannon Papers, American Philosophical Society Archived 14 August 2009 at the Wayback Machine
- ^ a b Discovery of Insulin Archived 30 September 2009 at the Wayback Machine
- ^ a b Thompson bio ref Archived 2009-02-10 at the Wayback Machine
- ^ a b Raventos J (1956) Br J Pharmacol 11, 394
- ^ a b Carrel A (1912) Surg. Gynec. Obst. 14: p. 246
- ^ a b Williamson C (1926) J. Urol. 16: p. 231
- ^ a b Woodruff H & Burg R (1986) in Discoveries in Pharmacology vol 3, ed Parnham & Bruinvels, Elsevier, Amsterdam
- ^ a b Moore F (1964) Give and Take: the Development of Tissue Transplantation. Saunders, New York
- ^ a b Gibbon JH (1937) Arch. Surg. 34, 1105
- ^ a b "Horton Corwin Hinshaw". www.rawbw.com. Retrieved 13 May 2025.
- ^ a b Fleming A (1929) Br J Exp Path 10, 226
- ^ a b Medical Research Council (1956) Br. Med. J. 2: p. 454
- ^ Fox MA (1986). The Case for Animal Experimention: An Evolutionary and Ethical Perspective. Berkeley and Los Angeles, California: University of California Press. ISBN 978-0-520-05501-8. OCLC 11754940 – via Google Books.
- ^ Allmon WD, Ross RM (December 2018). "Evolutionary remnants as widely accessible evidence for evolution: the structure of the argument for application to evolution education". Evolution: Education and Outreach. 11 (1) 1. doi:10.1186/s12052-017-0075-1. S2CID 29281160.
- ^ Slack JM (2013). Essential Developmental Biology. Oxford: Wiley-Blackwell. OCLC 785558800.
- ^ Chakraborty C, Hsu C, Wen Z, Lin C, Agoramoorthy G (1 February 2009). "Zebrafish: A Complete Animal Model for In Vivo Drug Discovery and Development". Current Drug Metabolism. 10 (2): 116–124. doi:10.2174/138920009787522197. PMID 19275547.
- ^ Kari G, Rodeck U, Dicker AP (July 2007). "Zebrafish: An Emerging Model System for Human Disease and Drug Discovery". Clinical Pharmacology & Therapeutics. 82 (1): 70–80. doi:10.1038/sj.clpt.6100223. PMID 17495877. S2CID 41443542.
- ^ a b c d e f g h i j k l A reference handbook of the medical sciences. William Wood and Co., 1904, Edited by Albert H. Buck.
- ^ a b Pu R, Coleman J, Coisman J, Sato E, Tanabe T, Arai M, Yamamoto JK (February 2005). "Dual-subtype FIV vaccine (Fel-O-Vax® FIV) protection against a heterologous subtype B FIV isolate". Journal of Feline Medicine and Surgery. 7 (1): 65–70. doi:10.1016/j.jfms.2004.08.005. PMC 10911555. PMID 15686976. S2CID 26525327.
- ^ a b Dryden MW, Payne PA (2005). "Preventing parasites in cats". Veterinary Therapeutics. 6 (3): 260–7. PMID 16299672.
- ^ a b Sources:
- P. Michael Conn (29 May 2013). Animal Models for the Study of Human Disease. Academic Press. p. 37. ISBN 978-0-12-415912-9.
...animal models are central to the effective study and discovery of treatments for human diseases.
- Lieschke GJ, Currie PD (May 2007). "Animal models of human disease: zebrafish swim into view". Nature Reviews Genetics. 8 (5): 353–367. doi:10.1038/nrg2091. PMID 17440532. S2CID 13857842.
Biomedical research depends on the use of animal models to understand the pathogenesis of human disease at a cellular and molecular level and to provide systems for developing and testing new therapies.
- Pierce K. H. Chow, Robert T. H. Ng, Bryan E. Ogden (2008). Using Animal Models in Biomedical Research: A Primer for the Investigator. World Scientific. pp. 1–2. ISBN 978-981-281-202-5.
Arguments regarding whether biomedical science can advance without the use of animals are frequently mooted and make as much sense as questioning if clinical trials are necessary before new medical therapies are allowed to be widely used in the general population [pg. 1] ...animal models are likely to remain necessary until science develops alternative models and systems that are equally sound and robust [pg. 2].
- Jann Hau, Steven J. Schapiro (2011). "The contribution of laboratory animals to medical progress". Handbook of Laboratory Animal Science, Volume I, Third Edition: Essential Principles and Practices. CRC Press. ISBN 978-1-4200-8456-6.
Animal models are required to connect [modern biological technologies] in order to understand whole organisms, both in healthy and diseased states. In turn, these animal studies are required for understanding and treating human disease [pg. 2] ...In many cases, though, there will be no substitute for whole-animal studies because of the involvement of multiple tissue and organ systems in both normal and aberrant physiological conditions [pg. 15].
- Royal Society of Medicine (24 May 2023). "Statement of the Royal Society's position on the use of animals in research".
At present the use of animals remains the only way for some areas of research to progress.
- P. Michael Conn (29 May 2013). Animal Models for the Study of Human Disease. Academic Press. p. 37. ISBN 978-0-12-415912-9.
- ^ a b Guela C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA (July 1998). "Aging renders the brain vulnerable to amyloid β-protein neurotoxicity". Nature Medicine. 4 (7): 827–831. doi:10.1038/nm0798-827. PMID 9662375. S2CID 45108486.
- ^ a b AIDS Reviews 2005;7:67-83 Antiretroviral Drug Studies in Nonhuman Primates: a Valid Animal Model for Innovative Drug Efficacy and Pathogenesis Experiments Archived 17 December 2008 at the Wayback Machine
- ^ a b Jameson BA, McDonnell JM, Marini JC, Korngold R (April 1994). "A rationally designed CD4 analogue inhibits experimental allergic encephalomyelitis". Nature. 368 (6473): 744–746. Bibcode:1994Natur.368..744J. doi:10.1038/368744a0. PMID 8152486. S2CID 4370797.
- ^ a b Lyuksyutova AL, Lu C-C MN, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, et al. (2003). "Anterior-posterior guidance of commissural axons by Wnt-Frizzled signaling". Science. 302 (5652): 1984–8. Bibcode:2003Sci...302.1984L. doi:10.1126/science.1089610. PMID 14671310. S2CID 39309990.
- ^ a b Taylor K, Alvarez LR (2019). "An Estimate of the Number of Animals Used for Scientific Purposes Worldwide in 2015". Alternatives to Laboratory Animals. 47 (5–6). SAGE Publications: 196–213. doi:10.1177/0261192919899853. ISSN 0261-1929. PMID 32090616. S2CID 211261775.
- ^ a b Seventh Report on the Statistics on the Number of Animals used for Experimental and other Scientific Purposes in the Member States of the European Union
- ^ a b Hedrich, Hans, ed. (21 August 2004). "The house mouse as a laboratory model: a historical perspective". The Laboratory Mouse. Elsevier Science. ISBN 978-0-08-054253-9.
- ^ Carbone, Larry. (2004). What Animals Want: Expertise and Advocacy in Laboratory Animal Welfare Policy.
- ^ "EU statistics show decline in animal research numbers". Speaking of Research. 2013. Retrieved 24 January 2016.
- ^ "U.S. Will No Longer Require Animal Testing for New Drugs". 13 January 2022.
- ^ Festing S, Wilkinson R (June 2007). "The ethics of animal research. Talking Point on the use of animals in scientific research". EMBO Reports. 8 (6): 526–530. doi:10.1038/sj.embor.7400993. ISSN 1469-221X. PMC 2002542. PMID 17545991.
- ^ Reddy N, Lynch B, Gujral J, Karnik K (September 2023). "Regulatory landscape of alternatives to animal testing in food safety evaluations with a focus on the western world". Regulatory Toxicology and Pharmacology. 143 105470. doi:10.1016/j.yrtph.2023.105470. ISSN 1096-0295. PMID 37591329. S2CID 260938742.
- ^ Petetta F, Ciccocioppo R (November 2021). "Public perception of laboratory animal testing: Historical, philosophical, and ethical view". Addiction Biology. 26 (6) e12991. doi:10.1111/adb.12991. ISSN 1369-1600. PMC 9252265. PMID 33331099.
- ^ Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA (May 2021). "Organs-on-chips: into the next decade". Nature Reviews. Drug Discovery. 20 (5): 345–361. doi:10.1038/s41573-020-0079-3. hdl:1887/3151779. ISSN 1474-1784. PMID 32913334. S2CID 221621465.
- ^ Löwa A, Jevtić M, Gorreja F, Hedtrich S (May 2018). "Alternatives to animal testing in basic and preclinical research of atopic dermatitis". Experimental Dermatology. 27 (5): 476–483. doi:10.1111/exd.13498. ISSN 1600-0625. PMID 29356091. S2CID 3378256.
- ^ Madden JC, Enoch SJ, Paini A, Cronin MT (July 2020). "A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications". Alternatives to Laboratory Animals. 48 (4): 146–172. doi:10.1177/0261192920965977. ISSN 0261-1929. PMID 33119417. S2CID 226204296.
- ^ Reddy N, Lynch B, Gujral J, Karnik K (September 2023). "Alternatives to animal testing in toxicity testing: Current status and future perspectives in food safety assessments". Food and Chemical Toxicology. 179 113944. doi:10.1016/j.fct.2023.113944. ISSN 1873-6351. PMID 37453475. S2CID 259915886.
- ^ a b Croce, Pietro (1999). Vivisection or Science? An Investigation into Testing Drugs and Safeguarding Health. Zed Books, ISBN 1-85649-732-1.
- ^ "Vivisection". Encyclopædia Britannica. 2007. Archived from the original on 1 January 2008.
- ^ "Vivisection FAQ" (PDF). British Union for the Abolition of Vivisection. Archived from the original (PDF) on 13 May 2015.
- ^ "Vivisection". Encyclopedia.com. Retrieved 5 May 2023.
- ^ "Vivisection". Definition of VIVISECTION. Merriam-Webster. Retrieved 5 May 2023.
- ^ a b Carbone, p. 22.
- ^ Paixão RL, Schramm FR (1999). "Ethics and animal experimentation: what is debated?". Cadernos de Saúde Pública. 15 (Suppl 1): 99–110. doi:10.1590/s0102-311x1999000500011. PMID 10089552.
- ^ Yarri, Donna (2005). The Ethics of Animal Experimentation, Oxford University Press U.S., ISBN 0-19-518179-4.
- ^ Cohen and Loew 1984.
- ^ "History of nonhuman animal research". Laboratory Primate Advocacy Group. Archived from the original on 13 October 2006.
- ^ Abdel-Halim RE (2005). "Contributions of Ibn Zuhr (Avenzoar) to the progress of surgery: a study and translations from his book Al-Taisir". Saudi Medical Journal. 26 (9): 1333–39. PMID 16155644.
- ^ Abdel-Halim RE (2006). "Contributions of Muhadhdhab Al-Deen Al-Baghdadi to the progress of medicine and urology. A study and translations from his book Al-Mukhtar". Saudi Medical Journal. 27 (11): 1631–41. PMID 17106533.
- ^ Mock M, Fouet A (2001). "Anthrax". Annu. Rev. Microbiol. 55: 647–71. doi:10.1146/annurev.micro.55.1.647. PMID 11544370.
- ^ Windholz G (1987). "Pavlov as a psychologist. A reappraisal". Pavlovian J. Biol. Sci. 22 (3): 103–12. doi:10.1007/BF02734662. PMID 3309839. S2CID 141344843.
- ^ Kohler, Lords of the Fly, chapter 5
- ^ Steensma DP, Kyle Robert A., Shampo Marc A. (November 2010). "Abbie Lathrop, the "Mouse Woman of Granby": Rodent Fancier and Accidental Genetics Pioneer". Mayo Clinic Proceedings. 85 (11): e83. doi:10.4065/mcp.2010.0647. PMC 2966381. PMID 21061734.
- ^ Pillai S (29 May 2012). "History of Immunology at Harvard". Harvard Medical School:About us. Harvard Medical School. Archived from the original on 20 December 2013. Retrieved 19 December 2013.
- ^ Gorden P (1997). "Non-insulin dependent diabetes – the past, present and future". Ann. Acad. Med. Singap. 26 (3): 326–30. PMID 9285027.
- ^ Ironside W, "John Frederick Joseph Cade (1912–1980)", Australian Dictionary of Biography, Canberra: National Centre of Biography, Australian National University, retrieved 13 May 2025
- ^ Whalen FX, Bacon DR & Smith HM (2005) Best Pract Res Clin Anaesthesiol 19, 323
- ^ "Developing a medical milestone: The Salk polio vaccine". Archived from the original on 11 March 2010. Retrieved 20 June 2015. Virus-typing of polio by Salk
- ^ "Tireless polio research effort bears fruit and indignation". Archived from the original on 5 September 2008. Retrieved 23 August 2008. Salk polio virus
- ^ "NMAH | Polio: Two Vaccines". americanhistory.si.edu. Archived from the original on 4 June 2011. Retrieved 13 May 2025.
- ^ "the work on [polio] prevention was long delayed by... misleading experimental models of the disease in monkeys" | ari.info
- ^ Walgate R (1981). "Armadillos fight leprosy". Nature. 291 (5816): 527. Bibcode:1981Natur.291..527W. doi:10.1038/291527a0. PMID 7242665.
- ^ Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL (2006). "The Continuing Challenges of Leprosy". Clin. Microbiol. Rev. 19 (2): 338–81. doi:10.1128/CMR.19.2.338-381.2006. PMC 1471987. PMID 16614253.
- ^ Jaenisch R, Mintz B (1974). "Simian Virus 40 DNA Sequences in DNA of Healthy Adult Mice Derived from Preimplantation Blastocysts Injected with Viral DNA". Proceedings of the National Academy of Sciences of the United States of America. 71 (4): 1250–54. Bibcode:1974PNAS...71.1250J. doi:10.1073/pnas.71.4.1250. PMC 388203. PMID 4364530.
- ^ a b Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH (1997). "Viable offspring derived from fetal and adult mammalian cells". Nature. 385 (6619): 810–13. Bibcode:1997Natur.385..810W. doi:10.1038/385810a0. PMID 9039911. S2CID 4260518.
- ^ "History of animal research". www.understandinganimalresearch.org.uk. Retrieved 8 April 2016.
- ^ PMPA blocks SIV in monkeys
- ^ PMPA is tenofovir
- ^ "Taste of Raspberries, Taste of Death. The 1937 Elixir Sulfanilamide Incident". FDA Consumer magazine. June 1981. Archived from the original on 4 June 2009.
- ^ Burkholz H (1 September 1997). "Giving Thalidomide a Second Chance". FDA Consumer. US Food and Drug Administration. Archived from the original on 21 April 1999.
- ^ Antoshechkin I, Sternberg PW (2007). "The versatile worm: genetic and genomic resources for Caenorhabditis elegans research". Nature Reviews Genetics. 8 (7): 518–32. doi:10.1038/nrg2105. PMID 17549065. S2CID 12923468.
- ^ Matthews KA, Kaufman TC, Gelbart WM (2005). "Research resources for Drosophila: the expanding universe". Nature Reviews Genetics. 6 (3): 179–93. doi:10.1038/nrg1554. PMID 15738962. S2CID 31002250.
- ^ Schulenburg H, Kurz CL, Ewbank JJ (2004). "Evolution of the innate immune system: the worm perspective". Immunological Reviews. 198: 36–58. doi:10.1111/j.0105-2896.2004.0125.x. PMID 15199953. S2CID 21541043.
- ^ Leclerc V, Reichhart JM (2004). "The immune response of Drosophila melanogaster". Immunological Reviews. 198: 59–71. doi:10.1111/j.0105-2896.2004.0130.x. PMID 15199954. S2CID 7395057.
- ^ Mylonakis E, Aballay A (2005). "Worms and flies as genetically tractable animal models to study host-pathogen interactions". Infection and Immunity. 73 (7): 3833–41. doi:10.1128/IAI.73.7.3833-3841.2005. PMC 1168613. PMID 15972468.
- ^ a b Kavanagh K, Reeves EP (2004). "Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens". FEMS Microbiology Reviews. 28 (1): 101–12. doi:10.1016/j.femsre.2003.09.002. PMID 14975532.
- ^ a b Antunes LC, Imperi F, Carattoli A, Visca P (2011). Adler B (ed.). "Deciphering the Multifactorial Nature of Acinetobacter baumannii Pathogenicity". PLOS ONE. 6 (8) e22674. Bibcode:2011PLoSO...622674A. doi:10.1371/journal.pone.0022674. PMC 3148234. PMID 21829642.
- ^ a b Aperis G, Fuchs BB, Anderson CA, Warner JE, Calderwood SB, Mylonakis E (2007). "Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain". Microbes and Infection / Institut Pasteur. 9 (6): 729–34. doi:10.1016/j.micinf.2007.02.016. PMC 1974785. PMID 17400503.
- ^ Waterfield NR, Sanchez-Contreras M, Eleftherianos I, Dowling A, Yang G, Wilkinson P, Parkhill J, Thomson N, Reynolds SE, Bode HB, Dorus S, Ffrench-Constant RH (2008). "Rapid Virulence Annotation (RVA): Identification of virulence factors using a bacterial genome library and multiple invertebrate hosts". Proceedings of the National Academy of Sciences of the United States of America. 105 (41): 15967–72. Bibcode:2008PNAS..10515967W. doi:10.1073/pnas.0711114105. PMC 2572985. PMID 18838673.
- ^ a b c d e f g "USDA Statistics for Animals Used in Research in the US". Speaking of Research. 20 March 2008.
- ^ Trull FL (1999). "More Regulation of Rodents". Science. 284 (5419): 1463. Bibcode:1999Sci...284.1463T. doi:10.1126/science.284.5419.1463. PMID 10383321. S2CID 10122407.
- ^ a b c d Rosenthal N, Brown S (2007). "The mouse ascending: perspectives for human-disease models". Nature Cell Biology. 9 (9): 993–99. doi:10.1038/ncb437. PMID 17762889. S2CID 4472227.
- ^ Mukerjee M (August 2004). "Speaking for the Animals". Scientific American. 291 (2): 96–97. Bibcode:2004SciAm.291b..96M. doi:10.1038/scientificamerican0804-96.
- ^ Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, Holmdahl R, Hubner N, Izsvák Z, Jacob HJ, Kuramoto T, Kwitek AE, Marrone A, Mashimo T, Moreno C, Mullins J, Mullins L, Olsson T, Pravenec M, Riley L, Saar K, Serikawa T, Shull JD, Szpirer C, Twigger SN, Voigt B, Worley K (2008). "Progress and prospects in rat genetics: a community view". Nature Genetics. 40 (5): 516–22. doi:10.1038/ng.147. PMID 18443588. S2CID 22522876.
- ^ Taylor K, Alvarez LR (November 2019). "An Estimate of the Number of Animals Used for Scientific Purposes Worldwide in 2015". Alternatives to Laboratory Animals. 47 (5–6): 196–213. doi:10.1177/0261192919899853. ISSN 0261-1929. PMID 32090616. S2CID 211261775.
- ^ Dog profile, The Humane Society of the United States
- ^ Smith D, Broadhead C, Descotes G, Fosse R, Hack R, Krauser K, Pfister R, Phillips B, Rabemampianina Y, Sanders J, Sparrow S, Stephan-Gueldner M, Jacobsen SD (2002). "Preclinical Safety Evaluation Using Nonrodent Species: An Industry/ Welfare Project to Minimize Dog Use". ILAR. 43 Suppl: S39-42. doi:10.1093/ilar.43.Suppl_1.S39. PMID 12388850.
- ^ Quianzon CC, Cheikh I (16 July 2012). "History of insulin". Journal of Community Hospital Internal Medicine Perspectives. 2 (2) 18701. doi:10.3402/jchimp.v2i2.18701. ISSN 2000-9666. PMC 3714061. PMID 23882369.
- ^ a b c d "Statistics of Scientific Procedures on Living Animals, Great Britain" (PDF). UK Home Office. 2017. Retrieved 23 July 2018.
- ^ "Germany sees 7% rise in animal research procedures in 2016". Speaking of Research. 6 February 2018.
- ^ "France, Italy and the Netherlands publish their 2016 statistics". Speaking of Research. 20 March 2018.
- ^ Li Z, Zheng W, Wang H, Cheng Y, Fang Y, Wu F, Sun G, Sun G, Lv C, Hui B (15 March 2021). "Application of Animal Models in Cancer Research: Recent Progress and Future Prospects". Cancer Management and Research. 13: 2455–2475. doi:10.2147/CMAR.S302565. ISSN 1179-1322. PMC 7979343. PMID 33758544.
- ^ Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham Da, Glennie MJ, Kelland LR (25 May 2010). "Guidelines for the welfare and use of animals in cancer research". British Journal of Cancer. 102 (11): 1555–1577. doi:10.1038/sj.bjc.6605642. ISSN 1532-1827. PMC 2883160. PMID 20502460.
- ^ Tsering J, Hu X (2018). "Triphala Suppresses Growth and Migration of Human Gastric Carcinoma Cells In Vitro and in a Zebrafish Xenograft Model". BioMed Research International. 2018 7046927. doi:10.1155/2018/7046927. ISSN 2314-6141. PMC 6311269. PMID 30643816.
- ^ International Perspectives: The Future of Nonhuman Primate Resources, Proceedings of the Workshop Held 17–19 April, pp. 36–45, 46–48, 63–69, 197–200.
- ^ "U.S. primate import statistics for 2014". International Primate Protection League. Archived from the original on 4 July 2017. Retrieved 9 July 2015.
- ^ a b Conlee KM, Hoffeld EH, Stephens ML (2004). "Demographic Analysis of Primate Research in the United States" (PDF). ATLA. 32 (Supplement 1): 315–22. doi:10.1177/026119290403201s52. PMID 23577480. Archived from the original (PDF) on 27 February 2008.
- ^ St Fleur N (12 June 2015). "U.S. Will Call All Chimps 'Endangered'". The New York Times. The New York Times. Retrieved 9 July 2015.
- ^ Lutz C, Well A, Novak M (2003). "Stereotypic and Self-Injurious Behavior in Rhesus Macaques: A Survey and Retrospective Analysis of Environment and Early Experience". American Journal of Primatology. 60 (1): 1–15. doi:10.1002/ajp.10075. PMID 12766938. S2CID 19980505.
- ^ Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G (2001). "Transgenic monkeys produced by retroviral gene transfer into mature oocytes". Science. 291 (5502): 309–12. Bibcode:2001Sci...291..309C. doi:10.1126/science.291.5502.309. PMID 11209082.
- ^ Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, Bachevalier J, Zola SM, Li SH, Li XJ, Chan AW (2008). "Towards a transgenic model of Huntington's disease in a non-human primate". Nature. 453 (7197): 921–24. Bibcode:2008Natur.453..921Y. doi:10.1038/nature06975. PMC 2652570. PMID 18488016.
- ^ a b The use of non-human animals in research: a guide for scientists The Royal Society, 2004, p. 1
- ^ a b Emborg ME (2007). "Nonhuman primate models of Parkinson's disease". ILAR Journal. 48 (4): 339–55. doi:10.1093/ilar.48.4.339. PMID 17712221.
- ^ McKie R (2 November 2008). "Ban on primate experiments would be devastating, scientists warn". The Observer. London.
- ^ "Statistics of Scientific Procedures on Living Animals, Great Britain" (PDF). British government. 2004. Retrieved 13 July 2012.
- ^ Statistics of Scientific Procedures on Living Animals, Great Britain, 1996 – UK Home Office, Table 13
- ^ "Annual Report Animals" (PDF). Aphis.usda.gov. Archived from the original (PDF) on 23 November 2020. Retrieved 6 August 2017.
- ^ Carbone, pp. 68–69.
- ^ Office of Laboratory Animal Welfare. Public Health Service Policy on Humane Care and Use of Laboratory Animals. nih.gov
- ^ Title 9 – Animals and Animal Products. Code of Federal Regulations. Vol. 1 (1 January 2008).
- ^ a b "Animal Testing and the Law – Animal Legal Defense Fund". Animal Legal Defense Fund. Archived from the original on 23 August 2017. Retrieved 14 June 2017.
- ^ Harden G. "USDA Inspector General Audit Report of APHIS Animal Care Program Inspection and Enforcement Activities" (PDF). United States Department of Agriculture Office of Inspector General (Report No. 33601–0001–41). Archived from the original (PDF) on 16 February 2015. Retrieved 7 July 2015.
- ^ Young R (September 2005). "Audit Report: APHIS Animal Care Program Inspection and Enforcement Activities" (PDF). USDA Office of Inspector General Western Region (Report No. 33002–3–SF). Archived from the original (PDF) on 8 November 2005. Retrieved 7 July 2015.
- ^ Hansen L, Goodman J, Chandna A (2012). "Analysis of animal research ethics committee membership at American institutions". Animals. 2 (1): 68–75. doi:10.3390/ani2010068. PMC 4494267. PMID 26486777.
- ^ Carbone, p. 94.
- ^ Plous S, Herzog H (2001). "Animal Research: Reliability of Protocol Reviews for Animal Research". Science. 293 (5530): 608–09. doi:10.1126/science.1061621. PMID 11474086. S2CID 33314019.
- ^ Nandi J (27 April 2012). "Scientists take on activists, want ban on live testing on animals lifted". The Times of India. Archived from the original on 27 October 2012. Retrieved 13 July 2012.
- ^ Commissioner Oo (10 April 2025). "FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs". FDA. Archived from the original on 10 April 2025. Retrieved 13 May 2025.
- ^ "Roadmap to Reducing Animal Testing in Preclinical Safety Studies". FDA.
- ^ Marshall LJ, Bailey J, Cassotta M, Herrmann K, Pistollato F (March 2023). "Poor Translatability of Biomedical Research Using Animals - A Narrative Review". Altern Lab Anim. 51 (2): 102–135. doi:10.1177/02611929231157756. PMID 36883244.
- ^ Director Oo. "NIH to prioritize human-based research technologies". NIH.
- ^ FDA & NIH Workshop on Reducing Animal Testing on YouTube
- ^ Taylor K, Gordon N, Langley G, Higgins W (2008). "Estimates for worldwide laboratory animal use in 2005". ATLA. 36 (3): 327–42. doi:10.1177/026119290803600310. PMID 18662096. S2CID 196613886.
- ^ Hunter, Robert G. (1 January 2014). "Alternatives to animal testing drive market". Gen. Eng. Biotechnol. News. Vol. 34, no. 1. p. 11.
While growth has leveled off and there have been significant reductions in some countries, the number of animals used in research globally still totals almost 100 million a year.

- ^ a b c d "The Ethics of research involving animals" (PDF). Nuffield Council on Bioethics. Archived from the original (PDF) on 25 June 2008.
- ^ "USDA publishes 2016 animal research statistics – 7% rise in animal use". Speaking of Research. 19 June 2017. Retrieved 10 December 2017.
- ^ Goodman J, Chandna A, Roe K (2015). "Trends in animal use at US research facilities". Journal of Medical Ethics. 41 (7): 567–69. doi:10.1136/medethics-2014-102404. PMID 25717142. S2CID 46187262. Retrieved 7 July 2015.
- ^ Rowan, A., Loew, F., and Weer, J. (1995) "The Animal Research Controversy. Protest, Process and Public Policy: An Analysis of Strategic Issues." Tufts University, North Grafton. cited in Carbone 2004, p. 26.
- ^ Alternatives to Animal Use in Research, Testing and Education, U.S. Congress Office of Technology Assessment, Washington, D.C.:Government Printing Office, 1986, p. 64. In 1966, the Laboratory Animal Breeders Association estimated in testimony before Congress that the number of mice, rats, guinea pigs, hamsters, and rabbits used in 1965 was around 60 million. (Hearings before the Subcommittee on Livestock and Feed Grains, Committee on Agriculture, U.S. House of Representatives, 1966, p. 63.)
- ^ a b "Animal research numbers in 2017". Understanding Animal Research. 2017.
- ^ "Home Office Statistics for Animals Used in Research in the UK". Speaking of Research. 23 October 2012.
- ^ a b Russell, W. M. S. (William Moy Stratton), Health JB (1992). The principles of humane experimental technique (Special ed.). South Mimms, Potters Bar, Herts, England: Universities Federation for Animal Welfare. ISBN 0-900767-78-2. OCLC 27347928. Archived from the original on 27 September 2011. Retrieved 16 August 2013.
- ^ Badyal D., Desai C. (2014). "Animal use in pharmacology education and research: The changing scenario". Indian Journal of Pharmacology. 46 (3): 257–65. doi:10.4103/0253-7613.132153. PMC 4071700. PMID 24987170.
- ^ "2009 CCAC Survey of Animal Use" (PDF). Canadian Council on Animal Care. December 2010. Archived from the original (PDF) on 7 June 2015. Retrieved 7 July 2015.
- ^ Merkes M, Buttrose R. "New code, same suffering: animals in the lab". ABC. The Drum. Retrieved 7 July 2015.
- ^ Even D (29 May 2013). "Number of animal experiments up for first time since 2008". Haaretz. Retrieved 7 July 2015.
- ^ "Rise in animal research in South Korea in 2017". Speaking of Research. 20 April 2018. Retrieved 23 July 2017.
- ^ "Number of laboratory animals in Germany". Max-Planck-Gesellschaft. Retrieved 7 July 2015.
- ^ Kong Q, Qin C (2009). "Analysis of current laboratory animal science policies and administration in China". ILAR. 51 (1): e1 – e11. doi:10.1093/ilar.51.1.e1. PMID 20075493.
- ^ Invertebrate Animal Resources Archived 25 October 2007 at the Wayback Machine. National Center for Research Resources. ncrr.nih.gov
- ^ "Who's Who of Federal Oversight of Animal Issues". Aesop-project.org. Archived from the original on 22 September 2007.
- ^ Collins FS, Rossant J, Wurst W (2007). "A mouse for all reasons". Cell. 128 (1): 9–13. doi:10.1016/j.cell.2006.12.018. PMID 17218247. S2CID 18872015.
- ^ a b Gillham, Christina (17 February 2006). "Bought to be sold", Newsweek.
- ^ Class B dealers Archived 29 April 2010 at the Wayback Machine, Humane Society of the United States.
- ^ "Who's Who of Federal Oversight of Animal Issues", Aesop Project.
- ^ Salinger, Lawrence and Teddlie, Patricia. "Stealing Pets for Research and Profit: The Enforcement (?) of the Animal Welfare Act" Archived 16 January 2013 at archive.today, paper presented at the annual meeting of the American Society of Criminology, Royal York, Toronto, 15 October 2006
- ^ Reitman, Judith (1995) Stolen for Profit, Zebra, ISBN 0-8217-4951-X.
- ^ Moran, Julio (12 September 1991) "Three Sentenced to Prison for Stealing Pets for Research," L.A. Times.
- ^ Francione, Gary. Animals, Property, and the Law. Temple University Press, 1995, p. 192; Magnuson, Warren G., Chairman. "Opening remarks in hearings prior to enactment of Pub. L. 89-544, the Laboratory Animal Welfare Act," U.S. Senate Committee on Commerce, 25 March 1966.
- ^ Notorious Animal Dealer Loses License and Pays Record Fine, The Humane Society of the United States
- ^ Animal Testing: Where Do the Animals Come From?. American Society for the Prevention of Cruelty to Animals. According to the ASPCA, the following states prohibit shelters from providing animals for research: Connecticut, Delaware, Hawaii, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, South Carolina, Vermont, and West Virginia.
- ^ Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes
- ^ Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes
- ^ Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Archived 31 July 2007 at the Wayback Machine Department for Environment, Food and Rural Affairs
- ^ a b ""Statistics of Scientific Procedures on Living Animals", Statistics of Scientific Procedures on Living Animals, Home Office" (PDF). 2004. p. 87.
- ^ U.S. Primate Imports Spike International Primate Protection League April 2007
- ^ Pain in Research Animals: General Principles and Considerations. NIH. 2009.
- ^ Duncan IJ, Petherick JC (1991). "The implications of cognitive processes for animal welfare". Journal of Animal Science. 69 (12): 5017–22. doi:10.2527/1991.69125017x. PMID 1808195.
- ^ Curtis SE, Stricklin WR (1991). "The importance of animal cognition in agricultural animal production systems: an overview". Journal of Animal Science. 69 (12): 5001–07. doi:10.2527/1991.69125001x. PMID 1808193.
- ^ Carbone, p. 149.
- ^ Rollin drafted the 1985 Health Research Extension Act and an animal welfare amendment to the 1985 Food Security Act: see Rollin, Bernard. "Animal research: a moral science. Talking Point on the use of animals in scientific research", EMBO Reports 8, 6, 2007, pp. 521–25
- ^ a b Rollin, Bernard. The Unheeded Cry: Animal Consciousness, Animal Pain, and Science. New York: Oxford University Press, 1989, pp. xii, 117–18, cited in Carbone 2004, p. 150.
- ^ Griffin DR, Speck GB (2004). "New evidence of animal consciousness". Animal Cognition. 7 (1): 5–18. doi:10.1007/s10071-003-0203-x. PMID 14658059. S2CID 8650837.
- ^ Allen C (1998). "Assessing animal cognition: ethological and philosophical perspectives". Journal of Animal Science. 76 (1): 42–47. doi:10.2527/1998.76142x. PMID 9464883.
- ^ "Smarter Than You Think: Renowned Canine Researcher Puts Dogs' Intelligence on Par with 2-Year-Old Human". www.apa.org. Retrieved 5 May 2023.
- ^ "Animal Welfare Act 1999". Parliamentary Counsel Office. 2015. Retrieved 23 January 2016.
- ^ "Norwegian animal welfare act". Animal Legal and Historical Center. 2011. Retrieved 25 January 2016.
- ^ "Guide for the Care and Use of Laboratory Animals", ILAR, National Research Council, National Academies Press, 1996, p. 64, ISBN 0-309-05377-3.
- ^ "How to Work With Your Institutional Animal Care and Use Committee (IACUC)". ori.hhs.gov.
- ^ Klabukov I, Shestakova V, Krasilnikova O, Smirnova A, Abramova O, Baranovskii D, Atiakshin D, Kostin AA, Shegay P, Kaprin AD (2023). "Refinement of Animal Experiments: Replacing Traumatic Methods of Laboratory Animal Marking with Non-Invasive Alternatives". Animals. 13 (22): 3452. doi:10.3390/ani13223452. ISSN 2076-2615. PMC 10668729. PMID 38003070.
- ^ Lindner E, Fuelling O (2002). "Marking methods in small mammals: ear-tattoo as an alternative to toe-clipping". Journal of Zoology. 256 (2): 159–163. doi:10.1017/S0952836902000195. ISSN 0952-8369.
- ^ Devolder K, Eggel M (2019). "No Pain, No Gain? In Defence of Genetically Disenhancing (Most) Research Animals". Animals. 9 (4): 154. doi:10.3390/ani9040154. PMC 6523187. PMID 30970545.
- ^ a b Flecknell P (2002). "Replacement, reduction and refinement". ALTEX. 19 (2): 73–78. PMID 12098013.
- ^ Animal Procedures Committee: review of cost-benefit assessment in the use of animals in research Archived 27 February 2008 at the Wayback Machine The Animal Procedures Committee, June 2003 p46-7
- ^ Carbone, Larry. "Euthanasia," in Bekoff, M. and Meaney, C. Encyclopedia of Animal Rights and Welfare. Greenwood Publishing Group, pp. 164–66, cited in Carbone 2004, pp. 189–90.
- ^ Cooper D (11 June 2017). ""Euthanasia Guidelines", Research animal resources". University of Minnesota.
- ^ Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C (1996). "Recommendations for euthanasia of experimental animals: Part 1". Laboratory Animals. 30 (4): 293–316 (295). doi:10.1258/002367796780739871. PMID 8938617.
- ^ "Guide for the Care and Use of Laboratory Animals", ILAR, National Research Council, National Academies Press, 1996, p. 65, ISBN 0-309-05377-3.
- ^ Diaz SL (2020). "Conducting and reporting animal experimentation: Quo vadis?". European Journal of Neuroscience. 52 (6): 3493–3498. doi:10.1111/ejn.14091. hdl:11336/88084. ISSN 0953-816X. PMID 30058230. S2CID 51865025.
- ^ "AVMA Guidelines on Euthanasia, June 2007 edition, Report of the AVMA Panel on Euthanasia" (PDF). Avma.org. Archived from the original (PDF) on 15 August 2011.
- ^ a b "Select Committee on Animals in Scientific Procedures Report", House of Lords, 16 July 2002. See chapter 3: "The purpose and nature of animal experiments." Retrieved 6 July 2010.
- ^ a b Job CK (2003). "Nine-banded armadillo and leprosy research". Indian Journal of Pathology & Microbiology. 46 (4): 541–50. PMID 15025339.
- ^ Venken KJ, Bellen HJ (2005). "Emerging technologies for gene manipulation in Drosophila melanogaster". Nature Reviews Genetics. 6 (3): 167–78. doi:10.1038/nrg1553. PMID 15738961. S2CID 21184903.
- ^ Sung YH, Song J, Lee HW (2004). "Functional genomics approach using mice". Journal of Biochemistry and Molecular Biology. 37 (1): 122–32. doi:10.5483/BMBRep.2004.37.1.122. PMID 14761310.
- ^ Janies D, DeSalle R (1999). "Development, evolution, and corroboration". The Anatomical Record. 257 (1): 6–14. doi:10.1002/(SICI)1097-0185(19990215)257:1<6::AID-AR4>3.0.CO;2-I. PMID 10333399. S2CID 23492348.
- ^ Akam M (1995). "Hox genes and the evolution of diverse body plans". Philosophical Transactions of the Royal Society B. 349 (1329): 313–19. Bibcode:1995RSPTB.349..313A. doi:10.1098/rstb.1995.0119. PMID 8577843.
- ^ Prasad BC, Reed RR (1999). "Chemosensation: Molecular mechanisms in worms and mammals". Trends in Genetics. 15 (4): 150–53. doi:10.1016/S0168-9525(99)01695-9. PMID 10203825.
- ^ Schafer WR (2006). "Neurophysiological methods in C. elegans: an introduction". WormBook: 1–4. doi:10.1895/wormbook.1.113.1. PMC 4780964. PMID 18050439.
- ^ Yamamuro Y (2006). "Social behavior in laboratory rats: Applications for psycho-neuroethology studies". Animal Science Journal. 77 (4): 386–94. doi:10.1111/j.1740-0929.2006.00363.x.
- ^ Marler P., Slabbekoorn H, Nature's Music: The Science of Birdsong, Academic Press, 2004. ISBN 0-12-473070-1[page needed]
- ^ For example "in addition to providing the chimpanzees with enrichment, the termite mound is also the focal point of a tool-use study being conducted", from the web page of the Lincoln Park Zoo. Retrieved 25 April 2007.
- ^ Festing, M., "Inbred Strains of Mice and their Characteristics", The Jackson Laboratory . Retrieved 30 January 2008.
- ^ Peichel CL (2005). "Fishing for the secrets of vertebrate evolution in threespine sticklebacks". Developmental Dynamics. 234 (4): 815–23. doi:10.1002/dvdy.20564. PMID 16252286.
- ^ Peichel CL, Nereng KS, Ohgi KA, Cole BL, Colosimo PF, Buerkle CA, Schluter D, Kingsley DM (2001). "The genetic architecture of divergence between threespine stickleback species" (PDF). Nature. 414 (6866): 901–05. Bibcode:2001Natur.414..901P. doi:10.1038/414901a. PMID 11780061. S2CID 4304296.
- ^ Ramaswamy S, McBride JL, Kordower JH (2007). "Animal models of Huntington's disease". ILAR Journal. 48 (4): 356–73. doi:10.1093/ilar.48.4.356. PMID 17712222.
- ^ Rees DA, Alcolado JC (2005). "Animal models of diabetes mellitus". Diabetic Medicine. 22 (4): 359–70. doi:10.1111/j.1464-5491.2005.01499.x. PMID 15787657.
- ^ Iwakuma T, Lozano G (2007). "Crippling p53 activities via knock-in mutations in mouse models". Oncogene. 26 (15): 2177–84. doi:10.1038/sj.onc.1210278. PMID 17401426.
- ^ Frese KK, Tuveson DA (2007). "Maximizing mouse cancer models". Nature Reviews Cancer. 7 (9): 645–58. doi:10.1038/nrc2192. PMID 17687385. S2CID 6490409.
- ^ Dunham SP (2006). "Lessons from the cat: development of vaccines against lentiviruses". Veterinary Immunology and Immunopathology. 112 (1–2): 67–77. doi:10.1016/j.vetimm.2006.03.013. PMID 16678276.
- ^ Vail DM, MacEwen EG (2000). "Spontaneously occurring tumors of companion animals as models for human cancer". Cancer Investigation. 18 (8): 781–92. doi:10.3109/07357900009012210. PMID 11107448. S2CID 32489790.
- ^ a b Tolwani RJ, Jakowec MW, Petzinger GM, Green S, Waggie K (1999). "Experimental models of Parkinson's disease: insights from many models". Laboratory Animal Science. 49 (4): 363–71. PMID 10480640.
- ^ Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I (2004). "Where is the evidence that animal research benefits humans?". BMJ. 328 (7438). Reviewing Animal Trials Systematically (RATS) Group: 514–47. doi:10.1136/bmj.328.7438.514. PMC 351856. PMID 14988196.
- ^ Langley, Gill (2006) next of kin...A report on the use of primates in experiments Archived 27 February 2008 at the Wayback Machine, BUAV.
- ^ The History of Deep Brain Stimulation Archived 31 March 2017 at the Wayback Machine. parkinsonsappeal.com
- ^ Platt JL, Lin SS (1998). "The future promises of xenotransplantation". Annals of the New York Academy of Sciences. 862 (1): 5–18. Bibcode:1998NYASA.862....5P. doi:10.1111/j.1749-6632.1998.tb09112.x. PMID 9928201. S2CID 72941995.
- ^ a b Schuurman HJ, Pierson RN (2008). "Progress towards clinical xenotransplantation". Frontiers in Bioscience. 13 (13): 204–20. doi:10.2741/2671. PMID 17981539.
- ^ Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, Elliott RB, Terán L, White DJ (2005). "Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study". European Journal of Endocrinology. 153 (3): 419–27. doi:10.1530/eje.1.01982. PMID 16131605.
- ^ Valdés-González RA, White DJ, Dorantes LM, Terán L, Garibay-Nieto GN, Bracho-Blanchet E, Dávila-Pérez R, Evia-Viscarra L, Ormsby CE, Ayala-Sumuano JT, Silva-Torres ML, Ramírez-González B (2007). "Three-yr follow-up of a type 1 diabetes mellitus patient with an islet xenotransplant". Clinical Transplantation. 21 (3): 352–57. doi:10.1111/j.1399-0012.2007.00648.x. PMID 17488384. S2CID 22668776.
- ^ Townsend, Mark (20 April 2003). "Exposed: secrets of the animal organ lab" , The Guardian.
- ^ Curtis, Polly (11 July 2003). "Home Office under renewed fire in animal rights row", The Guardian.
- ^ a b Household Product Tests Archived 27 February 2008 at the Wayback Machine BUAV
- ^ Fifth Report on the Statistics on the Number of Animals used for Experimental and other Scientific Purposes in the Member States of the European Union, Commission of the European Communities, published November 2007
- ^ a b c Abbott A (2005). "Animal testing: More than a cosmetic change" (PDF). Nature. 438 (7065): 144–46. Bibcode:2005Natur.438..144A. doi:10.1038/438144a. PMID 16281001. S2CID 4422086. Archived from the original (PDF) on 27 February 2008.
- ^ Watkins JB (1989). "Exposure of rats to inhalational anesthetics alters the hepatobiliary clearance of cholephilic xenobiotics". The Journal of Pharmacology and Experimental Therapeutics. 250 (2): 421–27. doi:10.1016/S0022-3565(25)22228-5. PMID 2760837.
- ^ Watt JA, Dickinson RG (1990). "The effect of diethyl ether, pentobarbitone and urethane anaesthesia on diflunisal conjugation and disposition in rats". Xenobiotica. 20 (3): 289–301. doi:10.3109/00498259009046848. PMID 2336839.
- ^ "Testing of chemicals – OECD". www.oecd.org. Retrieved 23 May 2022.
- ^ Walum E (1998). "Acute oral toxicity". Environmental Health Perspectives. 106 (Suppl 2): 497–503. doi:10.2307/3433801. JSTOR 3433801. PMC 1533392. PMID 9599698.
- ^ Inter-Governmental Organization Eliminates the LD50 Test, The Humane Society of the United States (2003-02-05)
- ^ "OECD guideline 405, Organisation for Economic Co-operation and Development" (PDF). Archived from the original (PDF) on 27 February 2008. Retrieved 6 April 2015.
- ^ Species Used in Research: Rabbit, Humane Society of the United States
- ^ Wilhelmus KR (2001). "The Draize eye test". Survey of Ophthalmology. 45 (6): 493–515. doi:10.1016/S0039-6257(01)00211-9. PMID 11425356.
- ^ Secchi A, Deligianni V (2006). "Ocular toxicology: the Draize eye test". Current Opinion in Allergy and Clinical Immunology. 6 (5): 367–72. doi:10.1097/01.all.0000244798.26110.00. PMID 16954791. S2CID 24972694.
- ^ a b Draize rabbit eye test replacement milestone welcomed. Dr Hadwen Trust (2009-09-21)
- ^ "Toxicity Testing for Assessment of Environmental Agents" National Academies Press, (2006), p. 21.
- ^ Hartung T (2009). "Toxicology for the twenty-first century". Nature. 460 (7252): 208–12. Bibcode:2009Natur.460..208H. doi:10.1038/460208a. PMID 19587762. S2CID 851143.
- ^ "Where is the toxicology for the twenty-first century?". Pro-Test Italia. 2013. Retrieved 30 January 2014.
- ^ Smith LL (2001). "Key challenges for toxicologists in the 21st century". Trends Pharmacol. Sci. 22 (6): 281–85. doi:10.1016/S0165-6147(00)01714-4. PMID 11395155.
- ^ Brown SL, Brett SM, Gough M, Rodricks JV, Tardiff RG, Turnbull D (1988). "Review of interspecies risk comparisons". Regul. Toxicol. Pharmacol. 8 (2): 191–206. doi:10.1016/0273-2300(88)90028-1. PMID 3051142.
- ^ Burden N, Sewell F, Chapman K (2015). "Testing Chemical Safety: What Is Needed to Ensure the Widespread Application of Non-animal Approaches?". PLOS Biol. 13 (5) e1002156. doi:10.1371/journal.pbio.1002156. PMC 4446337. PMID 26018957.
- ^ Moczko E, Mirkes EM, Cáceres C, Gorban AN, Piletsky S (2016). "Fluorescence-based assay as a new screening tool for toxic chemicals". Scientific Reports. 6 33922. Bibcode:2016NatSR...633922M. doi:10.1038/srep33922. PMC 5031998. PMID 27653274.
- ^ Stephens, Martin & Rowan, Andrew. An overview of Animal Testing Issues, Humane Society of the United States
- ^ "Cosmetics animal testing in the EU". Archived from the original on 30 December 2020. Retrieved 5 December 2018.
- ^ Engebretson, Monica (16 March 2014). "India Joins the EU and Israel in Surpassing the US in Cruelty-Free Cosmetics Testing Policy". The World Post.
- ^ "Cruelty Free International Applauds Congressman Jim Moran for Bill to End Cosmetics Testing on Animals in the United States" (Press release). 5 March 2014. Archived from the original on 18 March 2014.
- ^ Fox, Stacy (10 March 2014). "Animal Attraction: Federal Bill to End Cosmetics Testing on Animals Introduced in Congress" (Press release). Humane Society of the United States. Archived from the original on 11 March 2014.
- ^ a b Osborn, Andrew & Gentleman, Amelia."Secret French move to block animal-testing ban", The Guardian (19 August 2003). Retrieved 27 February 2008.
- ^ Mohan V (14 October 2014). "India bans import of cosmetics tested on animals". The Times of India. Retrieved 14 October 2014.
- ^ a b Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use, sub II. PERFORMANCE OF TESTS, A. Toxicity
- ^ Patronek G, Rauch A (1 January 2007). "Systematic review of comparative studies examining alternatives to the harmful use of animals in biomedical education". Journal of the American Veterinary Medical Association. 230 (1): 37–43. doi:10.2460/javma.230.1.37. PMID 17199490.
- ^ Hart L, Hart B, Wood M (2008). Why Dissection: Animal Use in Education. Westport: Greenwood Press. ISBN 978-0-313-32390-4.
- ^ Orlans B, Beauchamp T, Dresser R, Morton D, Gluck J (1998). The Human Use of Animals. Oxford University Press. pp. 213. ISBN 978-0-19-511908-4.
- ^ Downey M (25 June 2013). "Should students dissect animals or should schools move to virtual dissections?". The Atlanta Journal-Constitution. Retrieved 7 July 2015.
- ^ Pulla P (6 August 2014). "Dissections banned in Indian universities". Science. Retrieved 7 July 2015.
- ^ Shine N. "The Battle Over High School Animal Dissection". Pacific Standard. Retrieved 7 July 2015.
- ^ "Invertebrates in Education and Conservation Conference | Department of Neuroscience". Neurosci.arizona.edu. Archived from the original on 15 December 2018. Retrieved 6 April 2015.
- ^ Dalal R, Even M, Sandusky C, Barnard N (August 2005). "Replacement Alternatives in Education: Animal-Free Teaching" (Abstract from Fifth World Congress on Alternatives and Animal Use in the Life Sciences, Berlin). The Physicians Committee for Responsible Medicine. Archived from the original on 22 July 2014. Retrieved 9 April 2015.
- ^ "The NORINA database of alternatives". Oslovet.norecopa.no. Retrieved 6 April 2015.
- ^ "Welcome". Interniche.org. Retrieved 6 April 2015.
- ^ a b "Row over US mobile phone 'cockroach backpack' app". BBC News. 9 November 2013. Retrieved 9 November 2013.
- ^ Hamilton, Anita (1 November 2013). "Resistance is Futile: PETA Attempts to Halt the Sale of Remote-Controlled Cyborg Cockroaches". Time. Retrieved 10 November 2013.
- ^ Brook, Tom Vanden, "Brain Study, Animal Rights Collide", USA Today (7 April 2009), p. 1.
- ^ a b Kelly J (7 March 2013). "Who, What, Why: Does shooting goats save soldiers' lives?". BBC News Magazine.
- ^ Londoño E (24 February 2013). "Military is required to justify using animals in medic training after pressure from activists". The Washington Post. Archived from the original on 15 December 2013.
- ^ Vergakis B (14 February 2014). "Coast Guard reduces use of live animals in training". Archived from the original on 9 July 2015. Retrieved 7 July 2015.
- ^ Bender B (12 November 2014). "Military to curtail use of live animals in medical training". Boston Globe. Retrieved 7 July 2015.
- ^ Champaco B (15 August 2013). "PETA: Madigan Army Medical Center Has Stopped 'Cruel' Ferret-Testing". Patch. Retrieved 7 July 2015.
- ^ "Use of Laboratory Animals in Biomedical and Behavioral Research", Institute for Laboratory Animal Research, The National Academies Press, 1988 ISBN 0-309-07878-4.
- ^ Cooper, Sylvia (1 August 1999). "Pets crowd animal shelter" Archived 2 February 2014 at the Wayback Machine, The Augusta Chronicle.
- ^ "Science, Medicine, and Animals", Institute for Laboratory Animal Research, Published by the National Research Council of the National Academies 2004, p. 2
- ^ "About". Peta.org. Retrieved 6 April 2015.
- ^ "UK Legislation: A Criticism" (PDF). Archived from the original (PDF) on 25 June 2008. Retrieved 6 April 2015.
- ^ "FAQs: Vivisection" (PDF). British Union for the Abolition of Vivisection. Archived from the original (PDF) on 13 May 2015. Retrieved 6 April 2015.
- ^ "Biomedical Research: The Humane Society of the United States". Humanesociety.org. Archived from the original on 30 September 2020. Retrieved 6 April 2015.
- ^ "Animal Testing and Animal Experimentation Issues | Physicians Committee". Pcrm.org. Archived from the original on 23 July 2011. Retrieved 6 April 2015.
- ^ Rollin BE (2006). "The regulation of animal research and the emergence of animal ethics: A conceptual history" (PDF). Theoretical Medicine and Bioethics. 27 (4): 285–304. doi:10.1007/s11017-006-9007-8. PMID 16937023. S2CID 18620094. Archived from the original (PDF) on 8 October 2020. Retrieved 4 December 2019.
- ^ Riffkin R (18 May 2015). "In U.S., More Say Animals Should Have Same Rights as People". Gallup. Retrieved 7 July 2015.
- ^ Funk C, Rainie L (29 January 2015). "Public and Scientists' Views on Science and Society". Pew Research Center. Retrieved 7 July 2015.
- ^ Singer, Peter (ed.). "A Companion to Ethics". Blackwell Companions to Philosophy, 1991.
- ^ a b Chapter 14, Discussion of ethical issues, p . 244 Archived 28 September 2011 at the Wayback Machine in: The ethics of research involving animals Archived 29 April 2011 at the Wayback Machine at the Nuffield Council on Bioethics. Published 25 May 2005
- ^ George R. "Donald Watson 2002 Unabridged Interview" (PDF). Archived (PDF) from the original on 27 October 2019.
- ^ Rollin, Bernard E. (1998) "The moral status of animals and their use as experimental subjects," in Kuhse, Helga and Singer, Peter (eds.). "A Companion to Bioethics". Blackwell Publishing, ISBN 0-631-23019-X.
- ^ Bebarta V, Luyten D, Heard K (2003). "Emergency medicine animal research: does use of randomization and blinding affect the results?". Academic Emergency Medicine. 10 (6): 684–87. doi:10.1111/j.1553-2712.2003.tb00056.x. PMID 12782533.
- ^ Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA (2008). "Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality". Stroke. 39 (10): 2824–29. doi:10.1161/strokeaha.108.515957. PMID 18635842.
- ^ Sena E, Wheble P, Sandercock P, Macleod M (2007). "Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke". Stroke. 38 (2): 388–94. doi:10.1161/01.str.0000254462.75851.22. PMID 17204689.
- ^ Hirst JA, Howick J, Aronson J, Roberts N, Perera R, Koshiaris C, Heneghan C (2014). "The Need for Randomization in Animal Trials: An Overview of Systematic Reviews". PLOS ONE. 9 (6) e98856. Bibcode:2014PLoSO...998856H. doi:10.1371/journal.pone.0098856. PMC 4048216. PMID 24906117.
- ^ Van der Worp B, Sena E, Porritt M, Rewell S, O'Collins V, Macleod MR (2010). "Can Animal Models of Disease Reliably Inform Human Studies?". PLOS Med. 7 (3) e1000245. doi:10.1371/journal.pmed.1000245. PMC 2846855. PMID 20361020.
- ^ Gagneux P, Moore JJ, Varki A (2005). "The ethics of research on great apes". Nature. 437 (7055): 27–29. Bibcode:2005Natur.437...27G. doi:10.1038/437027a. PMID 16136111. S2CID 11500691.
- ^ Vermij P (2003). "Europe's last research chimps to retire". Nature Medicine. 9 (8): 981. doi:10.1038/nm0803-981b. PMID 12894144. S2CID 9892510.
- ^ St Fleur N (12 June 2015). "U.S. Will Call All Chimps 'Endangered'". The New York Times. Retrieved 7 July 2015.
- ^ Kaiser J (26 June 2013). "NIH Will Retire Most Research Chimps, End Many Projects". sciencemag.org. Retrieved 7 July 2015.
- ^ "Summary of House of Lords Select Committee on Animals in Scientific Procedures". UK Parliament. 24 July 2002. Retrieved 13 July 2012.
- ^ 韓国・食薬庁で「実験動物慰霊祭」挙行 Archived 29 August 2007 at the Wayback Machine
- ^ Huxley AF, Simmons RM (1971). "Proposed Mechanism of Force Generation in Striated Muscle". Nature. 233 (5321): 533–38. Bibcode:1971Natur.233..533H. doi:10.1038/233533a0. PMID 4939977. S2CID 26159256.
- ^ Gordon AM, Huxley AF, Julian FJ (1966). "The variation in isometric tension with sarcomere length in vertebrate muscle fibres". The Journal of Physiology. 184 (1): 170–92. doi:10.1113/jphysiol.1966.sp007909. PMC 1357553. PMID 5921536.
- ^ Ford LE, Huxley AF, Simmons RM (1985). "Tension transients during steady shortening of frog muscle fibres". The Journal of Physiology. 361 (1): 131–50. doi:10.1113/jphysiol.1985.sp015637. PMC 1192851. PMID 3872938.
- ^ Lutz GJ, Lieber RL (2000). "Myosin isoforms in anuran skeletal muscle: Their influence on contractile properties and in vivo muscle function". Microscopy Research and Technique. 50 (6): 443–57. doi:10.1002/1097-0029(20000915)50:6<443::AID-JEMT3>3.0.CO;2-5. PMID 10998635. S2CID 3477585.
- ^ Liber, R. L. (2002). Skeletal Muscle Structure, Function, and Plasticity: The Physiological Basis of Rehabilitation, 2nd ed. Lippincott Williams & Wilkins, ISBN 978-0-7817-3061-7.
- ^ Franklin, Ben A. (30 August 1987) "Going to Extremes for 'Animal Rights'", The New York Times.
- ^ Holden C (1986). "A pivotal year for lab animal welfare". Science. 232 (4747): 147–50. Bibcode:1986Sci...232..147H. doi:10.1126/science.3952503. PMID 3952503.
- ^ Laville, Sandra (8 February 2005). "Lab monkeys 'scream with fear' in tests", The Guardian.
- ^ "Columbia in animal cruelty dispute", CNN (2003-10-12)
- ^ Benz, Kathy and McManus, Michael (17 May 2005). PETA accuses lab of animal cruelty, CNN.
- ^ Scott, Luci (1 April 2006). "Probe leads to Covance fine", The Arizona Republic. Retrieved 8 March 2021.
- ^ Huggett B (2008). "When animal rights turns ugly". Nature Biotechnology. 26 (6): 603–05. doi:10.1038/nbt0608-603. PMID 18536673. S2CID 8006958.
- ^ Malone BJ, Kumar VR, Ringach DL (2007). "Dynamics of Receptive Field Size in Primary Visual Cortex". Journal of Neurophysiology. 97 (1): 407–14. CiteSeerX 10.1.1.133.3969. doi:10.1152/jn.00830.2006. PMID 17021020.
- ^ Epstein, David (22 August 2006). Throwing in the Towel Archived 27 November 2020 at the Wayback Machine, Inside Higher Education
- ^ Predators Unleashed, Investor's Business Daily (2006-08-24)
- ^ McDonald, Patrick Range (8 August 2007). UCLA Monkey Madness, LA Weekly.
- ^ "It's a Dog's Life", Countryside Undercover, Channel Four Television, UK (26 March 1997).
- ^ "It's a dog's life" Archived 8 March 2012 at the Wayback Machine, Small World Productions (2005). Retrieved 6 July 2010.
- ^ "A controversial laboratory". BBC News. 18 January 2001. Retrieved 13 July 2012.
- ^ Broughton, Zoe (March 2001). "Seeing Is Believing – cruelty to dogs at Huntingdon Life Sciences", The Ecologist.
- ^ "From push to shove" Archived 22 November 2009 at the Wayback Machine, Southern Poverty Law Group Intelligence Report, Fall 2002
- ^ Lewis, John E. "Statement of John Lewis", US Senate Committee on Environment and Public Works, 26 October 2005, accessed 17 January 2011.
- ^ Evers, Marco. "Resisting the Animal Avengers", Part 1, Part 2, Der Spiegel, 19 November 2007.
- ^ Weaver, Matthew. "Animal rights activists jailed for terrorising suppliers to Huntingdon Life Sciences", The Guardian, 25 October 2010.
- ^ Herbert, Ian (27 January 2007). "Collapse in support for animal rights extremist attacks", The Independent.
- ^ Knight A (May 2008). "Systematic reviews of animal experiments demonstrate poor contributions toward human healthcare". Reviews on Recent Clinical Trials. 3 (2): 89–96. doi:10.2174/157488708784223844. ISSN 1574-8871. PMID 18474018.
- ^ a b Greek R, Menache A (11 January 2013). "Systematic Reviews of Animal Models: Methodology versus Epistemology". International Journal of Medical Sciences. 10 (3): 206–21. doi:10.7150/ijms.5529. ISSN 1449-1907. PMC 3558708. PMID 23372426.
- ^ a b c Bracken MB (1 March 2009). "Why animal studies are often poor predictors of human reactions to exposure". Journal of the Royal Society of Medicine. 102 (3): 120–22. doi:10.1258/jrsm.2008.08k033. ISSN 0141-0768. PMC 2746847. PMID 19297654.
- ^ Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I (28 February 2004). "Where is the evidence that animal research benefits humans?". BMJ: British Medical Journal. 328 (7438): 514–17. doi:10.1136/bmj.328.7438.514. ISSN 0959-8138. PMC 351856. PMID 14988196.
- ^ Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS (25 January 2007). "Comparison of treatment effects between animal experiments and clinical trials: systematic review". BMJ. 334 (7586): 197. doi:10.1136/bmj.39048.407928.BE. ISSN 0959-8138. PMC 1781970. PMID 17175568.
- ^ Schulz KF, Chalmers I, Altman DG (5 February 2002). "The Landscape and Lexicon of Blinding in Randomized Trials". Annals of Internal Medicine. 136 (3): 254–59. doi:10.7326/0003-4819-136-3-200202050-00022. ISSN 0003-4819. PMID 11827510. S2CID 34932997.
- ^ Triunfol M, Gouveia FC (15 June 2021). Bero L (ed.). "What's not in the news headlines or titles of Alzheimer disease articles? #InMice". PLOS Biology. 19 (6) e3001260. doi:10.1371/journal.pbio.3001260. ISSN 1545-7885. PMC 8205157. PMID 34129637.
- ^ "White Coat Waste Project". Retrieved 8 March 2022.
- ^ "Should dogs be guinea pigs in government research? A bipartisan group says no". The Washington Post. 15 November 2016.
- ^ "WCW EXPOSÉ: FAUCI SPENT $424K ON BEAGLE EXPERIMENTS, DOGS BITTEN TO DEATH BY FLIES". 30 July 2021.
- ^ "PETA calls for Dr. Fauci to resign: 'Our position is clear'". Fox News. 5 November 2021.
- ^ "Experimenters Fed Puppies' Heads to Infected Flies, but That's Not All Fauci's NIH Funded". 25 October 2021.
- ^ a b Croce, Pietro. Vivisection or Science? An Investigation into Testing Drugs and Safeguarding Health. Zed Books, 1999, ISBN 1-85649-732-1 p. 11.
- ^ a b Bernard, Claude An Introduction to the Study of Experimental Medicine, 1865. First English translation by Henry Copley Greene, published by Macmillan & Co., Ltd., 1927; reprinted in 1949, p. 125.
- ^ Ryder, Richard D. (2000). Animal Revolution: Changing Attitudes Towards Speciesism. Berg Publishers, p. 54 ISBN 1-85973-330-1.
- ^ a b c "Animal Experimentation: A Student Guide to Balancing the Issues", Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART), accessed 12 December 2007, cites original reference in Maehle, A-H. and Tr6hler, U. Animal experimentation from antiquity to the end of the eighteenth century: attitudes and arguments. In N. A. Rupke (ed.) Vivisection in Historical Perspective. Croom Helm, London, 1987, p. 22.
- ^ Rudacille, Deborah (2000). The Scalpel and the Butterfly: The Conflict, University of California Press, p. 19 ISBN 0-520-23154-6.
- ^ "In sickness and in health: vivisection's undoing", The Daily Telegraph, November 2003
- ^ LaFollette, H., Shanks, N., Animal Experimentation: the Legacy of Claude Bernard Archived 10 January 2020 at the Wayback Machine, International Studies in the Philosophy of Science (1994) pp. 195–210.
- ^ Nicoll CS (1991). "A Physiologist's Views on the Animal Rights/Liberation Movement". The Physiologist. 34 (6): 303, 306–08, 315. PMID 1775539.
- ^ Mason, Peter. The Brown Dog Affair Archived 6 October 2020 at the Wayback Machine. Two Sevens Publishing, 1997.
- ^ "The Life and Letters of Charles Darwin, Volume II". Fullbooks.com.
- ^ Bowlby, John (1991). Charles Darwin: A New Life, W. W. Norton & Company, p. 420 ISBN 0-393-30930-4.
- ^ Ilman J (2008). Animal Research in Medicine: 100 years of politics, protest and progress. The Story of the Research Defence Society. Research Defence Society. p. 16. ISBN 978-0-9560008-0-4.
- ^ Publications of the Research Defence Society: March 1908–1909; Selected by the committee. London: Macmillan. 1909. p. xiv.
- ^ Buettinger, Craig (1 January 1993) Antivivisection and the charge of zoophil-psychosis in the early twentieth century. The Historian.
- ^ "What are the 3Rs?". NC3Rs. Archived from the original on 1 August 2014. Retrieved 16 December 2018.
- ^ Kolar R (2002). "ECVAM: desperately needed or superfluous? An animal welfare perspective". Altern Lab Anim. 30 (Suppl 2): 169–74. doi:10.1177/026119290203002S26. PMID 12513669.
- ^ Schuppli CA, Fraser D, McDonald M (2004). "Expanding the three Rs to meet new challenges in humane animal experimentation". Altern Lab Anim. 32 (5): 525–32. doi:10.1177/026119290403200507. PMID 15656775. S2CID 25015151.
- ^ Rusche B (2003). "The 3Rs and animal welfare – conflict or the way forward?". ALTEX. 20 (Suppl 1): 63–76. PMID 14671703.
- ^ Alexander FA, Eggert S, Wiest J (February 2018). "Skin-on-a-Chip: Transepithelial Electrical Resistance and Extracellular Acidification Measurements through an Automated Air-Liquid Interface". Genes. 9 (2): 114. doi:10.3390/genes9020114. PMC 5852610. PMID 29466319.
- ^ "Alternatives to Animal Testing | Animals Used for Experimentation | The Issues". Peta.org. 21 June 2010. Retrieved 6 April 2015.
- ^ Rhodes M (28 May 2015). "Inside L'Oreal's Plan to 3-D Print Human Skin". Wired. Retrieved 7 July 2015.
- ^ Watts G (27 January 2007). "Alternatives to animal experimentation". BMJ. 334 (7586): 182–84. doi:10.1136/bmj.39058.469491.68. PMC 1782004. PMID 17255608.
- ^ Edelman L, Eddy J, Price N (July–August 2010). "In silico models of cancer". Wiley Interdiscip Rev Syst Biol Med. 2 (4): 438–59. doi:10.1002/wsbm.75. PMC 3157287. PMID 20836040.
- ^ "Microdosing". 3Rs. Canadian Council on Animal Care in Science. Archived from the original on 7 June 2015. Retrieved 7 July 2015.
- ^ "What Is A PET Scan? How Does A PET Scan Work?". Medicalnewstoday.com. 23 June 2017.
- ^ Jiang J, Liu B, Nasca PC, Han W, Zou X, Zeng X, Tian X, Wu Y, Zhao P, Li J (2009). "Comparative study of control selection in a national population -based case-control study: Estimating risk of smoking on cancer deaths in Chinese men". International Journal of Medical Sciences. 6 (6): 329–37. doi:10.7150/ijms.6.329. PMC 2777271. PMID 19918375.
- ^ McNeil D (13 January 2014). "PETA's Donation to Help Save Lives, Animal and Human". The New York Times. Retrieved 7 July 2015.
- ^ Bernstein F (4 October 2005). "An On-Screen Alternative to Hands-On Dissection". The New York Times. Retrieved 7 July 2015.
- ^ "NTP Interagency Center for the Evaluation of Alternative Toxicological Methods – NTP". Iccvam.niehs.nih.gov. Archived from the original on 9 December 2013. Retrieved 6 April 2015.
- ^ ZEBET database on alternatives to animal experiments on the Internet (AnimAlt-ZEBET). BfR (30 September 2004). Retrieved on 2013-01-21.
- ^ About JaCVAM-Organization of JaCVAM Archived 11 May 2012 at the Wayback Machine. Jacvam.jp. Retrieved on 2013-01-21.
- ^ EPAA – Home Archived 1 November 2013 at the Wayback Machine. Ec.europa.eu. Retrieved on 2013-01-21.
- ^ ecopa – european consensus-platform for alternatives. Ecopa.eu. Retrieved on 2013-01-21.
- ^ Center for Alternatives to Animal Testing – Johns Hopkins Bloomberg School of Public Health. Caat.jhsph.edu. Retrieved on 2013-01-21.
- ^ "NC3Rs". NC3Rs.org.uk. Retrieved 6 April 2015.
Works cited
[edit]- Carbone L (2004). What animals want: expertise and advocacy in laboratory animal welfare policy. Oxford, UK: Oxford University Press. ISBN 978-0-19-972188-7. OCLC 57138138.
Further reading
[edit]- Carrie Friese (2025). A Mouse in a Cage: Rethinking Humanitarianism and the Rights of Lab Animals. New York University Press. ISBN 978-1-4798-3350-4.
- Guerrini, Anita (2003). Experimenting with humans and animals: from Galen to animal rights. Baltimore: The Johns Hopkins University Press. ISBN 978-0-8018-7197-9.
- 15 Companies That Still Test on Animals in 2022. Yahoo! Finance. 9 January 2023.
External links
[edit] Media related to Animal testing at Wikimedia Commons
Media related to Animal testing at Wikimedia Commons Quotations related to Animal testing at Wikiquote
Quotations related to Animal testing at Wikiquote
Animal testing
View on GrokipediaAnimal testing, also termed animal experimentation, entails the controlled use of non-human animals in scientific procedures to evaluate the safety, efficacy, and mechanisms of action of pharmaceuticals, medical treatments, biological processes, and consumer products.[1][2] This practice spans biomedical research, toxicology testing, and behavioral studies, employing species such as rodents, primates, dogs, and fish to model human physiology and disease states where ethical or practical constraints preclude direct human experimentation.[3][4] Globally, an estimated 100 to 150 million animals are utilized annually in laboratory settings, with the majority comprising mice and rats excluded from many regulatory reporting requirements, though precise figures remain uncertain due to incomplete international data collection.[5] Animal testing has underpinned key medical breakthroughs, including the refinement of insulin therapy through canine pancreatic studies, polio vaccine development via primate trials, and advancements in cardiovascular surgery modeled on animal anatomies, contributing to prolonged human lifespans and reduced disease mortality.[6][2] Notwithstanding these empirical successes, the methodology faces persistent scrutiny for inducing verifiable pain, distress, and mortality in subjects, prompting ethical frameworks like the 3Rs principle (replacement, reduction, refinement) to minimize harm, alongside causal critiques highlighting frequent translational failures—wherein physiological divergences between species yield drugs effective in animals but inefficacious or toxic in humans, as evidenced by high clinical trial attrition rates.[7][8][9] Regulatory bans on non-essential uses, such as cosmetics testing in regions including the European Union, reflect growing advocacy for alternatives like organ-on-chip technologies and computational modeling, though their scalability for complex systemic effects remains under validation.[10]
Definitions and Scope
Core Definition and Terminology
Animal testing, also termed animal experimentation or in vivo research, constitutes the utilization of non-human animals as subjects in controlled scientific procedures to investigate physiological mechanisms, evaluate therapeutic interventions, or assess potential hazards of substances. This practice encompasses a spectrum of methodologies, from behavioral observations to invasive interventions, primarily aimed at advancing biomedical knowledge applicable to human health, though also extending to veterinary, toxicological, and basic biological inquiries.[2][4][11] Central terminology distinguishes animal testing from narrower historical concepts like vivisection, which denotes surgical dissection or manipulation of living animals, often without anesthesia, to observe internal functions in real time—a method rooted in 19th-century physiology but now largely supplanted by refined protocols under modern welfare standards. In contrast, contemporary animal testing broadly includes non-surgical assays, such as pharmacological dosing or genetic modeling, conducted under ethical oversight to minimize distress. Key related terms include "model organisms," referring to species like rodents or zebrafish selected for their physiological similitude to humans and genetic tractability, and "in vivo" testing, denoting experiments within intact living systems as opposed to in vitro cellular studies.[12][3] Regulatory glossaries further delineate terms such as "animal procedure," defined as any intervention on a vertebrate that may cause pain, suffering, or distress exceeding routine husbandry, thereby triggering institutional review by bodies like Institutional Animal Care and Use Committees (IACUCs). The 3Rs framework—replacement (substituting non-animal models where feasible), reduction (minimizing animal numbers via statistical optimization), and refinement (enhancing procedures to lessen severity)—serves as a foundational ethical paradigm, originating from Russell and Burch's 1959 principles and embedded in global guidelines. These terms underscore the field's emphasis on empirical validation while navigating biological complexities inherent to extrapolating from animal models to human outcomes.[13][14]Types of Animal Testing
Basic biomedical research constitutes the largest category of animal testing, involving experiments to uncover fundamental physiological, genetic, and pathological processes. These studies often use rodents such as mice and rats to model cellular functions, organ development, or disease onset, with approximately 60-70% of U.S. laboratory animals dedicated to such purposes annually.[1] For instance, knockout mice genetically engineered to lack specific genes help identify protein roles in metabolism, contributing to over 10 million such animals used yearly worldwide.[15] This category relies on empirical observation of causal mechanisms, like how neural pathways respond to stimuli in controlled settings.[16] Toxicological testing evaluates the safety of chemicals, drugs, and environmental agents by assessing dose-response relationships in animals, typically through acute, subchronic, or chronic exposure protocols. Regulatory bodies like the FDA mandate such tests prior to human trials, using species like rats for LD50 determinations—measuring lethal doses for 50% of subjects—or rabbits for dermal irritation. In the U.S., this accounts for about 10-20% of animal use, with over 1 million rodents involved in chemical safety assessments in 2023.[17] These experiments prioritize causal inference from observed toxicities, such as organ damage from repeated dosing, though interspecies extrapolation to humans remains imperfect due to metabolic variances.[18] Preclinical efficacy testing assesses potential therapeutic interventions, including vaccines and biologics, by inducing disease states in animals and measuring outcomes like survival rates or symptom reduction. Dogs and nonhuman primates are common for cardiovascular or infectious disease models, as seen in the development of mRNA vaccines where rodents confirmed immune responses before primate validation. This type comprises roughly 15% of procedures, with efficacy endpoints grounded in quantifiable biomarkers rather than subjective welfare metrics.[19] Xenotransplantation research, a subset, tests cross-species organ viability, using pigs genetically modified for human compatibility, with ongoing trials reporting rejection rates above 50% in early 2025 models.[20] Behavioral and neuroscientific testing explores cognitive, sensory, and psychological responses, often employing conditioning paradigms in species like rats or zebrafish to map neural circuits. Techniques such as the Morris water maze quantify spatial learning deficits in aged or diseased animals, underpinning studies on Alzheimer's analogs where hippocampal lesions mimic memory loss. These account for under 10% of total use but provide causal insights into brain-behavior links, with data from over 500,000 rodents in such assays annually.[21] Regulatory and educational testing, including surgical training on anesthetized animals, forms a minor category, emphasizing procedural refinement over novel discovery.[22]Historical Development
Pre-Modern and Early Scientific Use
Animal experimentation originated in ancient civilizations, primarily for anatomical and physiological inquiry without modern ethical or methodological frameworks. In Greece during the 4th and 3rd centuries BCE, Aristotle systematically dissected diverse species, including fish and invertebrates, to develop theories of biological classification and function, marking early comparative anatomy efforts.[23] Erasistratus, working in Alexandria around 300 BCE, conducted vivisections on living animals to explore nerve impulses, valve functions in veins, and the distinction between arteries and veins, laying groundwork for physiological understanding.[24][25] In the Roman era, Galen (c. 129–c. 216 CE) extensively employed animal vivisections, as human dissection was prohibited, performing procedures on pigs, apes, goats, and dogs nearly daily to map anatomical systems. Notable experiments included severing the recurrent laryngeal nerve in live pigs to demonstrate its role in phonation and sectioning spinal cords to study paralysis, influencing Western medicine profoundly despite interspecies anatomical variances.[26][27] Medieval Europe saw diminished animal experimentation, constrained by Christian prohibitions against dissection reflecting divine creation, though Islamic scholars like Avicenna referenced Galenic animal-based knowledge in medical texts. The Renaissance revived direct observation, with Andreas Vesalius (1514–1564) dissecting animals alongside humans to rectify Galen's ape-derived errors, such as cardiac structure discrepancies, via parallel comparative methods.[28][29][30] Early scientific advancements in the 17th century integrated vivisection into mechanistic physiology. William Harvey (1578–1657) vivisected cold- and warm-blooded animals, ligating vessels and incising hearts in live subjects to quantify blood flow, culminating in his 1628 demonstration of systemic circulation against Galenic teleology.[31] Such practices extended to pneumatic experiments, where researchers like Robert Boyle used birds in air pumps to test respiration under reduced pressure, revealing gas dependencies in living systems.[32]19th and 20th Century Milestones
In the early 19th century, François Magendie pioneered systematic vivisection on animals such as dogs and frogs to elucidate physiological functions, laying the groundwork for modern experimental physiology.[33] This approach emphasized direct observation of living tissues over speculative anatomy, influencing subsequent researchers despite controversy over animal suffering. Claude Bernard, building on Magendie's methods, conducted extensive experiments on rabbits, dogs, and other species to study glycogenesis and the role of the pancreas in digestion, establishing the milieu intérieur concept.[34] In his 1865 treatise An Introduction to the Study of Experimental Medicine, Bernard defended vivisection as essential for verifying hypotheses, arguing that ethical qualms should not impede scientific progress grounded in observable causation. These works shifted animal testing from anecdotal to rigorous, hypothesis-driven inquiry, enabling causal insights into metabolic processes. Mid-century advancements included Louis Pasteur's 1881 demonstration of anthrax vaccination efficacy in sheep through controlled inoculation experiments, validating germ theory applications and reducing livestock mortality from 25% to near zero in treated herds.[35] Regulatory responses emerged with the 1876 Cruelty to Animals Act in Britain, the first law licensing vivisections and mandating inspections, prompted by public outcry over unregulated procedures yet preserving research utility.[36] By century's end, selective breeding of rodents for consistency began, with William Castle initiating mouse strains in 1902 to minimize genetic variability in studies.[37] The 20th century saw Ivan Pavlov's experiments on dogs from the 1890s, culminating in his 1904 Nobel Prize for elucidating digestive secretions and conditional reflexes; by surgically creating fistulas, he quantified salivary responses to stimuli, revealing learned associations independent of conscious intent.[38] In 1921, Frederick Banting and Charles Best isolated insulin via canine pancreatic extracts, reversing hyperglycemia in depancreatized dogs and enabling human treatment trials by 1922, a breakthrough confirmed through repeated survival extensions from days to months.[39] The 1937 Elixir Sulfanilamide tragedy, killing over 100 humans due to untested solvents, spurred U.S. mandates for animal toxicity screening, formalizing preclinical trials.[2] Post-World War II, Jonas Salk's inactivated polio vaccine development in the 1950s relied on rhesus monkey kidney cells for virus propagation and efficacy testing; over 9,000 primates were used in safety validations, contributing to the vaccine's 1955 licensure and subsequent U.S. case drop from 35,000 annually to near eradication.[40] Clarence Little's inbred mouse strains from 1909 facilitated genetic and cancer research, standardizing models for reproducibility.[37] These milestones underscored animal models' role in causal validation, from reflex arcs to vaccine attenuation, though debates persisted on translatability to human physiology.Post-WWII Expansion and Ethical Awakening
Following World War II, animal testing expanded dramatically due to increased government funding for biomedical research, particularly in the United States and United Kingdom, driven by advancements in pharmaceuticals, vaccines, and public health initiatives. The U.S. Public Health Service Act of 1944 laid groundwork for federal support, but the post-war period saw a surge in laboratory animal use as research institutions proliferated. In the UK, the number of scientific procedures on animals rose steadily from about 1 million in 1939 to a peak of approximately 5.5 million by the mid-1970s. This expansion paralleled the growth of the pharmaceutical industry and efforts to develop treatments for diseases like polio and cancer, with rodents, dogs, and primates becoming standard models.[33][41] Ethical concerns began to intensify in the 1950s and 1960s amid reports of poor laboratory conditions and public outrage over incidents such as the theft and sale of pets for research. These pressures culminated in the U.S. Animal Welfare Act of 1966, signed into law by President Lyndon B. Johnson on August 24, which established the first federal standards for the care of animals used in research, exhibition, and transport, initially covering dogs, cats, nonhuman primates, guinea pigs, hamsters, and rabbits but excluding rodents and birds. The Act was prompted by investigations revealing inadequate oversight and aimed to ensure humane handling, housing, and veterinary care, though enforcement initially relied on voluntary compliance by the U.S. Department of Agriculture. In the UK, similar unease grew, influenced by broader animal welfare advocacy.[42][43][44] The 1960s marked a pivotal "ethical awakening" with publications exposing animal suffering, extending concerns from factory farming to laboratory practices. Ruth Harrison's 1964 book Animal Machines detailed the dehumanizing conditions of intensive animal production, prompting the UK government's Brambell Committee investigation and the 1965 Report, which recommended welfare standards influencing subsequent legislation like the Agriculture Act 1968. Although focused on farming, it heightened public sensitivity to institutionalized animal exploitation, including in research. By 1975, philosopher Peter Singer's Animal Liberation argued against speciesism in experimentation, equating animal suffering to human moral considerations and catalyzing the modern animal rights movement, including organizations like PETA founded in 1980. These works shifted discourse from mere regulation to questioning the moral justification of animal use, leading to increased protests and demands for alternatives.[45][46][47]Scientific Foundations
Rationale for Using Animals in Research
Animals provide biologically relevant models for studying human physiology, disease mechanisms, and therapeutic responses due to shared genetic, anatomical, and physiological traits across mammalian species. For instance, rodents like mice exhibit approximately 85-95% genetic homology with humans, enabling researchers to investigate complex biological processes such as organ function, immune responses, and metabolic pathways in intact living systems that in vitro methods cannot replicate.[4][48] This homology facilitates causal inference about disease etiology and intervention efficacy, as animal models allow observation of dynamic, whole-organism interactions—including behavioral, neurological, and systemic effects—that isolated cell cultures or computational simulations often fail to capture accurately.[49][50] In drug development, animal testing is employed to assess pharmacokinetics, toxicity, and efficacy prior to human trials, addressing uncertainties that alternatives like organ-on-chip technologies or AI-driven predictions cannot fully resolve due to their inability to model long-term, multi-organ effects or individual variability. Regulatory frameworks, such as those from the U.S. Food and Drug Administration, mandate preclinical animal studies to minimize human risk, as evidenced by requirements under the Federal Food, Drug, and Cosmetic Act for demonstrating safety in at least two species before advancing to clinical phases.[51][52] Historical precedents underscore this utility: the development of insulin in 1921 via canine pancreatic extracts and the polio vaccine in the 1950s through primate and rodent trials prevented millions of human deaths, outcomes unattainable without in vivo validation.[53][54] Ethically, using animals aligns with principles of minimizing harm by prioritizing non-human subjects for initial safety assessments, given that direct human experimentation would violate non-maleficence without preliminary data on adverse effects like carcinogenicity or teratogenicity.[51][7] While non-animal alternatives, such as 3D tissue models, have advanced—reducing animal use in some toxicity screens by up to 30% in targeted assays—they remain supplementary rather than substitutive for systemic studies, as they lack vascularization, immune integration, and adaptive responses essential for predicting clinical translation.[55][56] Peer-reviewed analyses confirm that animal models, despite translational limitations (e.g., 90% of promising candidates failing in humans), provide indispensable empirical grounding for causal realism in biomedical advancement, outperforming alternatives in validating therapies for conditions like cardiovascular disease and cancer.[53][54]Model Organisms and Species Selection
Model organisms are non-human species selected for scientific research due to characteristics that facilitate the study of biological processes relevant to human health, such as genetic tractability, physiological similarities to humans, short reproductive cycles, and ease of maintenance in laboratory settings.[57] Selection criteria prioritize organisms that allow for reproducible experimentation, including the ability to induce specific pathologies or manipulate genes while minimizing variables like cost and ethical concerns associated with higher vertebrates.[58] These criteria ensure that findings can be generalized cautiously to humans, though translational success varies; for instance, genetic homology with humans ranges from about 60% in fruit flies to over 90% in mice.[59] Rodents, particularly mice and rats, dominate animal testing, comprising approximately 85-92% of vertebrates used in research globally.[1] [41] Mice (Mus musculus), such as the C57BL/6 strain, are favored for their small size, rapid breeding (gestation ~20 days, litters of 6-10), well-characterized genomes enabling CRISPR knockouts, and inbred lines that reduce genetic variability for consistent phenotypes.[60] Rats (Rattus norvegicus), like the Wistar strain developed in 1906, offer larger size for surgical procedures and behavioral studies, with similar advantages in genetic tools and disease modeling for conditions like hypertension or neurodegeneration.[54] These mammals provide physiological closeness to humans, including comparable immune systems and organ functions, justifying their prevalence despite limitations in replicating complex human behaviors.[61] Invertebrate models like the fruit fly (Drosophila melanogaster) and nematode (Caenorhabditis elegans) are selected for foundational genetic and developmental research due to their simplicity, short generation times (10 days for flies, 3 days for worms), and prolific reproduction (thousands of offspring per generation).[62] Drosophila has been instrumental since Thomas Hunt Morgan's 1910 chromosome theory work, with advanced tools like GAL4/UAS for targeted gene expression, though its distant phylogeny limits direct human disease applicability.[63] Zebrafish (Danio rerio) bridge invertebrates and mammals as a vertebrate model, valued for transparent embryos allowing real-time imaging of development, external fertilization yielding hundreds of embryos per clutch, and genetic similarity (70-80% human orthologs) for high-throughput screening of drugs or mutants.[64] These organisms enable rapid hypothesis testing at lower ethical and financial costs compared to mammals.[65] Larger species such as dogs, pigs, and non-human primates are chosen selectively when mammalian physiology, size, or cognitive complexity is essential, often for preclinical safety testing or neurology. Dogs, particularly beagles, have been used since ancient times for cardiovascular and toxicology studies due to their cooperative temperament and similar drug metabolism to humans, though they represent under 1% of U.S. research animals (e.g., 3,770 in recent EU data).[66] [67] Pigs model human anatomy for xenotransplantation and surgical trials owing to organ size similarity. Non-human primates, like macaques, account for about 0.1-0.2% of procedures but are critical for brain research due to 93-98% genetic overlap and advanced neural structures; their use is restricted by regulations emphasizing the 3Rs (replacement, reduction, refinement) amid ethical scrutiny.[68] [69] Selection balances scientific necessity against welfare, with alternatives pursued where feasible, as primate models have contributed to vaccines like polio but face criticism for variable predictivity to human outcomes.[70]Procedures and Welfare Standards
Laboratory Protocols and Handling
Laboratory protocols for handling animals in research facilities mandate standardized operating procedures (SOPs) to ensure consistency, minimize distress to animals, and protect personnel from injury or zoonotic transmission. These protocols, outlined in institutional guidelines aligned with the Guide for the Care and Use of Laboratory Animals, require trained personnel to approach animals calmly and confidently, avoiding sudden movements that could induce fear or flight responses, thereby reducing physiological stress indicators like elevated heart rates or cortisol levels.[71][72][73] Species-specific techniques are employed to prevent injury; for mice, traditional tail-lift methods are supplemented or replaced by low-stress alternatives such as tunnel handling or cupped support, which studies demonstrate lower anxiety responses compared to restraint by scruff or tail alone.[74][73] Rats are typically grasped by the loose skin at the nape or supported under the thorax and pelvis to avoid vertebral damage from tail pulling. Larger species, including rabbits or guinea pigs, require firm but gentle restraint by the scruff and hindquarters, while primates or dogs necessitate specialized devices like squeeze-back cages or pole-and-collar systems to facilitate safe transfer without bites or escapes.[72][75][76] Personal protective equipment (PPE), such as gloves, lab coats, and masks, is standard to mitigate allergen exposure, scratches, or pathogen transfer, particularly in facilities handling immunocompromised models or infectious agents. For invasive procedures, animals are often anesthetized using inhalants like isoflurane or injectables like ketamine, with monitoring of vital signs via pulse oximetry or capnography to maintain depth of anesthesia and prevent overdose. SOPs also incorporate post-handling observations for signs of distress, such as piloerection or hunching, prompting immediate adjustments or veterinary consultation.[71][77][78] Training programs, often coordinated by designated laboratory animal coordinators, certify staff in these techniques through hands-on sessions and competency assessments, ensuring compliance with oversight bodies like Institutional Animal Care and Use Committees (IACUCs). Protocols extend to transport within facilities, using ventilated carriers lined with absorbent bedding to control ammonia buildup and temperature fluctuations, with records maintained for traceability in auditing.[74][79][71]Pain, Suffering, and Mitigation Measures
Laboratory animals can experience pain and distress from procedures such as surgery, injections, or disease modeling, with rodents like mice and rats showing measurable behavioral indicators including reduced activity, vocalization, and facial grimacing.[80] Pain assessment relies on validated tools like the Mouse Grimace Scale and Rat Grimace Scale, which score orbital tightening, ear position, and whisker changes to quantify acute pain severity objectively, outperforming subjective observations alone.[81] These methods, developed from peer-reviewed studies, confirm pain responses in models like hot-plate tests where rodents withdraw paws from heated surfaces at temperatures above 42°C, mimicking nociceptive pathways relevant to human conditions.[82] Suffering extends beyond pain to include psychological distress from isolation or restraint, assessed via burrowing cessation or nesting impairment in rodents.[83] In the United States, the Animal Welfare Act classifies procedures into five pain/distress categories reported annually to the USDA: Category A (no pain/distress, e.g., breeding); B (momentary or slight pain, e.g., injections); C (minor pain relieved by anesthetics/analgesics); D (pain/distress relieved appropriately); and E (unrelieved pain/distress, permitted only when scientifically justified and unavoidable, comprising less than 5% of regulated procedures as of 2022 data).[84] [85] Similar severity classifications exist in the European Union under Directive 2010/63/EU, mandating prospective harm-benefit analysis and retrospective severity grading from non-recovery to severe.[86] These frameworks require institutional oversight, such as Institutional Animal Care and Use Committees (IACUCs), to approve protocols minimizing unrelieved suffering. Mitigation follows the 3Rs principle, with Refinement emphasizing techniques to minimize pain, such as pre-emptive multimodal analgesia combining opioids like buprenorphine (effective for 8-12 hours in rodents at 0.03-0.1 mg/kg subcutaneously) and NSAIDs to target inflammatory pathways.[87] [88] Humane endpoints—early intervention criteria like >20% body weight loss or persistent grimace scores—prevent escalation to severe distress, supported by evidence that timely euthanasia reduces cumulative suffering.[89] Long-acting formulations, such as lipid-encapsulated buprenorphine, extend relief up to 72 hours, reducing handling stress and improving welfare in post-surgical recovery.[90] Despite these measures, challenges persist: surveys indicate 92% of researchers use analgesics for surgical mice but only 34% for non-surgical models, with incomplete pain reporting in up to 25% of publications suggesting potential under-mitigation.[91] [92] Injectable over oral routes prove more effective for post-procedure pain, as demonstrated in craniotomy models where grimacing persisted without intervention.[93] Empirical data affirm that unmitigated pain alters physiological baselines, confounding research outcomes, thus reinforcing causal incentives for rigorous application of refinement strategies.[94]Sourcing, Housing, and Euthanasia Practices
Laboratory animals are primarily sourced from licensed commercial breeders or purpose-bred in institutional colonies to ensure genetic uniformity, pathogen-free status, and traceability, with over 95% of rodents used in U.S. research being purpose-bred.[52] Procurement must comply with the Animal Welfare Act, requiring animals from USDA-registered Class A dealers who maintain health records and transport standards, while avoiding random sources like pet stores to prevent disease introduction.[43] [71] Institutions implement quarantine upon arrival, typically 1-2 weeks, for veterinary health checks, with genetically modified strains requiring detailed pedigree documentation and periodic genetic monitoring.[71] For nonhuman primates, sourcing is restricted to purpose-bred colonies under regulations like the Endangered Species Act and CDC import rules, prohibiting most wild-caught imports since the 1970s to reduce zoonotic risks and ethical concerns.[95] [96] Housing standards, outlined in the 2011 NIH Guide for the Care and Use of Laboratory Animals, mandate species-specific environmental parameters to support physiological needs and minimize stress, including temperature ranges of 20-26°C for rodents and 18-29°C for primates, relative humidity of 30-70%, and 10-15 fresh air changes per hour.[97] [71] Minimum floor space varies by body weight and group size; for example, mice under 10g require at least 38.7 cm² per animal in groups, while adult rats over 500g need 451.5 cm², with cage heights ensuring postural freedom (e.g., 12.7 cm for mice).[71] Social housing is preferred for gregarious species like rodents and primates to promote natural behaviors, supplemented by environmental enrichment such as nesting materials, perches, or foraging devices, though single housing is permitted with IACUC justification for research compatibility.[71] Facilities must feature durable, sanitizable enclosures separated by species to prevent cross-contamination, with lighting cycles of 12 hours light/dark at 130-325 lux.[71] USDA inspections under the Animal Welfare Act enforce primary enclosure compliance, though coverage excludes rats and mice bred for research, comprising over 90% of U.S. lab animals.[43] [98] Euthanasia practices adhere to the AVMA Guidelines for the Euthanasia of Animals (2020 edition), prioritizing methods that induce rapid unconsciousness and death with minimal pain or distress, performed by trained personnel under veterinary oversight.[99] For rodents, carbon dioxide inhalation remains common, delivered via gradual chamber displacement at 30-70% volume per minute to achieve >70% concentration, followed by a 10-minute post-arrest observation, though it induces aversion from carbonic acid formation and requires adjunctive confirmation of death like cervical dislocation.[99] [100] Injectable barbiturates (e.g., pentobarbital at 60-100 mg/kg IV or IP) provide alternatives for precise dosing, especially in anesthetized animals, while physical methods like decapitation suit neonates or tissue harvest needs.[99] Rabbits typically receive IV barbiturates or CO2, primates anesthetic overdose via injection due to handling risks, and fish immersion in buffered tricaine methanesulfonate (250-500 mg/L) with pithing adjunct.[99] Protocols must specify methods and humane endpoints, with IACUC approval ensuring compliance and secondary verification of cardiopulmonary arrest.[101]Regulatory and Legal Framework
National and International Laws
The European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS No. 123), adopted by the Council of Europe in 1986, serves as a foundational international framework, requiring signatory states to ensure that animal procedures causing pain, suffering, distress, or lasting harm are only performed if no alternatives exist and if they align with humane endpoints.[102] This convention, ratified by over 30 countries including non-EU members like the UK and Turkey, mandates the application of the 3Rs principles—replacement, reduction, and refinement—implicitly through requirements for minimizing animal numbers, alleviating suffering, and preferring non-animal methods where feasible, though it lacks universal enforcement mechanisms.[103] No binding global treaty governs animal experimentation comprehensively, leaving gaps in harmonization, particularly in developing regions where standards vary widely.[104] In the European Union, Directive 2010/63/EU, enacted on September 22, 2010, harmonizes protections across member states by requiring project authorizations, ethical evaluations incorporating the 3Rs, and stringent standards for housing, veterinary care, and euthanasia to prevent unnecessary suffering.[105] The directive prohibits great apes in research except for exceptional cases like conservation or basic behavioral studies, mandates retrospective assessments of procedures, and sets severity classifications (non-recovery, mild, moderate, severe) to limit high-pain experiments, with member states required to transpose it into national law by 2013.[106] It builds on the 1986 convention but expands enforcement through national competent authorities and EU reporting, aiming for eventual replacement of animal use where scientifically possible, though full replacement remains aspirational as of 2025.[107] In the United States, the Animal Welfare Act of 1966, originally the Laboratory Animal Welfare Act and amended significantly in 1970, 1985, and 2008, regulates the care and use of warm-blooded animals in research, exhibition, and transport, excluding purpose-bred rats, mice, birds, and fish, which comprise the majority of research subjects.[43] Enforced by the USDA's Animal and Plant Health Inspection Service, it requires institutional animal care and use committees (IACUCs) to review protocols for humane treatment, including pain relief unless scientifically contraindicated, and minimum standards for facilities and veterinary oversight, with penalties for non-compliance up to $10,000 per violation.[108] Complementary guidelines, such as the Public Health Service Policy on Humane Care and Use of Laboratory Animals (last revised 2015), apply to federally funded research involving vertebrates and emphasize the 3Rs, though the AWA does not legally mandate them.[109] Other major nations exhibit diverse approaches: Canada's 1968 Animals for Scientific Purposes Regulations under the Criminal Code, updated via the Canadian Council on Animal Care guidelines, require institutional ethics reviews and 3Rs adherence for federally funded work.[110] The United Kingdom's Animals (Scientific Procedures) Act 1986, amended in 2012 to align with EU Directive 2010/63/EU and retained post-Brexit, licenses all regulated procedures on protected animals (excluding cold-blooded species below certain thresholds) and enforces cost-benefit analyses weighing animal suffering against scientific benefits.[106] In contrast, countries like China and India lack comprehensive federal laws equivalent to Western standards, relying on voluntary guidelines or sector-specific rules, leading to criticisms of lax oversight despite growing research volumes.[110] Globally, while cosmetics testing bans exist in the EU (full since 2013), Israel (2013), and India (2014), these do not extend to biomedical research, where animal use remains legally permitted under welfare constraints.[111]Oversight Mechanisms and Compliance
In the United States, Institutional Animal Care and Use Committees (IACUCs) serve as the primary institutional oversight body for animal research, mandated by the Animal Welfare Act (AWA) and Public Health Service (PHS) Policy. IACUCs review and approve research protocols, monitor animal care programs, inspect facilities semi-annually, and investigate concerns to ensure compliance with welfare standards, minimizing pain and distress while verifying scientific validity.[112][113] Federal enforcement falls under the U.S. Department of Agriculture's Animal and Plant Health Inspection Service (APHIS), which conducts unannounced inspections of registered facilities—totaling 10,595 site visits in fiscal year 2022, including 1,248 at research facilities—and issues citations for AWA violations such as inadequate veterinary care or housing. In fiscal year 2024, APHIS initiated 209 enforcement cases for alleged AWA breaches, including fines, stipulations for corrective actions, and license suspensions, though a 2025 study attributed reduced fine issuance to a Supreme Court ruling limiting public disclosure of inspection reports, potentially weakening deterrence.[114][115][116] Voluntary accreditation by AAALAC International supplements mandatory oversight, evaluating programs against the Guide for the Care and Use of Laboratory Animals and international standards; over 1,000 institutions participate, with accreditation requiring ongoing compliance demonstrations and often correlating with fewer citations. The Office of Laboratory Animal Welfare (OLAW) provides additional scrutiny for PHS-funded research, resolving non-compliance through assurance reviews and funding restrictions.[117][118] Internationally, oversight varies: the European Union enforces Directive 2010/63/EU through national competent authorities conducting inspections and project authorizations emphasizing the 3Rs (replacement, reduction, refinement), with non-compliance penalties including project suspensions. In the United Kingdom, the Animals in Science Regulation Unit (ASRU) reported 154,094 animals involved in 2023 non-compliance cases, a tenfold increase from prior years, prompting enhanced reporting mandates. Emerging frameworks, such as AAALAC's international principles, guide countries without robust laws, though enforcement gaps persist in regions with limited resources.[119][120][121]Recent Policy Evolutions (2020s)
In the United States, the FDA Modernization Act 2.0, enacted on December 29, 2022, amended the Federal Food, Drug, and Cosmetic Act by removing the requirement for animal testing in preclinical drug development, permitting alternatives such as organ chips, computer modeling, and real-world data to demonstrate safety and efficacy.[122][123] This shift aimed to accelerate innovation while maintaining rigorous standards, though the FDA emphasized that animal studies remain an option when non-animal methods are insufficient.[124] Building on this, in April 2025, the FDA outlined a roadmap to phase out mandatory animal testing for monoclonal antibodies and certain other biologics, prioritizing human-relevant approaches like advanced in vitro assays, with implementation guided by case-by-case validation of alternatives' predictive accuracy.[124][125] Concurrently, the National Institutes of Health (NIH) announced in July 2025 an initiative to prioritize human-based research methods, such as organoids and computational simulations, over traditional animal models where feasible, while clarifying that animal studies would continue to be funded if scientifically justified and ethically overseen.[126][127] The policy ended solicitations for animal-only research proposals but allowed integration of animals in hybrid human-animal studies, reflecting empirical evidence that non-animal tools often better predict human outcomes in areas like pharmacokinetics.[128] Effective October 1, 2025, NIH also expanded allowable grant costs to include rehoming or retirement of research animals, incentivizing welfare improvements post-study.[129] The Environmental Protection Agency (EPA) similarly advanced a strategic plan in the early 2020s to minimize vertebrate animal use in chemical toxicity testing via New Approach Methodologies (NAMs), including high-throughput screening and in silico predictions validated against historical data.[130] In the European Union, a 2021 European Parliament resolution endorsed phasing out animal use in research, testing, and education by 2025 where scientifically viable, prompting the Commission to integrate this into revisions of Directive 2010/63/EU on animal welfare standards, with evaluations ongoing since 2020 to modernize housing, reporting, and alternative validations.[131] The Commission's July 2024 plan targeted elimination of animal testing in chemical safety assessments under REACH regulations, favoring integrated approaches like read-across and in vitro assays, though implementation hinges on demonstrating equivalency to animal data in risk prediction.[132] EU statistics for 2022 showed a stabilization in animal numbers after a prior decline, underscoring challenges in scaling alternatives amid regulatory demands for causal evidence of toxicity.[133] Globally, cosmetics-specific policies advanced with Australia enacting a nationwide ban on animal testing for cosmetics and their importation in 2020, enforced through ingredient-level prohibitions unless mandated elsewhere.[111] Brazil followed in August 2025 by prohibiting sales of animal-tested cosmetics, joining over 40 jurisdictions including the pre-existing EU ban, though enforcement often permits testing abroad if not domestically conducted, reflecting pragmatic trade-offs over absolute phase-outs.[134] These evolutions prioritize validated non-animal methods but preserve animal testing for endpoints like chronic toxicity where alternatives lack sufficient empirical correlation to human outcomes, as determined by regulatory bodies.[135]Applications and Methodologies
Basic and Translational Research
Animal models facilitate basic research by allowing controlled examination of fundamental biological mechanisms that are ethically or practically infeasible in humans, such as genetic manipulations and longitudinal disease studies. Rodents, particularly mice and rats, are widely used due to their genetic similarity to humans, short generation times, and ease of handling, enabling insights into cellular processes, organ function, and disease etiology. For instance, knockout mouse models have elucidated gene functions in pathways like apoptosis and immune response, foundational to understanding cancer and autoimmunity.[136][54] Invertebrates like Drosophila melanogaster have contributed to discoveries in developmental biology, identifying conserved genes such as those in the hedgehog signaling pathway, which inform human congenital disorders.[37] Translational research employs animal models to bridge basic findings to potential clinical applications, testing hypotheses on efficacy, dosing, and safety before human trials. Xenograft models, where human tumor cells are implanted into immunocompromised mice, assess antitumor drug responses, predicting clinical outcomes with moderate success rates; for example, approximately 40% of oncology drugs advancing from such models show efficacy in phase I human trials.[137] Zebrafish (Danio rerio) models accelerate translational studies in cardiovascular and neurodevelopmental disorders due to their transparency for real-time imaging and high-throughput screening capabilities.[138] Non-human primates provide closer physiological analogs for complex neurological conditions, as seen in Parkinson's disease models using MPTP-induced toxicity in marmosets, which validated dopamine replacement therapies prior to human application.[49] Empirical evidence underscores the necessity of these models despite alternatives; for example, basic research in dogs led to the isolation of insulin in 1921 by Banting and Best, directly translating to diabetes treatment, while ongoing studies in genetically modified pigs advance xenotransplantation for organ shortages. Limitations exist, such as species-specific differences causing translational failures in about 90% of neuroscience candidates, yet refinements like humanized mice improve predictivity.[2][139] Overall, these approaches have driven causal understandings of disease, from viral pathogenesis in ferrets for influenza to metabolic pathways in worms (C. elegans) for aging, yielding verifiable advancements in human health.[140]Drug Development and Toxicology Testing
Animal testing constitutes a core component of preclinical drug development, where candidate compounds undergo evaluation for absorption, distribution, metabolism, excretion, toxicity, and preliminary efficacy in vivo. These studies, mandated under Good Laboratory Practice (GLP) standards by regulatory bodies such as the U.S. Food and Drug Administration (FDA), typically progress from rodents like mice and rats to non-rodent species such as dogs or non-human primates to assess cross-species consistency before advancing to human trials.[141] The process aims to identify potential adverse effects, including organ toxicity and carcinogenicity, through protocols like repeated-dose studies over durations mirroring intended human exposure.[142] In toxicology testing, animals are exposed to escalating doses to determine no-observed-adverse-effect levels (NOAELs) and margins of safety for humans, often involving endpoints such as histopathology, clinical chemistry, and hematology. Historically, the median lethal dose (LD50) test, introduced by J. W. Trevan in 1927, quantified the dose killing 50% of a test population, primarily rodents, to benchmark acute toxicity; however, due to ethical concerns over animal suffering and variable predictivity, its routine use has declined since the 1980s, with many jurisdictions favoring fixed-dose or up-and-down procedures that minimize animal numbers.[143] [144] Despite these refinements, species-specific metabolism—evident in cases like penicillin's lethality in guinea pigs versus safety in humans—limits direct extrapolation, as metabolic pathways diverge significantly across taxa.[142] Empirical data underscore the imperfect predictive value of animal models for human outcomes: over 90% of drugs demonstrating safety and efficacy in preclinical animal studies fail to gain FDA approval, predominantly due to unanticipated human toxicity or inefficacy during clinical phases.[123] A 2024 analysis of preclinical-to-clinical translation found only 5% of therapies advancing from animal studies achieve regulatory approval, with 50% progressing to human studies and 40% to randomized controlled trials, highlighting high attrition attributable to physiological discrepancies rather than mere statistical failure.[145] Notable failures include TGN1412, a monoclonal antibody safe in cynomolgus monkeys but inducing severe cytokine release syndrome in human volunteers in 2006, and fialuridine, which caused fatal hepatotoxicity in humans despite tolerability in mice, rats, dogs, and monkeys.[146] [142] Regulatory evolution reflects these limitations; in April 2025, the FDA outlined a roadmap to reduce, refine, or replace animal testing requirements for certain drugs, including monoclonal antibodies, leveraging human-relevant methods like organ-on-chip and computational modeling to enhance predictivity while maintaining safety thresholds.[124] Nonetheless, animal data remain integral for establishing initial safety profiles, as evidenced by their role in filtering compounds with overt toxicity, though critics argue the approach's causal fidelity to human biology is undermined by interspecies genomic and proteomic variances, with mouse-human response correlations often below 50% in toxicity assays.[147] [142] This tension drives ongoing refinement, balancing empirical risk mitigation against the inefficiencies of a system where animal successes rarely presage human ones.Specialized Uses (e.g., Cosmetics, Defense, Education)
Animal testing for cosmetics primarily involves assessing skin irritation, eye damage, and sensitization using methods such as the Draize test on rabbits' eyes or ears and guinea pig skin tests, though these are not mandated by the U.S. Food and Drug Administration (FDA) and have declined due to alternatives like in vitro models and computational toxicology.[148][18] Globally, the European Union prohibited animal testing for cosmetic ingredients since 2013 and sales of newly animal-tested cosmetics since 2013, influencing a shift toward non-animal methods.[111] By 2025, 12 U.S. states, including California, Hawaii, and Washington (effective January 1, 2025), have enacted bans on the sale of cosmetics developed using animal tests conducted after specific dates, with exemptions for legally required testing in other jurisdictions like China until policy relaxations.[149][150] The Humane Cosmetics Act, reintroduced in March 2025, seeks a federal U.S. ban on animal-tested cosmetics sales, permitting exceptions only where animal testing remains legally compelled abroad.[151] Despite reductions, residual use persists for products like sunscreens and anti-dandruff shampoos where human safety data gaps remain unaddressed by alternatives.[18] In defense and military applications, animals are employed to evaluate weapon effects, chemical and biological agent countermeasures, and trauma response training, with historical examples including exposure to AK-47 rifles, nuclear blasts, and extreme weather simulations to study physiological resilience.[152] The U.S. Department of Defense (DOD) utilizes live animals, such as goats and pigs, in combat trauma training to simulate battlefield injuries from gunshots, explosives, and burns, aiming to prepare medics for real-world hemorrhage control and wound management, though DOD guidance prioritizes alternatives when feasible.[153][154] Recent efforts include the Pentagon's 2023 funding of ferret studies to replicate directed-energy effects linked to Havana syndrome incidents among personnel.[155] Reforms have curtailed certain practices: the U.S. military banned dogs, cats, and primates from wound experiments following advocacy, and in June 2025, the U.S. Navy ceased all research involving cats and dogs.[156][157] In the UK, animal models are reserved for scenarios requiring whole-body systemic responses, with emerging non-animal technologies under evaluation.[158] These uses persist due to the irreplaceable value of live models in predicting kinetic and toxicological outcomes under combat conditions, despite ethical scrutiny and partial phase-outs.[153] Educational applications of animal testing center on dissections and live observations to teach anatomy, physiology, and surgical skills, predominantly using preserved specimens like fetal pigs, frogs, and rats in pre-college and veterinary curricula.[159] Approximately 85% of U.S. pre-college biology educators incorporate animal dissections, with millions of vertebrates euthanized annually for this purpose, though exact figures vary by jurisdiction due to decentralized reporting.[159][160] Student choice laws in states like California and Florida permit opt-outs with alternative assignments such as virtual dissections or models, reflecting a trend toward humane alternatives amid Next Generation Science Standards that do not mandate animal use.[161][162] Regulations prohibit live dissections in many U.S. elementary and middle schools, limiting them to supervised use of non-living mammals or birds from approved sources, while veterinary education emphasizes minimal live animal involvement beyond clinical cases.[163][164] Proponents argue dissections provide tactile, three-dimensional understanding superior to simulations for skill-building, yet critics highlight viable digital and plastinated alternatives that reduce animal sourcing without compromising learning outcomes, as evidenced by equivalent student performance in comparative studies.[165]Proven Contributions to Medicine and Science
Major Breakthroughs Enabled by Animal Models
The discovery of insulin in 1921 by Frederick Banting and Charles Best relied on experiments with depancreatized dogs, where pancreatic extracts from healthy dogs were injected to normalize blood glucose levels, paving the way for the first human treatments in 1922 that reversed fatal diabetic ketoacidosis.[166][167] In these studies, dogs served as models for type 1 diabetes after surgical removal of the pancreas induced hyperglycemia, allowing demonstration of insulin's efficacy before clinical translation, which has since saved millions of lives annually.[168] Howard Florey and Ernst Chain's 1940 experiments with mice demonstrated penicillin's ability to protect against lethal streptococcal infections; of eight mice injected with bacteria, the four treated with penicillin survived, while the untreated died, confirming the compound's antibacterial potential and enabling its rapid wartime scaling for human use by 1941.[169][170] This mouse model provided causal evidence of penicillin's therapeutic index, essential for purifying and producing the antibiotic that reduced mortality from bacterial infections like pneumonia and sepsis.[171] Development of the polio vaccine by Jonas Salk in the 1950s involved growing poliovirus in rhesus monkey kidney cells and testing inactivated vaccines in monkeys to confirm immunogenicity and safety, building on decades of primate, rat, and mouse research that identified viral strains and pathogenesis.[172][173] Similarly, Albert Sabin's oral vaccine used monkeys, rabbits, and rodents to refine attenuation, contributing to polio's near-eradication by enabling mass immunization that prevented paralysis in over 2.5 billion children worldwide.[174] Christiaan Barnard's 1967 human heart transplant followed extensive dog models for orthotopic transplantation techniques, including vascular anastomosis and immunosuppression, which established procedural feasibility and reduced operative risks after nearly 50 animal procedures.[175][176] These canine experiments provided empirical data on graft rejection timelines and hemodynamic stability, directly informing the first successful human orthotopic heart transplant and subsequent advances in solid organ transplantation, now routine with over 100,000 procedures annually.[177] More recently, animal models facilitated COVID-19 vaccine development; mRNA and viral vector platforms were validated in rodents and non-human primates for immunogenicity, efficacy against viral challenge, and safety profiles, accelerating regulatory approval and deployment that averted millions of deaths.[139] Mouse and ferret models, in particular, demonstrated protection via antibody neutralization and reduced lung pathology, underscoring the predictive value of cross-species testing for pandemic response.[6]Empirical Evidence of Predictive Value
A comprehensive analysis of 150 pharmaceutical compounds by Olson et al. in 2000 revealed that animal models predicted human toxicities with 71% concordance when results from both rodents and non-rodents (e.g., dogs, monkeys) were combined, demonstrating the added value of multi-species testing.[178] Rodent studies alone achieved 43% predictivity, while non-rodent models reached 63%, indicating species-specific differences influence reliability but overall support a non-negligible filtering role for preclinical safety assessment.[178] A 2019 systematic scoping review of reported concordances across 83 studies corroborated these findings, estimating 71% overall concordance for toxicity between animal and human data when all species were included, though efficacy translation remained lower at around 50-60% in aggregated cases.[179]| Study | Year | Concordance Rate for Toxicity | Key Details |
|---|---|---|---|
| Olson et al. | 2000 | 71% (multiple species) | Analyzed 150 compounds; rodents alone 43%, non-rodents 63%[178] |
| Scoping review (van der Worp et al. framework) | 2019 | 71% (all species) | 83 studies; highlights variability by endpoint and species[179] |
| Chandrasekera et al. (oncology focus) | 2020 | PPV 65%, NPV 50% | 165 drugs; moderate positive prediction but misses some human-specific risks[180] |
Broader Impacts on Human and Animal Health
Animal testing has significantly advanced human health by enabling the development of interventions that have eradicated or controlled major diseases. For instance, research on cows contributed to the first vaccine against smallpox, leading to its global eradication in 1980, while studies involving monkeys, dogs, and mice facilitated the polio vaccine, which has prevented an estimated 20 million cases of paralysis since 1988.[19] Similarly, animal models were instrumental in creating vaccines for meningitis and antibiotics, as well as therapies for diabetes, heart disease, and cancer, collectively reducing mortality rates from these conditions over decades.[139] Beyond direct medical breakthroughs, animal testing supports public health through insights into zoonotic diseases and pandemic preparedness. Experiments with animal models have elucidated transmission mechanisms for diseases like COVID-19, informing vaccine development that averted millions of deaths globally by 2023, with models replicating human immune responses to accelerate therapeutic testing.[184] This predictive capacity has also enhanced surveillance and control of emerging pathogens, reducing spillover risks from animal reservoirs to human populations.[185] Animal testing extends benefits to animal health via veterinary medicine, yielding treatments that improve welfare in companion animals, livestock, and wildlife. Techniques such as embryo transfer, parasitism elimination, and advanced anesthetics—developed through animal studies—have boosted survival rates and breeding success across species, including vaccines for distemper in dogs and feline leukemia.[186][53] Research on mammalian physiology has directly translated to therapies for conditions like heartworm in pets and respiratory diseases in livestock, enhancing overall animal populations' health and productivity.[187][188] While animal models exhibit limitations in fully predicting human outcomes—evidenced by approximately 94% of drugs succeeding in animals but failing in human trials—the empirical record of disease eradication and life-saving interventions demonstrates a net positive impact on both human and animal health, outweighing isolated predictive shortfalls that occasionally delay approvals or necessitate refinements.[189][9] These broader effects underscore animal testing's role in causal chains linking basic biological discovery to scalable health improvements, despite ongoing efforts to integrate non-animal methods where feasible.[15]Ethical and Philosophical Debates
Pro-Testing Arguments from Utilitarianism and Pragmatism
Utilitarian defenses of animal testing emphasize that the moral value of actions derives from their consequences in maximizing overall well-being, where the aggregate benefits to human health—such as the eradication of diseases and extension of lifespans—outweigh the harms inflicted on test subjects.[190][191] Proponents like Carl Cohen argue that the "incalculably great" advancements in medicine, including vaccines and therapies that have prevented widespread human suffering, justify the controlled use of animals, as abstaining would forfeit these gains without viable substitutes.[191] This calculus holds that the suffering of relatively few animals, often minimized through anesthetics and ethical protocols, pales against the prevention of pain, disability, and death for billions of humans; for instance, animal models contributed to insulin's development in 1921, enabling diabetes management and averting countless premature deaths.[191] Even utilitarian philosophers like Peter Singer, who advocate weighing animal sentience equally per capacity to suffer, concede that testing is defensible when human benefits demonstrably exceed animal costs, such as in research yielding treatments for cancer or heart disease that enhance long-term happiness for sentient beings across species.[190] This perspective frames animal testing not merely as permissible but potentially obligatory, as forgoing it could shift greater misery onto future human generations through untested interventions or stalled scientific progress.[190] Pragmatic arguments underscore animal testing's indispensable role in biomedical advancement due to animals' physiological parallels with humans, enabling reliable predictions of drug safety and efficacy that in vitro or computational methods cannot fully replicate.[4] Mice, sharing over 98% of their DNA with humans and exhibiting analogous diseases like cancer and diabetes, allow researchers to observe full disease progression, generational effects, and treatment responses in controlled settings infeasible with humans.[4] U.S. federal regulations, including FDA requirements for investigational new drugs, mandate such preclinical testing to ensure safety before human trials, reflecting pragmatic acknowledgment that ethical prohibitions on direct human experimentation necessitate animal proxies.[51] In practice, animal models facilitate breakthroughs unattainable otherwise, such as gene-editing studies in rodents that informed CRISPR applications, while their shorter lifespans accelerate data collection on long-term outcomes like toxicity or fertility impacts.[4] Critics of alternatives highlight their limitations in capturing whole-organism dynamics, such as immune responses or behavioral changes, affirming animal testing's empirical track record—evidenced by its role in over 90% of Nobel Prizes in Physiology or Medicine since 1901—as a cornerstone of evidence-based science rather than ideological preference.[51] This approach prioritizes tangible results, like veterinary treatments derived from human-focused research, yielding mutual health gains without compromising methodological rigor.[4]Anti-Testing Claims and Animal Rights Ideologies
Animal rights ideologies fundamentally oppose animal testing by positing that non-human animals possess moral status that precludes their exploitation in scientific experiments, often framing such practices as violations of intrinsic rights or unjustifiable suffering under utilitarian calculus. Proponents argue that vivisection—defined as surgical procedures on living animals without anesthesia—inflicts gratuitous pain, equating it to torture, and stems from "speciesism," a prejudice favoring human interests akin to racism or sexism.[192] These views gained philosophical articulation in the late 20th century, influencing activism that demands total abolition rather than reform.[193] Peter Singer, a utilitarian philosopher, advanced a preference-based framework in his 1975 book Animal Liberation, contending that moral consideration should extend to any being capable of suffering, rejecting species membership as a criterion for ethical priority. He maintains that animal experiments rarely yield benefits proportional to the harm inflicted, as most procedures produce negligible new knowledge while causing significant distress, and advocates weighing animal interests equally with human ones unless clear utilitarian gains justify otherwise.[194] Singer's position implies that speciesist biases in research ethics undervalue animal sentience, evidenced by physiological similarities in pain responses across mammals, though he concedes limited exceptions for high-stakes medical advances where no alternatives exist.[46] In contrast, deontological animal rights theorists like Tom Regan reject utilitarian trade-offs, asserting in The Case for Animal Rights (1983) that mammals are "subjects-of-a-life" with inherent value, entitling them to rights against being treated as means to human ends, including experimentation. Regan's ideology demands absolute prohibition of animal use in research, viewing it as inherently wrong irrespective of outcomes, such as potential cures, because it denies animals' status as ends-in-themselves with autonomy and welfare interests.[195] This rights-based absolutism, echoed by groups like the American Anti-Vivisection Society, holds that no regulatory framework can legitimize exploitation, prioritizing animal dignity over consequentialist benefits.[196] Historical anti-vivisection campaigns, originating in the 19th century, reinforced these ideologies by decrying experiments as cruel and pseudoscientific, with figures like Frances Power Cobbe arguing in 1863 that such practices degrade human morality and yield unreliable data due to interspecies physiological differences. Modern iterations, including claims by organizations like PETA, extend this to assert that testing perpetuates unnecessary suffering amid viable alternatives, though these assertions often overlook validation challenges for non-animal methods. Animal rights advocates thus frame opposition not merely as welfare reform but as a paradigm shift away from anthropocentrism, influencing policies like cosmetic testing bans in regions such as the European Union since 2013.[197][198]Scientific Critiques and Reliability Concerns
Scientific critiques of animal testing highlight its limited reliability in predicting human outcomes, primarily due to interspecies physiological, genetic, and metabolic differences that undermine translational validity. For instance, rodents, the most common models, exhibit divergent drug metabolism pathways, immune responses, and disease susceptibilities compared to humans, leading to discrepancies in efficacy and toxicity predictions.[199] A 2023 review in Alternatives to Laboratory Animals documented that the failure rate for translating drugs from animal testing to human treatments persists at over 92%, unchanged for decades, with particular shortcomings in oncology, Alzheimer's, and Parkinson's models where animal data poorly forecast human responses.[200] Empirical evidence underscores these limitations through high attrition in drug development pipelines. Approximately 92% of experimental drugs that succeed in animal safety and efficacy tests fail in human clinical trials, often on grounds of unexpected toxicity or lack of therapeutic benefit not anticipated from preclinical data.[201] This discordance is exemplified by the 2006 TGN1412 monoclonal antibody trial, where the drug, deemed safe after testing in rodents and cynomolgus macaques at doses up to 500 times the human equivalent, induced a life-threatening cytokine storm in all six human volunteers, resulting in multiorgan failure requiring intensive care.[202] Such cases illustrate how animal models can mask human-specific risks, as cytokine release profiles differ markedly between primates and humans despite genetic similarities.[203] Reproducibility challenges further erode confidence in animal model data. Preclinical studies suffer from low replication rates, with factors including inconsistent husbandry, genetic drift in inbred strains, small sample sizes, and publication bias favoring positive results contributing to variability.[204] A 2022 analysis in Frontiers in Behavioral Neuroscience identified methodological flaws, such as inadequate randomization and blinding, as systemic issues amplifying noise and hindering translation, with behavioral neuroscience models showing over 90% failure to replicate in humans.[205] These concerns are compounded by overreliance on simplified models that fail to capture human disease complexity, prompting calls for rigorous validation and integration with non-animal methods to mitigate false positives.[206]Activism, Controversies, and Societal Responses
Animal Rights Campaigns and Tactics
Animal rights campaigns against testing have employed a range of tactics, from public protests and media advocacy to direct actions including property damage and animal "liberations." Organizations such as People for the Ethical Treatment of Animals (PETA), founded in 1980, have conducted high-profile media campaigns, such as enlisting celebrities to oppose animal experiments and successfully lobbying the U.S. Department of Defense to close a wound ballistics laboratory in 1983 that involved shooting dogs and other animals.[207] PETA has also funded activist groups, including providing $42,000 to individuals convicted of animal rights-related offenses, as documented in reports on their financial ties to extremist actions.[208] The Stop Huntingdon Animal Cruelty (SHAC) campaign, launched in 1999 targeting Huntingdon Life Sciences (HLS), Europe's largest contract animal-testing firm, exemplified coordinated international efforts using demonstrations, home protests against employees, and economic pressure through boycotts and shareholder campaigns.[209] SHAC's tactics, which included harassment and intimidation, led to significant disruptions for HLS, including customer losses and relocations, though the company persisted despite convictions of over 30 activists under the U.S. Animal Enterprise Terrorism Act by 2006.[210] The Animal Liberation Front (ALF), emerging in Britain in the 1970s as a decentralized network, has focused on direct action tactics such as laboratory break-ins, equipment sabotage, and releasing animals destined for research. Notable actions include the 1981 Silver Spring raid, where activist Alex Pacheco documented conditions in a primate research facility, resulting in the seizure of 17 abused monkeys and the conviction of researcher Edward Taub on animal cruelty charges—the first such case against a scientist.[211] ALF claimed responsibility for over 2,000 actions globally by the 2000s, including a 2008 raid freeing 129 rabbits from a UK farm supplying labs, often justified by activists as non-violent property-focused interventions despite their classification as domestic terrorism by authorities.[210] Undercover investigations, another common tactic, involve activists infiltrating facilities to expose alleged abuses via hidden footage, as PETA did in campaigns against universities and pharmaceutical firms, generating media coverage but frequently contested for selective editing and legal violations like trespass.[212] While these efforts have influenced public opinion and contributed to bans on specific testing like cosmetics in some regions, empirical analyses indicate limited direct impact on reducing overall animal use in biomedical research, with protests showing minimal correlation to shifts in consumer behavior or policy beyond targeted industries.[213]Threats to Researchers and Industry Impacts
Animal rights extremists, including groups affiliated with the Animal Liberation Front (ALF), have employed tactics ranging from harassment and vandalism to arson and bombings against biomedical researchers since the 1970s, resulting in over 1,100 documented criminal acts in the United States alone by groups like ALF and the Earth Liberation Front (ELF), with estimated damages exceeding $110 million.[210] These actions often target researchers' homes and personal lives, including firebombings, flooding, and graffiti, as reported in incidents escalating through the 2000s, which have forced some scientists to relocate or abandon animal-based studies due to safety concerns.[214] For instance, in the 1980s, the Animal Rights Militia mailed bombs to researchers and politicians, marking a shift toward direct violence that the FBI classifies as domestic terrorism aimed at disrupting biomedical progress.[215] Such extremism has created a "chilling effect" in the scientific community, with researchers facing desecrated family graves, stolen ashes, and burned properties, as experienced by the CEO of Novartis in campaigns against pharmaceutical testing.[216][217] Specific attacks underscore the personal toll: in 1987, ALF arson at a University of California-Davis veterinary lab caused $3.5 million in damage, destroying research facilities and delaying studies on animal and human health.[218] By the early 2000s, tactics evolved to include cyber threats and infiltration, with activists endorsing violence explicitly; in 2004, an animal rights leader stated that harm to researchers would inevitably discourage others from pursuing animal-based work.[219][220] This has led to heightened security measures at universities and labs, including armed guards and restricted access, diverting resources from research—costs that peer-reviewed analyses attribute to extremism rather than mainstream advocacy.[221] Researchers in fields like neurology and addiction studies report ongoing intimidation, prompting some to self-censor publications or shift to non-animal models prematurely, potentially stalling empirical validation of findings.[216] On the industry side, these threats have imposed substantial economic burdens, including direct property losses and indirect effects like deterred investment; in 2004, UK pharmaceutical executives warned that activism was damaging the economy by scaring off foreign capital and slowing drug development timelines.[222] Campaigns targeting contract research organizations, such as those against Huntingdon Life Sciences in the 1990s and 2000s, involved boycotts and shareholder pressure that forced relocations and multimillion-dollar legal defenses, disrupting partnerships essential for preclinical testing.[223] Overall, the cumulative impact includes billions in foregone productivity, as violence hampers the infrastructure supporting regulatory-required animal studies, with FBI data linking extremism to sabotage that economically weakens sectors reliant on validated biomedical models.[210] While non-violent advocacy has influenced policy toward alternatives, empirical evidence from security reports indicates that extremist tactics primarily yield legal repercussions for perpetrators without advancing scientific alternatives, instead exacerbating caution in an industry already navigating high failure rates in translation to human therapies.[224]Legal Battles and Public Policy Influences
Public policy on animal testing has evolved through regulatory frameworks aimed at balancing scientific needs with welfare concerns, beginning with the UK's Cruelty to Animals Act of 1876, which first mandated licensing for experiments on vertebrates.[36] In the United States, the Animal Welfare Act of 1966 established federal oversight for laboratory animals, excluding birds, rats, and mice bred for research, with subsequent amendments expanding protections to warm-blooded species used in testing.[225] The European Union implemented phased bans on animal testing for cosmetics, prohibiting tests on finished products in 2004, ingredients in 2009, and marketing of tested products in 2013, though REACH regulations have permitted testing for non-cosmetic purposes like chemical safety, as upheld in court rulings.[226][227] Recent U.S. policy shifts reflect growing acceptance of alternatives, with the FDA Modernization Act 2.0, enacted in 2022, removing mandatory animal testing for new drugs by allowing non-animal methods like organ chips and computer modeling.[122] In April 2025, the FDA announced plans to phase out animal testing requirements for monoclonal antibodies and other biologics, citing advances in human-relevant technologies amid evidence that over 90% of drugs succeeding in animal models fail in human trials.[124][228] Similarly, the NIH in 2025 ended funding for animal-only studies and prioritized human-based research, influenced by empirical data on model limitations and innovation in alternatives.[229][230] Internationally, Brazil enforced a ban on cosmetic animal testing in July 2025, following Canada's 2023 prohibition and China's 2021 relaxation of import requirements, signaling a global trend driven by both ethical advocacy and validation of non-animal methods.[231][111] Legal battles have often stemmed from animal rights challenges to testing practices and regulatory enforcement. The 1981 Silver Spring monkeys case, involving USDA raids on a lab for neglect, resulted in the first U.S. conviction of a researcher for animal cruelty and galvanized organizations like PETA.[232] In 2024, Inotiv (formerly Envigo) pleaded guilty to Animal Welfare Act violations for mistreatment of beagles bred for testing, paying over $35 million in fines and restitution, leading to the rescue of nearly 4,000 dogs.[233] Ongoing litigation includes a 2025 Animal Legal Defense Fund suit against the USDA for a secret policy reducing lab inspections, argued to endanger animal welfare, and a federal appeals court ruling that the University of Wisconsin violated free speech by censoring social media comments on its animal research.[234][235] These cases highlight tensions between enforcement rigor and operational secrecy in labs, with courts occasionally favoring transparency over institutional protections.[236]Alternatives and Emerging Technologies
Existing Non-Animal Methods
Non-animal methods encompass a range of techniques designed to assess chemical safety, drug efficacy, and toxicity without relying on whole-animal models, including in vitro cell-based assays, computational (in silico) models, and in chemico approaches. These methods align with the 3Rs principles of replacement, reduction, and refinement, and several have achieved regulatory validation for specific endpoints such as skin corrosion and genotoxicity.[237][238] For instance, the U.S. Environmental Protection Agency (EPA) and Food and Drug Administration (FDA) incorporate new approach methodologies (NAMs) like in vitro assays using human-derived cells to evaluate endpoints including developmental toxicity and biocompatibility.[239][240] In vitro assays utilize isolated cells, tissues, or reconstructed human models to mimic physiological responses. The reconstructed human epidermis (RHE) test for skin corrosion (OECD Test Guideline 431), validated in 2019, applies test chemicals to multilayered skin equivalents derived from human keratinocytes and measures viability via MTT reduction to classify corrosivity, offering higher human relevance than animal dermal tests.[241] Similarly, OECD TG 439 assesses skin irritation using the same RHE models by evaluating cytotoxicity after short-term exposure.[242] For dermal absorption, OECD TG 428 employs excised human or animal skin mounted in diffusion cells to quantify penetration rates, accepted by the FDA and EU regulators since 2004.[238] These assays have been integrated into regulatory frameworks, reducing animal use for cosmetic and chemical safety testing.[243] Computational models, or in silico tools, predict toxicity through quantitative structure-activity relationship (QSAR) algorithms that correlate molecular structures with biological effects. The FDA employs QSAR for bacterial mutagenicity assessments in pharmaceutical screening, as outlined in its 2020 guidance, to flag potential genotoxins early in development.[244] The OECD QSAR Toolbox, updated as of 2024, supports read-across and profiling for endpoints like skin sensitization and acute toxicity, aiding agencies such as the European Chemicals Agency (ECHA) in grouping chemicals for risk assessment without experimental animals.[245][246] Tools like the FDA's CHemical RISk Calculator (CHRIS), qualified in 2022, evaluate biocompatibility of color additives via machine learning on chemical datasets.[240] Organ-on-a-chip and organoid systems replicate organ-level physiology using microfluidic devices or 3D stem cell-derived structures. Human organ chips, qualified by the FDA for radiation countermeasure evaluation as of 2023, simulate lung or intestine responses to drugs or toxins under flow conditions, providing dynamic data on absorption and inflammation superior to static 2D cultures.[240] Organoids, such as brain or liver models from induced pluripotent stem cells, enable disease modeling and toxicity screening; for example, hepatic organoids predict drug-induced liver injury with accuracy comparable to animal models in select studies.[247] While not yet universally validated for all regulatory endpoints, the FDA's ISTAND program accepted pilot tools in 2022 for off-target binding assessments, paving the way for broader adoption.[240] In chemico methods, such as direct peptide reactivity assays for skin sensitization (OECD TG 442C), measure covalent binding to proteins without cells, supporting tiered testing strategies.[239]| Method Category | Example | Endpoint | Regulatory Status |
|---|---|---|---|
| In Vitro | RHE Skin Corrosion (OECD 431) | Skin corrosivity | Validated 2019; FDA, OECD[241] |
| In Silico | QSAR Mutagenicity Models | Genotoxicity | FDA guidance 2020; OECD Toolbox[244] |
| Organ-on-Chip | Lung Chip for Toxicity | Drug absorption/inflammation | FDA qualified 2023[240] |


